Abstract
Background
Prior research demonstrates that Black Americans receive fewer health benefits at high levels of socioeconomic status (SES) relative to Whites. Yet, few studies have considered the role of lifetime SES (ie, changes in SES from childhood to adulthood) in shaping these patterns among older adults. This study investigates the extent to which racial disparities in allostatic load (AL), an indicator of accelerated physiological aging, vary across levels of lifetime SES among Black and White adults aged 50 and older.
Methods
With data from the Nashville Stress and Health Study, modified Poisson regression models were used to assess racial differences in the odds of high AL (4+ high-risk biomarkers) among Black and White older adults (N = 518) within each level of lifetime SES (ie, stable low SES, upward mobility, downward mobility, and stable high SES).
Results
Stable high SES was associated with greater odds of high AL; there was not a significant association between other lifetime SES trajectories and AL. However, the magnitude of racial disparities varied across levels of lifetime SES, with a significant Black–White difference in AL observed only among upwardly mobile (odds ratio [OR] = 1.76, 95% confidence interval [CI] = 1.24–2.51) and high SES groups (OR = 2.22, 95% CI = 1.37–3.58).
Conclusions
Our study demonstrates that racial disparities in AL among older adults depend on individuals’ lifetime SES trajectories and that older Black Americans receive fewer health benefits for achieving higher SES. These findings underscore the need to evaluate socioeconomic resources across the life course to clarify the extent of racial disparities among aging populations.
Keywords: Allostatic load, Black Americans, Lifetime SES, Social mobility
A vast literature documents large and persistent racial disparities in health among older adults in the United States. Relative to White adults, Black adults have higher rates of the leading causes of death (eg, heart disease, stroke, cancer, diabetes, and hypertension) (1–3) and experience accelerated physiological aging (4–7). The fundamental causes of disease theory (FCT), a prominent framework in the social sciences used to explain these disparities, posits that fundamental causes embody resources that can be used to avoid or alleviate health risks (8). Researchers have long recognized socioeconomic status (SES) as a fundamental cause of health, given the multilevel pathways through which SES shapes health, including influencing knowledge of and access to healthy behaviors, exposure to stressors, residence in (dis)advantaged neighborhoods, and the activation of biological systems (9–11). A plethora of research also indicates that racial differences in SES explain a large, but not total, portion of racial disparities in physical health among older adults (12–15).
While SES is an important determinant of health and health disparities, findings from prior work highlight inconsistencies in the expected relationship between SES and health. For example, studies have shown that Black Americans tend to have worse health than their White counterparts across all levels of SES (16,17) and, at times, Black–White disparities are greater among high SES individuals compared to those of low SES (18–21). Such findings do not support predictions made by the FCT and add to a growing body of evidence underscoring racial differences in the health benefits of SES across the life course (16,22–24). As such, research that clarifies the impact of SES on the health of older Blacks and Whites is needed to reduce disparities among this aging population.
Life course perspectives are critical for understanding the differential impact of SES on health for Black and White older adults. In particular, life course perspectives highlight the role of early-life conditions in shaping health later in life (25–27). Cumulative inequality theory, for example, posits that accumulating risks and resources influence health across the life course (28). Those who are advantaged in early life are able to acquire more resources (eg, income, wealth, access to quality health care) over time, providing benefits for health and aging. In contrast, those who are disadvantaged in early life tend to experience accumulating risks that may be harmful to health (eg, greater exposure to stressors, job insecurity, lack of health insurance) (28–30). Given that Black Americans are more likely to experience early-life adversity compared to Whites (16,31), it is likely that they also encounter more subsequent challenges to healthy living and accumulate disadvantages across the life course that negatively influence health, regardless of subsequent achievement of resources.
The important role of early-life conditions, in addition to later-life socioeconomic achievement, highlights the need to examine the relationship between lifetime SES (ie, changes in SES from childhood to adulthood) and health among older adults. Prior research has linked social mobility processes to later-life health (26,32,33). Specifically, upward or downward mobility—that is, achieving higher or lower levels of SES, respectively, in adulthood relative to one’s SES of origin—may be differentially associated with health at older ages compared to remaining in high or low SES groups throughout one’s life (34). While upward social mobility is typically associated with gains in health-protective resources such as access to safer housing, quality health care, and healthy foods (26,32,33), upwardly mobile individuals spend only a portion of their lives in advantaged positions. As such, it is possible that their health is poorer than those who spend their entire lives in high SES positions and who, consequently, experience prolonged accumulation of material advantages relevant for health (35). Similarly, while those who experience downward mobility would likely have worse health than their stable high SES and upwardly mobile counterparts, they may have better health than those who maintain low SES across the life course. This would stem from their early-life exposure to health-protective resources and the greater period of time they have these resources compared to those who experience low SES throughout their lives. Thus, studies that only measure SES in adulthood may obscure notable differences in the health of those who are upwardly mobile and those of high SES across the life course, as well as those who are downwardly mobile and those who remain in low SES groups. Prior work, however, has given less attention to the relationship between lifetime SES and health, particularly among older adults, leaving unclear whether and to what extent movement across SES levels is relevant for health at older ages.
In addition to combining the health experiences of those who are socioeconomically mobile and those who remain in the same SES groups across the life course, prior work examining the role of SES in explaining Black–White disparities in health tends to assume that the pathways to high or low SES are equivalent across racial groups. That is, many expect that the health consequences of achieving high SES are similar for Blacks and Whites. However, a growing body of research suggests that the experience of social mobility, including its causes and consequences, differs significantly across racial groups (18). Specifically, studies examining the “diminishing returns hypothesis” have documented the health costs of upward mobility among Black adults (36–38), largely stemming from historical and contemporary forms of systemic racism in the United States. Indeed, such forms of racism have resulted in Black Americans experiencing disproportionate rates of social and economic adversity in childhood and have led to enduring barriers to education, employment, and wealth across the life course among this population (39–43). Being upwardly mobile in such contexts requires sustained effort and energy, which can lead to the overactivation of bodily stress response systems and subsequently induce accelerated physiological aging and poor health (44–48). Moreover, Black adults across the SES spectrum are also more likely to live in under-resourced communities relative to their White counterparts, which contribute to limited educational and occupational opportunities, as well as greater exposure to environmental hazards and stressors (11,49,50).
Exposure to social stressors, including racial discrimination, throughout socioeconomic attainment processes and at higher socioeconomic contexts is another pathway through which Black adults experience diminished health returns to higher SES (21,46,51). Such exposures can take a cumulative toll on physical health via their impacts on biological systems, health behaviors, and mental health (4,38,44,45,49,52). Less understood, however, is whether the consequences of these diminishing returns have lasting effects on health and health disparities among older adults. That is, prior work leaves unclear the extent to which the processes underlying upward mobility, downward mobility, or stability in SES levels across the life course result in smaller or wider Black–White disparities in health at older ages. Documenting differences in the magnitude of racial disparities within levels of lifetime SES can provide insight into the potential pathways and factors contributing to the persistence of Black–White disparities across the life course, even after accounting for current SES.
This study integrates research on the diminishing returns hypothesis and life course perspectives to investigate the relationship between lifetime SES and allostatic load (AL) among older adults. Using data from the Nashville Stress and Health Study (NSAHS), we ask: (a) To what extent is lifetime SES associated with AL among Black and White older adults? and (b) Do Black–White disparities in AL vary within levels of lifetime SES? We focus on AL because it constitutes a global measure of physical health and represents the physiological toll of cumulative, adverse stress experiences and social inequalities on multiple bodily systems (33,53,54). As such, AL is ideal for capturing the cumulative health consequences of disparate life experiences among Black and White older adults and for understanding later-life disparities in health. Based on past literature described above, we hypothesize that AL will be lowest among older adults who experienced high SES across the life course, followed by those who were upwardly mobile and those who were downwardly mobile. Those who experienced low SES across the life course will have the highest AL. In addition, we expect that while Black older adults will have higher levels of AL than Whites across all levels of lifetime SES, the magnitude of the disparity will be largest among those who were upwardly mobile, followed by those who were consistently in higher SES groups across childhood and adulthood. These relationships would stem from the accumulation of stressors and strains across the life course among Blacks, particularly in their achievement of greater socioeconomic resources and in navigating higher SES contexts.
This study advances the literature on health disparities in aging by documenting whether and how well-known determinants of health differentially influence disparities in physiological aging among older adults. Understanding whether social mobility is linked to better health among older adults and the extent to which Black–White disparities in AL is conditional upon lifetime SES is needed to reduce racial health inequalities, as clarifying pathways to poor health will inform more effective public health interventions for older adults.
Method
Sample
We used data from the NSAHS, a community epidemiological survey of Black and White adults in Nashville, TN. A random sample was obtained using a multistage, stratified sampling approach. Although Black American households were oversampled, sampling weights allowed for generalizability to the county population. Between 2011 and 2014, 1252 respondents provided information about their personal and family backgrounds, stress and coping experiences, and health histories during 3-hour computer-assisted interviews with interviewers of the same race. The following day, clinicians made in-home visits, arriving before breakfast to retrieve 12-hour urine samples and collect blood samples. They also measured blood pressure, recorded waist, hip, height, and weight measurements, and documented prescription medication usage. Less than 1% of the sample was missing sociodemographic or biological data due to difficulty in drawing sufficient blood, specimen contamination, or clinician visit refusal. Upon completion of the interviewing period, American Association for Public Opinion Research rates were used to evaluate success across screening and interviewing phases (Response Rate 1 = 30.2, Cooperation Rate 1 = 74.2, Refusal Rate 1 = 30.2, Contact Rate 1 = 40.7). The NSAHS and all study procedures were approved by the Vanderbilt University Institutional Review Board and described in detail elsewhere (55). The analytic sample for this study included Black and White adults aged 50 and older with complete demographic, biomarker, and health factor data (N = 518).
Measures
AL was assessed using 10 biomarkers, including primary mediators and secondary mediators. Primary mediators refer to the substances released by the body in response to stress such as norepinephrine, epinephrine, cortisol, and dehydroepiandrosterone sulfate. Secondary mediators are the effects resulting from the actions of primary mediators, including systolic and diastolic blood pressure, total cholesterol, high-density lipids, glycated hemoglobin, and waist-to-hip ratio (6). Based on guidelines established by the MacArthur studies, each biomarker was designated as low risk (0) or high risk (1) based on established clinical risk levels (31,54,56); individuals taking blood pressure or cholesterol medication were also counted as “high risk” for those biomarkers. Total AL scores were based on a count of these high-risk biomarkers and ranged from 0 to 10, with higher scores indicating greater physiological dysregulation across bodily systems. Consistent with prior research (6), we used a cut-point of 4 to distinguish between individuals with low AL (0 = <4 high-risk biomarkers) and high AL (1 = 4+ high-risk biomarkers).
Race. Respondents self-identified as non-Hispanic White (reference category) or non-Hispanic Black (coded 1).
Lifetime SES was based on 2 measures: childhood SES (CSES) and adult SES (ASES). NSAHS respondents were asked to provide information about “the adult who provided major financial support for their family” during their childhood. Parent’s educational attainment and family’s financial situation (eg, often struggled to pay for food, clothing, and shelter) were assessed categorically. Parent’s occupation was a continuous variable based on the Nam-Powers-Boyd occupational status scale. Scores ranged from 0 to 100, and higher scores indicated more socially prestigious occupations (31). Parent’s education, family financial situation, and parent’s occupational prestige were standardized and summed to create a continuous CSES score with the mean score set to zero; values greater than zero corresponded with “above-average” CSES and values below zero indicated “below-average” CSES levels. ASES scores were based on 3 dimensions: (a) years of education completed, (b) gross annual household income, and (c) occupational prestige level. Each ASES dimension was then standardized, summed, and the total was divided by the number of available dimensions to avoid data loss (57). In this study, ASES was measured continuously, such that scores above zero corresponded with above-average ASES. Our measure of lifetime SES considered individuals’ socioeconomic trajectories and whether their SES levels were generally stable or changed from childhood to adulthood. Consistent with prior studies (32,33), we used a 2-step process to identify these trajectories. First, we dichotomized CSES and ASES scores into low and high based on the sample median. Then, we used these categories to create 4 social mobility trajectories: (0) stable low SES (low CSES and low ASES), (1) upward mobility (low CSES and high ASES), (2) downward mobility (high CSES and low ASES), and (3) stable high SES (high CSES and high ASES).
Sociodemographic characteristics. We also included the following characteristics as covariates. Age was measured continuously in years. Gender was self-reported: (0) women, (1) men. Marital status was assessed categorically (0 = married, 1 = never married, 2 = other [separated, widowed, and divorced]).
Health factors. Based on prior research (6), we also controlled for several health factors associated with AL. Smoking status was measured with a single item that asked, “Are you a current smoker, ex-smoker, or have you never been a smoker?” Non- and former smokers were categorized together, and response options were dichotomized (0 = nonsmoker, 1 = smoker). Respondents’ average alcohol consumption was measured using a single item that asked, “On the days you drank in the past 12 months, about how many drinks did you usually have per day?” Responses were categorized as (0) nondrinker (no drinks) and (1) drinker (1+ drinks). Physical activity was based on federal guidelines from the US Department of Health and Human Services (58), designating those who engaged in fewer than 75 minutes of vigorous activity in a week as “physically inactive” (coded 0) and those who engaged in at least 150 minutes of moderate activity or 75 minutes of vigorous activity per week as “physically active” (coded 1).
Statistical Analyses
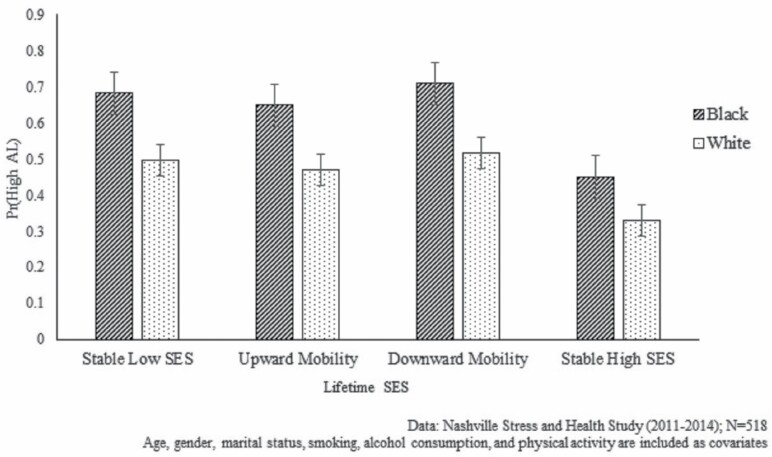
Sample characteristics (means and percentages) were assessed for the full sample and by race in Table 1. Significant Black–White differences in sample characteristics were determined using t-tests (for continuous variables) and chi-square tests of significance (for categorical variables). Second, we examined the relationship between lifetime SES and AL using modified Poisson regression models with robust standard errors (59,60), as the prevalence of our outcome variable (high AL) was greater than 10%. Estimated odds ratios (ORs) and corresponding 95% confidence intervals (95% CIs) are presented in Table 2. Model 1 assessed the association between race and lifetime SES among the full sample. To evaluate the extent to which racial differences in AL varied within levels of lifetime SES, Models 2–5 examined Black–White disparities in high AL within each of the 4 lifetime SES groups. Figure 1 illustrates the predicted probabilities of high AL for each racial group across levels of lifetime SES. Sociodemographic characteristics (age, gender, and marital status) and health factors (smoking status, alcohol consumption, and physical activity) were included as covariates in all models. All analyses were performed using STATA 15.1 (StataCorp LP, College Station, TX).
Table 1.
Sample Characteristics by Race, Nashville Stress and Health Study (2011–2014)
| All (N = 518) | Whites (n = 262) | Blacks (n = 256) | p | |
|---|---|---|---|---|
| Mean (SD) or % | Mean (SD) or % | Mean (SD) or % | ||
| Allostatic load (AL) | ||||
| Low AL (Ref.) | 50.07 | 56.43 | 32.78 | <.001 |
| High AL | 49.93 | 43.57 | 67.22 | |
| Lifetime socioeconomic status (SES) | ||||
| Stable low SES (Ref.) | 29.10 | 20.90 | 51.21 | <.001 |
| Upward mobility | 26.10 | 27.42 | 22.53 | |
| Downward mobility | 12.79 | 11.16 | 17.18 | |
| Stable high SES | 32.02 | 40.52 | 9.09 | |
| Sociodemographic characteristics | ||||
| Age (50–69) | 57.13 (5.05) | 57.42 (4.27) | 56.34 (6.29) | .02 |
| Gender | ||||
| Women (Ref.) | 54.66 | 54.14 | 56.09 | .72 |
| Men | 45.34 | 45.86 | 43.91 | |
| Marital status | ||||
| Married (Ref.) | 62.94 | 71.50 | 39.66 | <.001 |
| Never married | 9.85 | 7.65 | 15.85 | |
| Other | 27.20 | 20.85 | 44.49 | |
| Health factors | ||||
| Smoking | ||||
| Nonsmoker (Ref.) | 75.44 | 78.74 | 66.46 | .01 |
| Current smoker | 24.56 | 21.26 | 33.54 | |
| Alcohol consumption | ||||
| Nondrinker (Ref.) | 53.00 | 51.53 | 57.01 | .26 |
| Drinks alcohol | 47.00 | 48.47 | 42.99 | |
| Physical activity | ||||
| Nonactive (Ref.) | 46.28 | 39.43 | 64.91 | <.001 |
| Active | 53.72 | 60.57 | 35.09 |
Notes: Weighted means and percentages are shown; standard deviations are included in parentheses and ranges are included in brackets for continuous variables. Ref. = reference category.
Table 2.
Association Between Lifetime SES and Allostatic Load Among Older Adults, Nashville Stress and Health Study (2011–2014)
| Model 1 | Model 2 | Model 3 | Model 4 | Model 5 | |
|---|---|---|---|---|---|
| All (N = 518) | Stable low SES (n = 199) | Upward mobility (n = 115) | Downward mobility (n = 74) | Stable high SES (n = 130) | |
| OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | |
| Race | |||||
| White (Ref.) | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Black | 1.37** (1.10–1.70) | 1.02 (0.76–1.37) | 1.76** (1.24–2.51) | 1.38 (0.91–2.11) | 2.22*** (1.37–3.58) |
| Lifetime socioeconomic status (SES) | |||||
| Stable low SES (Ref.) | 1.00 | ||||
| Upward mobility | 0.95 (0.73–1.25) | ||||
| Downward mobility | 1.04 (0.75–1.44) | ||||
| Stable high SES | 0.66* (0.46–0.97) | ||||
| Sociodemographic characteristics | |||||
| Age | 1.02* (1.01–1.05) | 1.00 (0.97–1.03) | 1.07*** (1.03–1.11) | 0.98 (0.91–1.05) | 1.04 (0.98–1.11) |
| Gender | |||||
| Women (Ref.) | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Men | 1.20 (0.96–1.49) | 1.16 (0.87–1.54) | 0.99 (0.68–1.43) | 1.06 (0.63–1.79) | 2.06* (1.08–3.91) |
| Marital status | |||||
| Married (Ref.) | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Never married | 0.81 (0.56–1.16) | 0.90 (0.55–1.49) | 0.68* (0.47–0.97) | 0.91 (0.43–1.94) | 0.84 (0.40–1.77) |
| Other | 0.93 (0.70–1.24) | 1.24 (0.90–1.73) | 0.79 (0.44–1.40) | 0.71 (0.32–1.57) | |
| Health factors | |||||
| Smoking | |||||
| Nonsmoker (Ref.) | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Current smoker | 0.94 (0.74–1.20) | 0.71 (0.50–1.00) | 0.55 (0.28–1.08) | 1.54 (0.96–2.45) | 1.53 (0.76–3.11) |
| Alcohol consumption | |||||
| Nondrinker (Ref.) | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Drinks alcohol | 0.98 (0.77–1.24) | 1.32* (1.03–1.69) | 1.00 (0.66–1.50) | 0.98 (0.56–1.71) | 0.65 (0.37–1.12) |
| Physical activity | |||||
| Nonactive (Ref.) | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Active | 0.77** (0.63–0.95) | 0.82 (0.60–1.11) | 0.67* (0.47–0.96) | 0.97 (0.60–1.56) | 0.82 (0.48–1.40) |
| Intercept | 0.14** (0.03–0.64) | 0.60 (0.09–3.74) | 0.01*** (0.001–0.12) | 1.41 (0.03–72.74) | 0.03 (0.001–1.29) |
Note: Odds ratios (ORs) and 95% confidence intervals (CIs) are shown.
*p < .05, **p < .01, ****p < .001 (2-tailed tests).
Figure 1.
Racial differences in allostatic load (AL) by levels of lifetime SES among older adults. SES = socioeconomic status.
Results
The distribution of sample characteristics for the full sample and by race is displayed in Table 1. Nearly half (49.9%) of the sample had high AL. Among the sample, 29.1% remained in low SES groups across childhood and adulthood (stable low SES) and 32.0% remained in high SES groups (stable high SES); 26.10% were characterized as upwardly mobile while 12.8% were characterized as downwardly mobile. The average age was 57.1 years (SD = 5.05), and most respondents were married (63.0%) at the time of the survey; women comprised more than half of the sample (54.7%), and most were nonsmokers, (75.4%), did not consume alcohol (53.0%), and were physically active (53.7%).
There were also racial differences in the study characteristics. Compared to Whites, a higher percentage of Black older adults had high AL (p < .001). Moreover, additional chi-squared tests (not shown) indicated significant racial differences in lifetime SES, such that there were only significant Black–White differences in stable low SES (p < .001) and stable high SES (p < .001). Black and White older adults experienced upward and downward mobility at similar rates. However, White older adults were significantly older (p < .05), more likely to be married (p < .001), and more physically active (p < .001), while more Black adults were current smokers (p < .01). Levels of alcohol consumption were similar across racial groups.
The association between lifetime SES and AL among older adults is shown in Table 2, Model 1. Compared to those of stable low SES, older adults of stable high SES had significantly lower odds of high AL (OR = 0.66, 95% CI = 0.46–0.97). All other lifetime SES groups had similar odds of high AL as adults of stable low SES. Moreover, results from Model 1 indicate that on average, Black older adults had higher AL than Whites. Specifically, Blacks had 37% greater odds of high AL (OR = 1.37, 95% CI = 1.10–1.70) relative to Whites.
The next set of models (Table 2, Models 2–5) display Black–White disparities in AL among older adults within each category of lifetime SES. Results from Model 2 suggest no significant racial difference in AL among older adults who remained in low SES groups during childhood and adulthood. The same pattern was found for Black and White adults who were downwardly mobile (see Model 4). In contrast, there was a significant racial difference in AL (p < .01) among older adults who experienced upward mobility (ie, low CSES and high ASES). Upwardly mobile Black adults had 76% greater odds of high AL (OR = 1.76, 95% CI = 1.24–2.51) compared to upwardly mobile White adults (see Model 3). Additionally, among those who remained in high SES groups, Black adults had more than twice the odds (OR = 2.22, 95% CI = 1.37–3.58) of high AL relative to Whites (see Model 5). Collectively, these findings support our hypotheses.
Results from Models 2–5 are illustrated in Figure 1. While odds of high AL were generally lowest among the stable high SES group and highest among the stable low SES group among White older adults—consistent with expectations of the FCT—stable high SES did not confer the same benefits for Black older adults. For example, Black older adults with stable high SES or who had experienced upward mobility had greater odds of high AL than Whites who remained in low SES groups across the life course. Differences in the odds of high AL between lifetime SES groups were also smaller among Black older adults than among Whites. Taken together, results suggest fewer health returns to higher lifetime SES among Black older adults relative to White older adults.
Discussion
Black–White disparities in health persist across the life course, with Black adults experiencing shorter, sicker lives relative to Whites (1,3). While SES plays an important role in generating these inequalities, Black–White disparities in health often persist after taking SES into account (16,61). One possible explanation is that prior work tends to focus on SES at one point in time, leaving unclear whether and how changes in SES between childhood and adulthood may differentiate the extent of racial disparities in health at older ages. Examining whether the existence and magnitude of racial health disparities in older adulthood are contingent on lifetime SES provides critical insight into the underpinnings of persistent racial disparities in health and aging. Moreover, identifying the life course circumstances that result in larger Black–White disparities in health highlights priority areas to target for intervention. This study contributes to such knowledge by combining research on the diminishing returns hypothesis and life course perspectives to examine Black–White disparities in AL within categories of lifetime SES among older adults.
First, we assessed the relationship between lifetime SES and AL. Results indicated that among older adults, stable high SES was linked to lower AL relative to all other lifetime SES groups. Prior research has noted consistent SES gradients in AL among the general population (62) and suggests that changes in SES over time affect AL. For instance, a study by Singer and Ryff (34) compared the AL scores of individuals based on their socioeconomic mobility experiences. While they found that upwardly mobile individuals had AL scores that were consistent with those in the stable high SES group, those who experienced downward mobility had AL levels most like those with stable low SES. In contrast, results from this study suggest that SES mobility is not significantly associated with high AL among older adults. Prior research notes that individuals may receive fewer health benefits from SES resources in older adulthood than in early and middle adulthood (62). While all individuals are susceptible to age-associated increases in AL (62), the health benefits accumulated by older adults with high SES may diminish with age. For instance, some studies have reported that high SES older adults had AL scores similar to those of middle-aged adults with low SES (62), indicating that older adults may experience diminishing health despite access to socioeconomic resources. Taken together, these findings suggest that while lifetime SES importantly shapes AL among the general population, changes in socioeconomic resources, particularly at older ages, may have only a limited direct impact on the AL of older adults.
The central goal of this study, however, was to evaluate the extent to which Black–White disparities in AL varied across levels of lifetime SES. While Black older adults were more likely to report high AL compared to White older adults, regardless of lifetime SES (Table 2, Model 1), stratifying the association between race and AL by lifetime SES revealed additional nuances. For instance, there were no significant racial disparities observed among those with stable low SES. Such findings suggest that Black and White older adults who experienced low SES throughout their lives had similar levels of AL. Although prior research has noted worse health among Blacks relative to Whites at all levels of SES (16,17), our findings are more consistent with previous studies demonstrating that Blacks and Whites who experience similar levels of social disadvantage face comparable health risks (63). Specifically, these studies highlight the significance of place for shaping health disparities and suggest that social contextual factors may play a more important role in shaping SES-related health risks and benefits (63). Similarly, our results also suggest that Blacks and Whites who faced downward mobility—starting off with high SES during childhood and transitioning to low SES later in life—had comparable AL levels in older adulthood. Recent studies underscore the significance of social stress for shaping the links between social mobility and health among racial groups, noting that downward mobility confers heightened stress exposure among Whites, while Black Americans tend to experience high stress regardless of their social mobility status (64). Thus, the stress associated with the loss of high SES may trigger elevated AL among downwardly mobile Whites, such that their AL scores are comparable to those of their Black counterparts.
In contrast, findings from this study indicated there was a significant Black–White disparity in AL among those who experienced upward mobility and remained in high SES groups across childhood and adulthood. These patterns provide support for the diminishing returns hypothesis (38,64,65), as greater racial disparities were observed among high SES groups compared to low SES groups. Given the documented challenges associated with achieving high SES among Black Americans (65,66), it is possible that these disparities stem from Blacks’ heightened exposure to environmental and psychosocial stressors across the life course. In particular, the efforts required for older Black Americans to achieve or maintain high SES may contribute to greater stress and elevated AL relative to their White counterparts (38,47,67). While previous studies have documented the diminishing returns of higher SES for a range of physical and mental health outcomes (19,24,46), this study is among the first to demonstrate the impact of lifetime SES on AL among older adults. Understanding the significance of lifetime SES for physiological outcomes such as AL is important because it provides insight into the ways that social inequalities become embodied to shape health and later-life trajectories.
In addition, results from this study suggest that lifetime SES had a distinct impact on AL among Black and White older adults (Figure 1). A subtle SES gradient in AL was observed among Whites. While stable high SES was associated with the lowest odds of high AL, upward and downward mobility, as well as stable low SES, all conferred similar health risks among this group. Among Blacks, however, a different pattern emerged, as the odds of high AL were notably high among Black older adults, regardless of lifetime SES. These findings also lend support to the diminishing returns hypothesis (64), indicating that while lifetime SES significantly patterns racial disparities in AL, it does not account for SES differences in AL among older Black Americans. Specifically, the lack of a clear SES gradient in AL (ie, higher SES being associated with lower AL) suggests that higher SES—in childhood/adulthood or achieved through upward mobility—is related to few health benefits for Black Americans. As such, these findings are consistent with the notion that SES is not equivalent across racial groups (18). That is, due to systemic racism, Black Americans tend to have lower earnings than Whites at a given level of education, less wealth at a given level of income, and less purchasing power due to higher prices of goods and services in their communities (11,61). Such factors differentiate the ability of Black adults to acquire and activate potentially protective resources. Consequently, Black Americans may receive limited health benefits for their efforts; moreover, the stress associated with these challenges may undermine health. This may be particularly important for understanding racial inequalities in aging, as these diminishing returns likely contribute to increased disparities in later life. However, additional research is needed to identify the pathways undergirding these unexpected outcomes among older adults.
The results of this study should be considered within the context of several limitations. First, this study utilized cross-sectional data from a regional sample of Black and White older adults in the NSAHS. Thus, we are unable to make conclusions about causality. In addition, the use of a regional sample from Nashville, TN limits our ability to generalize these results to other contexts. As such, future studies should evaluate these relationships in nationally representative longitudinal data to clarify the relationships between race, lifetime SES, and AL among older adults over time. Second, our study focused on Black and White adults aged 50–69. Thus, we are unable to draw conclusions about the impact of lifetime SES among those aged 70 years and older. Nonetheless, we were able to assess these patterns among older adults within a racially and socioeconomically diverse sample. Future research should extend this work by evaluating the role of lifetime SES in shaping racial disparities among older samples. Third, as there were a limited number of respondents who reported experiencing downward mobility, findings among this group should be interpreted with caution. Finally, our study examines lifetime SES using an index of CSES measures (ie, parent’s education, parent’s occupation, and family financial situation) and ASES measures (ie, education, income, occupational prestige). Although these indicators provide a comprehensive assessment of SES throughout the life course, we did not assess measures such as wealth or homeownership. Given that prior research documents racial differences in the accumulation of wealth across the life course (41), future studies should also consider the ways that differences in these factors may pattern racial disparities in AL among older adults.
Nevertheless, this study advances our understanding of health disparities in aging in several ways. First, these findings contribute to accumulating evidence that socioeconomic resources do not translate into equivalent health benefits for Black and White adults, challenging the utility of traditional fundamental causes to explain racial disparities in health and aging. Future work should seek to identify and test unique factors that may play a role in shaping health across the life course above and beyond individual-level SES indicators (eg, cultural racism, intergenerational trauma). Second, we extend prior work on the diminishing returns hypothesis by considering the extent to which racial disparities in AL persist across levels of lifetime SES among older adults. Although a growing body of research has documented diminishing returns for higher SES among Black Americans (36), few studies have evaluated racial differences in the health benefits of SES among older adults. As such, it was unclear whether these patterns persist in later life. Indeed, results from the present study confirm that older Black Americans experience limited benefits of higher SES. Moreover, we find important nuances in the role of social mobility among this population, demonstrating the need to consider SES at multiple points during the life course. Failure to do so may obscure key differences in the relationship between SES and health across racial groups. Relatedly, few studies have evaluated the processes linking lifetime SES to older adult health (68,69), and most studies assessing diminishing returns among Blacks have not focused on physiological outcomes. Our study advances research on physiological aging by assessing racial and lifetime SES patterns in AL among older adults. AL captures the wear and tear on the body due to cumulative exposure to stress and is a significant predictor of mortality, co-occurring chronic conditions, and preclinical health status across multiple systems (33,55,62). It therefore provides important insight into health inequalities among older adults. Taken together, our findings suggest that lifetime SES and changes in socioeconomic resources across the life course are important for understanding the nature of racial disparities among older adults. This study also highlights the need for policy-based interventions that consider the unique health risks among this population, particularly among high SES and upwardly mobile older Black Americans.
Funding
Data collection for the Nashville Stress and Health Study was supported by a grant (R01-AG034067) from the Office of Behavioral and Social Science Research and the National Institute on Aging to R. Jay Turner. C.S.T.T. received support from the following sources: (1) The University of California, Los Angeles, Resource Centers for Minority Aging Research Center for Health Improvement of Minority Elderly (RCMAR/CHIME) under NIH/NIA Grant P30-AG021684; (2) The UCLA Clinical and Translational Science Institute (CTSI) under NIH/NCATS UCLA CTSI Grant Number UL1TR001881; (3) The California Center for Population Research at UCLA (CCPR), which receives core support (P2C- HD041022) from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD). T.W.H. was supported by the National Institute of Institute of Child Health and Human Development (NICHD) under award number P2C HD050924 and the National Institute on Aging (NIA) of the National Institutes of Health under award number P30 AG066615. The contents presented here are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health. Data collection for the Nashville Stress and Health Study was supported by a grant (R01-AG034067) from the Office of Behavioral and Social Science Research and the National Institute on Aging to R. Jay Turner. C.S.T.T. received support from the following sources: (1) The University of California, Los Angeles, Resource Centers for Minority Aging Research Center for Health Improvement of Minority Elderly (RCMAR/CHIME) under National Institutes of Health/National Institute on Aging Grant P30-AG021684; (2) The UCLA Clinical and Translational Science Institute (CTSI) under NIH/NCATS UCLA CTSI Grant Number UL1TR001881; (3) The California Center for Population Research at UCLA (CCPR), which receives core support (P2C- HD041022) from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD). T.W.H. was supported by the National Institute of Institute of Child Health and Human Development (NICHD) under award number P2C HD050924 and the National Institute on Aging (NIA) of the National Institutes of Health under award number P30 AG066615. The contents presented here are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
Conflict of Interest
None declared.
References
- 1. National Center for Health Statistics. With special feature on racial and ethnic health disparities . 2015. Accessed March 5, 2021. https://www.cdc.gov/nchs/data/nvsr/nvsr65/nvsr65_04.pdf
- 2. Kung HC, Hoyert DL, Xu J, Murphy SL. Deaths: final data for 2005. National vital statistics reports: from the Centers for Disease Control and Prevention, National Center for Health Statistics, Natl Vital Stat System. 2008;56(10):1–20. doi:https://stacks.cdc.gov/view/cdc/22317 [PubMed] [Google Scholar]
- 3. Thorpe RJ Jr, Fesahazion RG, Parker L, et al. Accelerated health declines among African Americans in the USA. J Urban Health. 2016;93:808–819. doi: 10.1007/s11524-016-0075-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Boen C. Death by a thousand cuts: stress exposure and Black–White disparities in physiological functioning in late life. J Gerontol B Psychol Sci Soc Sci. 2020;75:1937–1950. doi: 10.1093/geronb/gbz068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mitchell UA, Ailshire JA, Crimmins EM. Change in cardiometabolic risk among blacks, whites, and Hispanics: findings from the Health and Retirement Study. J. Gerontol A Biol Sci Med Sci. 2019;74:240–246. doi: 10.1093/gerona/gly026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Geronimus AT, Hicken M, Keene D, Bound J. “Weathering” and age patterns of allostatic load scores among blacks and whites in the United States. Am J Public Health. 2006;96:826–833. doi: 10.2105/AJPH.2004.060749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Levine ME, Crimmins EM. Evidence of accelerated aging among African Americans and its implications for mortality. Soc Sci Med. 2014;118:27–32. doi: 10.1016/j.socscimed.2014.07.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Phelan JC, Link BG. Is racism a fundamental cause of inequalities in health? Annu Rev Sociol. 2015;41:311–330. doi: 10.1146/annurev-soc-073014-112305 [DOI] [Google Scholar]
- 9. Adler NE, Stewart J. Health disparities across the lifespan: meaning, methods, and mechanisms. Ann N Y Acad Sci. 2010;1186:5–23. doi: 10.1111/j.1749-6632.2009.05337 [DOI] [PubMed] [Google Scholar]
- 10. Chen E, Miller GE. Socioeconomic status and health: mediating and moderating factors. Annu Rev Clin Psychol. 2013;9:723–749. doi: 10.1146/annurev-clinpsy-050212-185634 [DOI] [PubMed] [Google Scholar]
- 11. Williams DR, Collins C.. Racial Residential Segregation: A Fundamental Cause of Racial Disparities in Health. Los Angeles, CA: SAGE Publications; 2001. Accessed November 14, 2020. https://journals.sagepub.com/doi/abs/10.1093/phr/116.5.404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Williams DR, Priest N, Anderson NB. Understanding associations among race, socioeconomic status, and health: patterns and prospects. Health Psychol. 2016;35:407–411. doi: 10.1037/hea0000242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Brown T, Brown TH, Richardson LJ, Hargrove TW, Thomas CS. Health disparities over the life course using multiple-hierarchy stratification and life course approaches to understand health inequalities: the intersecting consequences of race, gender, SES, and age. J Health Soc Behav. 2016;57:200–222. doi: 10.1177/0022146516645165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hayward MD, Miles TP, Crimmins EM, Yang Y. The significance of socioeconomic status in explaining the racial gap in chronic health conditions. Am Sociol Rev. 2000;65:910–930. doi: 10.2307/2657519 [DOI] [Google Scholar]
- 15. Williams DR, Mohammed SA, Leavell J, Collins C. Race, socioeconomic status, and health: complexities, ongoing challenges, and research opportunities. Ann N Y Acad Sci. 2010;1186:69–101. doi: 10.1111/j.1749-6632.2009.05339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Williams DR, Priest N, Anderson N. Understanding associations between race, socioeconomic status, and health: patterns and prospects. In The Social Medicine Reader, Volume II, Third Edition. 2019.(pp. 258-267). Duke University Press. doi: 10.1037/hea0000242 [DOI] [Google Scholar]
- 17. Braveman PA, Cubbin C, Egerter S, Williams DR, Pamuk E. Socioeconomic disparities in health in the United States: what the patterns tell us. American journal of public health . 2010 Apr;100(S1):S186-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bell CN, Sacks TK, Thomas Tobin CS, Thorpe RJ Jr. Racial non-equivalence of socioeconomic status and self-rated health among African Americans and whites. SSM Popul Health. 2020;10:100561. doi: 10.1016/j.ssmph.2020.100561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Farmer MM, Ferraro KF. Are racial disparities in health conditional on socioeconomic status?. Social science & medicine. 2005. Jan 1; 60(1):191–204. Published online 2005. doi: 10.1016/j.socscimed.2004.04.026 [DOI] [PubMed] [Google Scholar]
- 20. Wilson KB, Thorpe RJ Jr, LaVeist TA. Dollar for dollar: racial and ethnic inequalities in health and health-related outcomes among persons with very high income. Prev Med (Baltim). 2017;96:149–153. doi: 10.1016/j.ypmed.2016.08.038 [DOI] [PubMed] [Google Scholar]
- 21. Colen CG, Ramey DM, Cooksey EC, Williams DR. Racial disparities in health among nonpoor African Americans and Hispanics: the role of acute and chronic discrimination. Soc Sci Med. 2018;199:167–180. doi: 10.1016/j.socscimed.2017.04.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Assari S, Nikahd A, Malekahmadi MR, Lankarani MM, Zamanian H. Race by gender group differences in the protective effects of socioeconomic factors against sustained health problems across five domains. J Racial Ethn Health Disparities. 2017;4:884–894. doi: 10.1007/s40615-016-0291-3 [DOI] [PubMed] [Google Scholar]
- 23. Kimbro RT, Bzostek S, Goldman N, Rodríguez G. Race, ethnicity, and the education gradient in health. Health Aff. 2008;27:361–372. doi: 10.1377/hlthaff.27.2.361 [DOI] [PubMed] [Google Scholar]
- 24. Boen C. The role of socioeconomic factors in Black–White health inequities across the life course: point-in-time measures, long-term exposures, and differential health returns. Soc Sci Med. Published online 2016. doi: 10.1016/j.socscimed.2016.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Friedman EM, Karas Montez J, McDevitt Sheehan C, Guenewald TL, Seeman TE. Childhood adversities and adult cardiometabolic health: does the quantity, timing, and type of adversity matter? J Aging Health. 2015;27:1311–1338. doi: 10.1177/0898264315580122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yang YC, Gerken K, Schorpp K, Boen C, Harris KM. Early-life socioeconomic status and adult physiological functioning: a life course examination of biosocial mechanisms. Biodemography Soc Biol. 2017;63:87–103. doi: 10.1080/19485565.2017.1279536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Shaefer HL, Lapidos A, Wilson R, Danziger S. Association of income and adversity in childhood with adult health and well-being. Soc Serv Rev. 2018;92:69–92. doi: 10.1086/696891 [DOI] [Google Scholar]
- 28. Ferraro, K. F., Shippee, T. P., & Schafer, M. H. Cumulative inequality theory for research on aging and the life course. In Bengston V. L., Gans D., Pulney N. M., & Silverstein M. (Eds.), Handbook of theories of aging. 2009. (pp. 413–433). Springer Publishing Company. Accessed November 14, 2020. https://psycnet.apa.org/record/2009-01257-022 [Google Scholar]
- 29. Ferraro KF, Kemp BR, Williams MM. Diverse aging and health inequality by race and ethnicity. Innov Aging. 2017;1:1–11. doi: 10.1093/geroni/igx002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Diprete TA, Eirich GM. Cumulative advantage as a mechanism for inequality: a review of theoretical and empirical developments. Annu Rev Sociol. 2006;32:271–297. doi: 10.1146/annurev.soc.32.061604.123127 [DOI] [Google Scholar]
- 31. Turner RJ, Thomas CS, Brown TH. Childhood adversity and adult health: evaluating intervening mechanisms. Soc Sci Med. 2016;156:114–124. doi: 10.1016/j.socscimed.2016.02.026 [DOI] [PubMed] [Google Scholar]
- 32. Luo Y, Waite LJ. The impact of childhood and adult SES on physical, mental, and cognitive well-being in later life. Accessed August 23, 2017. https://academic.oup.com/psychsocgerontology/article/60/2/S93/546786 [DOI] [PMC free article] [PubMed]
- 33. Gruenewald TL, Karlamangla AS, Hu P, et al. History of socioeconomic disadvantage and allostatic load in later life. Soc Sci Med. 2012;74:75–83. doi: 10.1016/j.socscimed.2011.09.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Singer B, Ryff CD. Hierarchies of life histories and associated health risks. Ann N Y Acad Sci. 1999;896(1):96–115. doi: 10.1111/j.1749-6632.1999.tb08108 [DOI] [PubMed] [Google Scholar]
- 35. Präg P, Richards L. To cite: Präg P, Richards L. J Epidemiol Community Health. 2019;73:100–105. doi: 10.1136/jech-2017-210171 [DOI] [PubMed] [Google Scholar]
- 36. Assari S. Health disparities due to diminished return among Black Americans: public policy solutions. Soc Issues Policy Rev. 2018;12:112–145. doi: 10.1111/sipr.12042 [DOI] [Google Scholar]
- 37. Cole ER, Omari SR. Race, class and the dilemmas of upward mobility for African Americans. J Soc Issues. 2003;59:785–802. doi: 10.1046/j.0022-4537.2003.00090 [DOI] [Google Scholar]
- 38. Hudson DL, Puterman E, Bibbins-Domingo K, Matthews KA, Adler NE. Race, life course socioeconomic position, racial discrimination, depressive symptoms and self-rated health. Soc Sci Med. 2013;97:7–14. doi: 10.1016/j.socscimed.2013.07.031 [DOI] [PubMed] [Google Scholar]
- 39. Bailey ZD, Feldman JM, Bassett MT. How structural racism works—racist policies as a root cause of U.S. racial health inequities. N Engl J Med. 2020;384:768–773. doi: 10.1056/nejmms2025396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Williams DR, Lawrence JA, Davis BA. Racism and health: evidence and needed research. Annu Rev Public Health. 2019;40:105–125. doi: 10.1146/annurev-publhealth-040218-043750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Brown TH. Diverging fortunes: racial/ethnic inequality in wealth trajectories in middle and late life. Race Soc Probl. 2016;8:29–41. doi: 10.1007/s12552-016-9160-2 [DOI] [Google Scholar]
- 42. Pager D, Shepherd H. The sociology of discrimination: racial discrimination in employment, housing, credit, and consumer markets. Annu Rev Sociol. 2008;34:181–209. doi: 10.1146/annurev.soc.33.040406.131740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Pager D, Western B, Sugie N. Sequencing disadvantage: barriers to employment facing young black and white men with criminal records. Ann Am Acad Pol Soc Sci. 2009;623:195–213. doi: 10.1177/0002716208330793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Brody GH, Lei MK, Chae DH, Yu T, Kogan SM, Beach SR. Perceived discrimination among African American adolescents and allostatic load: a longitudinal analysis with buffering effects. Child Dev. 2014;85(3):989–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hoggard LS, Volpe V, Thomas A, Wallace E, Ellis K. The role of emotional eating in the links between racial discrimination and physical and mental health. J Behav Med. 2019;42:1091–1103. doi: 10.1007/s10865-019-00044-1 [DOI] [PubMed] [Google Scholar]
- 46. Hudson DL, Puterman E, Bibbins-Domingo K, Matthews KA, Adler NE. Race, life course socioeconomic position, racial discrimination, depressive symptoms and self-rated health. Soc Sci Med. 2013;97:7–14. doi: 10.1016/j.socscimed.2013.07.031 [DOI] [PubMed] [Google Scholar]
- 47. Gaydosh L, Schorpp KM, Chen E, Miller GE, Harris KM. College completion predicts lower depression but higher metabolic syndrome among disadvantaged minorities in young adulthood. Proc Natl Acad Sci USA. 2018;115:109–114. doi: 10.1073/pnas.1714616114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Hudson DL, Neighbors HW, Geronimus AT, Jackson JS. Racial discrimination, John Henryism, and depression among African Americans. J Black Psychol. 2015;42:221–243. doi: 10.1177/0095798414567757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Williams DR, Mohammed SA. Discrimination and racial disparities in health: evidence and needed research. J Behav Med. 2009;32:20–47. doi: 10.1007/s10865-008-9185-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Williams DR, Lawrence JA, Davis BA. Racism and health: evidence and needed research. Annu Rev Public Health. 2019;40:105–125. doi: 10.1146/annurev-publhealth-040218-043750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Assari S, Lankarani MM. Association between stressful life events and depression: intersection of race and gender. J Racial Ethn Disparities. Published online 2015. Accessed September 24, 2017. https://www.researchgate.net/profile/Shervin_Assari/publication/283198644_Association_Between_Stressful_Life_Events_and_Depression_Intersection_of_Race_and_Gender/links/5631063208ae0530378cff91.pdf [DOI] [PubMed] [Google Scholar]
- 52. Lewis TT, Everson-Rose SA, Powell LH, et al. Chronic exposure to everyday discrimination and coronary artery calcification in African-American women: the SWAN Heart Study. Psychosom Med. 2006;68:362–368. doi: 10.1097/01.psy.0000221360.94700.16 [DOI] [PubMed] [Google Scholar]
- 53. Thorpe RJ Jr, Cobb R, King K, Bruce MA, Archibald P, Jones HP, Norris KC, Whitfield KE, Hudson D. The association between depressive symptoms and accumulation of stress among Black men in the Health and Retirement Study. Innovation in aging. 2020;4(5):igaa047-. doi: 10.1093/geroni/igaa047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. McEwen BS, Seeman T. Protective and damaging effects of mediators of stress elaborating and testing the concepts of allostasis and allostatic load. Ann N Y Acad Sci. 1999;896:30–47. doi: 10.1111/j.1749-6632.1999.tb08103 [DOI] [PubMed] [Google Scholar]
- 55. Brown TN, Turner RJ, Moore TR.. The multidimensionality of health: associations between allostatic load and self-report health measures in a community epidemiologic study. Health Sociol Rev. 2016;25:272–287. doi: 10.1080/14461242.2016.1184989 [DOI] [Google Scholar]
- 56. Crimmins EM, Johnston M, Hayward M, Seeman T. Age differences in allostatic load: an index of physiological dysregulation. In: Experimental Gerontology. Vol 38. doi: 10.1016/S0531-5565(03)00099-8 [DOI] [PubMed] [Google Scholar]
- 57. Erving CL, Thomas CS. Race, emotional reliance, and mental health. Soc Ment Health. 2018;8(1):69–83. doi: 10.1177/2156869317713552 [DOI] [Google Scholar]
- 58. Current Guidelines | health.gov. Accessed November 14, 2020. https://health.gov/our-work/physical-activity/current-guidelines
- 59. Zou G. A modified Poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159:702–706. doi: 10.1093/aje/kwh090 [DOI] [PubMed] [Google Scholar]
- 60. McNutt L-A. Estimating the relative risk in cohort studies and clinical trials of common outcomes. Am J Epidemiol. 2003;157:940–943. doi: 10.1093/aje/kwg074 [DOI] [PubMed] [Google Scholar]
- 61. Williams DR, Mohammed SA, Leavell J, Collins C. Race, socioeconomic status and health: Complexities, ongoing challenges and research opportunities. Annals of the New York Academy of Sciences. 2010;1186:69. doi: 10.1111/j.1749-6632.2009.05339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Seeman T, Epel E, Gruenewald T, Karlamangla A, McEwen BS. Socio‐economic differentials in peripheral biology: Cumulative allostatic load. Ann N Y Acad Sci. 2010;1186(1):223–239. doi: 10.1111/j.1749-6632.2009.05341 [DOI] [PubMed] [Google Scholar]
- 63. LaVeist T, Pollack K, Thorpe R, Fesahazion R, Gaskin D. Place, not race: disparities dissipate in Southwest Baltimore when blacks and whites live under similar conditions. Health Aff. 2011;30:1880–1887. doi: 10.1377/hlthaff.2011.0640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Assari S. Health disparities due to diminished return among Black Americans: public policy solutions. Soc Issues Policy Rev. 2018;12:112–145. doi: 10.1111/sipr.12042 [DOI] [Google Scholar]
- 65. Cole ER, Omari SR. Race, class and the dilemmas of upward mobility for African Americans. J Soc Issues. 2003;59(4):785–802. doi: 10.1046/j.0022-4537.2003.00090 [DOI] [Google Scholar]
- 66. Hudson D, Sacks T, Irani K, Asher A. The price of the ticket: health costs of upward mobility among African Americans. Int J Environ Res Public Health. 2020;17:1179. doi: 10.3390/ijerph17041179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Brody GH, Yu T, Chen E, Miller GE, Kogan SM, Beach SR. Is resilience only skin deep?: rural African Americans’ socioeconomic status-related risk and competence in preadolescence and psychological adjustment and allostatic load at age 19. Psychol Sci. 2013;24:1285–1293. doi: 10.1177/0956797612471954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Yang YC, Gerken K, Schorpp K, Boen C, Harris KM. Biodemography and social biology early-life socioeconomic status and adult physiological functioning: a life course examination of biosocial mechanisms. Biosoc Mech Biodemography Soc Biol. 2017;63:87–103. doi: 10.1080/19485565.2017.1279536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Yang YC, Schorpp K, Boen C, Johnson M, Harris KM. Socioeconomic status and biological risks for health and illness across the life course. J Gerontol B Psychol Sci Soc Sci. 2020;75:613–624. doi: 10.1093/geronb/gby108 [DOI] [PMC free article] [PubMed] [Google Scholar]