Abstract
Background
John Henryism (JH) is a form of active high-effort coping. Low-socioeconomic status (SES) African Americans adopting JH to deal with structural racism and other chronic stressors might be more likely to display cardiovascular disease risk factors. Previous tests of this hypothesis have mostly focused on the moderating role of current SES and hypertension as the outcome variable. Furthermore, most of the previous work has been conducted among young and middle-aged adults. This study aimed at extending work on the JH hypothesis by testing the combined effect of JH and childhood SES on metabolic syndrome and systemic inflammation among African American older adults.
Methods
One hundred seventy urban African American older adults (Mage = 67.64 years, 75.9% female) were recruited. Participants completed questionnaires assessing JH, childhood SES, and other variables used as covariates (ie, demographic information, chronic conditions, medication use, and health behaviors). Blood pressure, waist circumference, and blood were also collected. Triglycerides, high-density lipoprotein cholesterol, hemoglobin A1C, and C-reactive protein levels were measured from the blood samples.
Results
JH was positively associated with metabolic syndrome symptoms among participants reporting low childhood SES levels, but not among those reporting high childhood SES levels. The same pattern did not emerge when we considered current SES. Similar patterns of results did not emerge as far as systemic inflammation was concerned.
Conclusions
Our findings highlight the importance of considering the joint impact of objective conditions early in life and individual psychological proclivities in explaining increased risk for cardiovascular disease risk in this population.
Keywords: Childhood SES, John Henryism, Metabolic syndrome, Systemic inflammation
As highlighted in the coronavirus disease 2019 worldwide pandemic, health disparities for African Americans (AAs), particularly those concerning cardiovascular disease (CVD) (1), have a long, stable history (2). For example, rates of nonfatal stroke, fatal stroke, and heart disease are 1.3, 1.8, and 1.5 times higher, respectively, in AAs than Whites (1). Equally important, AA adults die earlier from CVD (3) and experience lower rates of survival after cardiac surgery than Whites (4). Notably, the prevalence of hypertension among AAs living in the United States is among the highest globally (57% vs 41% among Whites) (5). Age is a well-established risk factor for CVD; thus, it is not surprising that older AA adults bear an even greater share of CVD burden. Despite growing awareness of these inequities, disparities in CVD rates, processes, and outcomes persist and, in some cases, have even increased (6). Most of the research in this area has focused on proximate risks factors (eg, diabetes, dyslipidemia, medication adherence) (7) for CVD and their disproportionate incidence among AAs. Only recently, researchers have started to more consistently consider the role of distal risk factors, such as psychosocial stressors, particularly those related to racism (8).
Though the disparities mentioned above are disheartening, AAs are not helpless amidst these obstacles. In this regard, an important topic of investigation is the consideration of culturally relevant coping mechanisms. John Henryism (JH) refers to a form of active coping characterized by mental and physical vigor, determination, and commitment to hard work in the face of adversities (9). The concept of JH is taken from the legend of John Henry, the AA railroad worker who, through sheer grit, determination, and hard work, defeated a steam-powered drill in a steel-driving contest (9). From this perspective, JH is a paragon of the American Dream: Through hard work, anyone can obtain wealth, social success, and good health. However, like John Henry, who died shortly after his win due to psychological and physical fatigue, JH can take a toll on health when paired with enduring and unsurmountable stressors (9). JH is particularly relevant for AAs, who face the pervasive and pernicious consequences of structural (ie, “system of dominance, power, and privilege based on racial group designations” (10), p. 43) and cultural racism (ie, the dominant racial group’s values and belief system) (11).
The JH Hypothesis (JHH) proposes that JH can be toxic for health in the context of structural racism and barriers, particularly for those who lack resources for effective coping. In other words, although endorsing high levels of effortful coping might not be detrimental for health per se, it becomes so for individuals facing insurmountable economic adversities (9). In its original formulation, low socioeconomic status (SES) has been used as a proxy for reduced access to effective coping resources (9). Although the health implications of JH have been tested among non-AAs (12), this construct has been primarily conceptualized as a coping strategy relevant for AAs having to deal with the intergenerational economic and financial hardship caused by structural racism (9). Mechanistically, endorsing high JH in environments characterized by chronic stress has been associated with neuroendocrine dysregulations (eg, alterations in cortisol secretion; (13) see also (14)), elevated cardiovascular reactivity to stress (15), and engagement in unhealthy behaviors (eg, smoking) (16), all of which are implicated in CVD.
Overall, research on the JHH has yielded mixed results and has yet to clarify the moderating role played by early socioeconomic disadvantage and consider the concurrent impact on multiple health outcomes. Furthermore, most of the previous work on the JHH has been conducted among young and middle-aged adults. To address these gaps, the purpose of this study was to conduct a rigorous test of JHH by examining low childhood SES as a moderator of the effect of JH on cardiometabolic health (metabolic syndrome [MetS] and systemic inflammation) among urban AA older adults.
The majority of the research testing the JHH has focused on CVD risk factors, particularly hypertension, taking one of 2 approaches: independent versus interactive. The independent approach assesses the main effect of JH on health. Reviews of the literature show that results from studies using this approach are mixed, with research finding positive associations, negative associations, and null effects between JH and health outcomes (17,18). For example, Jackson and Adams-Campbell (19) did not find any association between JH and blood pressure in AA youth, while Dressler et al. (20) found JH to be associated with increased blood pressure in AA adult men, but decreased blood pressure in AA adult women. In a smaller sample of AA and White caregivers, Merritt et al. (13) found higher JH to be associated with dysregulations in diurnal cortisol secretion (ie, flatter cortisol slopes) among AAs but not Whites. On the other hand, the interactive approach tests whether and how SES moderates the main effect of JH on health. Results from this literature are similarly mixed, with studies finding no interaction between JH and SES in predicting blood pressure and hypertension prevalence in both AA only (21) and multiracial samples (22), and other studies finding significant interactions between JH and SES in predicting such outcomes, but not always in the expected direction (23,24).
Some gaps can be identified within the JHH literature. First, most of the existing studies have considered only one health outcome. The link between psychosocial stress and CVD is complex and involves several pathways. In this context, assessing multiple outcomes is imperative if psychosocially modulated biological pathways of CVD are to be established with certainty. Second, to date, the JHH has been overwhelmingly tested in relation to blood pressure and hypertension (but, see (25)). Although this approach is theoretically justified, studies are needed to push the field forward and test the JHH in relation to other risk factors for CVD, such as MetS and systemic inflammation. Third, most of the previous work has been conducted among young and middle-aged adults. Whether the JHH can be extended to older adults, who are at greater risk for CVD, has yet to be established. Fourth, not without exceptions (24), tests of the JHH have exclusively focused on current SES, overlooking the potential contribution of childhood SES. To further refine testing of the JHH, examining childhood SES and not just adult SES is vital. The theory of the JHH is predicated on the negative impact of prolonged effortful coping amidst disadvantages. With the effects of structural barriers on health compounding over time (26) and the early origins of CVD risk factors (27,28), assessing how low SES in childhood affects outcomes in AAs with low and high levels of JH can further delineate the potential long-term impact of structural inequality on health. In this regard, the only study to date that considered the interaction between childhood SES and JH in predicting MetS found support for the JHH (25). However, this study focused on rural AA youth; thus, whether these findings can be extended to older AA adults living in urban environments remains untested.
Our study addresses these gaps in the literature and aims at testing the JHH in urban AA older adults using childhood SES (vs current SES) as a moderator of the relationship between JH and MetS and systemic inflammation (as indexed by circulating levels of C-reactive protein [CRP]). We hypothesized that while controlling for adulthood SES, the association between JH and these outcomes would be moderated by childhood SES. The hypotheses and data analyses for this study were preregistered on the Open Science Framework, https://osf.io/f47hm/?view_only=83690a35cfe644809ccdb5e8b6aeefa0.
Method
Sample and Procedure
Participants were a subsample of 170 older adults (Mage = 67.64 ± 8.53 years, range = 50–89 years, 75.9% female) from the Health among Older Adults Living in Detroit study that ran from November 2017 to March 2020 (N = 211). Participants who did not have available data for both MetS and CRP were excluded from the analyses (N = 41). There were no differences between included and excluded participants in the predictors or covariates (ps > .10). One hundred five (61.8%) participants were recruited through the Institute of Gerontology’s Healthy Black Elders Center Participant Research Pool, a volunteer registry of African Americans adults willing to participate in the research of interest to them. In addition to recruitment through this registry, we used advertisements placed in the community as well as snowball sampling. The study consisted of 2 home visits intermitted by an at-home survey and daily diary segment. During the first home visit, participants provided written informed consent. Additionally, participants completed questionnaires concerning their demographic information, chronic conditions, medication use, and health behaviors. At the end of the first home visit, participants were given daily diaries and several questionnaires to complete independently at home over the following days. The JH scale and childhood SES questionnaire were collected during this time. The second home visit was conducted in the morning, and participants were instructed to fast. Upon arrival, a trained research assistant collected a series of anthropometric and health measurements, including blood pressure and waist circumference. Blood samples were collected via venipuncture by a certified phlebotomist. After collection, the blood vacutainers were immediately transported to the Detroit Medical Center to be analyzed for metabolic and inflammatory markers. Participants were compensated for their participation. The study was approved by the Institutional Review Board at Wayne State University.
Measures
John Henryism
The JH Scale for Active Coping (9) is a 12-item self-reported questionnaire that measures perceived determination in response to environmental challenges. Participants were asked to respond to how well they thought each item characterized themselves (eg, “When things don’t go the way I want them to, that makes me work even harder” and “I like doing things that other people thought could not be done”). Scores were reported using a Likert scale ranging from 1 (completely false) to 5 (completely true). Items were averaged to create the JH composite, with higher scores on this continuous variable indicating greater standing on the variable (α = 0.82).
Childhood SES
Childhood SES was assessed by asking participants to indicate their parents’ highest level of education when the participant was 15 years old on a 9-point scale, ranging from 1 (didn’t finish high school) to 9 (PhD, MD, or other higher degree). If a participant had missing data for parent education at age 15, the parental attainment at either 10 or 5 years old was used instead (N = 8). As done in previous seminal studies (29–31), the highest level of education between the parents was used (see Author Note 1).
Metabolic syndrome
MetS was derived by combining 5 indicators: blood pressure, waist circumference, triglycerides, high-density lipoprotein (HDL) cholesterol, and hemoglobin A1C. Blood pressure was obtained using the Omron BP786N blood pressure monitor. Blood pressure was recorded 3 times with a 2-minute resting period in between measurements. The values from the second and third measurements were averaged and used in the current analyses. Waist circumference was measured in centimeters using a standard tape measure that was placed between the ribcage and the belly button. Metabolic markers measured in blood samples (ie, triglycerides, HDL cholesterol) were analyzed through a series of coupled enzymatic reactions via a clinical chemistry analyzer (Beckman Coulter AU5800). Hemoglobin A1C testing was performed using high-performance liquid chromatography (Premier Hb9210; Trinity Biotech, Kansas City, MO, USA). The cutoff values for each risk factor were selected following the guidelines of the National Cholesterol Education Program Adult Treatment Panel III (32), except for the hemoglobin A1C. For each indicator, participants received a score of either 0 (if they did not exceed the cutoff value) or 1 (if they exceeded the cutoff value). Cutoff values were as follows: blood pressure ≥130/85 mm/Hg, waist circumference >102 cm for men and >88 cm for women, triglycerides ≥150 mg/dL, and HDL cholesterol <40 mg/dL for men and <50 mg/dL for women. In place of glucose, we used hemoglobin A1C, which is a valid surrogate when using a cutoff value of 5.7% or greater (33). As done in previous studies (34), scores on each indicator were summed to obtain a MetS composite for each participant.
C-reactive protein
High-sensitivity CRP concentrations were determined with assays conducted using the turbidimetry method (Beckman Coulter AU5800; high-sensitivity range = 0.2–80 mg/L). CRP values were natural log-transformed due to the skewed distribution.
Demographic covariates
Specific demographic, health, and behavioral covariates were accounted for in the analyses. Participants responded to a demographic questionnaire where items such as age, sex (0 = male, 1 = female), marital status (0 = others, 1 = married), and current SES (ie, operationalized as income and education) were collected. Participants’ annual income was measured on a 13-point scale from 1 (less than $500) to 13 ($150 000 or more). Education was measured on a 12-point scale from 1 (no schooling/some grade school) to 12 (PhD, EdD, MD, or other professional degree). Income and education were z-scored and then averaged to obtain an indicator of current SES.
Health status and health behavior covariates
Participants reported whether they had any of 16 chronic conditions (eg, autoimmune disorders, asthma) in the previous 12 months (0 = no, 1 = yes) and whether they were taking any over-the-counter or prescribed medications (0 = no, 1 = yes). Three health behaviors were also considered: smoking, alcohol use, and physical activity. Smoking and alcohol use were assessed using the Smoking and Alcohol Consumption Questionnaire used by Cohen et al. (35). For smoking status, participants were asked whether they were currently smoking cigarettes, cigars, or a pipe daily (0 = no, 1 = yes). As for alcohol consumption, participants indicated the number of weekdays and weekend days they consume alcohol. Scores on these 2 separate items were summed. Then, scores on this variable were coded as 0 (alcohol use less than 3 days per week) and 1 (alcohol use 3 or more days per week) (36). Physical activity was measured using the Minnesota Leisure Time Activity Questionnaire (37) (see Author Note 2). For each participant, we calculated the average number of activities (ie, walking, cycling, gardening, and up to 4 sports listed by the participant) performed per day in a typical week. This variable was used as a measure of individual differences in physical activity.
Statistical Analyses
Ordinary least square (OLS) regression for continuous variables (eg, CRP) and Poisson regression for count variables (ie, MetS) were employed to test our hypotheses. First, we tested the main effect of JH on MetS and CRP separately. Second, we tested the interactive effects of JH and childhood SES on MetS and CRP. The models were first carried out without covariates, controlling for current SES only, and controlling for current SES, demographic, health, and behavioral covariates.
As highlighted in the introduction section, most of the previous studies on the JHH have focused on the moderating role of current SES. To test this theoretically important prediction and determine whether the expected interaction between childhood SES and JH would extend to current SES in our sample, we conducted secondary analyses wherein we tested the interactive effects between JH and current SES on MetS and CRP while controlling for childhood SES. To consider the potential interaction between life course SES and JH, we also tested the 3-way interactive effects between JH, childhood SES, and current SES on MetS and CRP. The incidence of missing data was 2.9%, and missing data were dealt with multiple imputations (ie, 10 imputed data sets). For CRP, sensitivity analyses were run in which participants with CRP values greater than 10 mg/L were removed from the analyses (38). All analyses were performed using maximum likelihood with robust standard errors in Mplus 7.0.
Results
Table 1 displays the mean, standard deviation, and bivariate correlations among study variables. JH did not correlate with MetS (r = 0.09, p = .26) or CRP (r = 0.13, p = .13). JH also did not correlate with childhood SES (r = −0.00, p = .99). Childhood SES did not correlate with CRP (r = 0.07, p = .41), but there was a positive correlation between childhood SES and MetS (r = 0.22, p = .005).
Table 1.
Mean, Standard Deviation (SD), and Correlation Coefficients Among Study Variables
| Variable | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. CRP | — | ||||||||||||
| 2. MetS | 0.28*** | — | |||||||||||
| 3. John Henryism | 0.13 | 0.09 | — | ||||||||||
| 4. Childhood SES | 0.07 | 0.22** | −0.00 | — | |||||||||
| 5. Female | 0.04 | 0.10 | −0.08 | 0.07 | — | ||||||||
| 6. Age | −0.07 | −0.17* | −0.14 | −0.24** | 0.26** | — | |||||||
| 7. Married | 0.14 | 0.01 | 0.03 | −0.02 | −0.12 | 0.06 | — | ||||||
| 8. Chronic diseases | 0.11 | 0.05 | −0.06 | −0.05 | 0.18* | 0.21** | −0.07 | — | |||||
| 9. Medication use | 0.12 | 0.02 | −0.06 | −0.02 | 0.12 | 0.25** | 0.05 | 0.32*** | — | ||||
| 10. Smoking | 0.05 | 0.03 | 0.06 | 0.07 | −0.29*** | −0.30*** | −0.06 | −0.04 | −0.12 | — | |||
| 11. Alcohol use | −0.04 | −0.13 | 0.10 | 0.18* | −0.26** | −0.25** | −0.02 | −0.14 | 0.05 | 0.28*** | — | ||
| 12. Physical activity | −0.07 | −0.09 | 0.12 | 0.03 | −0.24** | −0.08 | −0.02 | −0.16* | −0.07 | 0.20* | 0.18* | — | |
| 13. Current SES | −0.13 | −0.12 | 0.03 | 0.03 | 0.10 | 0.30** | 0.17* | −0.03 | 0.08 | −0.21** | −0.14 | 0.14 | — |
| Mean | 4.35* | 2.42 | 3.90 | 3.44 | 129† | 67.64 | 25† | 109† | 155† | 30† | 23† | 1.05 | 0.00 |
| SD | 5.55 | 1.17 | 0.56 | 1.77 | 75.9‡ | 8.53 | 14.7‡ | 64.1‡ | 92.8‡ | 17.9‡ | 13.7‡ | 0.83 | 0.89 |
Note: CRP = C-reactive protein; MetS = metabolic syndrome; SES = socioeconomic status.
*Unit mg/L.
† N was displayed.
‡Percentage was displayed.
*p < .05, **p < .01, ***p < .001.
Main Effects of JH and Childhood SES
Results from our multiple linear regression models showed that there were no associations between JH and MetS (b = 0.08, SE = 0.07, p = .22) and between JH and CRP (b = 0.22, SE = 0.16, p = .18). The associations remained statistically nonsignificant, after controlling for current SES, demographic, health, and behavioral covariates (Table 2). Childhood SES was positively associated with MetS symptoms (b = 0.06, SE = 0.02, p = .006), but not CRP (b = 0.05, SE = 0.05, p = .27). These findings remained unchanged after controlling for current SES, demographic, health, and behavioral covariates (Table 2).
Table 2.
Main Effects of John Henryism on Metabolic Syndrome and C-Reactive Protein
| Metabolic Syndrome | C-Reactive Protein | |||||
|---|---|---|---|---|---|---|
| Variables | b | SE | p | b | SE | p |
| John Henryism | 0.09 | 0.06 | .13 | 0.23 | 0.16 | .13 |
| Childhood SES | 0.06 | 0.02 | .003 | 0.06 | 0.05 | .27 |
| Current SES | −0.07 | 0.05 | .22 | −0.19 | 0.09 | .047 |
| Female | 0.08 | 0.11 | .44 | 0.17 | 0.18 | .34 |
| Age | −0.01 | 0.01 | .10 | −0.01 | 0.01 | .62 |
| Married | 0.05 | 0.11 | .64 | 0.43 | 0.23 | .062 |
| Chronic diseases | 0.03 | 0.09 | .71 | 0.09 | 0.19 | .65 |
| Medication use | 0.13 | 0.19 | .50 | 0.58 | 0.30 | .050 |
| Smoking | 0.05 | 0.11 | .67 | 0.32 | 0.23 | .17 |
| Alcohol use | −0.32 | 0.15 | .029 | −0.31 | 0.28 | .26 |
| Physical activity | −0.02 | 0.06 | .74 | −0.03 | 0.09 | .73 |
Note: SES = socioeconomic status.
Interactive Effects of JH and Childhood SES
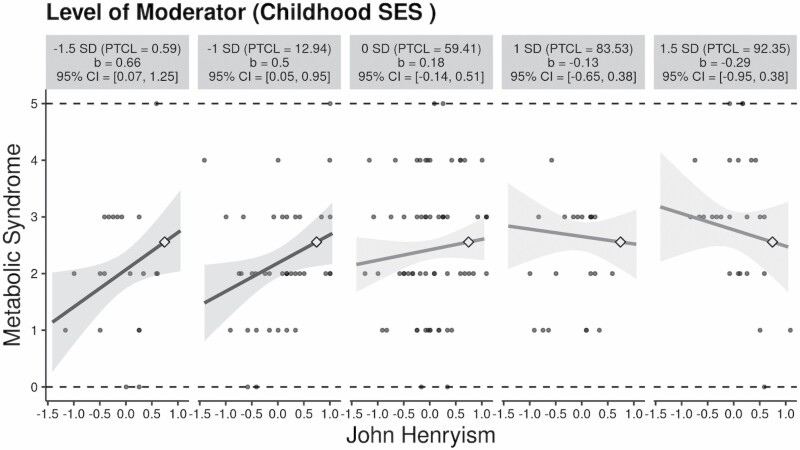
Moderation models showed that JH interacted with childhood SES in predicting MetS symptoms (b = −0.08, SE = 0.04, p = .041). The interactive effect remained significant after controlling for current SES (b = −0.09, SE = 0.04, p = .026) and further adjusting for demographic, health, and behavioral covariates (Table 3). To visualize this interactive effect, we used the interActive application developed by McCabe, Kim, and King (39), which relies on OLS regression. Given that the interActive application could not deal with missing data using multiple imputation, the first imputed data set was used to visualize the interactive effects. As shown in Figure 1, JH was associated with increased MetS symptoms for adults reporting low childhood SES (at −1.5 SD, b = 0.66, 95% confidence interval [CI] = [0.07, 1.25]; at −1 SD, b = 0.50, 95% CI = [0.05, 0.95]) but not high childhood SES (at 1 SD, b = −0.13, 95% CI = [−0.65, 0.38]; at 1.5 SD, b = −0.29, 95% CI = [−0.95, 0.38]).
Table 3.
Interactive Effects of John Henryism and Childhood SES on Metabolic Syndrome and C-Reactive Protein
| Metabolic Syndrome | C-Reactive Protein | |||||
|---|---|---|---|---|---|---|
| Variables | b | SE | p | b | SE | p |
| John Henryism (JH) | 0.09 | 0.06 | .15 | 0.21 | 0.16 | .18 |
| Childhood SES | 0.06 | 0.02 | .005 | 0.05 | 0.05 | .31 |
| JH × Childhood SES | −0.08 | 0.04 | .048 | −0.10 | 0.10 | .33 |
| Current SES | −0.07 | 0.05 | .20 | −0.19 | 0.09 | .046 |
| Female | 0.08 | 0.10 | .43 | 0.18 | 0.18 | .34 |
| Age | −0.01 | 0.01 | .070 | −0.01 | 0.01 | .55 |
| Married | 0.05 | 0.11 | .65 | 0.42 | 0.23 | .061 |
| Chronic diseases | 0.03 | 0.08 | .73 | 0.08 | 0.19 | .69 |
| Medication use | 0.07 | 0.18 | .68 | 0.52 | 0.32 | .10 |
| Smoking | 0.07 | 0.11 | .55 | 0.35 | 0.23 | .13 |
| Alcohol use | −0.29 | 0.14 | .037 | −0.29 | 0.28 | .31 |
| Physical activity | −0.01 | 0.06 | .83 | −0.02 | 0.09 | .80 |
Note: SES = socioeconomic status.
Figure 1.
The interactive effects of John Henryism and childhood socioeconomic status (SES) on metabolic syndrome. Note: PTCL = percentile. John Henryism was mean-centered. Current SES, age, sex, marital status, chronic diseases, medication use, smoking, alcohol use, and physical activity were included as covariates in the analysis.
The interactive effect between JH and childhood SES on CRP was not statistically significant (b = −0.12, SE = 0.09, p = .21). The interactive effect remained statistically nonsignificant after controlling for current SES (b = −0.13, SE = 0.09, p = .15) and further adjusting for, demographic, health, and behavioral covariates (Table 3).
Secondary Analyses
There were no interactive effects between JH and current SES on MetS symptoms (b = 0.04, SE = 0.07, p = .56) or CRP (b = 0.05, SE = 0.14, p = .72). These 2-way interactive effects remained statistically nonsignificant when adjusting for childhood SES, demographic, health, and behavioral covariates (b = 0.06, SE = 0.08, p = .41, for MetS symptoms; b = 0.13, SE = 0.16, p = .39, for CRP). Similarly, there was no significant 3-way interaction between JH, childhood SES, and current SES predicting MetS symptoms (b = −0.05, SE = 0.05, p = .37) or CRP (b = −0.07, SE = 0.09, p = .42). After adjusting for demographic, health, and behavioral covariates, results did not change (b = −0.05, SE = 0.05, p = .29, for MetS symptoms; b = −0.06, SE = 0.10, p = .55, for CRP). Sensitivity analyses for CRP (ie., removing participants with CRP values greater than 10 mg/L, N = 17) showed results similar to those reported above. Neither JH nor childhood SES was associated with CRP (b = 0.19, SE = 0.12, p = .10; b = 0.03, SE = 0.05, p = .47, respectively), above and beyond current SES, demographic, health, and behavioral covariates. There were also no interactive effects between childhood SES and JH on CRP (b = −0.02, SE = 0.08, p = .81).
Discussion
The purpose of this study was to test the interactive effects of childhood SES and JH on MetS and CRP levels in a sample of older urban-dwelling AA adults. Supporting our hypothesis concerning MetS, we found that JH was positively associated with MetS symptoms among participants reporting low levels of childhood SES, but not among those reporting high childhood SES levels. These results remained significant after controlling for current levels of SES and demographic, health, and behavioral covariates. Similar patterns of results did not emerge for systemic inflammation. Our statistical approach also allowed us to consider the main effects of JH and childhood SES (Table 2). Although no association was found between JH and any of the outcome variables, childhood SES was positively associated with MetS symptoms. Lastly, ancillary analyses revealed that JH did not interact with current SES (or both current and childhood SES) in predicting any of the outcomes under investigation. An interpretation of the primary and ancillary findings is presented in the paragraphs below.
According to the JHH, people endorsing sustained high-effort coping in the face of chronic stressors are at greater risk of stress-related health complications if they lack adequate resources, captured by SES, necessary to take advantage of this coping mechanism (9). Said differently, because low-SES individuals face more barriers and have fewer opportunities, they are less likely to benefit from persistent hard work and high efforts, which, if sustained (eg, from childhood to older adulthood) can take a physiological toll on them. Thus, endorsing high levels of effortful coping is not detrimental for health per se, but it becomes so for individuals facing chronic social and economic stressors. Findings testing the JHH in relation to CVD risk have yielded mixed findings (18). To the best of our knowledge, within this literature, only one study conducted among rural AA young adults (25) considered MetS. Furthermore, most of the previous studies considered current SES as a measure of resource availability. Our study’s novelty resides in testing the JHH in relation to MetS in a sample of urban AA older adults in combination with considering childhood SES (vs current SES) as a crucial measure of resource availability early in life.
In our sample, higher JH levels were associated with higher MetS symptoms among participants who reported low childhood SES; the same association was not found among those reporting high childhood SES. Notably, this effect was restricted to childhood SES as no interaction was found between JH and current SES. In this regard, our findings are comparable to those of Brody et al. (25), who found that AA young adults endorsing high levels of JH were more likely to be diagnosed with MetS if their childhood SES, which was assessed during adolescence, was low vs high. In 2013 (40), the same research group reported similar findings concerning allostatic load, a measure of biological risk that partially overlaps with MetS.
In our study, we found that lower resource availability early in life (ie, low childhood SES) rather than later in life (ie, low current SES) placed individuals endorsing high JH at greater risk of MetS. In other words, our findings suggest that an inexorable determination to overcome adversities early in life set the stage for MetS when combined with environments lacking opportunities and resources (ie, low childhood SES). It has been proposed that the origins of MetS can be traced back to childhood and adolescence (27,28). Within this perspective, it is possible that SES during middle and late adulthood, which reflects resources available after part of the cardiometabolic aging process has already taken place, was less relevant than childhood SES in moderating the effect of JH on health in our sample. This explanation, which echoes the Skin-Deep Resilience Hypothesis (40), is in line with those conceptual models that emphasize how early-life events shape interconnected behavioral (sedentary lifestyle, poor nutrition, smoking), endocrine (alternations in the activity of stress response system), and immunological (proinflammatory state) processes implicated in CVD risk and onset (41–43).
However, it is crucial to highlight that some of our findings seemed to deviate from this explanation. First, we did not find JH to interact with childhood SES to predict CRP levels. This null effect might be related to the age composition of our sample. Although relatively healthy (36% of participants reported no chronic conditions), CRP levels in the sample were high (45% of participants had CRP levels above 3 mg/L, 44), possibly leaving little to no room for the subtle net effects of JH and childhood SES to take place. Another reason as to why JH and childhood SES interacted in predicting MetS syndrome, but not CRP levels, might have been because MetS is a more integrated measure of cardiometabolic health. To the best of our knowledge, this is the first study testing the JHH in relation to CVD-related inflammation. Future studies using multiple immune biomarkers and larger samples are needed to corroborate our findings.
A second unexpected finding concerned the positive association between childhood SES and MetS symptoms, an association primarily driven by childhood SES being positively correlated with waist circumference (r = 0.16. p = .044), but not with the other MetS indicators (rs ranged from −0.06 to 0.13, ps > .10). Although few studies have found evidence in favor of a childhood SES gradient in MetS (45) and metabolic disturbances more broadly (46), it is worth noting that studies focusing exclusively on their analyses in AAs have found less consistent results. For example, Subramanyam et al. (24) found childhood SES to be inversely associated with hypertension among women (but not men) in the Jackson Heart Study, while James et al. (47) found that low childhood SES increased the odds of hypertension in a sample of only AA men. Within this narrow literature, the only study that specifically looked at MetS did not find any significant association (48). Some of these findings seem to suggest a weaker SES gradient in health among AAs, which is often explained in light of the Diminishing Returns Hypothesis (49), according to which high-SES AAs do not experience the same health benefits, including improved cardiovascular health, of high-SES Whites. For example, Farmer and Ferraro (49) found evidence for a flat slope between education and self-rated health among AAs. Diminished returns among AAs have also been found for indicators relevant for MetS, such as blood pressure (50) and obesity (51).
The Diminishing Returns Hypothesis, however, does not explain the positive association between childhood SES and MetS we found in our study. This surprising finding could be due to the fact that the older (vs younger) participants of our sample, who were also those who on average reported lower levels of parental education (ie, a negative correlation was found between age and childhood SES), were relatively healthy. Age, however, was controlled in the analysis. Another explanation might be related to the positive association between childhood SES and waist circumference. In this regard, it is worth noting that a reverse SES gradient in body mass index (52,53), and in some cases blood pressure (54,55), has been observed in medium- and low-development countries. One interpretation of these findings is that low-SES individuals in these countries perform physically taxing jobs while having less access to excess food. In comparison, high-SES individuals can avoid such professions and enjoy greater access to surplus food. The 10-State Nutrition Survey of 1968–1970 was the most comprehensive nutrition survey in the United States at the time (56). Germane to our study, this survey, which was conducted around the time the average participant in our sample was an adolescent, found that higher-income AA boys and girls evidenced higher fatness than their lower SES counterparts. These historical data combined with the dynamics observed in medium- and low-development countries might explain the pattern of results observed in our study. Although intriguing, this explanation should be considered with caution for 2 reasons. First, especially in medium-development countries, the reverse SES gradient has been found to be stronger for material indicators of SES (eg, income) than education, which was assessed in this study. Second, and most importantly, the critical cultural, lifestyle, and economic development differences between the United States and medium- and low-development countries render samples from these different countries hard to compare.
Our study relied on a modest sample size with potentially marginal statistical power. Future studies with larger sample sizes are needed to replicate these findings. Other limitations included the selection of a relatively healthy sample and the use of a single indicator of systemic inflammation and childhood SES, which was collected without providing participants with a clear definition of parental status (eg, no distinction between biological vs adoptive parents). We also acknowledge that childhood SES could have been susceptible to recall bias. Future tests of the JHH might want to consider other biomarkers of systemic inflammation and examine other relevant SES moderators measured across the life span, including longitudinal upward mobility and structural discrimination via neighborhood segregation, environmental racism, and increased police brutality. Another limitation of this study is its cross-sectional and correlational design. Future studies using a longitudinal design are warranted. One advantage of these designs is their ability of collecting biological samples at multiple time points, which can shed light on the long-term, developmental changes in objective CVD risk factors. Longitudinal designs can also lead to a better understanding of the temporal associations between our variables. For example, previous studies have shown that coping strategies develop over time and that early environments play an important role in this regard (57). In our study, we found a null association between childhood SES and JH; however, longitudinal studies can better elucidate the extent to which low childhood SES, along with other environmental factors early in life, contributes to shaping individual differences in JH over the life course. Another promising avenue for future research is the investigation of the broader context of stressors (eg, structural racism, racial discrimination, social isolation, caregiver burden) relevant to AA adults in which the discordance between JH and childhood SES relates to cardiometabolic health. Lastly, future studies on rural AA older adults are needed to complement our findings on urban AA older adults. Shedding light on these intricate relationships has the potential to unravel the conditions under which the interaction between JH and SES is most detrimental to health.
With these caveats in mind, this study is the first one to show that childhood SES interacts with JH in predicting MetS in a sample of urban AA older adults, highlighting the importance of considering the joint impact of objective conditions early in life and individual psychological proclivities in explaining increased risk for CVD risk in this population.
Author Notes
1. In the preregistration document, we proposed to assess childhood SES using parental education and 7 nontraditional SES indicators: out-of-town vacations, dental checkups, newspaper delivery, have a regular physician, number of bedrooms in the house, family homeownership, and family vehicle ownership. The internal consistency among these 7 nontraditional indicators was lower than expected (α = 0.57), and their correlations with parental education were relatively low (rs = 0.07–0.33). For these reasons and because parental education has been consistently used in the literature as the sole indicator of childhood SES, these indicators were not used in the analyses.
2. In the preregistration document, we proposed to calculate the total number of minutes per week (“days per week” × “minutes per day”) participants engaged in each physical activity. However, several participants did not provide valid data for the “minute per day” variable. Thus, we decided to calculate the average number of activities performed per day in a typical week.
Acknowledgments
The authors would like to thank the research participants for the time and effort they contributed to this research. The authors would also like to thank the Institute of Gerontology (IoG) at Wayne State University and all the research assistants in the Biopsychosocial Health Laboratory for their help in data collection and analyses.
Funding
This study was supported by a grant from the National Institutes of Health (P30 AG015281) and the Michigan Center for Urban African American Aging Research (MCUAAAR). The preparation of the manuscript was partly supported by a Faculty Competition for Postdoctoral Fellowship from Wayne State University (Samuele Zilioli).
Conflict of Interest
All authors declare that they have no conflict of interest.
Author Contributions
S.Z., J.M.G., and Y.J.: study conceptualization. Y.J.: data analyses. S.Z., J.M.G., Y.J., and J.R.-S.: writing.
References
- 1. Mozaffarian D, Benjamin EJ, Go AS, et al. Executive summary: heart disease and stroke statistics—2016 update: a report from the American Heart Association. Circulation. 2016;133:447–454. doi: 10.1161/CIR.0000000000000366 [DOI] [PubMed] [Google Scholar]
- 2. Williams DR, Yan Yu, Jackson JS, Anderson NB. Racial differences in physical and mental health: socio-economic status, stress and discrimination. J Health Psychol. 1997;2(3):335–351. doi: 10.1177/135910539700200305 [DOI] [PubMed] [Google Scholar]
- 3. Ingram DD, Montresor-Lopez JA.. Differences in Stroke Mortality Among Adults Aged 45 and Over: United States, 2010–2013. US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics; 2015. [Google Scholar]
- 4. Rangrass G, Ghaferi AA, Dimick JB. Explaining racial disparities in outcomes after cardiac surgery: the role of hospital quality. JAMA Surg. 2014;149(3):223–227. doi: 10.1001/jamasurg.2013.4041 [DOI] [PubMed] [Google Scholar]
- 5. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. J Am Coll Cardiol. 2018;71(19):e127–e248. doi: 10.1016/j.jacc.2017.11.006 [DOI] [PubMed] [Google Scholar]
- 6. Bild DE, McClelland R, Kaufman JD, et al. Ten-year trends in coronary calcification in individuals without clinical cardiovascular disease in the multi-ethnic study of atherosclerosis. PLoS One. 2014;9(4):e94916. doi: 10.1371/journal.pone.0094916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ferdinand KC, Yadav K, Nasser SA, et al. Disparities in hypertension and cardiovascular disease in blacks: the critical role of medication adherence. J Clin Hypertens (Greenwich). 2017;19(10):1015–1024. doi: 10.1111/jch.13089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Churchwell K, Elkind MSV, Benjamin RM, et al. ; American Heart Association. Call to action: structural racism as a fundamental driver of health disparities: a presidential advisory from the American Heart Association. Circulation. 2020;142(24):e454–e468. doi: 10.1161/CIR.0000000000000936 [DOI] [PubMed] [Google Scholar]
- 9. James SA. John Henryism and the health of African-Americans. Cult Med Psychiatry. 1994;18(2):163–182. doi: 10.1007/BF01379448 [DOI] [PubMed] [Google Scholar]
- 10. Harrell SP. A multidimensional conceptualization of racism-related stress: implications for the well-being of people of color. Am J Orthopsychiatry. 2000;70:42–57. doi: 10.1037/h0087722 [DOI] [PubMed] [Google Scholar]
- 11. Hicken MT, Kravitz-Wirtz N, Durkee M, Jackson JS. Racial inequalities in health: Framing future research. Soc Sci Med. 2018;199:11–18. doi: 10.1016/j.socscimed.2017.12.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Duijkers TJ, Drijver M, Kromhout D, James SA. “John Henryism” and blood pressure in a Dutch population. Psychosom Med. 1988;50(4):353–359. doi: 10.1097/00006842-198807000-00004 [DOI] [PubMed] [Google Scholar]
- 13. Merritt MM, McCallum TJ, Fritsch T. How much striving is too much? John Henryism active coping predicts worse daily cortisol responses for African American but not white female dementia family caregivers. Am J Geriatr Psychiatry. 2011;19(5):451–460. doi: 10.1097/JGP.0b013e3181eaffa4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Doan SN, Dich N, Evans GW. Stress of stoicism: low emotionality and high control lead to increases in allostatic load. Appl Dev Sci. 2016;20:310–317. doi: 10.1080/10888691.2016.1171716 [DOI] [Google Scholar]
- 15. Merritt MM, Bennett GG, Williams RB, Sollers JJ 3rd, Thayer JF. Low educational attainment, John Henryism, and cardiovascular reactivity to and recovery from personally relevant stress. Psychosom Med. 2004;66(1):49–55. doi: 10.1097/01.psy.0000107909.74904.3d [DOI] [PubMed] [Google Scholar]
- 16. Jackson JS, Knight KM, Rafferty JA. Race and unhealthy behaviors: chronic stress, the HPA axis, and physical and mental health disparities over the life course. Am J Public Health. 2010;100(5):933–939. doi: 10.2105/AJPH.2008.143446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bennett GG, Merritt MM, Sollers Iii JJ, et al. Stress, coping, and health outcomes among African-Americans: a review of the John Henryism hypothesis. Psychol Health. 2004;19:369–383. doi: 10.1080/0887044042000193505 [DOI] [Google Scholar]
- 18. Felix AS, Shisler R, Nolan TS, et al. High-effort coping and cardiovascular disease among women: a systematic review of the John Henryism hypothesis. J Urban Health. 2019;96(suppl 1):12–22. doi: 10.1007/s11524-018-00333-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jackson LA, Adams-Campbell LL. John Henryism and blood pressure in black college students. J Behav Med. 1994;17(1):69–79. doi: 10.1007/BF01856883 [DOI] [PubMed] [Google Scholar]
- 20. Dressler WW, Bindon JR, Neggers YH. John Henryism, gender, and arterial blood pressure in an African American community. Psychosom Med. 1998;60(5):620–624. doi: 10.1097/00006842-199809000-00019 [DOI] [PubMed] [Google Scholar]
- 21. Wiist WH, Flack JM. A test of the John Henryism hypothesis: cholesterol and blood pressure. J Behav Med. 1992;15(1):15–29. doi: 10.1007/BF00848375 [DOI] [PubMed] [Google Scholar]
- 22. McKetney EC, Ragland DR. John Henryism, education, and blood pressure in young adults. the CARDIA study. Coronary Artery Risk Development in Young Adults Study. Am J Epidemiol. 1996;143(8):787–791. doi: 10.1093/oxfordjournals.aje.a008816 [DOI] [PubMed] [Google Scholar]
- 23. Fernander AF, Durán RE, Saab PG, Schneiderman N. John Henry active coping, education, and blood pressure among urban blacks. J Natl Med Assoc. 2004;96(2):246–255. [PMC free article] [PubMed] [Google Scholar]
- 24. Subramanyam MA, James SA, Diez-Roux AV, et al. Socioeconomic status, John Henryism and blood pressure among African-Americans in the Jackson Heart Study. Soc Sci Med. 2013;93:139–146. doi: 10.1016/j.socscimed.2013.06.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Brody GH, Yu T, Miller GE, Ehrlich KB, Chen E. John Henryism coping and metabolic syndrome among young black adults. Psychosom Med. 2018;80(2):216–221. doi: 10.1097/PSY.0000000000000540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ferraro KF, Shippee TP. Aging and cumulative inequality: how does inequality get under the skin? Gerontologist. 2009;49(3):333–343. doi: 10.1093/geront/gnp034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gustafsson PE, Persson M, Hammarström A. Life course origins of the metabolic syndrome in middle-aged women and men: the role of socioeconomic status and metabolic risk factors in adolescence and early adulthood. Ann Epidemiol. 2011;21(2):103–110. doi: 10.1016/j.annepidem.2010.08.012 [DOI] [PubMed] [Google Scholar]
- 28. Umer A, Kelley GA, Cottrell LE, Giacobbi P Jr, Innes KE, Lilly CL. Childhood obesity and adult cardiovascular disease risk factors: a systematic review with meta-analysis. BMC Public Health. 2017;17(1):683. doi: 10.1186/s12889-017-4691-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Davis-Kean PE. The influence of parent education and family income on child achievement: the indirect role of parental expectations and the home environment. J Fam Psychol. 2005;19(2):294–304. doi: 10.1037/0893-3200.19.2.294 [DOI] [PubMed] [Google Scholar]
- 30. Phillips JE, Marsland AL, Flory JD, Muldoon MF, Cohen S, Manuck SB. Parental education is related to C-reactive protein among female middle-aged community volunteers. Brain Behav Immun. 2009;23(5):677–683. doi: 10.1016/j.bbi.2009.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Miller GE, Lachman ME, Chen E, Gruenewald TL, Karlamangla AS, Seeman TE. Pathways to resilience: maternal nurturance as a buffer against the effects of childhood poverty on metabolic syndrome at midlife. Psychol Sci. 2011;22(12):1591–1599. doi: 10.1177/0956797611419170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive summary of the third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486 [DOI] [PubMed] [Google Scholar]
- 33. Ong KL, Tso AW, Lam KS, Cherny SS, Sham PC, Cheung BM. Using glycosylated hemoglobin to define the metabolic syndrome in United States adults. Diabetes Care. 2010;33(8):1856–1858. doi: 10.2337/dc10-0190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chen E, Miller GE, Yu T, Brody GH. Unsupportive parenting moderates the effects of family psychosocial intervention on metabolic syndrome in African American youth. Int J Obes (Lond). 2018;42(4):634–640. doi: 10.1038/ijo.2017.246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cohen S, Tyrrell DA, Russell MA, Jarvis MJ, Smith AP. Smoking, alcohol consumption, and susceptibility to the common cold. Am J Public Health. 1993;83(9):1277–1283. doi: 10.2105/ajph.83.9.1277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Radler BT, Ryff CD. Who participates? Accounting for longitudinal retention in the MIDUS National Study of Health and Well-being. J Aging Health. 2010;22(3):307–331. doi: 10.1177/0898264309358617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Taylor HL, Jacobs DR Jr, Schucker B, Knudsen J, Leon AS, Debacker G. A questionnaire for the assessment of leisure time physical activities. J Chronic Dis. 1978;31(12):741–755. doi: 10.1016/0021-9681(78)90058-9 [DOI] [PubMed] [Google Scholar]
- 38. Pearson TA, Mensah GA, Alexander RW, et al. ; Centers for Disease Control and Prevention; American Heart Association. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: a statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003;107(3):499–511. doi: 10.1161/01.cir.0000052939.59093.45 [DOI] [PubMed] [Google Scholar]
- 39. McCabe CJ, Kim DS, King KM. Improving present practices in the visual display of interactions. Adv Method Pract Psychol Sci. 2018;1:147–165. doi: 10.1177/2515245917746792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Brody GH, Yu T, Chen E, Miller GE, Kogan SM, Beach SR. Is resilience only skin deep? Rural African Americans’ socioeconomic status-related risk and competence in preadolescence and psychological adjustment and allostatic load at age 19. Psychol Sci. 2013;24(7):1285–1293. doi: 10.1177/0956797612471954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat Rev Neurosci. 2009;10(6):434–445. doi: 10.1038/nrn2639 [DOI] [PubMed] [Google Scholar]
- 42. Miller GE, Chen E, Parker KJ. Psychological stress in childhood and susceptibility to the chronic diseases of aging: moving toward a model of behavioral and biological mechanisms. Psychol Bull. 2011;137(6):959–997. doi: 10.1037/a0024768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Nusslock R, Miller GE. Early-life adversity and physical and emotional health across the lifespan: a neuroimmune network hypothesis. Biol Psychiatry. 2016;80(1):23–32. doi: 10.1016/j.biopsych.2015.05.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ridker PM. Clinical application of C-reactive protein for cardiovascular disease detection and prevention. Circulation. 2003;107(3):363–369. doi: 10.1161/01.cir.0000053730.47739.3c [DOI] [PubMed] [Google Scholar]
- 45. Puolakka E, Pahkala K, Laitinen TT, et al. Childhood socioeconomic status in predicting metabolic syndrome and glucose abnormalities in adulthood: the Cardiovascular Risk in Young Finns Study. Diabetes Care. 2016;39(12):2311–2317. doi: 10.2337/dc16-1565 [DOI] [PubMed] [Google Scholar]
- 46. Tamayo T, Christian H, Rathmann W. Impact of early psychosocial factors (childhood socioeconomic factors and adversities) on future risk of type 2 diabetes, metabolic disturbances and obesity: a systematic review. BMC Public Health. 2010;10:525. doi: 10.1186/1471-2458-10-525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. James SA, Van Hoewyk J, Belli RF, Strogatz DS, Williams DR, Raghunathan TE. Life-course socioeconomic position and hypertension in African American men: the Pitt County Study. Am J Public Health. 2006;96(5):812–817. doi: 10.2105/AJPH.2005.076158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lucove JC, Kaufman JS, James SA. Association between adult and childhood socioeconomic status and prevalence of the metabolic syndrome in African Americans: the Pitt County Study. Am J Public Health. 2007;97(2):234–236. doi: 10.2105/AJPH.2006.087429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Farmer MM, Ferraro KF. Are racial disparities in health conditional on socioeconomic status? Soc Sci Med. 2005;60(1):191–204. doi: 10.1016/j.socscimed.2004.04.026 [DOI] [PubMed] [Google Scholar]
- 50. Assari S. Socioeconomic determinants of systolic blood pressure; minorities’ diminished returns. J Health Econ Dev. 2019;1:1–11. [PubMed] [Google Scholar]
- 51. Assari S. Family income reduces risk of obesity for white but not black children. Children. 2018;5:73. doi: 10.3390/children5060073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. McLaren L. Socioeconomic status and obesity. Epidemiol Rev. 2007;29:29–48. doi: 10.1093/epirev/mxm001 [DOI] [PubMed] [Google Scholar]
- 53. Pampel FC, Denney JT, Krueger PM. Obesity, SES, and economic development: a test of the reversal hypothesis. Soc Sci Med. 2012;74(7):1073–1081. doi: 10.1016/j.socscimed.2011.12.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Mendez MA, Cooper R, Wilks R, Luke A, Forrester T. Income, education, and blood pressure in adults in Jamaica, a middle-income developing country. Int J Epidemiol. 2003;32(3):400–408. doi: 10.1093/ije/dyg083 [DOI] [PubMed] [Google Scholar]
- 55. Fernald LC, Adler NE. Blood pressure and socioeconomic status in low-income women in Mexico: a reverse gradient? J Epidemiol Community Health. 2008;62(5):e8. doi: 10.1136/jech.2007.065219 [DOI] [PubMed] [Google Scholar]
- 56. Garn SM, Clark DC. Nutrition, growth, development, and maturation: findings from the ten-state nutrition survey of 1968-1970. Pediatrics. 1975;56(2):306–319. [PubMed] [Google Scholar]
- 57. Taylor SE, Stanton AL. Coping resources, coping processes, and mental health. Annu Rev Clin Psychol. 2007;3:377–401. doi: 10.1146/annurev.clinpsy.3.022806.091520 [DOI] [PubMed] [Google Scholar]