Abstract
Background
We aimed to examine if neighborhood social cohesion moderated longitudinal associations between baseline reports of discrimination and 10-year changes in leukocyte telomere length (LTL).
Methods
Data are from the Multi-Ethnic Study of Atherosclerosis (N = 1064; age range 45–84 years). Baseline discrimination was measured using the Major Experiences of Discrimination Scale (MDS; none, 1 domain, ≥2 domains) and the Experiences of Discrimination Scale (EDS; none, moderate, high). Neighborhood social cohesion at baseline was assessed via a community survey within census tract–defined neighborhoods. 10-year change in LTL was defined as regression to the mean-corrected 10-year difference in the ratio of telomeric DNA to a single-copy gene (T/S).
Results
In linear mixed-effects models, we found that neighborhood social cohesion modified the effect of baseline reports of MDS on 10-year changes in LTL, independent of sociodemographic characteristics, health behaviors, and health conditions (p(χ 2) = .01). Among those residing in neighborhoods with low social cohesion, experiencing major discrimination in ≥2 domains was associated with faster LTL attrition over 10 years, compared to reporting no discrimination (β = −0.03; 95% confidence interval: −0.06, −0.003). We found no main associations for either discrimination measure and no interaction between EDS and neighborhood social cohesion.
Conclusions
Results indicate that neighborhood social cohesion is an important dimension of the neighborhood context that may moderate the impact of major experiences of discrimination on telomere length attrition. These findings help advance our understanding of the integral role that neighborhood environments play in attenuating the effect of discrimination on accelerated cell aging.
Keywords: Discrimination, Multilevel stress, Neighborhood social cohesion, Telomeres
Discrimination, broadly defined as the unfair and unjust treatment of a group or an individual based on their sociopolitically ascribed identities, such as race, is a pervasive problem that continues to directly and indirectly hinder the health and well-being of marginalized populations (1). In addition to limiting an individual’s access to material and psychosocial resources that promote healthy behaviors, discrimination has been shown to influence numerous ill-health indicators, such as diabetes mellitus (2), hypertension (3,4), cardiovascular diseases (5), inflammation (6), and a range of poor mental health conditions (7). Chronic experience of unfair treatment and the repeated activation of biologic stress response over time lead to physiologic wear and tear and the dysregulation of multiple organ systems, which then put individuals at an elevated risk for a myriad of adverse health outcomes (8,9).
One measure of physiologic wear and tear that may elucidate the biological pathways through which discrimination is embodied is telomere length. Telomeres are protective caps made of DNA–protein complexes that prevent the ends of the human chromosome from deterioration. Telomere length is a known marker of biological aging, and short telomeres have been associated with increased risk of poor health, including several cardiometabolic disorders (10–12), cognitive decline (13), and even premature mortality (14–16). While telomeres naturally shorten due to age from cellular division, a significant body of research has consistently shown that telomere length may shorten as a result of sustained psychosocial stress via maladaptive physiologic stress response systems (17,18). Although findings for main-effects associations have been mixed, early evidence from studies investigating the link between discrimination and telomere length suggests that in specific population subgroups, experiencing discrimination may be associated with short telomeres (19). For example, in a recent cross-sectional study, Sullivan et al. (20) found that everyday discrimination was associated with short telomeres among Black and White women, while Lee et al. (21) found that major lifetime discrimination was cross-sectionally linked with short telomeres among older Black adults (mean age; 70 years). In addition to inconsistent findings, this body of work remains limited by the primary use of cross-sectional data, which restricts the ability to establish temporal relationships and explicate time-dependent mechanisms (19). Furthermore, the multilevel contexts that may play important roles in the relationship between discrimination and its adverse health consequences remain less well understood (1).
Neighborhood environments, especially neighborhood social environments, are important factors to consider in assessing the negative impacts of discrimination on disease risk and aging biomarkers. Positive neighborhood social environments, often characterized by strong social connectedness and collective efficacy, may have potential protective effects against chronic psychosocial stressors, including discrimination. Neighborhood social cohesion, a tenet of collective efficacy and a reflection of mutual trust within residents of a neighborhood, has been documented to have health rewards across a variety of outcomes (22,23). Neighborhoods with strong social cohesion allow their residents to garner a sense of community, belongingness, and support, creating a healthy coping environment from stressors both in and outside of their neighborhood (24,25). In other words, individuals who live in neighborhoods with high social cohesion may be able to practice beneficial coping strategies when faced with different social adversities, including discrimination, hence ameliorating their potential negative health consequences (26).
These findings underscore the need to more closely examine whether the relationship between discrimination and health manifests differently based on the multilevel contexts that surround individuals, in order to identify structural factors that may heighten the health impacts of discrimination, to promote healthy living and aging, and to ultimately eliminate pervasive health inequities. Furthermore, it is important to assess this link in racially and ethnically diverse populations using rich longitudinal data, to elucidate temporal associations, reduce study biases, and improve the generalizability of study findings (27,28). However, to the best of our knowledge, no study to date has assessed the relationship between experiences of discrimination, neighborhood social environments, and telomere length change over time. To address these important gaps, this study leveraged longitudinal and multilevel data from the Multi-Ethnic Study of Atherosclerosis (MESA) to determine whether the association between discrimination and the rate of leukocyte telomere length (LTL) attrition over 10 years was moderated by neighborhood social cohesion. We hypothesized that individuals reporting discrimination would experience faster telomere length attrition and that associations would be more pronounced among those residing in neighborhoods with low social cohesion, compared to those living in highly cohesive neighborhoods.
Method
Study Sample
We utilized data from the MESA. Details about MESA’s study design are described in depth elsewhere (29). In short, MESA is a cohort of 6814 older adults aged 45–84 years, recruited across 6 sites in the United States: Baltimore, MD; Chicago, IL; St. Paul, MN; Los Angeles, CA; New York, NY; and Forsyth County, NC, between the years 2000 and 2002. Participants were free of clinical cardiovascular disease at baseline and subsequent study waves occurred in approximate 2-year intervals. Data for the current analyses are from a random subset of MESA participants who were selected into the Stress Ancillary Study. This subset included White, Black, and Hispanic/Latinx participants from New York, NY and Los Angeles, CA with stored blood samples (N = 1295) at both Exam I (2000–2002) and Exam V (2010–2011). Participants were included in the final analytic subsample if they had 2 waves of data from both study waves (Exam I and Exam V) and did not have missing observations in the variables considered for these analyses (N = 1064). Across all study covariates, participants who had missing data had similar characteristics as those included in the analyses. The Institutional Review Boards of MESA field centers and the MESA coordinating center approved this study.
Study Variables
Change in LTL
Telomere length was assessed using quantitative polymerase chain reaction from leukocytes in peripheral blood samples collected at baseline (Exam I) and Exam V (30). Blood samples were stored at −80°C and assayed as a single batch at the University of California San Francisco. Telomere length was defined as the ratio of telomeric DNA (T) to a single-copy gene (S). Details regarding the protocol used to obtain telomere length are described elsewhere (31). Briefly, baseline and 10-year follow-up samples from each participant were assayed on the same plates and the same batch of assay reagents were used for all samples. Each of the DNA samples was assayed 3 different times on 3 separate days and each assay included 8 control DNA samples to normalize run-to-run variation. Each successfully assayed sample had 6 T/S values, of which the mean was obtained after removing outliers (0.2% of all the assayed observations). The intraassay coefficient of variation for the samples was 2.9%. Consistent with prior work, change in telomere length was calculated adjusting for regression to the mean, which could cause potential bias due to residual errors in baseline telomere length measurement (32). Given that controlling for baseline telomere length in our regression models may result in additional bias, we instead used the following formula developed by Verhulst et al. (33) to calculate 10-year changes in telomere length accounting for regression to the mean.
SD = standard deviation; r = Pearson correlation coefficient.
Discrimination
To assess participants’ reports of discrimination at baseline, we used modified versions of the Major Experiences of Discrimination Scale (MDS) and the Everyday Discrimination Scale (EDS), which were originally developed for the Detroit Area Study (34).
The MDS assesses the occurrence of lifetime discrimination in participants’ lives across 6 major domains: school, job/the workplace, housing, neighborhood, and encounters with the police. For example, participants are asked about being unfairly fired from a job or being physically threatened or abused by the police. Responses were recorded as yes/no, from which a summary score of participants’ affirmative replies was summed to create a total score ranging from 0 to 6. Based on the distribution of responses and consistent with prior work, we then categorized this score into 3 groups (did not experience discrimination, experienced discrimination in 1 domain, and experienced discrimination in 2 or more domains) (2,5,6).
The EDS asks participants about the frequency of unfair treatment in their everyday lives, such as being threatened/harassed and being treated less than others, using 9 items on a Likert scale. Responses ranged from almost every day (1) to never (6). Mean scores, of which higher values indicated increased discrimination, were obtained after each of the items of the scale were reverse coded, summed, and averaged. In order to capture both potential linear and nonlinear relationships, we use both the continuous and categorical version (None [mean score = 1], Moderate [mean score >1 and ≤2], and High [mean score >2]) of the EDS in the following analyses.
The MDS and the EDS are widely used measures that have been validated in racially/ethnically diverse populations to capture experiences of discrimination (34). They have both demonstrated good reliability in capturing the latent construct and have demonstrated good internal consistency in this population (EDS Cronbach’s alpha = 0.88).
Neighborhood social cohesion
Neighborhood social cohesion at baseline within census tracts was assessed using a community survey that asked individuals who lived in the same census tracts as MESA participants to rate the social environment within a mile from their place of residence using 4 items (35). Participants were asked about their perceptions on the presence of harmony, willingness to help, shared community values, and trust in their neighborhoods. Responses were on a Likert scale, ranged from strongly agree (1) to strongly disagree (5), and were reverse coded and summed. Conditional Empirical Bayes (CEB) estimates were utilized to average these items across all participants and to create a neighborhood-level measure of social cohesion, whereby higher values signified increased neighborhood social cohesion. The CEB estimate allows us to borrow information from other neighborhoods when data are sparse, shrinks less reliable scores on the scale toward the mean, and adjusts for respondent characteristics such as age, thereby increasing the validity of the measure. This measure has been shown to have good internal consistency, strong intraneighborhood correlation, and reliable ecometric property in appropriately capturing the area-level construct in this sample (Cronbach’s alpha = 0.72) (22). To facilitate a clearer interpretation, we categorized this measure into 2 groups (low neighborhood social cohesion and high neighborhood social cohesion) using a median split (36).
Study Covariates
In order to account for possible confounding, based on prior literature, we adjusted our estimates for the following self-reported covariates that were collected via study questionnaire at baseline: age, gender (man, woman), race/ethnicity (White, Black/African American, Hispanic/Latinx), educational status (high school or less, some college/technical degree, university graduate), employment (unemployed, employed), household income in US dollars (<$20 000, $20 000–49 999, $50 000–74 999, ≥$75 000), and marital status (married, not married) (37).
Consistent with previous studies on the possible links between health behaviors and health conditions that may be conceptualized as potential mediators of the relationship between discrimination, neighborhood social cohesion, and telomere length (38,39), we also additionally included the following baseline covariates in our analyses: body mass index (kg/m2), diabetes mellitus (yes, no), hypertension (yes, no), cancer (yes, no), pack-years of smoking (the product of the self-reported number of packs of cigarette smoked per day and the number of years smoked), moderate to vigorous physical activity (the metabolic equivalent task of physical activity minutes per week), Chronic Burden Score (range = 1–5), and Center for Epidemiologic Studies—Depression score (range = 0–60).
Statistical Analyses
To examine bivariate relationships between telomere length measurements at the 2 study waves and values of study covariates, we calculated descriptive statistics. We also examined the distribution of study covariates across major discrimination categories and determined mean everyday discrimination scores across population characteristics at Exam I. Linear regression models were used to examine associations between discrimination measures and telomere length change. To assess if the relationship between reports of discrimination and telomere length change varied across neighborhood social cohesion categories, we used hierarchical linear mixed-effects models, in which individuals are nested within census tracts. The use of 2-level models, with telomere length change, discrimination measures, and study covariates as Level-1 variables, and neighborhood social cohesion as a Level-2 variable, allows us to ensure the independence of individual observations that are clustered within neighborhoods. For each discrimination measure (categorized major experiences of discrimination, categorized everyday discrimination, and continuous everyday discrimination), we ran separate models with a 2-way interaction term between the measure of discrimination and neighborhood social cohesion. In both linear and mixed-effects regressions, the initial models adjusted for sociodemographic covariates (Model 1), and the subsequent fully adjusted models additionally included the health behaviors and health conditions specified above (Model 2).
For example, to investigate how the association of major discrimination and change in LTL varies across neighborhood social cohesion, the following model is specified:
where individuals (i) are nested within neighborhoods (j), with neighborhood-level and person-level error terms, which are each normally distributed with mean zero and variances ψ and θ, respectively. is a vector of all study covariates.
Based on these models, we then estimated the link between discrimination measures and telomere length within categories of neighborhood social cohesion.
In exploratory analyses, we examined if relationships between discrimination, neighborhood social cohesion, and telomere length change varied by race/ethnicity, given the fact that discrimination is often tied to characteristics such as race/ethnicity. For these analyses, we used linear mixed-effects models stratified by race/ethnicity and separately examined the interaction between each discrimination measure and neighborhood social cohesion.
All analyses were conducted using R statistical software version 3.5.0 at the University of California Berkeley. Statistical significance was defined at p < .05.
Results
Descriptive Results
Table 1 presents the overall distribution of population characteristics as well as the mean and standard deviation of LTL during both study waves (Exams I and V) within categories of baseline study covariates. The mean age in our study population was 60.6 years (SD = 9.37). The sample consisted of 30.1% Black/African American, 43.3% Hispanic/Latinx, and 26.6% White participants. 53.4% of our respondents were women and 46.6% were men.
Table 1.
Distribution of Baseline Population Characteristics and Leukocyte Telomere Length, the Multi-Ethnic Study of Atherosclerosis, 2000–2011
| Overall (N = 1064) | LTL Exam I | LTL Exam V | |
|---|---|---|---|
| N (%) | Mean (SD) | Mean (SD) | |
| Major Discrimination Scale (mean = 60.59, SD = 9.37) | |||
| None | 566 (53.20) | 0.92 (0.21) | 0.71 (0.14) |
| One domain | 260 (24.40) | 0.90 (0.20) | 0.70 (0.14) |
| ≥2 domains | 238 (22.40) | 0.92 (0.21) | 0.71 (0.16) |
| Everyday Discrimination Scale (mean = 1.61, SD = 0.71) | |||
| None | 314 (29.50) | 0.91 (0.21) | 0.69 (0.15) |
| Moderate | 517 (48.60) | 0.92 (0.20) | 0.71 (0.14) |
| High | 233 (21.90) | 0.92 (0.21) | 0.72 (0.14) |
| Neighborhood social cohesion | |||
| Low | 525 (49.30) | 0.90 (0.21) | 0.71 (0.15) |
| High | 539 (50.70) | 0.94 (0.20) | 0.70 (0.14) |
| Age (years) | |||
| 45–54 | 333 (31.30) | 0.98 (0.21) | 0.76 (0.15) |
| 55–64 | 337 (31.70) | 0.94 (0.19) | 0.71 (0.13) |
| 65 and older | 394 (37.00) | 0.85 (0.19) | 0.66 (0.13) |
| Race/ethnicity | |||
| White | 283 (26.60) | 0.94 (0.20) | 0.71 (0.15) |
| Black/African American | 320 (30.10) | 0.90 (0.20) | 0.72 (0.15) |
| Hispanic/Latinx | 461 (43.30) | 0.92 (0.21) | 0.70 (0.14) |
| Gender | |||
| Women | 568 (53.40) | 0.94 (0.20) | 0.72 (0.15) |
| Men | 496 (46.60) | 0.89 (0.20) | 0.69 (0.14) |
| Education | |||
| High school or less | 425 (39.90) | 0.92 (0.21) | 0.70 (0.15) |
| Some college/Technical school | 325 (30.50) | 0.93 (0.21) | 0.71 (0.14) |
| University graduate | 314 (29.50) | 0.91 (0.19) | 0.72 (0.14) |
| Income | |||
| Less than $20 000 | 253 (23.80) | 0.91 (0.21) | 0.69 (0.13) |
| $20 000–49 999 | 453 (42.60) | 0.91 (0.21) | 0.71 (0.14) |
| $50 000–74 999 | 166 (15.60) | 0.94 (0.20) | 0.72 (0.15) |
| More than $75 000 | 192 (18.00) | 0.93 (0.19) | 0.73 (0.16) |
| Employment status | |||
| Unemployed | 442 (41.50) | 0.90 (0.20) | 0.68 (0.14) |
| Employed | 622 (58.50) | 0.93 (0.21) | 0.73 (0.14) |
| Marital status | |||
| Not married | 461 (43.30) | 0.89 (0.20) | 0.70 (0.14) |
| Married | 603 (56.70) | 0.94 (0.20) | 0.72 (0.15) |
| Diabetes status | |||
| No | 930 (87.40) | 0.92 (0.20) | 0.71 (0.15) |
| Yes | 134 (12.60) | 0.91 (0.21) | 0.68 (0.13) |
| Hypertension | |||
| No | 600 (56.40) | 0.93 (0.21) | 0.72 (0.15) |
| Yes | 464 (43.60) | 0.90 (0.20) | 0.69 (0.14) |
| Cancer | |||
| No | 995 (93.50) | 0.92 (0.21) | 0.71 (0.14) |
| Yes | 69 (6.50) | 0.86 (0.17) | 0.69 (0.14) |
| Chronic Burden Score (mean = 1.31, SD = 1.18) | |||
| Low (0–1) | 666 (62.60) | 0.92 (0.21) | 0.71 (0.14) |
| Moderate (2) | 230 (21.60) | 0.91 (0.20) | 0.70 (0.14) |
| High (3 or more) | 168 (15.80) | 0.92 (0.21) | 0.72 (0.16) |
| BMI (mean = 29.08, SD = 5.47) | |||
| ≤24.9 | 241 (22.70) | 0.92 (0.21) | 0.72 (0.15) |
| 25.0–29.9 | 431 (40.50) | 0.92 (0.20) | 0.71 (0.15) |
| ≥30 | 392 (36.80) | 0.92 (0.21) | 0.70 (0.14) |
| CES-D (mean = 7.89, SD = 8.00) | |||
| No | 920 (86.50) | 0.92 (0.20) | 0.71 (0.15) |
| Yes | 144 (13.50) | 0.93 (0.21) | 0.70 (0.14) |
| Pack-years of smoking (mean = 8.70, SD = 15.94) | |||
| Nonsmoker (0) | 565 (53.10) | 0.93 (0.21) | 0.72 (0.14) |
| 1–10 | 220 (20.70) | 0.91 (0.18) | 0.71 (0.14) |
| >10–20 | 107 (10.10) | 0.93 (0.21) | 0.71 (0.16) |
| >20 | 172 (16.20) | 0.90 (0.20) | 0.68 (0.14) |
| Moderate to vigorous physical activity (mean = 6022.11, SD = 6353.43) | |||
| Low | 355 (33.40) | 0.92 (0.20) | 0.70 (0.14) |
| Moderate | 355 (33.40) | 0.91 (0.21) | 0.70 (0.15) |
| High | 354 (33.30) | 0.92 (0.21) | 0.72 (0.14) |
Note: LTL = leukocyte telomere length; SD = standard deviation; BMI = body mass index; CES-D = Center for Epidemiologic Studies—Depression.
Mean telomere length (T/S ratio) at Exam I in this population was 0.92 (SD = 0.20) and was 0.71 (SD = 0.14) 10 years later during Exam V. At baseline, those experiencing major discrimination in one domain, those reporting no everyday discrimination, and those residing in neighborhoods with low social cohesion had the shortest telomere length. This pattern remained relatively consistent during the follow-up period (Table 1).
The majority of our sample reported not having experienced major discrimination (53.2%), and the distribution of remaining study participants was relatively evenly split among those experiencing discrimination in one domain (24.4%) and in 2 or more domains (22.4%; Table 1). While distributions for most other study covariates were similar, compared to those reporting no major discrimination, participants who reported discrimination in 2 or more domains were younger (aged 45–54 years; 37.8% vs 29.2%), were more likely to identify as Black/African American (45.8% vs 22.2%), and were more likely to be a man (56.7% vs 40.8%; Table 2). Most study participants reported experiencing moderate everyday discrimination (48.6%), whereas 29.5% reported no everyday discrimination and 21.9% reported high everyday discrimination (Table 1). Mean everyday discrimination scores were higher for those aged 45–54, Black/African American participants, those who earned between $50 000 and $75 000, employed individuals, and those who resided in neighborhoods with low social cohesion (Table 2).
Table 2.
Distribution of Baseline Population Characteristics and Across Categories of Major Experiences of Discrimination, the Multi-Ethnic Study of Atherosclerosis, 2000–2011
| MDS (%) | Mean EDS | |||
|---|---|---|---|---|
| None | 1 Domain | ≥2 Domains | ||
| N = 566 | N = 260 | N = 238 | N = 1064 | |
| Age (years) | ||||
| 45–54 | 29.20 | 30.00 | 37.80 | 1.83 |
| 55–64 | 30.20 | 35.00 | 31.50 | 1.64 |
| 65 and older | 40.60 | 35.00 | 30.70 | 1.39 |
| Race/ethnicity | ||||
| White | 29.00 | 26.90 | 20.60 | 1.56 |
| Black/African American | 22.30 | 32.70 | 45.80 | 1.91 |
| Hispanic/Latinx | 48.80 | 40.40 | 33.60 | 1.44 |
| Gender | ||||
| Women | 59.20 | 50.00 | 43.30 | 1.6 |
| Men | 40.80 | 50.00 | 56.70 | 1.61 |
| Education | ||||
| High school or less | 47.20 | 37.30 | 25.60 | 1.48 |
| Some college/Technical school | 26.70 | 32.70 | 37.40 | 1.72 |
| University graduate | 26.10 | 30.00 | 37.00 | 1.67 |
| Income | ||||
| Less than $20 000 | 25.80 | 21.90 | 21.00 | 1.43 |
| $20 000–49 999 | 42.40 | 46.90 | 38.20 | 1.63 |
| $50 000–74 999 | 14.30 | 14.20 | 20.20 | 1.74 |
| More than $75 000 | 17.50 | 16.90 | 20.60 | 1.69 |
| Employment status | ||||
| Unemployed | 45.40 | 41.20 | 32.80 | 1.45 |
| Employed | 54.60 | 58.80 | 67.20 | 1.72 |
| Marital status | ||||
| Not married | 40.80 | 46.90 | 45.40 | 1.64 |
| Married | 59.20 | 53.10 | 54.60 | 1.58 |
| Diabetes status | ||||
| No | 89.20 | 83.50 | 87.40 | 1.62 |
| Yes | 10.80 | 16.50 | 12.60 | 1.55 |
| Hypertension | ||||
| No | 54.80 | 59.20 | 57.10 | 1.64 |
| Yes | 45.20 | 40.80 | 42.90 | 1.57 |
| Cancer | ||||
| No | 93.50 | 92.70 | 94.50 | 1.62 |
| Yes | 6.50 | 7.30 | 5.50 | 1.48 |
| Chronic Burden Score | ||||
| Low (0–1) | 73.00 | 55.40 | 45.80 | 1.50 |
| Moderate (2) | 17.70 | 26.20 | 26.10 | 1.66 |
| High (3 or more) | 9.40 | 18.50 | 28.20 | 1.95 |
| BMI | ||||
| ≤24.9 | 25.10 | 22.30 | 17.20 | 1.6 |
| 25.0–29.9 | 39.90 | 40.40 | 42.00 | 1.59 |
| ≥30 | 35.00 | 37.30 | 40.80 | 1.64 |
| CES-D | ||||
| No | 87.50 | 91.20 | 79.00 | 1.56 |
| Yes | 12.50 | 8.80 | 21.00 | 1.96 |
| Pack-years of smoking | ||||
| Nonsmoker (0) | 58.00 | 46.20 | 49.20 | 1.54 |
| 1–10 | 18.90 | 27.30 | 17.60 | 1.63 |
| >10–20 | 10.20 | 10.40 | 9.20 | 1.66 |
| >20 | 12.90 | 16.20 | 23.90 | 1.77 |
| Moderate to vigorous physical activity | ||||
| Low | 35.50 | 32.30 | 29.40 | 1.54 |
| Moderate | 32.90 | 35.00 | 32.80 | 1.56 |
| High | 31.60 | 32.70 | 37.80 | 1.73 |
| Neighborhood social cohesion | ||||
| Low | 50.20 | 48.80 | 47.90 | 1.67 |
| High | 49.80 | 51.20 | 52.10 | 1.55 |
Note: MDS = Major Discrimination Scale; EDS = Everyday Discrimination Scale; BMI = body mass index; CES-D = Center for Epidemiologic Studies—Depression.
Regression Results
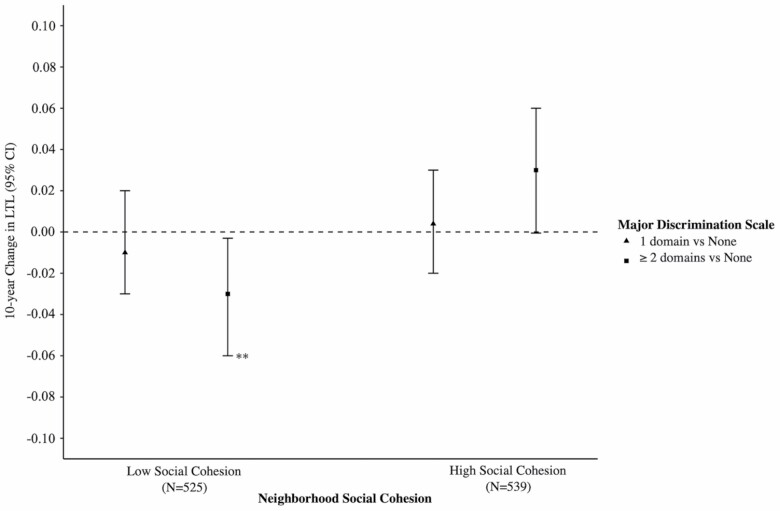
In the overall linear regression models, there was no association between major discrimination (1 domain; β = 0.0005; 95% CI: −0.02, 0.02; ≥2 domains; β = −0.01; 95% CI: −0.03, 0.02) or everyday discrimination (mean EDS; β = 0.004; 95% CI; −0.01, 0.02) and LTL change, after adjusting for all study covariates (Table 3). However, we did find that neighborhood social cohesion moderated the link between major discrimination and 10-year changes in LTL (p(χ 2) = .01; Table 3). In models adjusting for sociodemographic characteristics, among those residing in low social cohesion neighborhoods, individuals reporting major discrimination in 2 or more domains, experienced a 0.03-unit faster telomere length attrition after 10 years (β = −0.03; 95% CI: −0.06, −0.002) than those reporting no major discrimination (Model 1, Table 3). This association persisted after additional adjustment for health behaviors and health conditions (β = −0.03; 95% CI: −0.06, −0.003; Model 2, Table 3; Figure 1). Although not statistically significant, our findings were in the opposite direction for those residing in neighborhoods with high social cohesion, where neighborhoods with high social cohesion appeared to be protective against telomere attrition for those experiencing major discrimination in 2 or more domains (β = 0.03; 95% CI: −0.0005, 0.06; Model 2, Table 3; Figure 1).
Table 3.
Associations Between Baseline Experiences of Discrimination and 10-Year Change in Telomere Length Across Categories of Baseline Neighborhood Social Cohesion, the Multi-Ethnic Study of Atherosclerosis, 2000–2011
| Model 1 | Model 2 | |
|---|---|---|
| Beta (95% CI) | Beta (95% CI) | |
| Overall (N = 1064) a | ||
| Major Discrimination Scale | ||
| None | Ref | Ref |
| One domain | 0.002 (−0.02, 0.02) | 0.0005 (−0.02, 0.02) |
| ≥2 domains | −0.005 (−0.02, 0.02) | −0.01 (−0.03, 0.02) |
| Everyday Discrimination Scale | ||
| None | Ref | Ref |
| Low and moderate | −0.003 (−0.02, 0.02) | −0.001 (−0.02, 0.02) |
| High | −0.0004 (−0.02, 0.02) | −0.003 (−0.03, 0.02) |
| Mean Everyday Discrimination | 0.005 (−0.01, 0.02) | 0.004 (−0.01, 0.02) |
| Low social cohesion (N = 525) b | ||
| Major Discrimination Scale* | ||
| None | Ref | Ref |
| One domain | −0.004 (−0.03, 0.02) | −0.01 (−0.03, 0.02) |
| ≥2 domains | −0.03 (−0.06, −0.002) | −0.03 (−0.06, −0.003) |
| Everyday Discrimination Scale | ||
| None | Ref | Ref |
| Low and Moderate | −0.01 (−0.04, 0.02) | −0.01 (−0.04, 0.02) |
| High | −0.01 (−0.04, 0.02) | −0.01 (−0.04, 0.03) |
| Mean Everyday Discrimination | 0.001 (−0.01, 0.02) | 0.002 (−0.01, 0.02) |
| High social cohesion (N = 539) b | ||
| Major Discrimination Scale* | ||
| None | Ref | Ref |
| One domain | 0.01 (−0.02, 0.03) | 0.004 (−0.02, 0.03) |
| 2 or more domains | 0.03 (−0.002, 0.06) | 0.03 (−0.0005, 0.06) |
| Everyday Discrimination Scale | ||
| None | Ref | Ref |
| Low and moderate | −0.001 (−0.03, 0.03) | 0.003 (−0.02, 0.03) |
| High | −0.01 (−0.04, 0.03) | −0.004 (−0.04, 0.03) |
| Mean Everyday Discrimination | 0.003 (−0.02, 0.02) | 0.004 (−0.01, 0.02) |
Notes: CI = confidence interval. Model 1: adjusting for baseline sociodemographic covariates (age, race, gender, education, income, employment status, and marital status). Model 2: Model 1 + baseline health behaviors and conditions (exercise, smoking, body mass index, chronic burden, depression, diabetes, hypertension, and cancer).
aLinear regression models.
bLinear mixed-effects regression models.
*p(χ 2) < .05; boldface: p < .05.
Figure 1.
Associations between baseline reports of Major Experiences of Discrimination Scale (MDS) and 10-year change in telomere length across categories of baseline neighborhood social cohesion, the Multi-Ethnic Study of Atherosclerosis (MESA), 2000–2011. Note: **p < .05. Estimates are from linear mixed-effects regression models adjusted for baseline sociodemographic characteristics, health behaviors, and health conditions.
We found no association between everyday discrimination and LTL change in the overall models or within strata of neighborhood social cohesion (Table 3).
Results of our exploratory analyses models, where we stratified by race/ethnicity and tested 2-way interactions between discrimination measures and neighborhood social cohesion, did not reveal any significant differential associations between either discrimination measure, neighborhood social cohesion, and LTL change by race/ethnicity (data not shown).
Discussion
Despite early evidence documenting the negative health consequences of discrimination, the multilevel and time-dependent contexts that influence its effect on physiological wear and tear are not well understood. Utilizing longitudinal data on a multiethnic sample of older adults, this study investigated whether neighborhood social cohesion moderated the association between discrimination and 10-year telomere length attrition. Independent of a wide range of sociodemographic factors, health behaviors, and health conditions, we found that the link between major experiences of discrimination across multiple domains and 10-year changes in telomere length varied across levels of neighborhood social cohesion. Among those residing in neighborhoods with low social cohesion, experiencing discrimination in 2 or more domains resulted in faster telomere length attrition over 10 years, compared to those who reported no discrimination, and this decline in LTL was equivalent to the decline associated with a 15-year increase in age from our models. These results highlight the neighborhood social environment, more specifically, neighborhood social cohesion, as an important buffer that may mitigate the adverse and cumulative health impacts of discrimination on accelerated aging.
Rich psychosocial resources that come with residing in neighborhoods with high social cohesion are thought to reinforce healthy behaviors, the collective will to establish and maintain healthy physical environments, and strong community bonding (23,40). As such, studies have documented various physical and psychological health benefits associated with residing in socially cohesive neighborhoods (22,26,41). More specifically, in the context of experiencing discrimination, residing in neighborhoods with high social cohesion is thought to reinforce residents’ self-esteem and sense of belongingness, thus providing healthy coping and stress-buffering environments within which individuals are able to reappraise such unfair encounters as less threatening, recognize them as a collective experience, and distract themselves from the distresses that arise from them (42). Rather than engaging in maladaptive coping strategies such as self-blame, rumination, and attributing experiences of discrimination as warranted, strong social cohesion facilitates a venue for processing and contextualizing discrimination, and for seeking guidance on how to contend with these experiences, attenuating its psychological and physiological impacts (43,44). Although not statistically significant, our findings for those residing neighborhoods with high social cohesion highlight this potentially protective nature of social cohesion against the adverse health impacts of discrimination. However, more research is needed to more closely assess highly socially cohesive neighborhoods in relation to discrimination and telomere length attrition.
On the other hand, neighborhoods characterized by low social cohesion may present additional challenges to their residents and exacerbate the effect of discrimination on health. As a result of historic inequitable policies and practices in the United States that have orchestrated racial and economic residential segregation and subsequent sustained disinvestment, individuals who live in such neighborhoods bear the clustering of spatial disenfranchisement. In these settings, the lack of protective social environments, particularly low social cohesion, may pose as a source of chronic stress through its links with increased social disorder, weakened social fabric, and the lack of health-promoting physical environments (eg, parks) (24,36,45). Such concentration of stressful neighborhood environments can then compound together with discrimination to heighten physiological wear and tear (38,46–48). These types of environments may also constrain individuals’ opportunities to process and cope with discrimination. The limited availability of social support networks, with whom to garner a sense of social capital and connectedness in less socially cohesive neighborhoods, leaves individuals with depleted psychosocial resources and therefore may lead them to internalize unfair treatment and accept it as a deserved treatment, further enhancing its ill-health effects. In a prior cross-sectional study, although we did not find associations between discrimination and baseline telomere length in the full sample, we showed that among participants with low individual-level social support, reporting moderate and high everyday discrimination versus no discrimination was associated with shorter telomeres. In these analyses, there was no association between discrimination and telomere length for those with moderate and high social support (37). The current study extends this work by including longitudinal measurements of telomere length to assess the influence of discrimination on telomere length attrition over time and by additionally examining neighborhood social cohesion, which is integral in shaping stress-coping mechanisms. Our results are also consistent with Saleem et al. (49), who found that neighborhood social cohesion attenuated the impact of racial discrimination on depressive symptoms among African American adolescents.
The findings of this study provided evidence that neighborhood social cohesion moderated the impact of major discrimination, rather than everyday discrimination, on telomere length change. While contrary to prior studies that have found significant associations between everyday hassles and poor health outcomes (7), our results are consistent with studies that have documented inverse links between lifetime (major) discrimination and telomere length, as well as with previous findings in MESA showing negative relationships between major discrimination and incident cardiovascular disease and diabetes (2,5,19,21,39). More specifically, our findings are aligned with a recent study by Chae et al. (39) that used latent change score analyses to document that African Americans reporting racial discrimination across multiple major domains experienced faster telomere length attrition. There are also several reasons that may explain why we saw stronger associations for major discrimination instead of everyday unfair treatment. First, major experiences of discrimination capture the presence of lifetime adversity across multiple institutions that lead to sustained socioeconomic and psychosocial deprivation. Experiences of discrimination that are more blatant, such as unfairly being denied housing, losing a job or a promotion, and being harassed by law enforcement, have a long-lasting impact on quality of life and limit the availability of resources that aid in establishing adaptive coping behaviors and consequently healthy living and aging (1). By assessing the presence of unjust treatment across a broader set of institutions, the major discrimination scale captures deep-seated inequalities that burden marginalized populations. Such inequitable and unfair practices can also be conceptualized as more upstream indicators of day-to-day hassles, as they reinforce social norms that manifest in experiences of interpersonal discrimination (50). Alternatively, because we were only able to assess discrimination at baseline, the major experiences of discrimination may have been better suited to assess long-term unfair treatment, as opposed to responses to the everyday discrimination scale, which may have changed throughout the 10 years. Finally, because our sample primarily includes individuals who were alive during the Jim Crow era, when de jure discrimination was widely practiced, the major discrimination scale may be more salient in capturing the health effects of such extreme experiences of discrimination. As such, more research is warranted to further understand the complex nature of major versus everyday experiences of discrimination and their health effects.
Limitations
The results of this study should be interpreted within the context of its limitations. First, our study sample may not be representative of all older adults in the United States, as our participants were selected from 2 study sites, and are more educated, healthier, and wealthier than the general US population in the same age range. Hence, more nationally representative studies should be conducted to corroborate our findings. Second, utilizing census tracts as a way to define neighborhoods may have limited our ability to capture participants’ geographic definition of a neighborhood that may be more relevant to their health. Our results are also subject to possible biases such as neighborhood self-selection. In order to address this possibility, consistent with prior work, we adjusted for a range of sociodemographic characteristics that may influence self-selection. However, we cannot rule out residual confounding due to unmeasured factors. Our study was also not powered to detect the potential differential manifestation of discrimination and neighborhood environments across racial/ethnic groups, which is needed for a more nuanced comprehension of their joint health impacts. Furthermore, because discrimination measures were only available at baseline, we were not able to assess how changes in reports of discrimination may influence changes in telomere length. We also only examined baseline measures of social cohesion in this study. Thus, future work should assess how longitudinal changes in discrimination and neighborhood social cohesion may be related to telomere length change. Additionally, in this study, we did not examine the attribution of experiences of discrimination or more explicit forms of unfair treatment such as racism. Hence, studies should more closely examine how experiences of discrimination that are attributed to social identities (eg, race/ethnicity) influence telomere length attrition. Lastly, the measure of telomere length available in this sample is specific to leukocytes and may not be generalizable to other tissue samples. Further investigations are needed to corroborate our results using other markers of aging that may further elucidate the biological embodiment of discrimination.
Strengths
Despite these limitations, our study has several strengths. By assessing telomere length at 2 time points with enough time to observe meaningful changes, this study provides evidence on how discrimination may be related to the rate of cellular aging over time. This study also additionally incorporates information on neighborhood social cohesion, a strong indicator of social connectedness at the neighborhood level. To the best of our knowledge, this is the first study to examine the extent to which neighborhood contexts may moderate the link between discrimination and longitudinal changes in telomere length. Studies such as this one that jointly investigate individual-level stressors and neighborhood environments more accurately represent the complex multilevel realities of people, as beings nested within both time and space and interacting with structural and environmental factors in their day-to-day lives. They also further explicate the multifaceted psychosocial processes through which discrimination, across multiple institutions, affects health. By accounting for a range of covariates, including sociodemographic characteristics, health behaviors, and health conditions, the results of this study are able to better explain the differential consequences of discrimination on telomere length across neighborhood contexts. Additionally, the rigor of measures utilized in this study increases the validity of our findings. Both our discrimination and neighborhood social cohesion measures have been previously validated and have demonstrated good test–retest reliability (33,34). By utilizing CEB estimates, our neighborhood social cohesion measure additionally accounts for data sparsity and differential self-report. Furthermore, MESA is a well-characterized cohort that is racially and socioeconomically diverse, making the results of this study notable.
Conclusions
By leveraging longitudinal and neighborhood-level data in a well-ascertained and racially/ethnically diverse cohort, our study is the first of its kind to show that residing in neighborhoods with low social cohesion may make individuals vulnerable to the adverse effects of discrimination on telomere length. In addition to highlighting the need for transformational change across institutions that harbor discriminatory practices, this study provides evidence for intervening at the neighborhood level with the intent to promote social connectedness and collective agency as a way to mitigate the negative health consequences of discrimination.
Acknowledgments
The authors thank the investigators, staff, and participants of the Multi-Ethnic Study of Atherosclerosis (MESA) for their valuable contributions. A full list of participating MESA investigators and institutions is available at http://www.mesa-nhlbi.org.
Funding
This work was supported by contracts N01-HC-95159 through N01-HC-95169 and by grants R01HL101161 and R01HL076831 (PI: Ana Diez Roux) from the National Heart, Lung, and Blood Institute at the National Institutes of Health.
Conflict of Interest
None declared.
References
- 1. Williams DR, Lawrence JA, Davis BA. Racism and health: evidence and needed research. Annu Rev Public Health. 2019;40(1):105–125. doi: 10.1146/annurev-publhealth-040218-043750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Whitaker KM, Everson-Rose SA, Pankow JS, et al. Experiences of discrimination and incident type 2 diabetes mellitus: the Multi-Ethnic Study of Atherosclerosis (MESA). Am J Epidemiol. 2017;186(4):445–455. doi: 10.1093/aje/kwx047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Krieger N. Racial and gender discrimination: risk factors for high blood pressure? Soc Sci Med. 1990;30(12):1273–1281. doi: 10.1016/0277-9536(90)90307-e [DOI] [PubMed] [Google Scholar]
- 4. Sims M, Diez-Roux AV, Dudley A, et al. Perceived discrimination and hypertension among African Americans in the Jackson Heart Study. Am J Public Health. 2012;102(suppl 2):S258–S265. doi: 10.2105/AJPH.2011.300523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Everson-Rose SA, Lutsey PL, Roetker NS, et al. Perceived discrimination and incident cardiovascular events: the Multi-Ethnic Study of Atherosclerosis. Am J Epidemiol. 2015;182(3):225–234. doi: 10.1093/aje/kwv035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kershaw KN, Lewis TT, Diez Roux AV, et al. Self-reported experiences of discrimination and inflammation among men and women: the Multi-Ethnic Study of Atherosclerosis. Health Psychol. 2016;35(4):343–350. doi: 10.1037/hea0000331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Williams DR, Mohammed SA. Racism and health I: pathways and scientific evidence. Am Behav Sci. 2013;57(8):1152–1173. doi: 10.1177/0002764213487340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Geronimus AT, Hicken MT, Pearson JA, Seashols SJ, Brown KL, Cruz TD. Do US black women experience stress-related accelerated biological aging? A novel theory and first population-based test of black–white differences in telomere length. Hum Nat. 2010;21(1):19–38. doi: 10.1007/s12110-010-9078-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Epel ES, Prather AA. Stress, telomeres, and psychopathology: toward a deeper understanding of a triad of early aging. Annu Rev Clin Psychol. 2018;14:371–397. doi: 10.1146/annurev-clinpsy-032816-045054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Brouilette S, Singh RK, Thompson JR, Goodall AH, Samani NJ. White cell telomere length and risk of premature myocardial infarction. Arterioscler Thromb Vasc Biol. 2003;23(5):842–846. doi: 10.1161/01.ATV.0000067426.96344.32 [DOI] [PubMed] [Google Scholar]
- 11. D’Mello MJ, Ross SA, Briel M, Anand SS, Gerstein H, Paré G. Association between shortened leukocyte telomere length and cardiometabolic outcomes: systematic review and meta-analysis. Circ Cardiovasc Genet. 2015;8(1):82–90. doi: 10.1161/CIRCGENETICS.113.000485 [DOI] [PubMed] [Google Scholar]
- 12. Willeit P, Willeit J, Brandstätter A, et al. Cellular aging reflected by leukocyte telomere length predicts advanced atherosclerosis and cardiovascular disease risk. Arterioscler Thromb Vasc Biol. 2010;30(8):1649–1656. doi: 10.1161/ATVBAHA.110.205492 [DOI] [PubMed] [Google Scholar]
- 13. Yaffe K, Lindquist K, Kluse M, et al. ; Health ABC Study . Telomere length and cognitive function in community-dwelling elders: findings from the Health ABC Study. Neurobiol Aging. 2011;32:2055–2060. doi: 10.1016/j.neurobiolaging.2009.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bekaert S, De Meyer T, Van Oostveldt P. Telomere attrition as ageing biomarker. Anticancer Res. 2005;25:3011–3022. [PubMed] [Google Scholar]
- 15. Bakaysa SL, Mucci LA, Slagboom PE, et al. Telomere length predicts survival independent of genetic influences. Aging Cell. 2007;6(6):769–774. doi: 10.1111/j.1474-9726.2007.00340.x [DOI] [PubMed] [Google Scholar]
- 16. Marioni RE, Harris SE, Shah S, et al. The epigenetic clock and telomere length are independently associated with chronological age and mortality. Int J Epidemiol. 2016;45:424–432. doi: 10.1093/ije/dyw041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Epel ES, Blackburn EH, Lin J, et al. Accelerated telomere shortening in response to life stress. Proc Natl Acad Sci U S A. 2004;101(49):17312–17315. doi: 10.1073/pnas.0407162101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Oliveira BS, Zunzunegui MV, Quinlan J, Fahmi H, Tu MT, Guerra RO. Systematic review of the association between chronic social stress and telomere length: a life course perspective. Ageing Res Rev. 2016;26:37–52. doi: 10.1016/j.arr.2015.12.006 [DOI] [PubMed] [Google Scholar]
- 19. Coimbra BM, Carvalho CM, Ota VK, et al. A systematic review on the effects of social discrimination on telomere length. Psychoneuroendocrinology. 2020;120:104766. doi: 10.1016/j.psyneuen.2020.104766 [DOI] [PubMed] [Google Scholar]
- 20. Sullivan S, Hammadah M, Al Mheid I, et al. An investigation of racial/ethnic and sex differences in the association between experiences of everyday discrimination and leukocyte telomere length among patients with coronary artery disease. Psychoneuroendocrinology. 2019;106:122–128. doi: 10.1016/j.psyneuen.2019.03.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lee DB, Kim ES, Neblett EW. The link between discrimination and telomere length in African American adults. Health Psychol. 2017;36:458–467. doi: 10.1037/hea0000450 [DOI] [PubMed] [Google Scholar]
- 22. Mujahid MS, Diez Roux AV, Morenoff JD, et al. Neighborhood characteristics and hypertension. Epidemiology. 2008;19(4):590–598. doi: 10.1097/EDE.0b013e3181772cb2 [DOI] [PubMed] [Google Scholar]
- 23. Kawachi I, Berkman L. Social cohesion, social capital, and health. In: Berkman L, Kawachi I, eds. Social Epidemiology. 1st ed. New York: Oxford University Press; 2000. [Google Scholar]
- 24. Sampson RJ, Raudenbush SW, Earls F. Neighborhoods and violent crime: a multilevel study of collective efficacy. Science. 1997;277(5328):918–924. doi: 10.1126/science.277.5328.918 [DOI] [PubMed] [Google Scholar]
- 25. Ross CE, Mirowsky J. Neighborhood disadvantage, disorder, and health. J Health Soc Behav. 2001;42:258–276. doi: 10.2307/3090214 [DOI] [PubMed] [Google Scholar]
- 26. Stockdale SE, Wells KB, Tang L, Belin TR, Zhang L, Sherbourne CD. The importance of social context: neighborhood stressors, stress-buffering mechanisms, and alcohol, drug, and mental health disorders. Soc Sci Med. 2007;65:1867–1881. doi: 10.1016/j.socscimed.2007.05.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lewis TT, Cogburn CD, Williams DR. Self-reported experiences of discrimination and health: scientific advances, ongoing controversies, and emerging issues. Annu Rev Clin Psychol. 2015;11:407–440. doi: 10.1146/annurev-clinpsy-032814-112728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kubzansky LD, Seeman TE, Glymour MM. Biological pathways linking social conditions and health: plausible mechanisms and emerging Puzzles. In: Berkman L, Kawachi I, Glymour M, eds. Social Epidemiology. 2nd ed. Oxford University Press; 2014. [Google Scholar]
- 29. Bild DE, Bluemke DA, Burke GL, et al. Multi-Ethnic Study of Atherosclerosis: objectives and design. Am J Epidemiol. 2002;156(9):871–881. doi: 10.1093/aje/kwf113 [DOI] [PubMed] [Google Scholar]
- 30. Cawthon RM. Telomere measurement by quantitative PCR. Nucleic Acids Res. 2002;30(e47). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lin J, Epel E, Cheon J, et al. Analyses and comparisons of telomerase activity and telomere length in human T and B cells: insights for epidemiology of telomere maintenance. J Immunol Methods. 2010;352(1–2):71–80. doi: 10.1016/j.jim.2009.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Meier HCS, Hussein M, Needham B, et al. Cellular response to chronic psychosocial stress: ten-year longitudinal changes in telomere length in the Multi-Ethnic Study of Atherosclerosis. Psychoneuroendocrinology. 2019;107:70–81. doi: 10.1016/j.psyneuen.2019.04.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Verhulst S, Aviv A, Benetos A, Berenson GS, Kark JD. Do leukocyte telomere length dynamics depend on baseline telomere length? An analysis that corrects for ‘regression to the mean’. Eur J Epidemiol. 2013;28(11):859–866. doi: 10.1007/s10654-013-9845-4 [DOI] [PubMed] [Google Scholar]
- 34. Williams DR, Yu Y, Jackson JS, Anderson NB. Racial differences in physical and mental health socio-economic status, stress and discrimination. J Health Psychol. 1997;2:335–351. journals.sagepub.com/doi/pdf/10.1177/135910539700200305. [DOI] [PubMed]
- 35. Mujahid MS, Diez Roux AV, Morenoff JD, Raghunathan T. Assessing the measurement properties of neighborhood scales: from psychometrics to ecometrics. Am J Epidemiol. 2007;165(8):858–867. doi: 10.1093/aje/kwm040 [DOI] [PubMed] [Google Scholar]
- 36. Barber S, Hickson DA, Kawachi I, Subramanian SV, Earls F. Double-jeopardy: the joint impact of neighborhood disadvantage and low social cohesion on cumulative risk of disease among African American men and women in the Jackson Heart Study. Soc Sci Med. 2016;153:107–115. doi: 10.1016/j.socscimed.2016.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hailu EM, Needham BL, Lewis TT, et al. Discrimination, social support, and telomere length: the Multi-Ethnic Study of Atherosclerosis (MESA). Ann Epidemiol. 2020;42:58–63.e2. doi: 10.1016/j.annepidem.2019.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Needham BL, Carroll JE, Diez Roux AV, Fitzpatrick AL, Moore K, Seeman TE. Neighborhood characteristics and leukocyte telomere length: the Multi-Ethnic Study of Atherosclerosis. Health Place. 2014;28:167–172. doi: 10.1016/j.healthplace.2014.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Chae DH, Wang Y, Martz CD, et al. Racial discrimination and telomere shortening among African Americans: the Coronary Artery Risk Development in Young Adults (CARDIA) study. Health Psychol. 2020;39:209–219. doi: 10.1037/hea0000832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Echeverría S, Diez-Roux AV, Shea S, Borrell LN, Jackson S. Associations of neighborhood problems and neighborhood social cohesion with mental health and health behaviors: the Multi-Ethnic Study of Atherosclerosis. Health Place. 2008;14(4):853–865. doi: 10.1016/j.healthplace.2008.01.004 [DOI] [PubMed] [Google Scholar]
- 41. Kim ES, Chen Y, Kawachi I, VanderWeele TJ. Perceived neighborhood social cohesion and subsequent health and well-being in older adults: an outcome-wide longitudinal approach. Health Place. 2020;66(July):102420. doi: 10.1016/j.healthplace.2020.102420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Brondolo E, Brady Ver Halen N, Pencille M, Beatty D, Contrada RJ. Coping with racism: a selective review of the literature and a theoretical and methodological critique. J Behav Med. 2009;32(1):64–88. doi: 10.1007/s10865-008-9193-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Krieger N, Sidney S. Racial discrimination and blood pressure: the CARDIA Study of young black and white adults. Am J Public Health. 1996;86(10):1370–1378. doi: 10.2105/ajph.86.10.1370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Clark R. Self-reported racism and social support predict blood pressure reactivity in blacks. Ann Behav Med. 2003;25(2):127–136. doi: 10.1207/S15324796ABM2502_09 [DOI] [PubMed] [Google Scholar]
- 45. Ross CE. Neighborhood disadvantage and adult depression. J Health Soc Behav. 2000;41:177–187. doi: 10.2307/2676304 [DOI] [Google Scholar]
- 46. Yen IH, Kaplan GA. Neighborhood social environment and risk of death: multilevel evidence from the Alameda County Study. Am J Epidemiol. 1999;149:898–907. doi: 10.1093/oxfordjournals.aje.a009733 [DOI] [PubMed] [Google Scholar]
- 47. Lynch SM, Mitra N, Ravichandran K, et al. Telomere length and neighborhood circumstances: evaluating biological response to unfavorable exposures. Cancer Epidemiol Biomarkers Prev. 2017;26(4):553–560. doi: 10.1158/1055-9965.EPI-16-0554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Thierry AD. Association between telomere length and neighborhood characteristics by race and region in US midlife and older adults. Health Place. 2020;62(November 2019):102272. doi: 10.1016/j.healthplace.2019.102272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Saleem FT, Busby DR, Lambert SF. Neighborhood social processes as moderators between racial discrimination and depressive symptoms for African American adolescents. J Community Psychol. 2018;46(6):747–761. doi: 10.1002/jcop.21970 [DOI] [Google Scholar]
- 50. Krieger N. Discrimination and health inequities. Int J Health Serv. 2014;44(4):643–710. doi: 10.2190/HS.44.4.b [DOI] [PubMed] [Google Scholar]