Abstract
Background
Little is known about how adverse, midlife metabolic profiles affect future physical functioning. We hypothesized that a higher number of midlife metabolic syndrome (MetS) components are associated with poorer physical performance in early old age for multiethnic women.
Methods
MetS status from 1996 to 2011 (8 visits) and objective physical performance in 2015/2016 (Short Physical Performance Battery [SPPB; 0–12], 40-foot walk [meter/second], 4-meter gait speed [meter/second], chair stands [seconds], stair climb [seconds]) were assessed in the Study of Women’s Health Across the Nation (SWAN; n = 1722; age 65.4 ± 2.7 years; 26.9% African American, 10.1% Chinese, 9.8% Japanese, 5.5% Hispanic). Poisson latent class growth modeling identified MetS component trajectory groups: none (23.9%), 1 = low-MetS (28.7%), 2 = mid-MetS (30.9%), and ≥3 = high-MetS (16.5%). Adjusted linear regression related MetS groups to physical performance outcomes.
Results
High-MetS versus none had higher body mass index, pain, financial strain, and lower physical activity and self-reported health (p < .0001). Compared with White, African American and Hispanic women were more likely to be in the high-MetS groups and had worse physical functioning along with Chinese women (SPPB, chair stand, stair climb, and gait speed—not Hispanic). After adjustments, high-MetS versus none demonstrated significantly worse 40-ft walk (β: −0.08; 95% CI: −0.13, −0.03), gait speed (β: −0.09; 95% CI: −0.15, −0.02), SPPB (β: −0.79; 95% CI: −1.15, −0.44), and chair stands (β: 0.69; 95% CI: 0.09, 1.28), but no difference in stair climb.
Conclusions
Midlife MetS groups were related to poor physical performance in early old age multiethnic women. Midlife management of metabolic function may improve physical performance later in life.
Keywords: Longitudinal, Metabolic, Physical functional performance, Racial disparities, Successful aging
Whether changes in metabolic function among midlife adults are predictive of physical performance declines in early old age is unknown. Metabolic syndrome (MetS) is clinically defined as having at least 3 of the following cardiometabolic risk factors including hypertension, abdominal obesity, impaired fasting glucose, low high-density lipoprotein cholesterol level, and hypertriglyceridemia (1). The relationship between MetS and cardiovascular disease, diabetes, and premature mortality is well documented (2,3). In the United States, over one-third of adults have MetS (4) and the prevalence increases dramatically with age (5). Women have higher MetS prevalence than men, though there is variation by race/ethnicity with non-Hispanic Black, Mexican American, and Hispanic adults more likely to have MetS versus White counterparts (4,6). Although MetS has been associated with loss of mobility among adults aged ≥70 years (7,8), and despite evidence that the midlife is a critical window for changes in MetS (9), the impact of midlife MetS changes on physical function in early old age among diverse populations is unclear.
Women have longer life expectancies than men, yet often live with more disability (10) and experience steeper declines in functioning throughout old age (11). Additionally, midlife and older African American and Hispanic women experience greater functional limitations versus White women (12,13). Importantly, whether similar functional disparities exist between Asian subgroups relative to White women is currently not known. The multiethnic Study of Women’s Health Across the Nation (SWAN) examined self-reported function during and after the menopausal transition among White, African American, Chinese, Japanese, and Hispanic women (14,15). From 40 to 55 years, 10% of women self-reported some functional limitations and 9% reported substantial limitations (14), with 50% reporting some limitations by 56–66 years (16). Midlife women experience both onset and increases in physical function limitations, yet the highly dynamic patterns may make it an ideal time for risk factor prevention (16). Multiple biological mechanisms (eg, chronic inflammatory processes (17); hyperglycemia related (7); obesity related due to pain, osteoarthritis, and reduced physical activity levels; (18,19)) in which MetS contributes to decreases in muscle mass and strength have been investigated. Additionally, midlife functional decline has been associated with greater risk for early old age disability (20). Therefore, identifying targets for early intervention in midlife women is a priority to help tailor strategies to delay the onset of disability.
Previous studies examining the contribution of MetS and its components on function have only included old age adults who are identified as White or Black, used one assessment of MetS, and/or used self-reported measures of mobility as a proxy of objective function with lack of objective physical performance outcomes (7,8). We used a prospective design to assess how changes in the number of components of MetS across midlife are associated with objective physical performance among early old age women from 5 racial/ethnic groups (White, African American, Chinese, Hispanic, and Japanese). We hypothesize that (i) higher total components of MetS will be associated with worse physical performance in midlife to early old age women and (ii) women from some racial/ethnic groups will experience worse physical performance compared with White counterparts.
Method
Study Design and Participants
The Study of Women’s Health Across the Nation (SWAN) is an ongoing, multiethnic, multicenter, prospective community-based cohort study of the menopausal transition. A full description of the SWAN recruitment and methodology has been published previously (21). Women were eligible for SWAN if they were aged 42–52 years at baseline (1996/1997), had an intact uterus and at least 1 ovary, were premenopausal or early perimenopausal (ie, had at least 1 menstrual period in the past 3 months), and were not pregnant, lactating, or breastfeeding. A total of 3302 women were recruited from 7 sites across the United States: Boston, MA; Chicago, IL; Detroit, MI; Oakland, CA; Los Angeles, CA; Hudson County, NJ; and Pittsburgh, PA. All sites recruited White participants; additionally, each site recruited a non-White sample including African American (Boston, Chicago, Detroit, and Pittsburgh), Japanese (Los Angeles), Hispanic (Newark), and Chinese (Oakland) women. Prior to visit 15, 149/3302 = 4.5% deaths occurred. Overall retention for those alive at the visit 15 follow-up exam in 2015/2016 was 75% (2366/[3302–149]). Participants provided written informed consent before enrolling and at each follow-up visit, and all protocols were approved from each site’s Institutional Review Board.
Of those who completed a clinic visit at visit 15 (2091/2366 = 88%), 97% (2029/2091) were available to participate in the Physical Functioning Assessment and 307/2029 did not complete any physical function measures (267 = unwilling/unable to attend visit, 5 = refused, 35 = other reasons, eg, out of state). Prior to visit 15, women were excluded for having less than 2 time points for MetS (n = 232; collection at baseline, visit 1, 3, 4, 5, 6, 7, and 12). A total of 1722 women were included in this analytic sample from baseline (1996/1997) to follow-up visit 15 (2015/2016). Excluded women were more likely to be African American or Hispanic, have ≤high school diploma, fair/poor self-rated health, higher mean body mass index (BMI), bodily pain, and self-report physical function difficulties (visit 4 SF-36; all p < .05).
Study Variables
Metabolic syndrome
Using clinically accepted and current diagnostic criteria of MetS (22), the 5 MetS components were defined as follows: hypertriglyceridemia (fasting triglycerides ≥ 150 mg/dL), abdominal obesity (if Japanese/Chinese, obese if waist circumference ≥ 80 cm; otherwise, obese if waist circumference > 88 cm), impaired fasting glucose (glucose ≥ 100 mg/dL fasting value), hypertension (systolic blood pressure ≥ 130 mm Hg or diastolic blood pressure ≥ 85 mm Hg or taking any blood pressure medication), and low high-density lipoprotein (<50 mg/dL). Fasting blood draws were taken in the morning and assayed for triglycerides, total cholesterol, and high-density lipoprotein (23). Standardized protocols were used to measure waist circumference and blood pressure. Blood pressure was measured with readings taken on the right arm, with the respondent seated and feet flat on the floor for at least 5 minutes prior to measurement. Two sequential blood pressure measures were completed, with a minimum 2-minute rest period between measures. Respondents had not smoked or consumed any caffeinated beverage within 30 minutes of blood pressure measurement. Waist circumference at the umbilicus was measured by a trained technician with participants wearing nonrestrictive undergarments. The present analysis summed total MetS components (range 0–5) with 5 being the most severe.
Physical performance
During the visit 15 exam (2015/2016), SWAN participants completed physical performance tasks following standardized protocols conducted by trained clinic staff. The tests included the timed 40-ft walk, timed stair climb test and the Short Physical Performance Battery (SPPB; includes standing balance, timed 4-m gait speed, and timed 5-repeated chair stand).
Forty-foot walk:
The walking course was set up on a level floor with 2 tape markers indicating the start and end points. Participants were instructed to complete the walk in a “comfortable but steady, brisk pace as in the manner of showing purpose, but not being late.” Timing was stopped when both feet crossed the end line. If necessary, use of a walking assistive device was permitted. The 40-ft walk protocol was conducted twice, and the faster time (meter/second) was used in analysis.
Stair climb test:
The timed stair climb test measured balance, strength, and endurance and was comprised of 4 standard stairs of steps (10 inches deep and 6 inches high). Participants ascended and descended the stairs for 3 consecutive cycles, using a hand rail for assistance if necessary (24). Total time (seconds) to complete the 3 consecutive cycles was used for analysis. A slower time indicates worse time to complete the stair climb.
Four-meter gait speed:
The course was set up on a level floor with markers at the start and stop point (25). Participants were instructed to walk at their usual pace and timing was stopped when the first foot completely crossed the end line, with use of assistive devices if needed. The 4-meter walk was completed twice, and the faster time (meter/second) was used in analysis.
Repeated chair stands:
A standard height chair or bench with a back was placed on a level floor (25). While seated, women were asked to sit and place their arms across their chest and stand without using their arms. Time (seconds) taken to complete 5 consecutive repetitions was used in analysis.
Short Physical Performance Battery:
The standard SPPB protocol consisted of 3 physical performance measures: 4-m usual gait speed, 5-repeated chair stands, and a series of balance tests (side-by-side, semitandem, tandem, and 1-foot stands, each held for 10 seconds). This measure has documented validity and reliability (25). For analysis, the traditional scoring cutoffs for the balance component were used, but with SWAN-specific time quartiles to score gait speed and chair stand components (26). The total SPPB score ranges from 0 (worst performance) to 12 (best performance), and the continuous score was used.
Covariates
Covariates of interest were measured at visit 15 (2015/2016) unless otherwise indicated. They included demographic characteristics and health conditions associated with cardiovascular disease and/or physical performance. Self-reported sociodemographic variables included age (years); education assessed at baseline (≤high school, some college, ≥college degree); self-reported difficulty paying for basics (very hard/somewhat hard vs not very hard representing financial difficulty); and marital status (single/never married, married/living as married, separate/widowed/divorced). Health indices included self-rated health status (excellent/very good, good, or fair/poor), objectively measured BMI (kg/m2), menopausal status (natural postmenopausal, postmenopausal by bilaterial salpingo-oophorectomy, or pre- or early/late perimenopausal), hormone user (ever use), presence of depressive symptoms (≥16 on Center for Epidemiological Studies Depression [CES-D] scale) (27), and physician-diagnosed self-reported comorbidities (osteoarthritis, heart attack, and/or stroke). Although different designations of race/ethnicity may be used currently, recruitment for this longitudinal study occurred in 1996/1997. Race/ethnicity was defined during a screening interview prior to the baseline examination from participants’ response to the question, “How would you describe your primary racial or ethnic group?” The response categories included Black/African American; Puerto Rican/Dominican/Central American/Cuban or Cuban American/South American/Spanish or other Hispanic (all categorized as Hispanic); Chinese/Chinese American; Japanese/Japanese American; and Caucasian/White Non-Hispanic (referent group). Participants who identified as Mexican/Mexican American, Mixed, or Other were not included in the cohort (12). Bodily pain severity in the past 4 weeks and self-reported physical function (assessed at visits 4 and 15) were estimated from the Short Form Health Survey (SF-36; score 0–100) (28,29). Self-reported physical activity was assessed using the Kaiser Physical Activity Survey (30). The total physical activity score ranges from 3 to 15, with higher scores indicating higher levels of physical activity in sports/exercise, active living, and household/caregiving domains (31). Smoking status at visit 15 was defined as current smoking (yes/no). Clinical site was also included in analyses based on potential site differences confounding the main relationships of interest.
Statistical Methods
Baseline characteristics for those included versus excluded in the analysis were compared using 2 sample t-tests or analysis of variance and chi-square tests for continuous and categorical variables, respectively. Variable distributions were assessed for normality. Analysis of variance was used to describe racial/ethnic differences across each objective physical performance measure (40-ft walk, 4-m gait speed, 5-repeated chair stand, SPPB, and stair climb).
Latent class group modeling was used to identify subgroups of women following similar patterns of trajectories in counts of MetS components in midlife. Models were compared based on Bayesian information criteria and varying degrees of polynomial trends for each group (32). After the trajectory groups were determined, women were assigned to the group that reflected their highest probability (33). Trajectory groups were created from total number of MetS components across the midlife period. Information for MetS trajectories was available from baseline (1996–1997) and visits 1 (1997–1999), 3 (1999–2001), 4 (2000–2002), 5 (2001–2003), 6 (2002–2004), 7 (2003–2005), and 12 (2009–2011). Once MetS component trajectory groups based on patterns of MetS during midlife were determined, groups were entered into separate models as predictors for physical function outcomes. Means and SD and frequencies and percentages were used to describe the analytic sample by MetS groups. Differences by MetS groups in demographic and health characteristics, MetS individual components (visit 12), and physical performance outcomes were examined using analysis of variance for continuous variables and chi-square for categorical variables.
Linear regression was used to model each continuous objective physical performance measure in early old age as a function of the MetS groups throughout midlife. The referent group was designated as the healthiest trajectory: MetS = none. Unadjusted linear regression models were fit followed by minimally adjusted models that included age, race, site, difficulty paying for basics, self-rated health, and BMI. Then, fully adjusted models added additional health risk factors and comorbidities including bodily pain, self-reported physical activity, current smoking status, hormone use, menopause status, osteoarthritis, and depressive symptoms to the above list of covariates. Covariates were determined based on prior literature and associations with the physical performance outcomes at p < .10 with collinearity of covariates assessed. In each model, only covariates that reached significance of p < .10 were retained. Because some racial/ethnic groups were enrolled at only one site so race and site may be collinear, models with age and site were compared with models with age and race. Model assumptions and goodness of fit tests were assessed to determine the best predictive model. All analyses were conducted using 2-sided hypothesis testing and SASv9.4 software (SAS Institute, Inc., Cary, NC).
We conducted sensitivity analyses to ensure the robustness of the findings by adjusting for baseline self-reported physical function at the first possible time point (visit 4), excluding women who reported moderate or substantial functional limitation (n = 589 excluded); substantial limitation: <50 (n = 140, 8.8%); moderate limitation: 51–85 (n = 449, 28.2%); no limitations in physical function: 86–100 (n = 1001, 63%) based on the SF-36 (14), and adjusting for comorbidities (eg, cumulative self-reported stroke or heart attack from baseline until visit 15).
Results
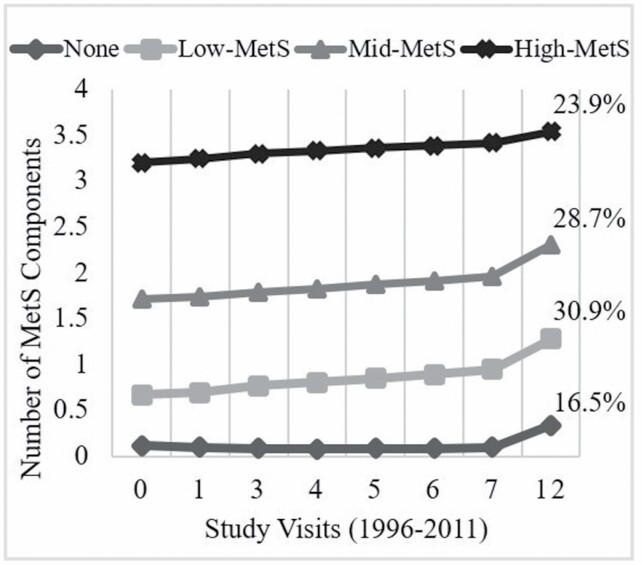
Four MetS groups were determined from the latent class growth model with patterns reflecting the number of MetS components: none (16.5%), low-MetS (1 component; 30.9%), mid-MetS (2 components; 28.7%), and high-MetS (≥3 components; 23.9%; Figure 1). The mean group membership probability of women assigned to none, low-MetS, mid-MetS, and high-MetS was >80% for each group. On examination of the trajectories, we defined MetS groups based on the number of components rather than change in the number of components over time, since the number of MetS components in each group remained relatively stable with only slight average increases over time.
Figure 1.
Metabolic syndrome (MetS) component groups by study visits from 1996 to 2011 based on a Poisson latent class growth model. Four MetS groups were determined with patterns reflecting the number of MetS components: none (16.5%), low-MetS (1 component; 30.9%), mid-MetS (2 components; 28.7%), and high-MetS (3 or more components; 23.9%).
Characteristics of participants by MetS groups are shown in Table 1. Compared with White women, Hispanic and African American women were more likely to be in higher MetS groups. White, Chinese, and Japanese women were more likely to be in the none-MetS group. The high-MetS group exhibited the poorest health. The high-MetS group had more class 2 obesity (34.9 kg/m2), fair/poor self-reported health, current smoking, bodily pain, osteoarthritis, lower self-reported physical activity, lower education, and had somewhat/very hard time paying for basics. Previously self-reported physical function was lower in the high-MetS versus none (63.0 ± 28.2 vs 86.9 ± 16.9; p < .0001). In addition, the mid-MetS group had higher BMI, worse self-reported overall health, more osteoarthritis, more bodily pain, and lower physical activity versus none and low-MetS groups. Low-MetS group had more prevalent hypertension and abdominal obesity versus other MetS components. As expected, each progressive MetS group had higher prevalence in all the individual MetS components (Table 1).
Table 1.
Characteristics of Study of Women’s Health Across the Nation (SWAN) Participants at Visit 15 (When Physical Performance Measures Were Assessed, 2015/2016) by Metabolic Syndrome Component Group
| Metabolic Syndrome Component Groups | |||||
|---|---|---|---|---|---|
| Participant Characteristics (N = 1,722) | None | Low-MetS | Mid-MetS | High-MetS | p-Value |
| N = 412 (23.9%) | N = 494 (28.7%) | N = 532 (30.9%) | N = 284 (16.5%) | ||
| Baseline demographics | |||||
| Age, mean ± SD | 65.2 ± 2.6 | 65.4 ± 2.6 | 65.8 ± 2.7 | 65.7 ± 2.8 | .003 |
| Race/ethnicity | |||||
| White | 247 (60.0%) | 246 (49.8%) | 214 (40.2%) | 114 (40.1%) | <.0001 |
| African American | 35 (8.5%) | 121 (24.5%) | 198 (37.2%) | 109 (38.4%) | |
| Hispanic | 10 (2.4%) | 24 (4.9%) | 36 (6.8%) | 25 (8.8%) | |
| Chinese | 61 (14.8%) | 50 (10.1%) | 43 (8.1%) | 20 (7.0%) | |
| Japanese | 59 (14.3%) | 53 (10.7%) | 41 (7.7%) | 16 (5.6%) | |
| Education | |||||
| ≤ High school | 64 (15.7%) | 92 (18.7%) | 116 (22.0%) | 70 (24.8%) | <.0001 |
| Some college | 94 (23.0%) | 158 (32.2%) | 180 (34.2%) | 100 (35.5%) | |
| ≥ College degree | 250 (61.3%) | 241 (49.1%) | 231 (43.8%) | 112 (39.7%) | |
| Marital status | |||||
| Single/never married | 48 (11.7%) | 49 (10.0%) | 65 (12.2%) | 52 (18.3%) | <.0001 |
| Married/living as married | 277 (67.2%) | 305 (62.0%) | 280 (52.6%) | 126 (44.4%) | |
| Separate/widowed/divorced | 87 (21.1%) | 138 (28.1%) | 187 (35.2%) | 106 (37.3%) | |
| Difficulty paying for basics, somewhat/ very hard | 52 (12.9%) | 92 (19.1%) | 130 (25.0%) | 104 (38.2%) | <.0001 |
| Health characteristics | |||||
| BMI, mean ±SD | 23.6 ± 3.9 | 27.2 ± 5.1 | 32.4 ± 6.8 | 34.9 ± 6.7 | <.0001 |
| Menopause status | |||||
| Natural post | 386 (93.7%) | 455 (92.1%) | 482 (90.8%) | 244 (85.9%) | .007 |
| Post by BSO | 26 (6.3%) | 39 (7.9%) | 48 (9.0%) | 40 (14.1%) | |
| Pre/early/late peri | 0 | 0 | 1 (0.2%) | 0 | |
| Hormone user, ever | 198 (48.1%) | 248 (50.2%) | 251 (47.2%) | 143 (50.4%) | .73 |
| Current smoking | 13 (3.2%) | 37 (7.6%) | 36 (6.8%) | 23 (8.2%) | 0.02 |
| Overall health | |||||
| Excellent/very good | 282 (69.3%) | 268 (54.9%) | 204 (38.6%) | 69 (24.7%) | <.0001 |
| Good | 92 (22.6%) | 159 (32.6%) | 28 (43.2%) | 118 (42.3%) | |
| Fair/poor | 33 (8.1%) | 61 (12.5%) | 96 (18.2%) | 92 (33.0%) | |
| Depression, CES-D score ≥ 16 | 40 (9.71%) | 59 (11.9%) | 67 (12.6%) | 50 (17.6%) | .02 |
| SF-36 Bodily Pain (0–100), mean ±SD | 75.9 ± 19.8 | 69.7 ± 21.6 | 64.5 ± 23.3 | 59.5 ± 24.9 | <.0001 |
| KPAS Physical Activity Score, mean ± SD | 8.31 ± 1.72 | 7.70 ± 1.78 | 7.17 ± 1.72 | 6.66 ± 1.80 | <.0001 |
| Osteoarthritis | 103 (25.3%) | 170 (34.6%) | 224 (42.4%) | 145 (51.2%) | <.0001 |
| Metabolic syndrome components | |||||
| Hypertension | 52 (13.0%) | 214 (45.7%) | 347 (69.1%) | 241 (86.7%) | <.0001 |
| Abdominal obesity | 32 (8.0%) | 194 (42.1%) | 406 (82.0%) | 261 (96.3%) | <.0001 |
| Impaired fasting glucose | 19 (4.9%) | 69 (15.3%) | 191 (39.2%) | 211 (75.9%) | <.0001 |
| Low high-density lipoprotein | 6 (1.6%) | 51 (11.3%) | 148 (30.6%) | 160 (58.2%) | <.0001 |
| Hypertriglyceridemia | 21 (5.4%) | 60 (13.36%) | 102 (21.2%) | 140 (51.7%) | <.0001 |
| Physical function measures | |||||
| SF-36 Physical Function score (0–100), mean ± SD | 86.9 ± 16.9 | 80.7 ± 20.9 | 71.7 ± 25.0 | 63.0 ± 28.2 | <.0001 |
| 40 ft gait speed (m/s), mean ± SD | 1.49 ± 0.25 | 1.41 ± 0.25 | 1.31 ± 0.27 | 1.19 ± 0.24 | <.0001 |
| 4-m gait speed (m/s), mean ± SD | 1.06 ± 0.37 | 1.00 ± 0.33 | 0.92 ± 0.42 | 0.82 ± 0.27 | <.0001 |
| SPPB (0–12), mean ± SD | 9.71 ± 1.84 | 9.10 ± 1.98 | 8.15 ± 2.20 | 7.13 ± 2.45 | <.0001 |
| 5-Repeated chair stand (s), mean ± SD | 9.87 ± 2.80 | 10.73 ± 3.29 | 11.55 ± 3.56 | 12.72 ± 3.60 | <.0001 |
| Total stair climb time (s), mean ± SD | 18.89 ± 3.77 | 20.17 ± 4.56 | 22.83 ± 7.31 | 25.51 ± 8.20 | <.0001 |
Notes: Continuous variables are represented as mean ± SD, and categorical variables are represented as frequency (percentage). Metabolic syndrome components are from visit 12. BMI = body mass index (kg/m2); BSO = bilateral salpingo-oophorectomy; CES-D = Center for Epidemiologic Studies Depression Scale; KPAS = Kaiser Physical Activity Questionnaire; MetS = metabolic syndrome; SD = standard deviation; SPPB = Short Physical Performance Battery.
Physical performance was significantly different by racial/ethnic groups (all p < .0001; Table 2). Japanese women had the highest function on gait speed, SPPB, chair stand, and stair climb versus all other racial/ethnic groups. African American, Hispanic, and Chinese women had worse physical functioning versus White on the SPPB (African American: 14.5%, Hispanic: 21.3%, Chinese: 8.2% worse, respectively), chair stand (13.1%, 19.8%, 2.1% worse, respectively), and stair climb (13.5%, 31.7%, 4.6% worse, respectively). African American and Chinese women had a 14% and 16%, respectively, slower gait speed versus White.
Table 2.
Mean and Standard Deviation of Physical Performance Measures by Self-identified Racial/Ethnic Group
| Sample Size, Mean ± SD | Racial/Ethnic Groups | p-Value | ||||
|---|---|---|---|---|---|---|
| White | African American | Hispanic | Chinese | Japanese | ||
| 40-ft walk speed, m/s | 1.39 ± 0.26 n = 657 |
1.27 ± 0.27 n = 439 |
— | 1.46 ± 0.28 n = 173 |
— | <.0001 |
| 4-m gait speed, m/s | 1.00 ± 0.34 n = 798 |
0.86 ± 0.19 n = 445 |
1.05 ±1.11 n = 80 |
0.84 ± 0.16 n = 173 |
1.13 ± 0.18 n = 164 |
<.0001 |
| SPPB, 0–12 | 9.02 ± 2.07 n = 805 |
7.71 ± 2.36 n = 455 |
7.10 ± 2.06 n = 84 |
8.28 ± 1.93 n = 174 |
10.38 ± 1.75 n = 168 |
<.0001 |
| 5-Repeated chair stand, s | 10.70 ± 2.99 n = 787 |
12.10 ± 3.63 n = 423 |
12.82 ± 6.36 n = 83 |
10.92 ± 2.88 n = 172 |
9.61 ± 2.33 n = 167 |
<.0001 |
| Total stair climb time, s | 20.61 ± 5.48 n = 626 |
23.40 ± 8.10 n = 332 |
27.15 ± 7.00 n = 76 |
21.56 ± 6.00 n = 171 |
18.80 ± 3.40 n = 165 |
<.0001 |
Notes: Sites recruiting Hispanic and Japanese women did not perform the 40-ft walk speed. SPPB = Short Physical Performance Battery.
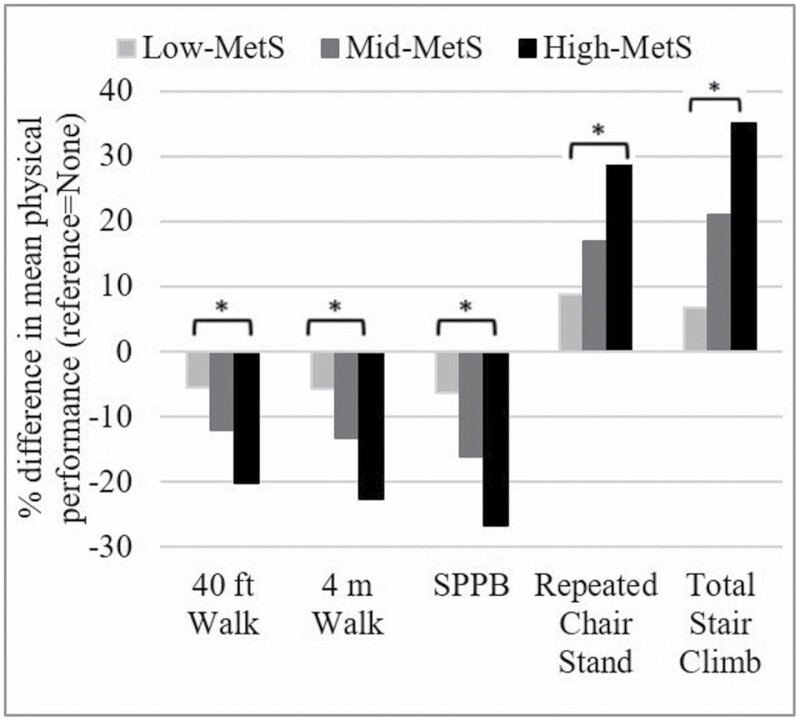
The unadjusted mean difference in all physical performance outcomes was worse with each higher MetS group versus none, indicating a possible dose–response relationship (all outcomes: p-value for trend < .0001; Figure 2). Women in the high-MetS group versus none had 20.1% slower (worse) 40-ft walk time, 22.6% slower 4-m gait speed, 28.9% slower repeated chair, 35% higher (worse) stair climb. In addition, women in the high- and mid-MetS groups had a 26.6% lower and 16.1% lower (worse) total SPPB score, respectively, versus none. Finally, the mid-MetS group versus none had 13.2% slower (worse) gait speed.
Figure 2.
Percent difference in mean objective physical performance outcomes by metabolic syndrome component group. % difference is calculated as follows: [(mean MetS group (high-MetS, mid-MetS, low-MetS) – mean none-MetS (referent))/mean none-MetS group] × 100. Negative values indicate lower means for low-/mid-/high-MetS group versus none (eg, participants in high-MetS group took 20% longer to complete the 40-ft walk (m/s) vs those with no MetS components). MetS = metabolic syndrome. *p-Value for trend <0.0001 for all physical performance outcomes.
In unadjusted models, the magnitude of worsening for each physical performance outcome relative to the none-MetS group approximately doubled with each MetS group (all p-value for trend < .0001; Table 3). In fully adjusted multivariable models (Table 3), the high-MetS group had significantly consistently worse physical performance on the 40-ft walk, 4-m gait speed, SPPB, and chair stand versus none-MetS group (β = −0.08, β = −0.09, β = −0.79, β = 0.69, respectively). The high-MetS group had slower total stair climb versus none-MetS group although was not statistically significant (β = 1.10, 95% CI: −0.04, 2.23). The mid-MetS group also had poorer SPPB performance and chair stand time versus none-MetS group but was not statistically significant. Race/ethnicity remained significant in final adjusted models. For all outcomes, the strength of association increased with each additional MetS component in a gradient pattern.
Table 3.
β Coefficients and 95% Confidence Intervals From Univariate and Multivariable Linear Regression Models of Metabolic Syndrome Component Groups (1996–2011) and Objective Physical Performance Outcomes (2015/2016)
| 40-ft Walk Speed, m/s | 4-m Gait Speed, m/s | SPPB, 0–12 | 5-Repeated Chair Stand, s | Total Stair Climb Time, s | |
|---|---|---|---|---|---|
| Model 1 | |||||
| High-MetS | −0.30 (−0.34, −0.25)*** | −0.24 (−0.29, −0.18)*** | −2.58 (−2.90, −2.26)*** | 2.85 (2.33, 3.37)*** | 6.61 (5.58, 7.65)*** |
| Mid-MetS | −0.18 (−0.21, −0.14)*** | −0.13 (−0.18, −0.08)*** | −1.56 (−1.84, −1.29)*** | 1.68 (1.24, 2.11)*** | 3.94 (3.06, 4.82)*** |
| Low-MetS | −0.07 (−0.11, −0.03)** | −0.05 (−0.10, −0.00)* | −0.61 (−0.88, −0.33)*** | 0.86 (0.41, 1.30)*** | 1.27 (0.39, 2.16)** |
| None | Ref. | Ref. | Ref. | Ref. | Ref. |
| Model 2 | |||||
| High-MetS | −0.08 (−0.13, −0.03)** | −0.09 (−0.15, −0.02)** | −0.79 (−1.15, −0.44)*** | 0.69 (0.09, 1.28)* | 1.10 (−0.04, 2.23) |
| Mid-MetS | −0.01 (−0.06, 0.03) | −0.01 (−0.06, 0.04) | −0.26 (−0.55, 0.02) | 0.17 (−0.31, 0.65) | −0.17 (−1.10, 0.75) |
| Low-MetS | 0.02 (−0.02, 0.06) | 0.007 (−0.04, 0.05) | 0.01 (−0.24, 0.27) | 0.00 (−0.42, 0.43) | −0.54 (−1.36, 0.28) |
| None | Ref. | Ref. | Ref. | Ref. | Ref. |
Notes: Unadjusted and fully adjusted models (all significant predictors across outcomes: age, body mass index, site, overall health, bodily pain, difficulty paying for basics, physical activity, current smoking status, hormone use, and arthritis) are presented in 1,772 Study of Women’s Health Across the Nation (SWAN) participants. β Estimates (per unit, eg, m/s) and 95% confidence intervals are presented above. Model 1 = unadjusted model. Model 2 = fully adjusted model: age, body mass index (kg/m2), race/ethnicity, site, overall health, bodily pain, difficulty paying for basics, KPAS physical activity, current smoking status, hormone use, and osteoarthritis. MetS = metabolic syndrome; KPAS = Kaiser Physical Activity Questionnaire; SPPB = Short Physical Performance Battery.
*p < .05; **p < .01; ***p < .0001.
When excluding women with previous moderate/substantial physical limitations, results remained consistent in final models. In the sensitivity analysis adjusting for previous self-reported physical function, β coefficients were largely consistent and all groups remained statistically significant with high-MetS becoming significant for stair climb (β = 1.30; 95% CI: 0.15, 2.45; p < .05). In addition, results were consistent after adjusting for comorbid conditions (stroke and heart attack) throughout the study period (baseline through visit 15). Finally, to ensure relationships with race/ethnicity were not site specific (eg, sites recruiting Hispanic and Japanese women did not perform the 40-ft walk), we created models with race only versus site only with estimates also consistent to the fully adjusted model with no changes in significance except the mid-MetS group reaching significance at p < .05 for SPPB in site-only and race-only models.
Discussion
Midlife women clinically classified has having MetS (with ≥3 components) were more likely to have worse objective physical performance in early old age across all outcomes (ie, 40-ft walk, 4-m gait speed, SPPB, 5-repeated chair stand, and stair climb performance) versus women with no MetS components. Women in the mid-MetS group (approximately 2 components), typically not considered a clinical syndrome (22), also demonstrated worse performance with respect to the SPPB and the chair stand, though these results were not statistically significant after adjustments. In addition, after excluding women with moderate or substantial self-reported physical limitations, the main results were consistent. Importantly, African American and Hispanic women were more likely to be in the higher MetS groups. Our findings contribute to the understanding of preventable and treatable factors in midlife adults, particularly in Black and Hispanic racial/ethnic populations, known to have higher late-life mobility disability (4,12).
Even prior to clinical classification of MetS, metabolic changes throughout midlife likely have important implications on early old age physical performance among women. Our results indicate that early old age physical performance is worse in African American, Hispanic, and Chinese women versus White and suggest that this performance for African American and Hispanic women may be associated with their generally higher numbers of MetS components earlier in midlife. Although African American and Hispanic women have more late-life disability versus White women (4), less is known regarding prevalence of disability among U.S. Asian subgroups (ie, Chinese and Japanese). Previous work in SWAN found that midlife Chinese women experienced an apparent disparity in a composite physical performance decile score derived from grip strength, gait speed, and chair stand compared with White women, but Japanese women did not (12). Our current study was consistent with these findings, as Chinese women had worse SPPB, gait speed, chair stand, and stair climb performance than White women. Future research should include more focus on differences among race/ethnic groups to further understand early risk factors for physical function and disability across these populations.
Evidence suggests that the onset of disability begins in midlife, while women have decades of life expectancy (34). Worse physical function in old age predicts several future poor health outcomes (eg, lower quality of life and increased mortality rates) (35,36), which are more prevalent in African American and Hispanic women. Life course factors probably contribute to old age physical function and should be considered particularly among African American and Hispanic older adults (37). The midlife high-MetS group had significantly worse objective physical performance in early old age except stair climb. In addition, midlife women with 2 components of MetS had worse early old age physical performance, though the relationships were not as consistent. Therefore, by the time adults are clinically diagnosed with MetS, functional changes have already occurred during midlife, and prevention efforts for disability should be initiated at an earlier timepoint, particularly in at-risk racial/ethnic minority women with MetS.
Measuring function among midlife adults (especially high-risk midlife adults, eg, women, racial/ethnic minorities, adults with MetS components) in clinical settings using simple, inexpensive, noninvasive tests such as the SPPB could be a strategy to identify early signs of physical limitations and help maintain independence with aging (38,39). Certain functional tests such as SPPB or gait speed (37) that are able to be performed quickly could be used as a “sixth vital sign” (40,41) for midlife women and particularly for those with cardiometabolic-related risk factors who may be at risk for functional decline as they age. We found significant associations with MetS and SPPB, a feasible test that could be implemented in clinical settings for midlife women (38,42). However, it is currently unknown which test is most appropriate to assess early functional decline in midlife (38), and identifying appropriate tests for this age group is important to assess functional decline and initiate early prevention efforts.
Common midlife cardiometabolic health conditions, especially diabetes (43), obesity (44), and MetS (7), may affect age-related outcomes including physical function and disability (45). The highest prevalence of severe obesity is among adults 40–59 years, women (9.2% higher rates vs men), and specifically non-Hispanic Black women (56.9% vs 39.8% among non-Hispanic White) (46). Almost 40% of midlife adults are classified as having MetS, which increases with age (5). Previous research showed that SWAN women with worse self-reported physical function were more likely to have MetS and almost 50% aged 55–66 years self-reported having functional limitations (16,47). Therefore, slower gait speed may be a marker for early cardiovascular disease risk (48). Although the relationship between function and cardiometabolic-related outcomes is likely bidirectional, our work suggests that MetS evaluated longitudinally over midlife contributes to function decline and the timing of the onset of limitations in early old age (15).
Our findings are consistent with previous studies that were either cross-sectional in nature (8) or used self-reported measures of physical function (49,50) and showed MetS was related to functional impairment (ie, Rosow and Breslau scale and limitations in instrumental/basic activities of daily living). However, these could not determine onset or changes in objective physical function. One study of older diverse adults (age = 73.6 ± 2.9 years; 52.5% women; 41.3% Black) found MetS was associated with increased risk of incident mobility limitations, defined as 2 consecutive self-reports of inability to climb 10 steps without rest and/or walk 1/4 mile (7). We observed consistent findings, though with objective performance measures instead of self-report. Although previous cohort studies used one measurement of MetS at baseline in late life (49,50), our study demonstrated that MetS (2 or ≥3 components) measured longitudinally during midlife was an independent predictor of worse physical performance after adjusting for lifestyle and other comorbid conditions.
Midlife MetS counts of 2 (preclinical MetS) or ≥3 (MetS clinical syndrome) should be viewed as a potential risk factor for future functional decline in old age. Abdominal obesity was the most frequent component of MetS with 86% of SWAN women having abdominal obesity. Notably, among SWAN women with only obesity at study enrollment, 30% progressed to preclinical MetS counts (2 components) and 15% developed the clinical syndrome (≥3 components) (9). Our results indicate that in mid- and high-MetS groups, there were not 1 or 2 components that appeared to be most common, but rather all individual components were prevalent in midlife. In addition, prevalence for each MetS component increased with each progressive MetS group. Thus, midlife women experience changes in preventable/treatable risk factors (eg, blood pressure, lipids, and glucose) that are related to developing MetS. Therefore, midlife is a critical point for implementing preventive strategies aimed at adopting healthy behaviors for risk reduction of future MetS.
The current study had several limitations. Only women during midlife to early old age were assessed, so results may not be generalizable to men or other ages. In addition, we did not examine how each individual component of MetS during midlife affects future functional changes. Due to the longitudinal study design, not all women initially enrolled in SWAN participated in the visit 15 clinic visit, which should be taken into consideration when interpreting the results. Understanding which MetS components are associated with functional changes could guide intervention efforts and is an important future research direction. Also, not all sites measured 40-ft walk and stair climb, which may have attenuated the findings slightly due to lower numbers of women. However, many notable strengths of our study exist. To our knowledge, this is the first assessment of MetS in midlife and early old age objective physical performance in a multiethnic population of women. Our study had 26.9% African American and 5.5% Hispanic women. Women are at higher risk for old age disability than men, with African American and Hispanic women at the highest risk among women (12). The multiethnic composition of the sample allowed us to identify important racial/ethnic disparities among midlife MetS component patterns and physical performance, though since our recruitment occurred in 1996/1997, racial/ethnic groupings, and terminology may differ from current preferred classifications. Women excluded due to incomplete measures were more likely to be African American and Hispanic with poorer health status, which may have attenuated race/ethnic differences in our results. It also may be important to investigate the impact of MetS component groups within specific racial/ethnic groups at greater risk for early functional decline. A longitudinal design enabled us to establish temporality between MetS component trajectories and functional outcomes. The importance of onset of function limitation is overlooked in many studies of older adults, in which temporality cannot be determined and in which life course factors in disability are understudied. Furthermore, we examined several measures of objective physical performance, which may capture early subclinical changes versus self-report, allowing us to better understand which measures are most sensitive to change in early old age.
Given the potential clinical utility of our findings, future studies should examine the relationship of individual MetS components over time in midlife adults with functional decline and by racial/ethnic subgroups. Both initial prevention of comorbidity and slowing comorbidity severity might reduce the number of years living with disability in late life. Focusing efforts on the preliminary stages of the disablement process will allow clinical care and preventive efforts to be tailored for midlife women at high risk for future functional decline with the goal of compressing years of morbidity, delaying the onset of old age disability, and extending independence with aging.
Acknowledgments
We thank the study staff at each site and all the women who participated in SWAN.
Funding
J.M.N. is supported by the National Institute on Aging (NIA) of the National Institutes of Health (NIH) under Award Number NIA T32-AG000181-28. The Study of Women’s Health Across the Nation (SWAN) has grant support from NIH, U.S. Department of Health and Human Services (DHHS), through NIA, the National Institute of Nursing Research (NINR), and the NIH Office of Research on Women’s Health (ORWH) (grant numbers U01NR004061, U01AG012505, U01AG012535, U01AG012531, U01AG012539, U01AG012546, U01AG012553, U01AG012554, and U01AG012495). The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the NIA, NINR, ORWH, or the NIH.
Clinical Centers: University of Michigan, Ann Arbor—Siobán Harlow, PI 2011–present, MaryFran Sowers, PI 1994–2011; Massachusetts General Hospital, Boston, MA—Joel Finkelstein, PI 1999–present; Robert Neer, PI 1994–1999; Rush University, Rush University Medical Center, Chicago, IL—Howard Kravitz, PI 2009–present; Lynda Powell, PI 1994–2009; University of California, Davis/Kaiser—Ellen Gold, PI; University of California, Los Angeles—Gail Greendale, PI; Albert Einstein College of Medicine, Bronx, NY—Carol Derby, PI 2011–present, Rachel Wildman, PI 2010–2011; Nanette Santoro, PI 2004–2010; University of Medicine and Dentistry – New Jersey Medical School, Newark—Gerson Weiss, PI 1994–2004; and the University of Pittsburgh, Pittsburgh, PA—Karen Matthews, PI.
NIH Program Office: National Institute on Aging, Bethesda, MD—Chhanda Dutta 2016–present; Winifred Rossi 2012–2016; Sherry Sherman 1994–2012; Marcia Ory 1994–2001; National Institute of Nursing Research, Bethesda, MD—Program Officers.
Central Laboratory: University of Michigan, Ann Arbor—Daniel McConnell (Central Ligand Assay Satellite Services).
Coordinating Center: University of Pittsburgh, Pittsburgh, PA—Maria Mori Brooks, PI 2012–present; Kim Sutton-Tyrrell, PI 2001–2012; New England Research Institutes, Watertown, MA—Sonja McKinlay, PI 1995–2001.
Steering Committee: Susan Johnson, Current Chair; Chris Gallagher, Former Chair.
Author Contributions
J.M.N., E.S.S., and R.M.B. conceived/designed the manuscript; J.M.N. performed statistical analyses; J.M.N., E.S.S., and R.M.B. interpreted the data; and all coauthors contributed to and revised the manuscript for important intellectual content and provided final approval.
Conflict of Interest
None declared.
References
- 1. Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults E and T of HBC in A. Executive summary of the third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA J Am Med Assoc. 2001;285(19):2486–2497. doi: 10.1001/jama.285.19.2486 [DOI] [PubMed] [Google Scholar]
- 2. Mottillo S, Filion KB, Genest J, et al. The metabolic syndrome and cardiovascular risk a systematic review and meta-analysis. J Am Coll Cardiol. 2010;56:1113–1132. doi: 10.1016/j.jacc.2010.05.034 [DOI] [PubMed] [Google Scholar]
- 3. Hui WS, Liu Z, Ho SC, Ho SC. Metabolic syndrome and all-cause mortality: a meta-analysis of prospective cohort studies. Eur J Epidemiol. 2010;25(6):375–384. doi: 10.1007/s10654-010-9459-z [DOI] [PubMed] [Google Scholar]
- 4. Moore JX, Chaudhary N, Akinyemiju T. Metabolic syndrome prevalence by race/ethnicity and sex in the United States, National Health and Nutrition Examination Survey, 1988–2012. Prev Chronic Dis. 2017;14(3):160287. doi: 10.5888/pcd14.160287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Benjamin EJ, Muntner P, Alonso A, et al. Heart disease and stroke statistics – 2019 update: a report from the American Heart Association. Circulation. 2019;139:e56–e528. doi: 10.1161/CIR.0000000000000659 [DOI] [PubMed] [Google Scholar]
- 6. Aguilar M, Bhuket T, Torres S, Liu B, Wong RJ. Prevalence of the metabolic syndrome in the United States, 2003–2012. JAMA. 2015;313:1973–1974. doi: 10.1001/jama.2015.4260 [DOI] [PubMed] [Google Scholar]
- 7. Penninx BW, Nicklas BJ, Newman AB, et al. Metabolic syndrome and physical decline in older persons: results from the health, aging and body composition study. J Gerontol A Biol Sci Med Sci. 2009;64:96–102. doi: 10.1093/gerona/gln005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Blazer DG, Hybels CF, Fillenbaum GG. Metabolic syndrome predicts mobility decline in a community-based sample of older adults. J Am Geriatr Soc. 2006;54:502–506. doi: 10.1111/j.1532-5415.2005.00607.x [DOI] [PubMed] [Google Scholar]
- 9. Ward E, Gold EB, Johnson WO, et al. Patterns of cardiometabolic health as midlife women transition to menopause: a prospective multiethnic study. J Clin Endocrinol Metab. 2019;104:1404–1412. doi: 10.1210/jc.2018-00941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Freedman VA, Wolf DA, Spillman BC. Disability-free life expectancy over 30 years: a growing female disadvantage in the US population. Am J Public Health. 2016;106:1079–1085. doi: 10.2105/AJPH.2016.303089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Samson MM, Meeuwsen IB, Crowe A, Dessens JA, Duursma SA, Verhaar HJ. Relationships between physical performance measures, age, height and body weight in healthy adults. Age Ageing. 2000;29:235–242. doi: 10.1093/ageing/29.3.235 [DOI] [PubMed] [Google Scholar]
- 12. Sternfeld B, Colvin A, Stewart A, et al. Understanding racial/ethnic disparities in physical performance in midlife women: findings from SWAN (Study of Women’s Health Across the Nation). J Gerontol B Psychol Sci Soc Sci. 2020;75:1961–1971. doi: 10.1093/geronb/gbz103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Okoro CA, Hollis ND, Cyrus AC, Griffin-Blake S. Prevalence of disabilities and health care access by disability status and type among adults – United States, 2016. MMWR Morb Mortal Wkly Rep. 2018;67:882–887. doi: 10.15585/mmwr.mm6732a3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tseng LA, El Khoudary SR, Young EA, et al. The association of menopause status with physical function: the Study of Women’s Health Across the Nation. Menopause. 2012;19:1186–1192. doi: 10.1097/gme.0b013e3182565740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. El Khoudary SR, Greendale G, Crawford SL, et al. The menopause transition and women’s health at midlife: a progress report from the Study of Women’s Health Across the Nation (SWAN). Menopause. 2019;26:1213–1227. doi: 10.1097/GME.0000000000001424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ylitalo KR, Karvonen-Gutierrez CA, Fitzgerald N, et al. Relationship of race-ethnicity, body mass index, and economic strain with longitudinal self-report of physical functioning: the Study of Women’s Health Across the Nation. Ann Epidemiol. 2013;23:401–408. doi: 10.1016/j.annepidem.2013.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Schaap LA, Pluijm SM, Deeg DJ, Visser M. Inflammatory markers and loss of muscle mass (sarcopenia) and strength. Am J Med. 2006;119:526.e9–526.17. doi: 10.1016/j.amjmed.2005.10.049 [DOI] [PubMed] [Google Scholar]
- 18. Blaum CS, Xue QL, Michelon E, Semba RD, Fried LP. The association between obesity and the frailty syndrome in older women: the Women’s Health and Aging Studies. J Am Geriatr Soc. 2005;53:927–934. doi: 10.1111/j.1532-5415.2005.53300.x [DOI] [PubMed] [Google Scholar]
- 19. Visser M, Goodpaster BH, Kritchevsky SB, et al. Muscle mass, muscle strength, and muscle fat infiltration as predictors of incident mobility limitations in well-functioning older persons. J Gerontol A Biol Sci Med Sci. 2005;60:324–333. doi: 10.1093/gerona/60.3.324 [DOI] [PubMed] [Google Scholar]
- 20. Brown RT, Diaz-Ramirez LG, Boscardin WJ, Lee SJ, Steinman MA. Functional impairment and decline in middle age: a cohort study. Ann Intern Med. 2017;167:761–768. doi: 10.7326/M17-0496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sowers M, Crawford SL, Sternfeld B, et al. SWAN: a multicenter, multiethnic, community-based cohort study of women and the menopausal transition. In: Lobo RA, Kelsey J, Marcus RBT-M, eds. Menopause: Biology and Pathobiology. Academic Press; 2000:175–188. doi: 10.1016/B978-012453790-3/50012-3 [DOI] [Google Scholar]
- 22. Grundy SM, Cleeman JI, Daniels SR, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute scientific statement. Circulation. 2005;112:2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404 [DOI] [PubMed] [Google Scholar]
- 23. El Khoudary SR, Brooks MM, Thurston RC, Matthews KA. Lipoprotein subclasses and endogenous sex hormones in women at midlife. J Lipid Res. 2014;55:1498–1504. doi: 10.1194/jlr.P049064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lange-Maia BS, Karvonen-Gutierrez CA, Strotmeyer ES, et al. Factors influencing longitudinal stair climb performance from midlife to early late life: the study of women’s health across the nation Chicago and Michigan sites. J Nutr Health Aging. 2019;23:821–828. doi: 10.1007/s12603-019-1254-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49:M85–M94. doi: 10.1093/geronj/49.2.m85 [DOI] [PubMed] [Google Scholar]
- 26. Miller DK, Wolinsky FD, Andresen EM, Malmstrom TK, Miller JP. Adverse outcomes and correlates of change in the Short Physical Performance Battery over 36 months in the African American health project. J Gerontol A Biol Sci Med Sci. 2008;63:487–494. doi: 10.1093/gerona/63.5.487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Radloff LS. The CES-D scale. Appl Psychol Meas. 1977;1(3):385–401. doi: 10.1177/014662167700100306 [DOI] [Google Scholar]
- 28. Ware JEJ. The status of health assessment 1994. Annu Rev Public Health. 1995;16:327–354. doi: 10.1146/annurev.pu.16.050195.001551 [DOI] [PubMed] [Google Scholar]
- 29. Sherman SE, Reuben D. Measures of functional status in community-dwelling elders. J Gen Intern Med. 1998;13:817–823. doi: 10.1046/j.1525-1497.1998.00245.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ainsworth BE, Sternfeld B, Richardson MT, Jackson K. Evaluation of the Kaiser Physical Activity Survey in women. Med Sci Sports Exerc. 2000;32:1327–1338. doi: 10.1097/00005768-200007000-00022 [DOI] [PubMed] [Google Scholar]
- 31. Baecke JA, Burema J, Frijters JE. A short questionnaire for the measurement of habitual physical activity in epidemiological studies. Am J Clin Nutr. 1982;36:936–942. doi: 10.1093/ajcn/36.5.936 [DOI] [PubMed] [Google Scholar]
- 32. Jones BL, Nagin DS, Roeder K. A SAS procedure based on mixture models for estimating developmental trajectories. Sociol Methods Res. 2001;29(3):374–393. doi: 10.1177/0049124101029003005 [DOI] [Google Scholar]
- 33. Andruff H, Carraro N, Thompson A, Gaudreau P. Latent class growth modelling: a tutorial. Quant Methods Psychol. 2009;5(1):11–24. doi: 10.20982/tqmp.05.1.p011 [DOI] [Google Scholar]
- 34. Dugan SA, Gabriel KP, Lange-Maia BS, Karvonen-Gutierrez C. Physical activity and physical function: moving and aging. Obstet Gynecol Clin North Am. 2018;45:723–736. doi: 10.1016/j.ogc.2018.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wu LW, Chen WL, Peng TC, et al. All-cause mortality risk in elderly individuals with disabilities: a retrospective observational study. BMJ Open. 2016;6:e011164. doi: 10.1136/bmjopen-2016-011164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Motl RW, McAuley E. Physical activity, disability, and quality of life in older adults. Phys Med Rehabil Clin N Am. 2010;21:299–308. doi: 10.1016/j.pmr.2009.12.006 [DOI] [PubMed] [Google Scholar]
- 37. Alvidrez J, Castille D, Laude-Sharp M, Rosario A, Tabor D. The National Institute on Minority Health and Health Disparities research framework. Am J Public Health. 2019;109(suppl 1):S16–S20. doi: 10.2105/AJPH.2018.304883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kritchevsky SB, Forman DE, Callahan KE, et al. Pathways, contributors, and correlates of functional limitation across specialties: workshop summary. J Gerontol A Biol Sci Med Sci. 2019;74:534–543. doi: 10.1093/gerona/gly093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Beaudart C, Rolland Y, Cruz-Jentoft AJ, et al. Assessment of muscle function and physical performance in daily clinical practice: a position paper endorsed by the European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases (ESCEO). Calcif Tissue Int. 2019;105:1–14. doi: 10.1007/s00223-019-00545-w [DOI] [PubMed] [Google Scholar]
- 40. Richardson J, Letts L, Chan D, et al. Monitoring physical functioning as the sixth vital sign: evaluating patient and practice engagement in chronic illness care in a primary care setting – a quasi-experimental design. BMC Fam Pract. 2012;13:29. doi: 10.1186/1471-2296-13-29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bierman AS. Functional status: the six vital sign. J Gen Intern Med. 2001;16:785–786. doi: 10.1111/j.1525-1497.2001.10918.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Studenski S, Perera S, Wallace D, et al. Physical performance measures in the clinical setting. J Am Geriatr Soc. 2003;51:314–322. doi: 10.1046/j.1532-5415.2003.51104.x [DOI] [PubMed] [Google Scholar]
- 43. Wong E, Backholer K, Gearon E, et al. Diabetes and risk of physical disability in adults: a systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2013;1:106–114. doi: 10.1016/S2213-8587(13)70046-9 [DOI] [PubMed] [Google Scholar]
- 44. Batsis JA, Zagaria AB. Addressing obesity in aging patients. Med Clin North Am. 2018;102:65–85. doi: 10.1016/j.mcna.2017.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. El Khoudary SR, Chen X, Nasr A, et al. Greater periaortic fat volume at midlife is associated with slower gait speed later in life in women: The SWAN Cardiovascular Fat Ancillary Study. J Gerontol A Biol Sci Med Sci. 2019;74:1959–1964. doi: 10.1093/gerona/glz095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hales C, Carroll M, Fryar C, Ogden C.Prevalence of Obesity and Severe Obesity Among Adults: United States, 2017–2018. NCHS Data Brief. 2020;360:1–8. [PubMed] [Google Scholar]
- 47. Ylitalo KR, Karvonen-Gutierrez C, McClure C, et al. Is self-reported physical functioning associated with incident cardiometabolic abnormalities or the metabolic syndrome? Diabetes Metab Res Rev. 2016;32:413–420. doi: 10.1002/dmrr.2765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. El Khoudary SR, Chen HY, Barinas-Mitchell E, et al. Simple physical performance measures and vascular health in late midlife women: the Study of Women’s Health across the nation. Int J Cardiol. 2015;182:115–120. doi: 10.1016/j.ijcard.2014.12.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Carriere I, Pérès K, Ancelin ML, et al. Metabolic syndrome and disability: findings from the prospective three-city study. J Gerontol A Biol Sci Med Sci. 2014;69:79–86. doi: 10.1093/gerona/glt101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Laudisio A, Bandinelli S, Gemma A, Ferrucci L, Incalzi RA. Metabolic syndrome and functional ability in older age: the InCHIANTI study. Clin Nutr. 2014;33:626–633. doi: 10.1016/j.clnu.2013.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]