Abstract
All human Shiga toxin-producing Escherichia coli (STEC) non-O157 strains (n = 56) isolated in Finland from 1990 to August 2000 were characterized for the O:H serotype, stx1 and stx2 genes, production of enterohemolysin, and sensitivity to 12 antimicrobial agents. Strains of the same serotype were genotyped by pulsed-field gel electrophoresis (PFGE) after XbaI restriction of total DNA. The 56 non-O157 isolates belonged to 29 serotypes. Two of the serotypes (O102:H7 and OX181:H49) have not previously been described as being associated with STEC infections in humans or isolated from animals. Thirty-four strains (61%) within seven serotypes (O103:H2 [14 isolates], O26:H11 [6 isolates], O145:H28 [4 isolates], O145:HNM [3 isolates], O15:HNM [3 isolates], OX174:H21 [2 isolates], and O Rough:HNM [2 isolates]) were represented by more than one isolate. Of these strains, O103:H2 isolates were divided into seven, O26:H11 isolates were divided into four, and the rest within a serotype were divided into two genotypes in PFGE. In PCR, 31 (55%) of the 56 strains were positive for the stx2 gene only and 24 strains (43%) were positive for stx1 only. One strain (O43:H2) carried both stx1 and stx2. Forty-two strains (75%) produced enterohemolysin, and 39 strains (70%) possessed the eae gene. Of the latter 39 strains, 36 (92%) were enterohemolytic, whereas only 6 (35%) of the 17 isolates lacking the eae gene were enterohemolytic (P < 0.001). The majority of the strains (44 strains, 79%) were sensitive to all 12 antimicrobials tested. Of the 56 strains, 20 (36%) were associated with small family outbreaks in nine families and 14 (25%) were associated with recent travel abroad.
Shiga toxin-producing Escherichia coli (STEC) is a serologically diverse group of foodborne, zoonotic pathogens, of which the serotype O157:H7 has been epidemiologically significant worldwide (11; http://www.who.int/emc-documents/zoonooses/whocsraph988.c.html). However, altogether about 250 non-O157 STEC serotypes have been reported and more than 100 of them have been associated with human illness (http://www.microbionet.com.au/frames/feature/vtec/brief01.html). In some geographic areas, non-O157 strains are more commonly isolated from persons with diarrhea or hemolytic-uremic syndrome (HUS) than are O157 STEC strains (32). From the epidemiologic point of view, the most important non-O157 STEC serotypes have been O26:H11/HNM, O103:H2, O111:HNM, and O145:HNM (http://www.who.int/emc-documents/zoonooses/whocsraph988.c.html). All STEC strains have stx1 and/or stx2 genes determining the production of Shiga toxin (Stx). The carriage of the stx1 gene is most common among O26, O103, and O111 strains (4, 12, 20, 28, 31), even though some of the O26 and O111 strains possess stx2, emphasizing the genetic diversity of the STEC non-O157 bacteria (17, 36). Several outbreaks caused by strains of these O groups have been described in various European countries (6). In Australia, infections caused by STEC O160 strains producing Stx1 and Stx2 have been reported (22). In the United States, O4:HNM, O45:H2, O111:HNM, and O145:HNM strains were isolated from sporadic cases of hemorrhagic colitis in the early 1980s (34). There are also a few reports of an association of STEC O17:H18, O103:H2, O145:H28, and OX3:H2 strains with urinary tract infections (30, 33).
For rapid and sensitive detection of STEC non-O157 strains from clinical samples, PCR has proven to be of great diagnostic value in the detection of stx genes (22). In the epidemiologic research, pulsed-field gel electrophoresis (PFGE) has been very discriminative in the molecular comparison of STEC strains independent of the serotype (28, 36). The antimicrobial resistance patterns may be an additional epidemiologic marker for surveys of non-O157 STEC strains (9).
In Finland, all suspected STEC isolates from humans have been referred to the Laboratory of Enteric Pathogens of the National Public Health Institute, since the 1980s for verification and further investigations. In the present study, we utilized PCR for the stx genes, O:H serotyping, enterohemolysin (Ehly) detection, and antimicrobial susceptibility testing and we used PFGE for the characterization of all E. coli non-O157 strains isolated from Finns with STEC infection during a 10-year period.
[The data were partly presented as a poster at the 4th International Symposium and Workshop on Shiga Toxin (Verocytotoxin)-Producing Escherichia coli Infections, Kyoto, Japan, 29 October to 2 November 2000].
MATERIALS AND METHODS
Bacterial strains.
E. coli non-O157 fecal isolates (n = 56) from 55 Finns with STEC infection found from May 1990 to August 2000 were characterized. The suspected STEC cultures were submitted to the Laboratory of Enteric Pathogens of the National Public Health Institute from Finnish clinical microbiological laboratories mainly as primary cultures of fecal specimens taken from patients with clinical symptoms suggesting STEC infection. At the Laboratory of Enteric Pathogens, the presence or absence of the stx1, stx2 and eae genes was investigated by PCR (see below) and specific STEC colonies were subsequently purified as previously described (15). The isolates were identified biochemically by API 20 E (BioMérieux SA, Marcy l'Etoile, France). The ability to ferment sorbitol was confirmed on sorbitol MacConkey agar and in tubes containing 0.5% sorbitol. The relevant patient data, including information on recent foreign travel, were collected on a special form that always accompanied the isolate.
PCR amplification methods.
The stx1, stx2 and eae genes were investigated as described previously (15) with the following modifications. For stx1, 1.5 μl of boiled bacterial supernatant was used as template and 0.5 μl of AmpliTaq Gold (Perkin Elmer, Roche Molecular Systems, Inc., Branchburg, N.J.) was used as the polymerase. The steps from denaturation to elongation were repeated 35 times. The PCR amplification mixture for the detection of stx2 contained 26.2 μl of sterile water, 3.5 μl of 10× PCR buffer solution (Finnzymes, Espoo, Finland), 0.98 μl of 4 dNTP mix (containing 5 mM dATP, 5 mM dCTP, 5 mM dTTP, and 5 mM dGTP), 1.0 μl each of forward and reverse primers (10.0 μM), and 0.7 μl of DNA polymerase (Dynazyme II; Finnzymes). Predenaturation was carried out for 4.5 min at 95°C, and annealing was carried out for 1 min at 62°C. The eae gene was amplified by using 27.0 μl of sterile water, 3.5 μl of 10× PCR buffer solution, 0.98 μl of 4 dNTP mix 0.59 μl each of the forward and reverse primers (10.0 μM), and 0.6 μl of DNA polymerase (Dynazyme II).
Serotyping.
O:H serotyping of the strains was performed by bacterial agglutination as described previously (21, 26). Strains giving clumping with 4% saline were defined as O Rough. The O157 antigen was also tested with the E. coli O157 antigen kit (Oxoid, Basingstoke, England). Strains which did not move through a semisolid agar tube after cultivation for 5 days were defined as nonmotile. O antisera were from the Statens Serum Institute, Copenhagen, Denmark. H antisera were kindly received from Yuli Ratiner (the Mechnikov Research Institute for Vaccines and Sera, Russian Academy of Medical Science, Moscow, Russia).
Detection of Ehly.
Enterohemolytic activity of the strains was detected on tryptose agar plates (Difco Laboratories, Detroit, Mich.) supplemented with 10 mM CaCl2 and 5% defibrinated sheep blood washed three times in phosphate-buffered saline, (pH 7.2) as described previously (2). The inoculated plates were observed for hemolysis after 3 h of incubation (for detection of alpha-hemolysis) and after overnight incubation in ambient air at 37°C (for detection of enterohemolysis or nonhemolysis).
Antimicrobial susceptibility testing.
The antimicrobial susceptibilities of the strains were determined by the agar diffusion method (19) on Iso-Sensitest agar (Oxoid) to the following 12 antimicrobial agents (Oxoid): ampicillin, chloramphenicol, streptomycin, sulfonamide, tetracycline, ciprofloxacin, trimethoprim, gentamicin, nalidixic acid, cefotaxime, mecillinam, and imipenem.
PFGE.
PFGE was performed for all strains as described previously (29), except that the PFGE images were analyzed by BioNumerics version 2.0 software (Applied Maths bvba). A library of the different profiles of the strains belonging to the same serotype was collected. A single-fragment difference was defined as significant, and the subtypes were coded referring to the O group (O26a-O26e, O103a-O103h, etc.). The PFGE patterns of the strains of a distinct serotype were coded as single.
Calculation of similarity indices.
The BioNumerics software version 2.0 (Applied Maths bvba) was used for calculating the Dice similarity indices (tolerance 1.0%, unweighted pair group method using arithmetic averages) in the cluster analysis.
Statistical methods.
Fisher's exact test was used for statistical analysis. P < 0.05 indicated statistical significance.
RESULTS
From May 1990 to August 2000, 56 non-O157 STEC human isolates were found in Finland. The isolates belonged to 20 different O groups and 29 O:H serotypes (Table 1). Thirty-four (61%) of the strains belonged to the following seven serotypes: O103:H2 (14 isolates), O26:H11 (6 isolates), O145:H28 (4 isolates), O145:HNM (3 isolates), O15:HNM (3 isolates), OX174:H21 (2 isolates), and O Rough:HNM (2 isolates). Each of the remaining 22 isolates belonged to a serotype of its own (Table 1).
TABLE 1.
E. coli non-O157 strains isolated in Finland from January 1990 to August 2000
| Yr | Country where infection acquired | Serotype | PCRa result | Antimicrobial resistanceb | PFGE subtypec | Ehlyd | Familye |
|---|---|---|---|---|---|---|---|
| 1998 | Italy | O15:HNM | stx1 +, eae + | s | O15a | + | A |
| 1998 | Italy | O15:HNM | stx1 +, eae + | s | O15a | + | A |
| 1998 | Finland | O15:HNM | stx2 +, eae + | Ampr, Strr, Sulr | O15b | + | Spor. |
| 1997 | Finland | O26:H11 | stx1 +, eae + | s | O26a | + | Spor. |
| 1997 | Finland | O26:H11 | stx1 +, eae + | s | O26b | + | B |
| 1997 | Finland | O26:H11 | stx1 +, eae + | s | O26b | + | B |
| 1996 | Singapore | O26:H11 | stx1 +, eae + | Strr | O26c | + | Spor. |
| 1998 | The Netherlands | O26:H11 | stx1 +, eae + | Strr | O26d | + | C |
| 1998 | Finland | O26:H11 | stx1 +, eae + | Strr | O26d | + | C |
| 1997 | Finland | O26:HNM | stx1 +, eae + | Strr, Sulr | O26e | − | Spor. |
| 1998 | Finland | O103:H2 | stx2 +, eae + | s | O103a | + | Spor. |
| 1998 | Finland | O103:H2 | stx2 +, eae + | s | O103a | + | D |
| 1998 | Finland | O103:H2 | stx2 +, eae + | s | O103a | + | D |
| 1998 | Finland | O Rough:H2 | stx2 +, eae + | s | O103a | + | D |
| 1998 | Finland | O103:H2 | stx1 +, eae + | Tetr | O103b | + | Spor. |
| 1999 | Finland | O103:H2 | stx1 +, eae + | s | O103b | + | E |
| 1999 | Finland | O103:H2 | stx1 +, eae + | s | O103b | + | E |
| 1999 | Finland | O103:H2 | stx1 +, eae + | s | O103c | + | F |
| 1999 | Finland | O103:H2 | stx1 +, eae + | s | O103c | + | F |
| 1999 | Finland | O103:H2 | stx1 +, eae + | s | O103c | + | F |
| 1999 | Finland | O103:H2 | stx1 +, eae + | s | O103d | + | Spor. |
| 1999 | Finland | O103:H2 | stx1 +, eae + | s | O103e | + | G |
| 1999 | Finland | O103:H2 | stx1 +, eae + | s | O103e | + | G |
| 2000 | Finland | O103:H2 | stx1 +, eae + | s | O103f | + | Spor. |
| 2000 | Finland | O103:H2 | stx1 +, eae + | Tetr | O103g | + | Spor. |
| 2000 | Tunisia | O103:HNM | stx1 +, eae + | s | O103h | + | Spor. |
| 1999 | Finland | O145:H28 | stx2 +, eae + | s | O145a | + | H |
| 1999 | Finland | O145:H28 | stx2 +, eae + | s | O145a | + | H |
| 1999 | Finland | O145:H28 | stx2 +, eae + | s | O145a | + | I |
| 1999 | Finland | O145:H28 | stx2 +, eae + | s | O145a | + | I |
| 1999 | Finland | O145:HNM | stx2 +, eae + | s | O145b | + | Spor. |
| 1999 | Finland | O145:HNM | stx2 +, eae + | s | O145b | + | Spor. |
| 1999 | Switzerland | O145:HNM | stx2 +, eae + | Chlr, Strr, Sulr, Tetr | O145c | + | Spor. |
| 1996 | Finland | OX174:H21 | stx2 +, eae − | s | Oxa | − | Spor. |
| 1999 | Finland | OX174:H21 | stx2 +, eae − | s | Oxb | − | Spor. |
| 1998 | The Netherlands | O Rough:HNM | stx2 +, eae − | Strr, Sulr, Tetr | Ra | + | Spor. |
| 1999 | Tunisia | O Rough:HNM | stx1 +, eae + | s | Rb | + | Spor. |
| 1997 | Finland | O2:H29 | stx2 +, eae − | s | Single | − | Spor. |
| 1999 | Finland | O8:H9 | stx2 +, eae − | s | Single | − | Spor. |
| 1998 | Spain | O20:H7 | stx2 +, eae − | s | Single | − | Spor. |
| 1990 | Finland | O43:H2 | stx1,2,+ eae− | s | Single | + | Spor. |
| 1999 | Finland | O76:H19 | stx2 +, eae − | s | Single | + | Spor. |
| 1997 | Finland | O91:H21 | stx2 +, eae − | s | Single | − | Spor. |
| 1996 | Turkey | O91:H40 | stx2 +, eae + | Tetr | Single | − | Spor. |
| 1997 | Finland | O101:HNM | stx2 +, eae + | s | Single | − | Spor. |
| 1996 | Finlandf | O102:H7 | stx2 +, eae − | s | Single | − | Spor. |
| 1997 | France | O107:H27 | stx2 +, eae − | s | Single | − | Spor. |
| 2000 | Morocco | O111:H8 | stx1 +, eae + | Ampr, Strr, Sulr, Tetr, Tmpr, Nalr | Single | + | Spor. |
| 1996 | Abroadg | O116:H21 | stx2 +, eae − | s | Single | + | Spor. |
| 2000 | Finland | O156:H25 | stx1 +, eae + | s | Single | + | Spor. |
| 1997 | Finland | O165:H25 | stx2 +, eae + | s | Single | + | Spor. |
| 1998 | Finland | OX174:H2 | stx2 +, eae − | s | Single | + | Spor. |
| 1999 | Finland | OX181:H49 | stx2 +, eae − | s | Single | + | Spor. |
| 1996 | Abroadh | O Rough:H18 | stx2 +, eae − | s | Single | − | Spor. |
| 1999 | Finland | O Rough:H21 | stx2 +, eae − | s | Single | − | Spor. |
| 1996 | Finland | O Rough:H49 | stx2 +, eae − | Strr | Single | − | Spor. |
Carriage of stx1 or stx2 or eae gene; +, positive; −, negative.
s, sensitive; r, resistant. The following antimicrobial agents were used in the susceptibility test: ampicillin (Amp), chloramphenicol (Chl), streptomycin (Str), sulfonamide (Sul), tetracycline (Tet), ciprofloxacin, trimethoprim (Tmp), gentamicin, nalidixic acid (Nal), cefotaxime, mecillinam, and imipenem.
PFGE subtypes are coded on the basis of the serotype. Single, a PFGE subtype dissimilar to all other subtypes.
Ehly production: +, Ehly positive; − Ehly negative (no hemolysis or alpha-hemolysis).
Capital letters (A to I), infection within a family; Spor, sporadic infection.
Isolated from a subject before the subject participated in a round-the-world trip.
Isolated from another subject on return from a round-the-world trip.
Isolated from the subject in footnote f on return from the round-the-world trip.
In PCR, 31 (55%) of the 56 strains were positive for the stx2 gene only and 24 (43%) were positive for the stx1 gene only (Table 1). One strain (O43:H2) carried both stx1 and stx2. The stx1 gene was highly associated with O26 (7 of 7 isolates were stx1 positive), O103 (12 of 15) and O15 (2 of 3), whereas all seven O145 isolates were positive for stx2 only. Thirty-nine strains (70%) representing 10 O groups (O15, O26, O91, O101, O103, O111, O145, O156, O165, and O Rough) carried the eae gene, and 42 strains (75%) produced Ehly. Of the 39 isolates possessing eae, 36 (92%) were enterohemolytic, whereas only 6 (35%) of the 17 isolates lacking eae were enterohemolytic (P < 0.001). All except one strain (O76:H19) fermented sorbitol, and one strain (O Rough:HNM) produced urease. The majority of the strains (44 of 56 [79%]) were sensitive to all 12 antimicrobial agents tested (Table 1).
A total of 14 of the 56 strains (25%) belonging to 11 serotypes were associated with a recent trip of more than 4 days abroad: to France (O107:H27), Italy (O15:HNM, two strains), Morocco (O111:H8), The Netherlands (O Rough:HNM and O26:H11), Tunisia (O103:HNM and O Rough:HNM), Singapore (O26:H11), Spain (O20:H7), Switzerland (O145:HNM), Turkey (O91:H41), or a round-the-world trip (O116:H21 and O Rough:H18).
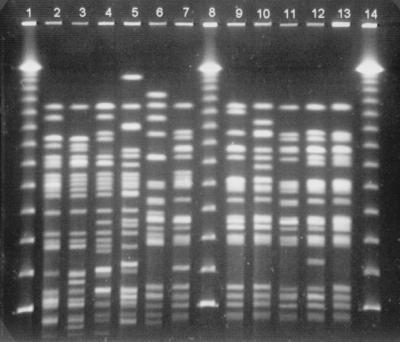
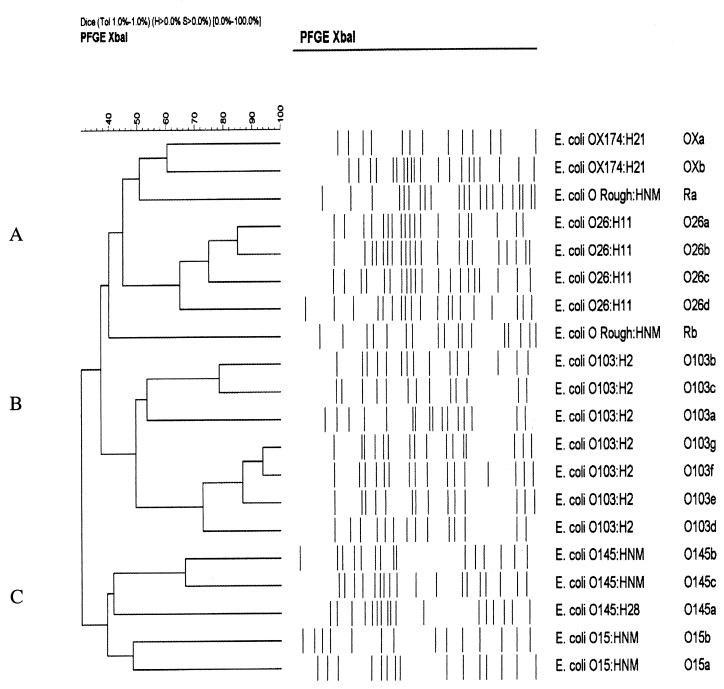
In the molecular comparison of the strains within the same serotype, several PFGE subtypes were identified (Fig. 1): seven subtypes among 14 O103:H2 strains, four among 6 O26:H11 strains, two among 2 OX174:H21 strains, two among 3 O145:HNM strains, two among 3 O15:HNM strains, and two among 2 O Rough:HNM strains (Table 1). Only one PFGE subtype was detected among four O145:H28 strains. Isolates from members of the same family had the same O:H serotype and PFGE subtype, except for one family D isolate, which had lost the O antigen. In the cluster analysis of the subtypes belonging to the same serotype, three DNA fingerprint profile clusters (A, B, and C) were identified (Fig. 2). The most closely related isolates were the four strains of O26:H11 (62 to 85% similarity) within cluster A; within cluster B, the subtypes of seven isolates of O103:H2 had a similarity of 50 to 92%. The two isolates of OX174:H21 had a 60% similarity in cluster A. Cluster C included five different subtypes from serotypes O15 and O145, which were the most heterogeneous (40% similarity).
FIG. 1.
PFGE fingerprint patterns of the STEC O26:H11 and STEC O103:H2 isolates. Lanes: 1, 8, 14, bacteriophage lambda ladder PFG marker 340 (New England Biolabs Inc., Beverly, Mass.); 2, subtype O26a; 3, subtype O26b; 4, subtype O26c; 5, subtype O26d; 6, subtype O103a; 7, subtype O103b; 9, subtype O103c; 10, subtype O103d; 11, subtype O103e; 12, subtype O103f; 13, subtype O103g.
FIG. 2.
Cluster analysis of PFGE subtypes of STEC non-O157 isolates of the same serotype.
DISCUSSION
In Finland, 56 STEC non-O157 strains were isolated from humans. During approximately the same time period, the number of STEC O157 strains isolated was 105 (29). Among the 56 STEC non-O157 strains, this study revealed a wide spectrum of 29 STEC O:H serotypes isolated from humans. Of these, 24 serotypes (83%) have been isolated from humans previously (http://www.microbionet.com.au/frames/feature/vtec/brief01.html). Of the remaining serotypes, three, O8:H9, O43:H2, and O Rough:H18, have previously been linked only to nonhuman sources, i.e., cattle or some other animal reservoir (10, 18, 27). All these isolates carried stx2, but the strain of serotype O43:H2 also possessed stx1; none carried the eae gene. In addition to these three serotypes, we found two other serotypes, O102:H7 and OX181:H49, that have never before been isolated from humans, or animals, or other nonhuman sources. Both isolates carried stx2 only but not the eae gene. Interestingly, the infections caused by four strains of these rare serotypes (O8:H9, O43:H2, O102:H7, and OX181:H49) were indigenously acquired.
The majority (53%) of the strains found in this study belonged to O groups O26, O103, and O145. In Continental Europe, the most common STEC non-O157 O groups are O111, O26, O103, and O145, which have been reported in 11, 10, 7, and 5 countries, respectively (6). In Hungary, serogroup O26 was retrospectively recognized as a cause of a nosocomial outbreak as early as 1977 (6). In the Czech Republic, strains of this serogroup were causative agents in 31% of STEC infections in a nursery from 1988 to 1995 (4). All of our O26 strains carried the stx1 gene. Similar associations have also been described in previous reports (3, 17, 31). However, both the stx1 and stx2 genes have also been found among this O group (28), and O26 strains possessing only the stx2 gene have been described (25, 36). The number of infections caused by strains of serotype O103:H2 in Finland during 1998 to 2000 was considerable (25% of all 56 non-O157 STEC findings). All these infections were of indigenous origin. In addition, 79% of 14 strains carried the stx1 gene. Among O103 strains, the distribution of the stx genes has is closely similar to that of the O group O26 (31).
On the basis of cluster analysis between O26 and O103 strains, we also observed a similarity of about 40%. Strains of O103:H2 have been associated with human infections worldwide (http://www.microbionet.com.au/frames/feature/vtec/brief01.html). A nonmotile strain of this O group (O103:HNM) isolated during the study period was of foreign origin (Tunisia). Only three previous cases of human disease due to O103:HNM have been reported in the literature: in the United States, Australia, and Germany (1)(http://www.microbionet.com.au /frames/feature/vtec/brief01.html). The O group O145 was the second most common STEC non-O157 O group in Finland. All seven O145 strains possessed only the stx2 gene. Human strains of O145 have been reported to carry stx1 and/or stx2 (12, 25). One of the three nonmotile O145 strains was associated with a trip to Switzerland. In Japan, a similar strain caused an outbreak with 100 cases (16).
On the basis of the data collected on the form accompanying each isolate, only 25% of the infections were of foreign origin. Although O111 has been the major group of strains responsible for non-O157 STEC infections reported (1) and has also caused outbreaks in Europe (6), only one strain of this O group (O111:H8) was found in Finland, and it was linked to a recent trip to Morocco.
In routine diagnostics, there is no definitive biochemical characteristic, such as sorbitol fermentation, which would distinguish STEC strains belonging to serogroups other than O157 from commensal E. coli members of the flora. However, nearly all O157 STEC strains and a significant proportion of non-O157 strains have been observed to produce Ehly (24), a putative virulence factor that also facilitates the detection of various STEC isolates (2, 35). In our study, 75% of the non-O157 strains produced Ehly, and we used this phenotypic characteristic to facilitate the isolation of sorbitol-positive non-O157 STEC strains. It also seems that the Ehly production is associated with carriage of the eae gene; strains possessing the eae gene were statistically significantly more likely to be enterohemolytic than were the strains which did not carry this gene (92 and 35%, respectively, were enterohemolytic). A previous study similarly suggested an association among the locus of enterocyte effacement (i.e., the location of the eae gene), the enterohemorrhagic E. coli hemolysin plasmid, and the hemolysin itself among STEC non-O157 isolates (5). In our study, 70% of the strains carried the eae gene. All strains within a certain O group were always either eae negative or eae positive, excluding the O groups O91 and O Rough, which contained eae-positive and eae-negative strains. In other studies, strains belonging to serogroup O113 have been described as being associated with human disease, often with HUS, even though they do not possess the eae gene (7, 23). Furthermore, in our previous studies, STEC strains of serotypes O Rough:H49 and OX3:H21, both negative for the eae gene, were causative agents of HUS (13, 14). The data obtained in the present study emphasize the need for additional research into the role of the eae gene or other putative factors affecting the virulence of STEC strains.
Resistance to antimicrobial agents can be a useful epidemiological marker for STEC strains (http://www.who.int/emc-documents/zoonooses/whocsraph988.c.html) as suggested also by Izumikawa et al. (9). In the present study, the majority (79%) of the 56 strains were sensitive to all 12 antimicrobials tested. However, the strains associated with foreign travels were often multiresistant.
This study revealed a wide variety of non-O157 STEC serotypes, including serotypes O102:H7 and OX181:H49, that have not previously been isolated from humans, animals, or other nonhuman sources. Without efficient Stx toxin and stx gene detection, these infections would not have been diagnosed. Thus, the data emphasize continued, comprehensive detection and epidemiologic research in STEC diagnostics in Finland and worldwide to detect these infections efficiently.
Despite the epidemic potential of STEC bacteria, the infections in Finland were sporadic, even though 20 infections were associated with small family clusters in nine families. This might be due to easy spread of non-O157 infections by person-to-person contact, by consumption of food items, or via other vehicles of transmission. Thus, to find the clusters of STEC infections, the epidemiologic study of the infecting strains should be as precise as possible. The standardization of the PFGE method and computer-based submission of the genomic profiles enables very discriminative and rapid comparison of STEC strains among laboratories. In the United States a national network (PulseNet) of public health laboratories that performs DNA fingerprinting has been developed for this purpose (8). In Finland, a similar electronic database for comparison of human and animal STEC isolates has been generated for STEC O157 (29). However, on the European level, the genomic profiles of STEC isolates can at present be compared only on an intralaboratory basis. Judging from the vast number of STEC non-O157 findings of same serotypes (http://www.microbionet.com.au/frames/feature/vtec/brief01.html), the standardization of the PFGE method for STEC non-O157 would be needed for efficient international epidemiologic research.
ACKNOWLEDGMENTS
We are grateful to Yuli Ratiner at the Mechnikov Research Institute for Vaccines and Sera, Russian Academy of Medical Science, Moscow, Russia, for kindly providing the H antisera. We thank Tarja Heiskanen and Sirkku Waarala for excellent laboratory assistance.
REFERENCES
- 1.Bettelheim K A. Role of non-O157 VTEC. J Appl Microbiol Suppl. 2000;88:38S–50S. doi: 10.1111/j.1365-2672.2000.tb05331.x. [DOI] [PubMed] [Google Scholar]
- 2.Beutin L, Montenegro M A, Ørskov I, Ørskov F, Prada J, Zimmermann S, Stephan R. Close association of verotoxin (Shiga-like toxin) production with enterohemolysin production in strains of Escherichia coli. J Clin Microbiol. 1989;27:2559–2564. doi: 10.1128/jcm.27.11.2559-2564.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beutin L, Aleksic S, Zimmermann S, Gleier K. Virulence factors and phenotypical traits of verotoxigenic strains of Escherichia coli isolated from human patients in Germany. Med Microbiol Immunol. 1994;183:13–21. doi: 10.1007/BF00193627. [DOI] [PubMed] [Google Scholar]
- 4.Bielaszewska M, Janda J, Blahova K, Feber J, Potuznik V, Souckova A. Verocytotoxin-producing Escherichia coli in children with hemolytic uremic syndrome in the Czech Republic. Clin Nephrol. 1996;46:42–22. [PubMed] [Google Scholar]
- 5.Boerling P, Chen S, Colbourne J K, Johnson R, De Grandis S, Gyles C L. Evolution of enterohemorrhagic Escherichia coli hemolysin plasmids and the locus for enterocyte effacement in Shiga toxin-producing E. coli. Infect Immun. 1998;66:2553–2561. doi: 10.1128/iai.66.6.2553-2561.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caprioli A, Tozzi A E. Epidemiology of Shiga toxin-producing Escherichia coli infections in continental Europe. In: Kaper J B, O'Brien A D, editors. Escherichia coli O157:H7 and other Shiga toxin-producing E. coli strains. Washington, D.C.: American Society for Microbiology; 1998. pp. 38–48. [Google Scholar]
- 7.Dytoc M T, Ismaili A, Philpott D J, Soni R, Brunton J L, Sherman P. Distinct binding properties of eaeA-negative verocytotoxin-producing Escherichia coli of serotype O113:H21. Infect Immun. 1994;62:3494–3505. doi: 10.1128/iai.62.8.3494-3505.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hilborn E D, Mermin J H, Mshar P A, Hadler J L, Voetsch A, Wojtkunski C, Swartz M, Mshar R, Lambert-Fair M A, Farrar J A, Glynn M K, Slutsker L. A multistate outbreak of Escherichia coli O157:H7 infections associated with consumption of mesclun lettuce. Arch Intern Med. 1999;159:1758–1764. doi: 10.1001/archinte.159.15.1758. [DOI] [PubMed] [Google Scholar]
- 9.Izumikawa K, Hirakata Y, Yamaguchi T, Yoshida R, Nakano M, Matsuda J, Mochida C, Maesaki S, Tomono K, Yamada Y, Tashiro T, Kohno S, Kamihira S. Analysis of genetic relationships and antimicrobial susceptibility of Verotoxin-producing Escherichia coli strains isolated in Nagasaki Prefecture, Japan in 1996. Microbiol Immunol. 1998;42:677–681. doi: 10.1111/j.1348-0421.1998.tb02339.x. [DOI] [PubMed] [Google Scholar]
- 10.Johnson W M, Pollard D R, Lior H, Tyler D, Rozee K R. Differentiation of genes coding for Escherichia coli verotoxin 2 and the verotoxin associated with porcine edema disease (VTe) by the polymerase chain reaction. J Clin Microbiol. 1990;28:2351–2353. doi: 10.1128/jcm.28.10.2351-2353.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karmali M A. Infection by verocytotoxin-producing Escherichia coli. Clin Microbiol Rev. 1989;2:15–38. doi: 10.1128/cmr.2.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karch H, Schubert S, Zhang D, Zhang W, Schimdt H, Ölschläger T, Hacker J. A genomic island, termed high-pathogenicity island is present in certain non-O157 Shiga toxin-producing Escherichia coli clonal lineages. Infect Immun. 1999;67:5994–6001. doi: 10.1128/iai.67.11.5994-6001.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Keskimäki M, Ikäheimo R, Kärkkäinen P, Scheutz F, Puohiniemi R L, Siitonen A. Shiga toxin producing Escherichia coli serotype OX3:H21 causing hemolytic uremic syndrome. Clin Infect Dis. 1997;24:1278–1279. doi: 10.1086/513668. [DOI] [PubMed] [Google Scholar]
- 14.Keskimäki M, Ratiner Y, Oinonen S, Leijala E, Nurminen M, Saari M, Siitonen A. E. coli Rough:K:H49 causing HUS. Scand J Infect Dis. 1998a;31:141–144. doi: 10.1080/003655499750006173. [DOI] [PubMed] [Google Scholar]
- 15.Keskimäki M, Saari M, Heiskanen T, Siitonen A. Shiga toxin-producing Escherichia coli in Finland from 1990 through 1997: prevalence and characteristics of isolates. J Clin Microbiol. 1998b;36:3641–3646. doi: 10.1128/jcm.36.12.3641-3646.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kudoh Y, Kai A, Obata H, Kusunoki J, et al. Epidemiological surveys on verocytotoxin-producing Escherichia coli infections in Japan. In: Karmali M A, Goglio A G, editors. Recent advances in verocytotoxin-producing Escherichia coli infections. (Excerpta Medica International Congress Series 1072.) Amsterdam, The Netherlands: Elsevier Science; 1994. pp. 53–56. [Google Scholar]
- 17.Law D. Virulence factors of Escherichia coli O157 and other Shiga toxin-producing E. coli. J Appl Microbiol. 2000;88:729–745. doi: 10.1046/j.1365-2672.2000.01031.x. [DOI] [PubMed] [Google Scholar]
- 18.Montenegro M M, Bülte M, Trumpf T, Aleksic S, Reuter G, Bulling E, Helmuth R. Detection and characterization of fecal verotoxin-producing Escherichia coli from healthy cattle. J Clin Microbiol. 1990;28:1417–1421. doi: 10.1128/jcm.28.6.1417-1421.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.National Committee for Clinical Laboratory Standards. Performance standards for antimicrobial disk susceptibility tests—approved standard, 6th ed. NCCLS Document M2–A6. Villanova, Pa: NCCLS; 1997. [Google Scholar]
- 20.Ojeda A, Prado V, Martinez J, Arellano C, Borczyk A, Johnson W, Lior H, Levine M M. Sorbitol-negative phenotype among enterohemorrhagic Escherichia coli strains of different serotypes and from different sources. J Clin Microbiol. 1995;33:2199–2201. doi: 10.1128/jcm.33.8.2199-2201.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ørskov F, Ørskov I. Serotyping of Escherichia coli. Methods Microbiol. 1984;14:43–112. [Google Scholar]
- 22.Paton A W, Ratcliff R M, Doyle R M, Seymour-Murray J, Davos D, Lanser J A, Paton J. Molecular microbiological investigation of an outbreak of hemolytic-uremic syndrome caused by dry fermented sausage contaminated with Shiga-like toxin-producing Escherichia coli. J Clin Microbiol. 1996;34:1622–1627. doi: 10.1128/jcm.34.7.1622-1627.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paton A, Woodrow M C, Doyle R M, Lanser J A, Paton J. Molecular characterization of a Shiga toxigenic Escherichia coli O113:H21 strain lacking eae responsible for a cluster of cases of hemolytic-uremic syndrome. J Clin Microbiol. 1999;37:3357–3361. doi: 10.1128/jcm.37.10.3357-3361.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Paton J, Paton A. Pathogenesis and diagnosis of Shiga toxin-producing Escherichia coli infections. Clin Microbiol Rev. 1998;11:450–479. doi: 10.1128/cmr.11.3.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pierard D, Cornu G, Proesmans W, Dediste A, Jacobs F, Van de Walle J, Mertens A, Ramet J, Lauwers S the Belgian Society for Infectiology and Clinical Microbiology HUS Study Group. Hemolytic uremic syndrome in Belgium: incidence and association with verocytotoxin-producing Escherichia coli infection. Clin Microbiol Infect. 1999;5:16–22. doi: 10.1111/j.1469-0691.1999.tb00093.x. [DOI] [PubMed] [Google Scholar]
- 26.Ratiner Y. Serotyping of Escherichia coli flagellar antigens. In: Stain G, Fünfstück R, editors. Harnwegsinfektion. Aktuelle Gesichtspunkte zur Pathogenese, Diagnostic und Therapie. II. Wissenschftliches Symposium. Frankfurt am Main, Germany: pmi-Verlag GmbH; 1991. pp. 47–51. [Google Scholar]
- 27.Richter H, Klie H, Timm M, Gallien P, Steinruck H, Perlberg K W, Protz D. Verotoxin-producing E. coli (VTEC) in feces from cattle slaughtered in Germany: Berl. Munch Tieraerztl Wochenschr. 1997;110:121–127. [PubMed] [Google Scholar]
- 28.Rios M, Prado V, Trucksis M, Arellano C, Borie C, Alexandre M, Fica A, Levine M M. Clonal diversity of Chilean isolates of enterohemorrhagic Escherichia coli from patients with hemolytic-uremic syndrome, asymptomatic subjects, animal reservoirs, and food products. J Clin Microbiol. 1999;37:778–781. doi: 10.1128/jcm.37.3.778-781.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saari M, Cheasty T, Leino K, Siitonen A. Phage and genotypes of Shiga toxin-producing Escherichia coli O157 in Finland. J Clin Microbiol. 2000;39:1140–1143. doi: 10.1128/JCM.39.3.1140-1143.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Scheutz F, Olesen B, Nørgaard A. Two cases of human urinary tract infection complicated by hemolytic uremic syndrome caused by verotoxin-producing Escherichia coli. Clin Infect Dis. 2000;31:815–816. doi: 10.1086/314019. [DOI] [PubMed] [Google Scholar]
- 31.Schmidt H, Geitz C, Tarr P I, Frosch M, Karch H. Non-O157:H7 pathogenic Shiga toxin-producing Escherichia coli: phenotypic and genetic profiling of virulence traits and evidence for clonality. J Infect Dis. 1999;179:115–123. doi: 10.1086/314537. [DOI] [PubMed] [Google Scholar]
- 32.Strockbine N A, Wells J G, Bopp C A, Barrett T J. Overview of detection and subtyping methods. In: Kaper J B, O'Brien A D, editors. Escherichia coli O157:H7 and other Shiga toxin-producing E. coli strains. Washington, D.C.: American Society for Microbiology; 1998. pp. 331–356. [Google Scholar]
- 33.Tarr P I, Fouser L S, Stapleton A E, Wilson R A, Kim H H, Vary J C, Jr, Clausen C R. Hemolytic-uremic syndrome in a six-year-old girl after a urinary tract infection with Shiga-toxin-producing Escherichia coli O103:H2. N Engl J Med. 1996;335:635–638. doi: 10.1056/NEJM199608293350905. [DOI] [PubMed] [Google Scholar]
- 34.Tzipori S, Wachsmuth K I, Smithers J, Jackson C. Studies in gnotobiotic piglets on non-O157:H7 Escherichia coli serotypes isolated from patients with hemorrhagic colitis. Gastroenterology. 1988;94:590–597. doi: 10.1016/0016-5085(88)90228-4. [DOI] [PubMed] [Google Scholar]
- 35.Wieler L H, Busse B, Steinrück H, Beutin L, Weber A, Karch H, Baljer G. Enterohemorrhagic Escherichia coli (EHEC) strains of serogroup O118 display three distinctive clonal groups of EHEC pathogens. J Clin Microbiol. 2000;38:2162–2169. doi: 10.1128/jcm.38.6.2162-2169.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang W-L, Bielaszewska M, Liesegang A, Tschäpe H, Schmidt H, Bitzan M, Karch H. Molecular characteristics and epidemiological significance of Shiga toxin-producing Escherichia coli O26 strains. J Clin Microbiol. 2000;38:2134–2140. doi: 10.1128/jcm.38.6.2134-2140.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]