Abstract
Previous research supports the distinction between proactive and reactive control. Although the dorsolateral prefrontal cortex (DLPFC) has been consistently related to these processes, lateralization of proactive and reactive control is still under debate. We manipulated brain activity to investigate the role of the left and right DLPFC in proactive and reactive cognitive control. Using a single-blind, sham-controlled crossover within-subjects design, 25 young healthy females performed the ‘AX’ Continuous Performance Task after receiving sham vs active high-frequency repetitive transcranial magnetic stimulation (HF-rTMS) to increase left and right DLPFC activity. Reaction times (RTs) and pupillometry were used to assess patterns of proactive and reactive cognitive control and task-related resource allocation, respectively. We observed that, compared to sham, HF-rTMS over the left DLPFC increased proactive control. After right DLPFC HF-rTMS, participants showed slower RTs on AX trials, suggesting more reactive control. However, this latter result was not supported by RTs on BX trials (i.e. the trial that specifically assess reactive control). Pupil measures showed a sustained increase in resource allocation after both active left and right HF-rTMS. Our results with RT data provide evidence on the role of the left DLPFC in proactive control and suggest that the right DLPFC is implicated in reactive control.
Keywords: proactive cognitive control, reactive control, HF-rTMS, neurostimulation, AX-CPT
Introduction
Cognitive control can be defined as the collection of mental processes that allow flexible adaptation of information processing and behaviour depending on the individual’s current goals. Within the context of cognitive control, Braver et al. (2007, 2012) have recently proposed the Dual Mechanisms of Control framework. This framework proposes that cognitive control operates via two distinct operating modes, namely proactive control and reactive control. Proactive control occurs before the onset of a stimulus, and it comprises anticipatory and sustained maintenance of task-relevant information that enhances coping with conflict when it is presented. Reactive control, instead, is a corrective mechanism that involves recruiting processing resources to resolve conflict when it occurs. Affective neuroscience research has demonstrated that cognitive control plays a critical role in emotion regulation processes (Ochsner and Gross, 2005) and that it is closely associated with affective disorders (e.g. Austin et al., 2001; Vanderhasselt and De Raedt, 2009). Moreover, it has recently been proposed that proactive and reactive cognitive controls are two critical mechanisms in the process of stress regulation and the development of depression and other stress-related disorders (De Raedt and Hooley, 2016). Importantly, the dorsolateral prefrontal cortex (DLPFC) is considered to play a critical role in proactive and reactive control (Braver et al., 2009; Vanderhasselt et al., 2009). However, the exact role of this region and the lateralization of the process remain under debate. In this context, understanding the role of the right and left DLPFC in proactive and reactive control is crucial to further comprehend their implications in emotion and stress regulation and the development of stress-related disorders.
Most of the empirical evidence supporting the implication of the DLPFC in proactive and reactive cognitive control comes from studies using the ‘AX’ Continuous Performance Task (AX-CPT; Rosvold et al., 1956; MacDonald, 2008). The AX-CPT comprises a cue-probe-response task in which subjects have to maintain cue (context) information actively and flexibly respond to probe targets. Importantly, in comparison to other cognitive control tasks, the AX-CPT allows for a direct contrast between reactive and proactive processes in the same experimental paradigm (Ryman et al., 2019). Studies in healthy participants have consistently shown a sustained activation of the DLPFC during the cue phase of the AX-CPT, reflecting recruitment of proactive control (e.g. Barch et al., 1997, 2001; MacDonald and Carter, 2003; Holmes et al., 2005; Paxton et al., 2008; Lopez-García et al., 2016). Results from Braver et al. (2009) suggest that increased anticipatory/sustained activity of the left DLPFC and the right inferior frontal junction are observed when proactive control is recruited, whereas a transient activation of the same areas is observed during reactive control. However, proactive and reactive control have been associated with left DLPFC in some studies (e.g. MacDonald and Carter, 2003), but with right DLPFC in others (e.g. Holmes et al., 2005; Paxton et al., 2008). Therefore, although the current evidence supports the idea that the DLPFC is implicated in proactive and reactive control, the results regarding lateralization are far from conclusive.
Neurostimulation techniques to transiently modulate brain activity may offer critical information to establish causal links between the left and right DLPFC and proactive and reactive control. Within this context, Vanderhasselt et al. (2009) carried out an extensive literature review of the effects on different versions of the Stroop task of high-frequency repetitive transcranial magnetic stimulation (HF-rTMS), a stimulation protocol that increases cortical excitability (Hoogendam et al., 2010). Based on this review, the authors proposed that the left DLPFC is active when there is foreknowledge of upcoming conflict, leading to attentional preparation. In contrast, the right DLPFC is proposed to participate in top-down attentional control when conflict is occurring at stimulus level (Vanderhasselt et al., 2009). Following this idea, we could expect that HF-rTMS (to increase cortical excitability) over the left DLPFC would improve proactive control, whereas HF-rTMS over the right DLPFC would improve reactive cognitive control. So far, no previous studies have systematically investigated the effect of an experimental manipulation of the DLPFC using rTMS over the left and right DLPFC to test its role in proactive and reactive control using the AX-CPT. Two recent studies, using transcranial direct current stimulation (tDCS), examined the effect of online (i.e. while stimulation is delivered) and offline (i.e. immediately after stimulation completion) stimulation on this task, showing mixed results (Goméz-Ariza et al., 2017; Boudewyn et al., 2019). Anodal tDCS is used to increase brain activity, whereas cathodal tDCS would decrease brain activity. In accordance with Vanderhasselt et al. (2009), Boudewyn et al. (2019) observed that offline anodal tDCS over the left DLPFC increases proactive control using the dot-pattern version of the AX-CPT (Jones et al., 2010). However, Goméz-Ariza et al. (2017) observed that tDCS over the left DLPFC did not affect performance on the AX-CPT, whereas offline cathodal tDCS over the right DLPFC and online anodal tDCS over the right inferior frontal junction led participants to adopt a more reactive strategy. It is important to note that in contrast to TMS, tDCS can manipulate the membrane potential of neurons, but does not directly activate the neurons (Paulus, 2011). Therefore, a critical gap in the literature is whether HF-rTMS to experimentally increase brain activity of the left and right DLPFC would have different effects on proactive and reactive cognitive control.
Using a sham-controlled, single-blind, crossover design we investigated whether HF-rTMS over the left or right DLPFC affects the performance on the AX-CPT. Following Vanderhasselt et al. (2009) and Braver et al. (2009), we hypothesized that a sustained increase in left DLPFC activity (i.e. HF-rTMS over the left DLPFC) would lead to more proactive cognitive control. In contrast, participants would show more reactive cognitive control after HF-rTMS over the right DLPFC (Vanderhasselt et al., 2009). Previous studies have demonstrated that changes in pupil size can be used to investigate the allocation of cognitive resources or mental effort during the AX-CPT (Chiew and Braver, 2013, 2014; Mäki-Marttunen et al., 2018). Chiew and Braver (2013) showed that an increase in proactive control provokes a sustained increase in pupil size measured in the period immediately before the cue is presented, reflecting sustained resource allocation during the task. Moreover, an increase in proactive control also provoked a transient increase in pupil size during the proactive phase of each trial (when participants had to maintain cue information actively), but only when the cue gave information about the specific response that had to be provided to the probe (i.e. B-cue trials, see methods for a detailed description of the task) (Chiew and Braver, 2013). Accordingly, we also used pupillometry to assess changes in pupil size during the proactive phase and expected, in line with Chiew and Braver (2013), an increase in pupil size when proactive cognitive control is increased in our participants (i.e. when HF-rTMS is applied over the left DLPFC). If reactive control is increased (i.e. when HF-rTMS is applied over the right DLPFC), accordingly, we do not expect differences in pupil size during the proactive phase between the sham and the active condition.
Methods
Participants
A total of 28 healthy female undergraduates from Ghent University between 18 and 30 years old were recruited for this study. Two participants were excluded from the final study sample due to problems during the stimulation protocol. One participant decided not to continue the experiment after the first session. Therefore, the final sample of this study consisted of 25 participants (Mean age = 21.80, SD = 2.10). Participants were selected according to the following criteria: medication-free with the exception of birth-control medication; no current (or history of) neurological, psychiatric, cardiovascular or endocrine disease; no family history of epilepsy, smoking <10 cigarettes per day, and no eye problems that could not be corrected with glasses or contact lenses. Participants who had a history of severe head injuries, a pacemaker or other electronic implants, inner ear prostheses, metal or magnetic objects in the brain and a skin condition at the level of the head were also excluded from the study.
AX-Continuous Performance Task
The AX-CPT paradigm (Rosvold et al., 1956; MacDonald, 2008) was used to investigate the effect of rTMS on proactive and reactive cognitive control (see Figure 1). The participants were presented with four type of trials: (I) AX trials: a cue ‘A’ (i.e. the letter A) followed by a probe ‘X’ (i.e. the letter X); (II) AY trials: a cue ‘A’ (i.e. the letter A) followed by a probe ‘Y’ (i.e. any letter of the alphabet except A, X or K); (III) BX trials: a cue ‘B’ (i.e. any letter of the alphabet except A, X or K) followed by a probe ‘X’ (i.e. the letter X); and (IV) BY trials: a cue ‘B’ (i.e. any letter of the alphabet except A, X or K) followed by a probe ‘Y’ (i.e. any letter of the alphabet except A, X or K). The reason that the letter K is not presented is to avoid confusions due to its similarity with the letter X. The participants were instructed to give a target response to AX trials and to give a non-target response to any of the other trials (i.e. AY, BX or BY). Critically, in the version of the AX-CPT used in this study (Barch et al., 2003, 2005), the AX pair is presented in 70% of the trials, whereas the other type of trials (BX, AY and BY) occurred with a 10% frequency each. In the AX-CPT, the participants’ performance on AY and BX trials (i.e. conflict trials) is used to assess proactive and reactive control. If participants use a proactive strategy, after a cue ‘A’ they tend to expect a probe ‘X’ due to the high frequency of AX trials (70%) and have to inhibit the prepotent target response. Therefore, the interference in AY trials (i.e. slower response) reflects proactive control (i.e. the slower the response, the higher the proactive control). On the other hand, if the participants use a reactive strategy, they give slower responses to BX trials because participants react to the probe X without being able to use the context given by the cue ‘B’. Therefore, interference in BX trials (i.e. slower response) reflects reactive control (i.e. the slower the response, the higher the reactive control). BY trials are considered a control condition used to determine whether participants understood and followed the instructions. The frequency of the various trial types replicates those used in most previous studies with the AX-CPT paradigm (e.g. Barch et al., 1997, 2003; Cohen et al., 1999; Braver et al., 2005). In this version of the AX-CPT, young adults tend to use a proactive strategy to solve the task (see Cooper et al., 2017), a bias that is due to the fact that a proactive strategy is the most efficient strategy in 90% of the trials (AX, BX and BY trials).
Fig. 1 .
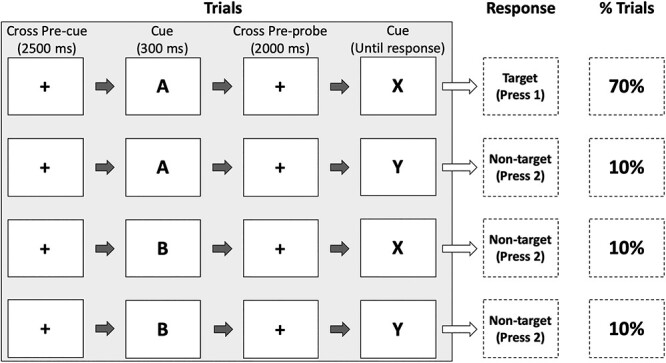
Description of the AX-CPT. Each trial started with a pre-cue fixation cross (2500 ms), followed by the cue (300 ms). After the cue, a pre-probe fixation cross was presented for 2000 ms. Then the probe appeared until a response was given by the participants. Each trial was followed by an inter-trial interval of 400 ms. The AX trials were presented 140 times (70%). The other type of trials (i.e. AY, BX and BY) were presented 20 times each (10% each). In BX, BY and AY trials, ‘B’ and ‘Y’ represent any letter of the alphabet except A, X or K.
In total, 200 trials were randomly presented. Each trial started with a pre-cue fixation cross (2500 ms). Then, the cue was presented for 300 ms, followed by a pre-probe fixation cross of 2000 ms. After the inter-stimulus interval, the probe appeared until a response was given by the participants. Each trial was followed by an inter-trial interval of 400 ms. Participants were instructed to press the target button with the middle finger of their right hand as quickly as possible whenever they observed the probe. The fixation cross, cue and target were all presented in the centre of a 24-inch screen and displayed in black ink and bold on grey background in font size 80 pt. Trials in which reaction times (RTs) were <200 ms or >1500 ms were excluded from the analyses (Reimer et al., 2015). In this study, we focused on RTs because, as observed by Cooper et al. (2017), they show better psychometric properties (higher reliability and less ceiling effect) than accuracy when healthy young participants perform the AX-CPT.
Pupillometry
A Tobii TX300 infrared eye-tracker system with eye-gaze coordinates and pupil size sampling at 300 Hz and a 9-point calibration procedure was used to investigate pupil size during the task. Participants were seated at approximately 60 cm of distance from the eye tracker. The data were pre-processed using published scripts (Kret and Sjak-Shie, 2019). The data were first automatically inspected for common occurring artefacts (e.g. eye blinks) and these were removed using a 4-pass deviation filter. Then, the data of the left and right eye were aggregated, and this signal was upsampled (1000 Hz) to increase the temporal resolution and smoothness. The data were then interpolated to remove missing data gaps. However, interpolation was not performed over gaps of missing data that were >250 ms. The resulting signal was then smoothed using a zero-phase low-pass 4 Hz filter. Following Chiew and Braver (2013), the pupil size of the last 200 ms of the pre-cue fixation cross period immediately before the appearance of the cue was used as a measure of sustained resource allocation during the task1. The change in pupil size during the anticipation of the probe, reflecting transient changes in resource allocation, was computed as the subtractive baseline-corrected (i.e. last 500 ms before the start of the cue) pupil size during the anticipation phase (the period from the start of the cue until the start of the probe in each trial). Segments that contained over 25% of missing data were removed from subsequent analysis (5.24% of the trials were removed).
Transcranial magnetic brain stimulation
The stimulation was performed over left and right DLPFC using a Magstim Rapid2 Plus stimulator (Magstim Company Limited, Whitland, UK) connected to an eight-shaped coil. The coil was located over the left or right DLPFC using the adjusted BeamF3 algorithm (see Mir-Moghtadaei et al., 2015 for a detailed description). Based on the distances between nasion, inion, tragus and vertex as landmarks, this algorithm corrects the coordinates for the approximate F4 (right DLPFC) and F3 (left DLPFC) electrode sites developed by Beam et al. (2009). Previous studies have used this method to place the coil over the left and right DLPFC (e.g. Lan et al., 2016; Trapp et al., 2019a). At the beginning of each stimulation session, we estimated the resting motor threshold (rMT), defined as the intensity that induced visually perceptible movement of the right abductor pollicus brevis 50% of the time. The rMT was estimated by stimulating the cortex at low frequency (1 Hz) and device output (starting at 30%), advancing the power and repositioning the coil to elicit a reliable muscle twitch (cf., Schutter and van Honk, 2006). The high-frequency (20 Hz) stimulation sessions consisted of 40 trains of 2 s duration, separated by an intertrain interval of 12 s (1600 pulses per session, with a duration of 9.33 min) at a stimulation intensity of 110% of the subject’s MT. Similar parameters have been used in previous studies to increase cortical excitability and to investigate the role of the DLPFC in cognitive performance and stress regulation (Herremans et al., 2013; Baeken et al., 2014; Remue et al., 2015). As in previous studies from our lab (Pulopulos et al., 2019; Poppa et al., 2020), for the sham session we used the Magstim 70 mm Double Air Film sham coil, a coil that is identical in all aspects to its active variant, but without stimulation output. The sham coil stimulates the peripheral nerves of the face and scalp, and it looks, sounds and feels like an active coil, but it does not deliver active stimulation of cortical neurons. The participants were randomly allocated to receive sham stimulation over the left or the right DLPFC. During stimulation, participants were blindfolded and wore headphones. The study conforms to current safety guidelines (Rossi et al., 2009).
Procedure
A randomized sham-controlled, single-blind, crossover design was used. To avoid carry-over effects, an interval between sessions of 7−14 days was used (M = 9.40 days, SD = 2.83). The order of the rTMS sessions (active right DLPFC, active left DLPFC and sham) were counterbalanced across participants. The sessions started at 11:30 h or 16:00 h, and the participants started the three sessions always at the same time to control for the circadian regulation of arousal (Aston-Jones et al., 2001). At the beginning of the sessions, participants were asked to sit and relax for 10 min to habituate to the room. After this phase, they were asked to fill out a series of demographic and psychological questionnaires (data not shown here)2. Following these questionnaires, participants received sham or active HF-rTMS over the left or right DLPFC. After the active or sham stimulation, the participants were asked to rest for 5 min. Then, they eye-tracker was calibrated, and the participants received the instruction for the AX-CPT (duration: mean = 4.91 min, SD = 2.21). After the instructions, the participants were asked to perform the AX-CPT while the pupil size was measured. The participants were allowed to rest for a short period after the first 100 trials. E-prime Professional Software was used for stimulus presentation and recording of the pupil size. The task lasted between 18.30 and 20.38 min (Mean = 18.62 min), and there were no differences between sessions in the duration of the task (P = 0.358). It is important to note that previous research using HF-rTMS protocols with similar number of pulses have shown an effect up to 60 min after the end of the stimulation on different measures (e.g. electroencephalography, cognitive tasks; for reviews see Hoogendam et al., 2010; Thut and Pascual-Leone, 2010). Therefore, the task was performed within the influence of the stimulation effects.
To check for a possible effect of rTMS on mood, participants were asked to fill in several visual analogue scales (VAS) (i) immediately before, (ii) after the sham/rTMS stimulation and (iii) at the end of the AX-CPT to assess changes in worry, anxiety, stress, tension, tiredness, happiness, depression and anger.
The study was approved by the medical ethical committee of the Gent University Hospital (UZ Gent), and all the participants provided written informed consent, and received 50 euros for their participation.
Statistical analyses and data management
The data from the right HF-rTMS session from two participants could not be recorded because the participant left the session before the cognitive task due to personal reasons, and the pupil data from the sham session from another participant could not be recorded due to technical issues.
To investigate the effects of rTMS on mood, we performed a repeated measure multivariate analysis of variance (MANOVA) with time (pre-rTMS, post-rTMS and post-AX-CPT) and stimulation (left HF-rTMS, right HF-rTMS and sham) as within-subject factors using SPSS 24.0 (IBM SPSS Statistics 24.0). The eight VAS scales (i.e. worry, anxiety, stress, tension, tiredness, happiness, depression and anger) were used as the multiple dependent variables. Higher scores indicate more negative affect (scores in happiness were reversed). Where necessary, Greenhouse−Geisser correction was applied to ensure the assumption of sphericity. Partial Eta squared (ηp2) was computed as a measure of the effect size of the MANOVA.
Linear Mixed Models were used to investigate the effects of rTMS on the behavioural and pupil size during the AX-CPT task. Stimulation (left HF-rTMS, right HF-rTMS and sham) and trial (AX, AY, BX and BY) were used as fixed effects. Subject and the number of the trial were used as random effects (random intercept). RTs on each trial of the AX-CPT were used as the dependent variable. Linear Mixed Models were used to investigate the effects of rTMS on the sustained and phasic changes in resource allocation indexed via pupil size. For the sustained measure of resource allocation, stimulation (left HF-rTMS, right HF-rTMS and sham) was used as a fixed effect, subject was used as a random effect (random intercept) and the average pupil size during the last 200 ms before cue presentation was used as the dependent variable. For the phasic changes in resource allocation during the anticipation phase, stimulation (left HF-rTMS, right HF-rTMS and sham) and cue (A and B) were used as fixed effects, subject and the number of the trial were used as random effects (random intercept) and the baseline-corrected average change in pupil size from the start of the cue until the start of the probe were used as the dependent variable. Linear Mixed Models were performed in R 3.5.0 (R Core Team, 2013) in conjunction with RStudio 1.1.453 (RStudio, 2012), using linear mixed-effects regression models fitted via the ‘lmerTest’ package (Kuznetsova et al., 2017). ‘lmerTest’ produces P-values for the fixed effects using the Satterthwaite approximations to degrees of freedom. In the results section, only differences between stimulation sessions within each trial are described. Using the MuMIn package (Nakagawa and Schielzeth, 2013; Johnson, 2014; Nakagawa et al., 2017), we derived the conditional r squared (rc2) values, a measure of the proportion of variance explained by both the fixed and random effects. The statistical significance level was set to P < 0.05.
We estimated a sample size of 28 participants for a medium effect size (f = 0.25, alpha = 0.05 and power = 0.80). However, due to exclusions, the final sample included in the analyses was 25. Given that we use Linear Mixed Model with fixed and random effects, a statistical analysis with larger statistical power than the one estimated by G*Power for repeated measured analysis of variance (ANOVA), we did not continue recruiting more participants even though our sample was slightly smaller than estimated.
Results
Mood
The repeated measures MANOVA revealed a significant multivariate effect of time (Pillai’s Trace = 0.64, F(16,80) = 2.37, P = 0.006, ηp2 = 0.33). The factor stimulation and the interaction between stimulation and time were not statistically significant (stimulation: Pillai’s Trace = 0.29, F(16,80) = 0.86, P = 0.614, ηp2 = 0.14; stimulation*time: Pillai’s Trace = 0.40, F(32,352) = 1.23, P = 0.186, ηp2 = 0.10). Follow-up ANOVAs indicated a significant univariate effect of time on the subscales ‘tiredness’ (F(1.58355.377) = 18.75, P < 0.001, ηp2 = 0.48) and ‘worry’ (F(2,1013.56) = 6.40, P = 0.004, ηp2 = 0.21). Overall, participants reported being more tired after the active or sham stimulation and after the AX-CPT than before the active or sham stimulation, and more tired at the end of the AX-CPT than after the active or sham stimulation (all Ps < 0.014). Moreover, they reported being less worried at the end of the AX-CPT than before and after the active or sham stimulation (all Ps < 0.018). None of the other univariate ANOVAs showed a significant effect of time (all Ps > 0.125). See Table 1 for the scores on the VAS.
Table 1.
Mean (SD) for the VAS scales
| Before rTMS | After rTMS | After AX-CPT | ||
|---|---|---|---|---|
| Anger | Left rTMS | 5.04(6.90) | 6.96(13.82) | 5.21(10.08) |
| Right rTMS | 5.42(10.69) | 10.71(23.33) | 9.54(19.57) | |
| Sham | 6.96(9.83) | 3.63(5.72) | 4.00(5.60) | |
| Left rTMS | 7.46(11.86) | 7.00(11.90) | 4.25(6.22) | |
| Anxiety | Right rTMS | 8.21(14.54) | 10.54(21.44) | 5.00(5.78) |
| Sham | 6.92(9.62) | 3.75(6.77) | 4.96(7.20) | |
| Left rTMS | 4.33(6.40) | 6.67(12.40) | 4.67(10.47) | |
| Depression | Right rTMS | 7.13(14.86) | 9.37(21.16) | 6.46(9.72) |
| Sham | 11.29(15.57) | 4.67(10.28) | 4.79(7.34) | |
| Left rTMS | 64.71(19.66) | 61.04(21.86) | 60.29(20.63) | |
| Happiness | Right rTMS | 64.58(22.80) | 64.08(22.70) | 58.92(24.57) |
| Sham | 57.83(25.00) | 66.50(20.18) | 62.46(20.04) | |
| Left rTMS | 16.25(20.52) | 19.88(20.99) | 12.08(13.75) | |
| Stress | Right rTMS | 20.92(21.94) | 18.38(23.10) | 16.17(20.22) |
| Sham | 17.83(18.68) | 14.25(13.81) | 15.25(15.91) | |
| Left rTMS | 12.75(13.22) | 14.29(16.70) | 21.13(21.88) | |
| Tension | Right rTMS | 17.25(17.40) | 23.83(24.75) | 19.71(19.71) |
| Sham | 19.04(15.83) | 14.37(13.88) | 20.92(20.96) | |
| Left rTMS | 27.29(22.87) | 34.79(20.68) | 44.54(25.41) | |
| Tirednessa | Right rTMS | 26.75(22.57) | 34.29(24.69) | 46.13(28.23) |
| Sham | 23.79(17.09) | 33.88(18.81) | 43.04(23.44) | |
| Left rTMS | 18.58(20.30) | 13.50(16.74) | 10.58(13.51) | |
| Worrya | Right rTMS | 18.00(20.32) | 21.87(24.63) | 13.38(16.26) |
| Sham | 23.54(20.43) | 18.42(18.57) | 14.29(15.55) |
The repeated measure MANOVA revealed a significant effect of time only for the subscales ‘tiredness’ and ‘worry’. Overall, participants were more tired after than before the stimulation, and more tired at the end of the AXCPT than after the stimulation (all Ps < 0.014). Also, they reported being less worried at the end of the AX-CPT than before and after the active or sham stimulation (all Ps < 0.018). The factor stimulation and the interaction between stimulation and time were not statistically significant (all Ps > 0.186).
AX-CPT
Reaction times
The results of the Linear Mixed Models with RT as the dependent variable showed a significant effect of trial (F(3,13 988) = 729.91, P < 0.001), and a significant interaction between stimulation and trial (F(6,13 988) = 2.30, P = 0.032). The main effect of stimulation was not statistically significant (F(2,13 960) = 0.10, P = 0.903). The rc2 of this model was 0.33. Overall, the RTs were slower in the AY trials (mean = 491.15, SD = 115.18) than in the AX (mean = 375.08, SD = 117.82), BX (mean = 320.83, SD = 139.79) and BY (mean = 321.52, SD = 132.31) trials (P < 0.001). The RT in the AX trials was slower than in the BX and BY trials (P < 0.001). No differences were observed between the BX and BY trials, (P = 0.762).
Regarding the interaction between stimulation and trial, the results showed that after active HF-rTMS over the left DLPFC the participants were slower on the AY (P = 0.028), and faster on the BX trials (P = 0.049), when compared to the sham condition. After the active HF-rTMS over the right DLPFC, the participants were slower on the AX trials than after the sham (P = 0.012), and left HF-rTMS (P = 0.017). Moreover, a marginally significant effect was observed for the BX trials, being the participants slightly faster after the active HF-rTMS over the right DLPFC than after the sham session (P = 0.065). No other significant differences between stimulation sessions were observed for each trial (P > 0.154). See Table 2 for the RTs on the AX-CPT.
Table 2.
Mean and SD for the RTs and percentage of errors for each trial and stimulation session
| RT | Trial | Left HF-rTMS | Sham | Right HF-rTMS |
|---|---|---|---|---|
| AXa,b | 372.63 (112.42) | 373.03 (113.36) | 379.71 (127.28 | |
| AYc | 499.35 (116.07) | 483.50 (111.12) | 490.23 (117.97) | |
| BX | 315.30 (122.68) | 331.19 (162.92) | 315.87 (129.99) | |
| BY | 321.86 (137.32) | 319.01 (129.78) | 323.67 (129.94) | |
| % Errorsd | ||||
| AX | 1.03 (1.09) | 1.06 (1.19) | 1.16 (1.30) | |
| AY | 13.60 (8.96) | 16.80 (11.26) | 17.92 (11.88) | |
| BX | 1.00 (2.04) | 0.60 (2.20) | 2.29 (4.89) | |
| BY | 0.80 (2.36) | 0.40 (1.38) | 0.00 (0.00) |
aStatistically significant difference between the left HF-rTMS and the right HF-rTMS session.
bStatistically significant difference between the right HF-rTMS and the sham session.
cStatistically significant difference between the left HF-rTMS and the sham session. None of the other comparison within trials was statistically different between stimulation sessions.
dThe main factor stimulation (F(2,262.17) = 1.15, P = 0.317) and the interaction between stimulation and trial (F(6,260.21) = 1.16, P = 0.326) were not statistically significant. The factor trial was significant (F(3,262.17) = 136.14, P < 0.001). Post hoc analyses show that the participants made more mistakes in AY trials in comparison with the other three trials (AX, BX and BY) (all Ps < 0.001). No significant differences between the AX, BX and BY trials were observed (all Ps > 0.330).
For informative purposes, we also report the percentage of errors for each type of trial (AX, AY, BX and BY) and stimulation sessions in Table 2. No effects of stimulation were observed for the percentage of errors.
Pupillometry3
The results of the Linear Mixed Models using as a dependent variable the pupil size during the last 200 ms before cue presentation (reflecting sustained resource allocation during the task) showed a significant main effect of stimulation (F(2,13 162) = 381.76, P < 0.001). The rc2 of this model was 0.67. The pupil size was greater after the active HF-rTMS over the left DLPFC than after the sham (P < 0.001) or right active HF-rTMS (P < 0.001). Moreover, the pupil size was greater after the right HF-rTMS than after the sham stimulation (P < 0.001) (see Figure 2).
Fig. 2 .
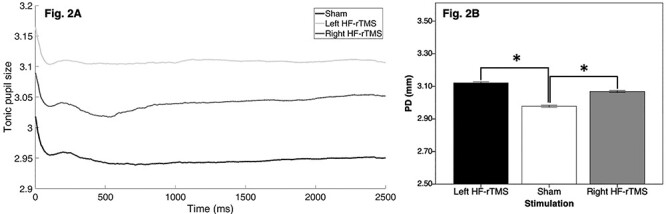
Pupil size during the pre-cue fixation cross (Figure 2A) and average pupil size during the last 200 ms of the pre-cue interval (Figure 2B) for the sham, left HF-rTMS and right HF-rTMS sessions. *P < 0.05.
The results of the Linear Mixed Models using the baseline-corrected changes in pupil size from the start of the cue until the start of the probe (a marker of phasic changes in resource allocation during the anticipation phase) as the dependent variable showed a significant main effect of cue (F(1,13 838) = 136.66, P < 0.001). Overall, the baseline-corrected average change in pupil size was greater during the B-cue trials than during the A-cue trials. The main effect of stimulation (F(2,13 751) = 0.98, P = 0.374) and the interaction between stimulation and cue were not statistically significant (F(2,13 825) = 0.37, P = 0.692). The rc2 of this model was 0.08. See Figure 3 for the baseline-corrected pupil size during the anticipation phase.
Fig. 3 .
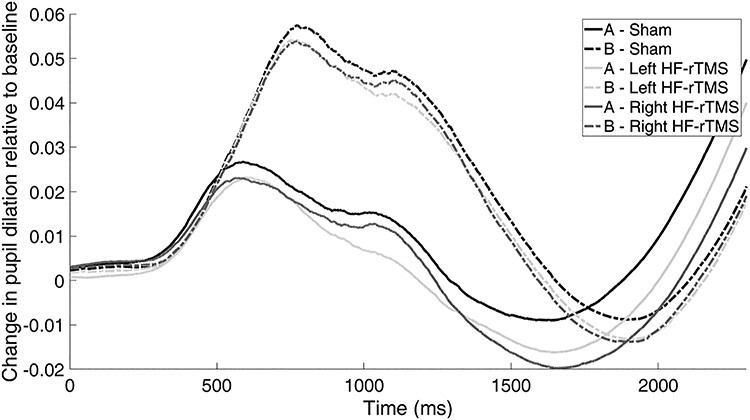
Baseline-corrected average change in pupil size from the start of the cue (‘A’ and ‘B’) until the start of the probe for the sham, left HF-rTMS and right HF-rTMS sessions. No differences between sessions were observed.
Discussion
This study aimed to investigate the effect of HF-rTMS over the left and right DLPFC on the performance in the AX-CPT, a cognitive task that permits relative examination of both proactive and reactive cognitive control. After HF-rTMS over the left DLPFC, we observed an increase in proactive cognitive control (reflected in slower RTs in AY and faster RTs in BX trials) when compared to the performance after the sham stimulation. After right DLPFC HF-rTMS, the participants showed slower RTs on AX trials, reflecting more reactive cognitive control. However, the increase in reactive control in this session was not supported by the results in the BX trials (i.e. the trial condition that specifically assesses reactive control). No differences in BY trials were observed, indicating that the participants understood and followed the instructions of the task during the three sessions. The results of the pupil data showed an increase in sustained resource allocation (assessed with pupil size at the beginning of each trial, before the cue appeared) after the active stimulation of both the left and right DLPFC than after the sham stimulation. Regarding transient changes in pupil size during the anticipation phase of the task, the pupil size was greater during B-cue trials than during A-cue trials, but we did not observe differences between sessions.
This is the first study that systematically investigated the effect of rTMS over the left and right DLPFC on proactive and reactive cognitive control. In accordance with our hypothesis, HF-rTMS over the left DLPFC increased proactive cognitive control, reflected in slower RTs in AY trials and faster RTs in BX trials. This result is in line with prior research using experimental manipulations and showing that the left DLPFC is involved in proactive control (Braver et al., 2009; Vanderhasselt et al., 2009; Boudewyn et al., 2019; but see Goméz-Ariza et al., 2017). Based on a review of studies investigating the effects of HF-rTMS on the Stroop task, Vanderhasselt et al. (2009) proposed that the left DLPFC is involved in the implementation of proactive control, by representing and actively maintaining the attentional demands of the task. Braver et al. (2009) investigated changes in brain activity in older people, a population that tends to use reactive strategies in the AX-CPT. After being trained to use proactive control during the task, a sustained increase in left DLPFC activity during the anticipatory phase was observed. Finally, using the dot-pattern version of the AX-CPT, Boudewyn et al. (2019) demonstrated that anodal tDCS over the left DLPFC increased proactive control. Our observations support the idea proposed by Dual Mechanisms of Control framework that proactive control is associated with a sustained activation of the lateral prefrontal cortex. Importantly, rTMS does not only modulate brain activity at the stimulation target, but also has network-wide effects (e.g. Tik et al., 2017; Corlier et al., 2019). Along this line, it has been shown that rTMS over the DLPFC also modulates the activity of the anterior cingulate cortex (ACC), a brain region that proactively signals the need for control, that recruits other regions of the central executive network to implement cognitive control (Ide et al., 2013; Shenhav et al., 2013), and that is involved in the process of a reactive-to-proactive shift (Braver, 2012). Thus, the effect of HF-rTMS on proactive control may also be driven by interaction of the DLPFC and the ACC, facilitating the active maintenance of task goals and the processing of expected high cognitive demand (Braver et al., 2009).
After right DLPFC stimulation, the performance on AX trials was faster than during the sham session. Although this result may suggest that HF-rTMS over the right DLPFC increased reactive control, this is not confirmed by the result on the BX trial (i.e. the trial condition that specifically assesses reactive control). Three explanations can be considered for these mixed results. First, the Dual Mechanisms of Control framework proposes that, in contrast to proactive control that is associated with early sustained activation during the anticipation phase, reactive control would be reflected in a transient activation of the lateral prefrontal cortex during the detection of conflict (a moment-to-moment adjustment of neural activity) (Braver, 2012). Moreover, neuroimaging studies have shown an activation of the right DLPFC during both proactive and reactive phases of the AX-CPT (MacDonald and Carter, 2003; Holmes et al., 2005; Paxton et al., 2008). Since HF-rTMS is expected to provoke a sustained increase in cortical excitability of the right DLPFC during both the proactive and reactive phases of the task, the stimulation may have influenced both strategies. Thus, our results suggest that the left DLPFC may play a more specific role in proactive control, whereas the role of the right DLPFC might be less specific and it could be involved in both proactive and reactive control.
Second, another possible explanation for the mixed results with the right DLPFC would be related to the fact that, besides the DLPFC, other brain regions such as the ventromedial prefrontal cortex and the inferior frontal junction also participate in proactive and reactive cognitive control (Braver et al., 2009; Braver, 2012; Goméz-Ariza et al., 2017). Along this line, Goméz-Ariza et al. (2017) showed that online anodal tDCS over the right inferior frontal junction improved reactive control in healthy adults. Thus, it is also possible that the right DLPFC interacts with of other brain areas during reactive control processes.
A third possible explanation for the results with the right DLPFC may be related to the task used in this experiment. In line with previous studies using a similar version of the task (e.g. Barch et al., 1997, 2003; Cohen et al., 1999; Braver et al., 2005; Chaillou et al., 2018; Boudewyn et al., 2019), the results showed that, overall, the participants were slower for the AY trials when compared to the other trials (AX, BX and BY). This is a common result in healthy adults and indicates that our participants used a proactive strategy during the three sessions (i.e. the RTs on AY trials was significantly slower than in AX, BX and BY trials). Considering these results, it is possible that right HF-rTMS did not show a clear effect on reactive control because the participants were more prone to use a proactive strategy during the task. Thus, a question remains whether HF-rTMS over the right DLPFC will show a stronger effect on reactive control if a version of the AX-CPT in which participants are encouraged to use reactive cognitive control is used. Further studies are needed to investigate whether HF-rTMS over the left and right DLPFC have different effects on different proactive and reactive versions of the AX-CPT task.
After both left and right DLPFC stimulation, we observed an increase in pupil size during the inter-trial period prior to the beginning of the cue. As proposed by Chiew and Braver (2013), this result can be interpreted as an increase in the allocation of cognitive resources or mental effort during the AX-CPT. Given that this effect was observed after both left and right stimulation, it would reflect a general increase in cognitive resources. Moreover, our results do not show an association with an increase in proactive or reactive cognitive control (no significant correlation between pupillometry and behavioural data was observed, results not shown). In contrast to these effects, we did not observe significant differences between sessions in the pupil response before the probe (i.e. during the delay period after the cue). This finding is in accordance with a recent study showing the same results in individuals with high and low proactive cognitive control (Maki-Marttunen et al., 2018). Replicating previous studies, we observed larger pupil changes in response to B than to A cues (Chiew and Braver, 2013, 2014; Maki-Marttunen et al., 2018). It has been proposed that this cue-related difference may reflect the suppression of the dominant target response since the target trials (i.e. AX trials) are more frequent (70%) than non-target trials starting with a B cue (20%). However, another plausible explanation is that B-cue-related increase in pupil size reflects the detection of infrequent stimuli (Chiew and Braver, 2013; Maki-Marttunen et al., 2018). Taking all together, HF-rTMS over the left and right DLPFC lead to an increase in the sustained resource allocation during the cognitive task. However, in this study, measures of pupil size do not provide specific information regarding processes associated with proactive and reactive cognitive control.
Our results are also in line with a recent systematic review (Remue et al., 2016) showing that a single session of HF-rTMS over the DLPFC does not affect self-reported mood. It is well-known that the DLPFC participates in both cognitive control and emotion regulation processes, and that changes in mood can affect flexibility and the ability to evaluate performance in the AX-CPT (Baeken et al., 2010; Martin and Kerns, 2011; van Wouwe et al., 2011). Therefore, it is crucially important that our results indicate that the effect of rTMS on cognitive control and pupil size observed in this study cannot be explained by rTMS-related short-term changes in mood.
The results of this study are of interest for affective neuroscience research. Recent findings indicate that patients with major depressive disorder have abnormal proactive and reactive control (Vanderhasselt et al., 2014). Moreover, depression has been related to decreased activity of the left DLPFC, and the use of rTMS to increase the activity of this region has shown to be an effective technique for the treatment of stress-related psychopathology, including depression (Schutter, 2009; De Raedt et al., 2015). Our results highlight the critical role of the left and right DLPFC in proactive and reactive cognitive control and provide important evidence to future studies investigating the neurocognitive mechanisms behind the development of depression and the effects of rTMS treatment in disorders in which cognitive control is compromised. Within this context, it may be important to investigate whether reductions in depressive symptoms after rTMS treatments are associated with changes in proactive control.
Despite the important findings of this study, some limitations should be considered. Only women were included in this study and therefore, more research is needed to investigate these effects in men. Also related to the study sample, some studies have reported that the performance on prefrontal cortex-related cognitive tasks may be affected by oestrogen and progesterone levels (Solis-Ortiz et al., 2004; Amin et al., 2006). Future studies investigating the role of the prefrontal cortex in proactive and reactive cognitive control may benefit from controlling for the phase of the menstrual cycle of the participants.
In conclusion, our results provide experimental evidence on the role of the left DLPFC in proactive cognitive control processes. Moreover, our findings suggest that the right DLPFC may be implicated in reactive control. Furthermore, this study shows that an increase in left and right DLPFC provokes an increase in the allocation of cognitive resources during cognitive control processes. This study provides important evidence for future research trying to understand the neurocognitive mechanism behind the clinical beneficial effects of rTMS on stress-related disorders.
Acknowledgment
The authors would like to thank Stefanie De Smet for her help during data collection.
Footnotes
The statistical conclusions are the same if the analyses are performed using the averaged pupil size during the 2500 ms of the fixation cross before the cue.
We measured stress perception during the month before the beginning of the first session using the Perceived Stress Scale (PSS; Cohen et al., 1983), rumination using the Ruminative Response Scale (RRS; Nolen-Hoeksema and Morrow, 1991; Dutch translation by Raes et al., 2003), self-esteem using the Rosenberg Self-Esteem Questionnaire (RSEQ; Rosenberg, 1965; Dutch translation by Franck et al., 2008) and Depressive Symptoms using the Beck Depression Inventory-II (BDI; Beck et al., 1996; Dutch translation by Van der Does, 2002). The statistical conclusions of this study remain the same if these variables are included as covariates in the models.
Before the AX-CPT, we asked the participants to stare at a black fixation cross against a grey background for 1 min. This part of the protocol was designed to assess the effect of HF-rTMS on pupil size before the cognitive task (see Tsukahara et al., 2016). However, due to technical issues, only the first 13.65 s of all the participants could be recorded. To investigate differences between sessions in pupil size before the AX-CPT, we performed Linear Mixed Models with stimulation (left HF-rTMS, right HF-rTMS and sham) as a fixed effect, and subject as a random effect. As dependent variable, we used the average pupil diameter (mm) during the last 10 s of the fixation cross (we use the last 10 s of the 13.65 s to exclude the initial phasic pupil response due to the beginning of the task and to the initial change in the luminance of the screen). The results showed that there were no differences in pupil size before the AX-CPT task during the three session (F(2,46.09) = 2.02, P = 0.145). Moreover, the statistical conclusions of the study remain the same if this measure is used as a covariate in all the analyses with pupil data during the AX-CPT.
Contributor Information
Matias M Pulopulos, Department of Experimental Clinical and Health Psychology, Ghent University, 9000 Ghent, Belgium.
Jens Allaert, Department of Experimental Clinical and Health Psychology, Ghent University, 9000 Ghent, Belgium; Department of Head and Skin, University Hospital Ghent (UZ Ghent), Ghent University, 9000 Ghent, Belgium; Ghent Experimental Psychiatry (GHEP) Lab, Ghent University, 9000 Ghent, Belgium.
Marie-Anne Vanderhasselt, Department of Experimental Clinical and Health Psychology, Ghent University, 9000 Ghent, Belgium; Department of Head and Skin, University Hospital Ghent (UZ Ghent), Ghent University, 9000 Ghent, Belgium; Ghent Experimental Psychiatry (GHEP) Lab, Ghent University, 9000 Ghent, Belgium.
Alvaro Sanchez-Lopez, Department of Clinical Psychology, Universidad Complutense de Madrid, 28040 Madrid, Spain.
Sara De Witte, Department of Experimental Clinical and Health Psychology, Ghent University, 9000 Ghent, Belgium; Department of Head and Skin, University Hospital Ghent (UZ Ghent), Ghent University, 9000 Ghent, Belgium; Ghent Experimental Psychiatry (GHEP) Lab, Ghent University, 9000 Ghent, Belgium.
Chris Baeken, Department of Head and Skin, University Hospital Ghent (UZ Ghent), Ghent University, 9000 Ghent, Belgium; Ghent Experimental Psychiatry (GHEP) Lab, Ghent University, 9000 Ghent, Belgium; Department of Psychiatry, University Hospital Brussels (UZBrussel), Jette, Belgium.
Rudi De Raedt, Department of Experimental Clinical and Health Psychology, Ghent University, 9000 Ghent, Belgium.
Funding
This study was supported by grant BOF16/GOA/017 for a Concerted Research Action of Ghent University (awarded to R.D.R. and C.B.) and by grant G044019N from the Research Foundation Flanders (awarded to M.A.V. and R.D.R.). M.M.P. is a postdoctoral research fellow, supported by the Research Foundation Flanders (FWO18/PDO/174). A.S.L. is currently supported by the Program for the Attraction of Scientific Talent of the Community of Madrid.
Declaration of conflicts of interest
None.
References
- Amin, Z., Epperson, C.N., Constable, R.T., Canli, T. (2006). Effects of estrogen variation on neural correlates of emotional response inhibition. NeuroImage, 32(1), 457–64. [DOI] [PubMed] [Google Scholar]
- Aston-Jones, G., Chen, S., Zhu, Y., Oshinsky, M.L. (2001). A neural circuit for circadian regulation of arousal. Nature Neuroscience, 4(7), 732. [DOI] [PubMed] [Google Scholar]
- Austin, M.P., Mitchell, P., Goodwin, G.M. (2001). Cognitive deficits in depression—possible implications for functional neuropathology. British Journal of Psychiatry, 178, 200–6. [DOI] [PubMed] [Google Scholar]
- Baeken, C., De Raedt, R., Van Schuerbeek, P., Vanderhasselt, M.A., De Mey, J., Bossuyt, A., et al. (2010). Right prefrontal HF-rTMS attenuates right amygdala processing of negatively valenced emotional stimuli in healthy females. Behavioural Brain Research, 214(2), 450–5. [DOI] [PubMed] [Google Scholar]
- Baeken, C., Vanderhasselt, M.A., Remue, J., Rossi, V., Schiettecatte, J., Anckaert, E., De Raedt, R. (2014). One left dorsolateral prefrontal cortical HF-rTMS session attenuates HPA-system sensitivity to critical feedback in healthy females. Neuropsychologia, 57, 112–121. [DOI] [PubMed] [Google Scholar]
- Bandelow, B., Michaelis, S. (2015). Epidemiology of anxiety disorders in the 21st century. Dialogues in Clinical Neuroscience, 17(3), 327–35. https://doi.org/10.1016/j.siny.2015.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barch, D.M., Braver, T.S., Nystom, L.E., Forman, S.D., Noll, D.C., Cohen, J.D. (1997). Dissociating working memory from task difficulty in human prefrontal cortex. Neuropsychologia, 35, 1373–80. [DOI] [PubMed] [Google Scholar]
- Barch, D.M., Carter, C.S., Braver, T.S., et al. (2001). Selective deficits in prefrontal cortex function in medication-naive patients with schizophrenia. Archives of General Psychiatry, 58, 280–8. [DOI] [PubMed] [Google Scholar]
- Barch, D.M., Carter, C.S., Cohen, J.D. (2003). Context processing deficit in schizophrenia: diagnostic specificity, 4-week course, and relation- ships to clinical symptoms. Journal of Abnormal Psychology, 112, 132–43. [PubMed] [Google Scholar]
- Beam, W., Borckardt, J.J., Reeves, S.T., George, M.S. (2009). An efficient and accurate new method for locating the F3 position for prefrontal TMS applications. Brain Stimulation, 2(1), 50–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck, A.T., Steer, R.A., Brown, G. (1996). Beck Depression Inventory-II, San Antonio: Pearson. [Google Scholar]
- Boudewyn, M., Roberts, B.M., Mizrak, E., Ranganath, C., Carter, C.S. (2019). Prefrontal transcranial direct current stimulation (tDCS) enhances behavioral and EEG markers of proactive control. Cognitive Neuroscience, 10(2), 57–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braver, T.S. (2012). The variable nature of cognitive control: a dual mechanisms framework. Trends in Cognitive Sciences, 16(2), 106–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braver, T.S., Cohen, J.D. (2001). Working memory, cognitive control, and the prefrontal cortex: computational and empirical studies. Cognitive Processing, 2(1), 25–55. [Google Scholar]
- Braver, T.S., Satpute, A.B., Keys, B.A., Racine, C.A., Barch, D.M. (2005). Context processing and context maintenance in healthy aging and early-stage dementia of the Alzheimer’s type. Psychology and Aging., 20, 33–46. [DOI] [PubMed] [Google Scholar]
- Braver, T.S., Gray, J.R., Burgess, G.C. (2007). In: Conway, A.R.A., Jarrold, C., Kane, M.J., Miyake, A., Towse, J.N., editors. Explaining the Many Varieties of Working Memory Variation: Dual Mechanisms of Cognitive Control, New York: Oxford University Press, pp. 75–106. [Google Scholar]
- Braver, T.S., Paxton, J.L., Locke, H.S., Barch, D.M. (2009). Flexible neural mechanisms of cognitive control within human prefrontal cortex. Proceedings of the National Academy of Sciences, 106, 7351–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaillou, A.C., Giersch, A., Hoonakker, M., et al. (2018). Evidence of impaired proactive control under positive affect. Neuropsychologia, 114, 110–7. [DOI] [PubMed] [Google Scholar]
- Chiew, K.S., Braver, T.S. (2013). Temporal dynamics of motivation and cognitive control interactions revealed by high-resolution pupillometry. Frontiers in Psychology, 4, 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiew, K.S., Braver, T.S. (2014). Dissociable influences of reward motivation and positive emotion on cognitive control. Cognitive, Affective, & Behavioral Neuroscience, 14(2), 509–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen, S., Kamarck, T., Mermelstein, R. (1983). A global measure of perceived stress. Journal of Health and Social Behavior, 24, 385–96. [PubMed] [Google Scholar]
- Cohen, J.D., Barch, D.M., Carter, C., Servan-Schreiber, D. (1999). Context- processing deficits in schizophrenia: converging evidence from three theoretically motivated cognitive tasks. Journal of Abnormal Psychology, 108, 120–33. [DOI] [PubMed] [Google Scholar]
- Cooper, S.R., Gonthier, C., Barch, D.M., Braver, T.S. (2017). The role of psychometrics in individual differences research in cognition: a case study of the AX-CPT. Frontiers in Psychology, 8, 1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corlier, J., Wilson, A., Hunter, A.M., et al. (2019). Changes in functional connectivity predict outcome of repetitive transcranial magnetic stimulation treatment of major depressive disorder. Cerebral Cortex, 29(12), 4958–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Raedt, R., Hooley, J.M. (2016). The role of expectancy and proactive control in stress regulation: a neurocognitive framework for regulation expectation. Clinical Psychology Review, 45, 45–55. [DOI] [PubMed] [Google Scholar]
- De Raedt, R., Vanderhasselt, M.A., Baeken, C. (2015). Neurostimulation as an intervention for treatment resistant depression: from research on mechanisms towards targeted neurocognitive strategies. Clinical Psychology Review, 41, 61–9. [DOI] [PubMed] [Google Scholar]
- Franck, E., De Raedt, R., Barbez, C., Rosseel, Y. (2008). Psychometric properties of the Dutch Rosenberg self-esteem scale. Psychologica Belgica, 48(1), 25–35. [Google Scholar]
- Gómez-Ariza, C.J., Martín, M.C., Morales, J. (2017). Tempering proactive cognitive control by transcranial direct current stimulation of the right (but not the left) lateral prefrontal cortex. Frontiers in Neuroscience, 11, 282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herremans, S.C., Vanderhasselt, M.A., De Raedt, R., Baeken, C. (2013). Reduced intra-individual reaction time variability during a go–NoGo task in detoxified alcohol-dependent patients after one right-sided dorsolateral prefrontal HF-rTMS session. Alcohol and Alcoholism, 48(5), 552–7. [DOI] [PubMed] [Google Scholar]
- Holmes, A.J., MacDonald, A., III, Carter, C.S., Barch, D.M., Stenger, V.A., Cohen, J.D. (2005). Prefrontal functioning during context processing in schizophrenia and major depression: an event related fMRI study. Schizophrenia Research, 76, 199–206. [DOI] [PubMed] [Google Scholar]
- Hoogendam, J.M., Ramakers, G.M., Di Lazzaro, V. (2010). Physiology of repetitive transcranial magnetic stimulation of the human brain. Brain Stimulation, 3(2), 95–118. [DOI] [PubMed] [Google Scholar]
- Ide, J.S., Shenoy, P., Yu, A.J., Li, C.S. (2013). Bayesian prediction and evaluation in the anterior cingulate cortex. The Journal of Neuroscience, 33, 2039–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones, J.A., Sponheim, S.R., MacDonald, A.W., 3rd (2010). The dot pattern expectancy task: reliability and replication of deficits in schizophrenia. Psychological Assessment, 22, 131–41. [DOI] [PubMed] [Google Scholar]
- Johnson, P. C. (2014). Extension of Nakagawa & Schielzeth’s R2GLMM to random slopes models. Methods in Ecology and Evolution, 5(9), 944–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler, R.C., McGonagle, K.A., Nelson, C.B., Hughes, M., Swartz, M., Blazer, D.G. (1994). Sex and depression in the National Comorbidity Survey. II: cohort effects. Journal of Affective Disorders, 30(1), 15–26. [DOI] [PubMed] [Google Scholar]
- Kozel, F.A., Van Trees, K., Larson, V., et al. (2019). One hertz versus ten hertz repetitive TMS treatment of PTSD: a randomized clinical trial. Psychiatry Research, 273, 153–62. [DOI] [PubMed] [Google Scholar]
- Kret, M.E., Sjak-Shie, E.E. (2019). Preprocessing pupil size data: guidelines and code. Behavior Research Methods, 51(3), 1336–7342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuznetsova, A., Brockhoff, P.B., Christensen, R.H.B. (2017). lmerTest package: tests in linear mixed effects models. Journal of Statistical Software, 82(13), 1–26. [Google Scholar]
- Lan, M.J., Chhetry, B.T., Liston, C., Mann, J.J., Dubin, M. (2016). Transcranial magnetic stimulation of left dorsolateral prefrontal cortex induces brain morphological changes in regions associated with a treatment resistant major depressive episode: an exploratory analysis. Brain Stimulation, 9(4), 577–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Garcia, P., Lesh, T.A., Salo, T., et al. (2016). The neural circuitry supporting goal maintenance during cognitive control: a comparison of expectancy AX-CPT and dot probe expectancy paradigms. Cognitive, Affective, & Behavioral Neuroscience, 16(1), 164–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald, A.W. (2008). Building a clinically relevant cognitive task: case study of the AX paradigm. Schizophrenia Bulletin, 34, 619–28. doi: 10.1093/schbul/sbn038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald, A.W., III, Carter, C.S. (2003). Event-related fMRI study of context processing in dorsolateral prefrontal cortex of patients with schizophrenia. Journal of Abnormal Psychology, 112, 689–97. [DOI] [PubMed] [Google Scholar]
- Mäki-Marttunen, V., Hagen, T., Aminihajibashi, S., et al. (2018). Ocular signatures of proactive versus reactive cognitive control in young adults. Cognitive, Affective, & Behavioral Neuroscience, 18(5), 1049–63. [DOI] [PubMed] [Google Scholar]
- Martin, E.A., Kerns, J.G. (2011). The influence of positive mood on different aspects of cognitive control. Cognition and Emotion, 25(2), 265–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mir-Moghtadaei, A., Caballero, R., Fried, P., et al. (2015). Concordance between BeamF3 and MRI-neuronavigated target sites for repetitive transcranial magnetic stimulation of the left dorsolateral prefrontal cortex. Brain Stimulation, 8(5), 965–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa, S., Johnson, P.C., Schielzeth, H. (2017). The coefficient of determination R 2 and intra-class correlation coefficient from generalized linear mixed-effects models revisited and expanded. Journal of the Royal Society Interface, 14(134), 20170213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa, S., Schielzeth, H. (2013). A general and simple method for obtaining R2 from generalized linear mixed?effects models. Methods in ecology and evolution, 4(2), 133–142. [Google Scholar]
- Nolen-Hoeksema, S., Morrow, J. (1991). A prospective study of depression and posttraumatic stress symptoms after a natural disaster: the 1989 Loma Prieta earthquake. Journal of Personality and Social Psychology, 61(1), 115–21. [DOI] [PubMed] [Google Scholar]
- Ochsner, K.N., Gross, J.J. (2005). The cognitive control of emotion. Trends in Cognitive Sciences, 9(5), 242–9. [DOI] [PubMed] [Google Scholar]
- Paulus, W. (2011). Transcranial electrical stimulation (tES - tDCS; tRNS, tACS) methods. Neuropsychological Rehabilitation, 21(5), 602–17. doi: 10.1080/09602011.2011.557292. [DOI] [PubMed] [Google Scholar]
- Paxton, J.L., Barch, D.M., Racine, C.A., Braver, T.S. (2008). Cognitive control, goal maintenance, and prefrontal function in healthy aging. Cerebral Cortex, 18, 1010–28. doi: 10.1093/cercor/bhm135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poppa, T., De Witte, S., Vanderhasselt, M.A., Bechara, A., Baeken, C. (2020). Theta-burst stimulation and frontotemporal regulation of cardiovascular autonomic outputs: the role of state anxiety. International Journal of Psychophysiology, 149, 25–34. [DOI] [PubMed] [Google Scholar]
- Pulopulos, M.M., De Witte, S., Vanderhasselt, M.A., et al. (2019). The influence of personality on the effect of iTBS after being stressed on cortisol secretion. PLoS One, 14(10), e0223927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remue, J., Vanderhasselt, M.-A., Baeken, C., Rossi, V., Tullo, J., De Raedt, R. (2016). The effect of a single HF-rTMS session over the left DLPFC on the physiological stress response as measured by heart rate variability. Neuropsychology, 30(6), 756–766. [DOI] [PubMed] [Google Scholar]
- R Core Team (2013). R: A language and environment for statistical computing. [Google Scholar]
- Raes, F., Hermans, D., Eelen, P. (2003). De Nederlandstalige versie van de ruminative response scale en de rumination on sadness scale (the Dutch version of the rumination response scale and the rumination on sadness scale). Gedragstherapie, 36, 97–104. [Google Scholar]
- Reimer, J.F., Radvansky, G.A., Lorsbach, T.C., Armendarez, J.J. (2015). Event structure and cognitive control. Journal of Experimental Psychology, 41(5), 1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remue, J., Baeken, C., De Raedt, R. (2016). Does a single neurostimulation session really affect mood in healthy individuals? A systematic. Neuropsychologia, 85, 184–98. [DOI] [PubMed] [Google Scholar]
- Rosenberg, M. (1965). Society and the Adolescent Self-Image, Princeton, NJ: Princeton University Press. [Google Scholar]
- Rossi, S., Hallett, M., Rossini, P.M., Pascual-Leone, A. (2009). Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clinical Neurophysiology, 120(12), 2008–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosvold, H.E., Mirsky, A.F., Sarason, I., Bransome, E.D., Jr., Beck, L.H. (1956). A continuous performance test of brain damage. Journal of Consulting Psychology, 20(5), 343. [DOI] [PubMed] [Google Scholar]
- RStudio , 2012. Rstudio: Integrated Development Environment for R (version 0.96.122) [computer software] http://www.rstudio.org/
- Ryman, S.G., El Shaikh, A.A., Shaff, N.A., et al. (2019). Proactive and reactive cognitive control rely on flexible use of the ventrolateral prefrontal cortex. Human Brain Mapping, 40(3), 955–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schutter, D. (2009). Antidepressant efficacy of high-frequency transcranial magnetic stimulation over the left dorsolateral prefrontal cortex in double-blind sham-controlled designs: a meta-analysis. Psychological Medicine, 39(1), 65–75. doi: 10.1017/s0033291708003462. [DOI] [PubMed] [Google Scholar]
- Schutter, D.J., van Honk, J. (2006). A standardized motor threshold estimation procedure for transcranial magnetic stimulation research. The Journal of ECT, 22(3), 176–8. [DOI] [PubMed] [Google Scholar]
- Shenhav, A., Botvinick, M.M., Cohen, J.D. (2013). The expected value of control: an integrative theory of anterior cingulate cortex function. Neuron, 79, 217–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh, A., Erwin-Grabner, T., Sutcliffe, G., Antal, A., Paulus, W., Goya-Maldonado, R. (2019). Personalized repetitive transcranial magnetic stimulation temporarily alters default mode network in healthy subjects. Scientific Reports, 9(1), 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solis-Ortiz, S., Guevara, M.A., Corsi-Cabrera, M. (2004). Performance in a test demanding prefrontal functions is favored by early luteal phase progesterone: an electroencephalographic study. Psychoneuroendocrinology, 29(8), 1047–57. [DOI] [PubMed] [Google Scholar]
- Thut, G., Pascual-Leone, A. (2010). A review of combined TMS-EEG studies to characterize lasting effects of repetitive TMS and assess their usefulness in cognitive and clinical neuroscience. Brain Topography, 22(4), 219–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tik, M., Hoffmann, A., Sladky, R., et al. (2017). Towards understanding rTMS mechanism of action: stimulation of the DLPFC causes network-specific increase in functional connectivity. NeuroImage, 162, 289–96. [DOI] [PubMed] [Google Scholar]
- Trapp, N.T., Uitermarkt, B., Johnson, M.K., et al. (2019a). A new device to improve target localization for transcranial magnetic stimulation therapy. Brain Stimulation, 12(6), 1600–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapp, N.T., Uitermarkt, B., Johnson, M.K., et al. (2019b). A new device to improve target localization for transcranial magnetic stimulation therapy. Brain Stimulation, 12(6), 1600–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukahara, J.S., Harrison, T.L., Engle, R.W. (2016). The relationship between baseline pupil size and intelligence. Cognitive Psychology, 91, 109–23. [DOI] [PubMed] [Google Scholar]
- Valero-Cabré, A., Amengual, J.L., Stengel, C., Pascual-Leone, A., Coubard, O.A. (2017). Transcranial magnetic stimulation in basic and clinical neuroscience: a comprehensive review of fundamental principles and novel insights. Neuroscience & Biobehavioral Reviews, 83, 381–404. [DOI] [PubMed] [Google Scholar]
- Van der Does, A.J.W. (2002). Manual of the Dutch Version of the BDI-II, San Antonio, TX: Amst NL Harcourt. [Google Scholar]
- Vanderhasselt, M.A., De Raedt, R. (2009). Impairments in cognitive control persist during remission from depression and are related to the number of past episodes: an event related potentials study. Biological Psychology, 81(3), 169–76. [DOI] [PubMed] [Google Scholar]
- Vanderhasselt, M.A., De Raedt, R., Baeken, C. (2009). Dorsolateral prefrontal cortex and Stroop performance: tackling the lateralization. Psychonomic Bulletin & Review, 16(3), 609–12. http://dx.doi.org/10.3758/pbr.16.3.609. [DOI] [PubMed] [Google Scholar]
- Vanderhasselt, M.A., De Raedt, R., De Paepe, A., et al. (2014). Abnormal proactive and reactive cognitive control during conflict processing in major depression. Journal of Abnormal Psychology, 123(1), 68. [DOI] [PubMed] [Google Scholar]
- van Wouwe, N.C., Band, G.P., Ridderinkhof, K.R. (2011). Positive affect modulates flexibility and evaluative control. Journal of Cognitive Neuroscience, 23(3), 524–39. [DOI] [PubMed] [Google Scholar]


