Abstract
Background
Racial disparities in cognitive function are well documented, but factors driving these disparities remain underexplored. This study aims to quantify the extent to which cumulative stress exposures across the life course explain Black–White disparities in executive function and episodic memory in middle-aged and older adults.
Method
Data were drawn from the 2004–2006 wave of the Midlife Development in the United States Study (MIDUS 2) and the MIDUS Refresher study (N = 5,947; 5,262 White and 685 Black). Cumulative stress exposures were assessed by 10 stressor domains (ie, childhood stress, stressful life events in adulthood, financial stress, work psychological stress, work physical stress, work–family conflicts, neighborhood disorder, relationship stress, perceived inequality, and perceived discrimination). Cognitive function was assessed using the Brief Test of Adult Cognition by Telephone. Marginal structural models were used to quantify the proportion of the effect of race/ethnicity status on cognitive function mediated through cumulative stress exposures.
Results
After adjusting for age, sex, and sample, on average, Black participants had lower levels of executive function (difference: −0.83 SD units, 95% CI: −0.91, −0.75) and episodic memory (difference: −0.53 SD units, 95% CI: −0.60, −0.45) scores than White participants. Cumulative stress exposures accounted for 8.4% of the disparity in executive function and 13.2% of the disparity in episodic memory.
Conclusions
Cumulative stress exposures across the life course explained modest proportions of Black–White disparities in cognitive function in this large cross-sectional study.
Keywords: Episodic memory, Executive function, Health disparities, Race, Stressors
Alzheimer’s disease and related dementias (ADRD) affect 5.8 million adults in the United States and pose significant burdens on patients, families, and society as a whole (1). Racial disparities in ADRD in the United States are widely documented, with prior research showing that Black Americans are more likely to develop ADRD than their White counterparts (2,3). Poor cognitive functioning is a strong predictor of future ADRD risk and has been linked to poor quality of life and functional status (4,5). A large body of research has consistently shown that Black older adults perform worse on tests of cognitive function than non-Hispanic White older adults (6–8). Socioeconomic factors (eg, income, education, education quality, literacy) (6,9–12) and cardiovascular risk factors (eg, diabetes, hypertension) (13) have been proposed to explain these disparities. However, these factors only partially account for the disparities, and a substantial fraction remains unexplained.
Recently, investigators have turned their attention to the role that stress exposures may play in explaining racial disparities in cognitive outcomes (14,15). Stress exposures have been long hypothesized as a potential mechanism underlying racial disparities in health (16–18). Much of this work is guided by the “Weathering Hypothesis,” which posits that exposures to cumulative social and economic disadvantages across the life course lead to acceleration of normal aging processes and earlier onset of diseases for Black Americans compared with White Americans (19). Stressful experiences take place within the context of social structures (18). Due to racism and residential segregation, Black Americans are more likely than White Americans to live in areas where unemployment, pollution, and violence are disproportionately concentrated; as a result, they are more likely to experience elevated psychological and social stressors in the form of material deprivation, neighborhood disorder, discrimination, and other adversities both in childhood and adulthood (16). Moreover, compared with White Americans, Black Americans tend to have more limited access to personal, social, educational, and material resources, resulting in higher vulnerability to the threats associated with stress exposures (20). Finally, the accumulation of exposures across multiple domains of stressors has been found to be more common among Black Americans than among White Americans (16,21). This phenomenon of stress clustering may add to the overall burden of stress exposure among racial/ethnic minorities and exacerbate racial disparities in health (22).
Stress exposures have been found to be associated with a range of adverse health outcomes including poor cognitive functioning (23–25). An emerging body of research has suggested that exposure to stressors (eg, financial strain, discrimination) activates the hypothalamic–pituitary–adrenal axis, resulting in elevated inflammation (26,27). Heightened chronic inflammation, in turn, may disrupt brain structures and impair brain health, leading to poorer cognitive function (28). Stress exposures might also influence a person to adopt unhealthy stress coping behaviors (eg, smoking, alcohol consumption, substance use) as well as induce biobehavioral responses (eg, sleep disturbances), which are themselves considered risk factors for cognitive impairment and future risk of dementia (29).
Given that higher stress exposures have been linked to both minority status and poor cognitive function, it is plausible that they could mediate racial disparities in cognitive function. However, to our knowledge, this hypothesis has only been tested in 2 prior studies to date (13,14). In a recent study using data from the Wisconsin Registry for Alzheimer’s Prevention, after accounting for acute stressful life events (eg, involuntary unemployment, the death of a child), the difference between Black and White participants’ performances on tests of cognitive speed and flexibility were attenuated by 6.9% (14). However, the study included few Black participants (N = 50), who were selected according to whether they had a family history of Alzheimer’s disease. This study was also unable to take into account effects of potentially prevalent chronic stress experiences, such as financial stress and discrimination. As some studies suggest, chronic stress may have an even stronger effect on health than acute stress (30); thus, focusing on acute stressful events alone may underestimate the effect of stress exposures on racial disparities in cognitive function. In another study using data from the Midlife Development in the United States Study (MIDUS), Zahodne et al. focused on perceived discrimination and did not find evidence of discrimination as a mediator for racial disparities in executive function and episodic memory (13). As stress exposures across the life course are likely to influence cognitive function in late life interactively and cumulatively, examinations of a single type of stressor may not capture the full spectrum of the effect of stress exposures on disparities. Our study builds on this prior work by using the MIDUS sample to consider the cumulative effects of both acute and chronic stressors across the life course.
Methodological challenges can make it difficult to investigate the pathways underlying racial disparities in health outcomes. Traditional mediation approaches compare the effect of the exposure with and without adjusting for the mediator. This method requires strict assumptions, one of which is that confounders of the mediator–outcome association are not affected by the exposure (31). However, this assumption is hard to satisfy in studies of racial disparities in health given the complex relationships between race/ethnicity status and a variety of social (eg, income, education), behavioral (eg, smoking), and health (eg, diabetes, hypertension) factors. Therefore, traditional mediation analyses are likely to yield biased estimates. Recent research has suggested the use of g-methods, such as marginal structural models, to handle exposure-induced mediator–outcome confounders (31). Instead of the traditional approach of regressing the outcome on all the covariates in the models, marginal structural models use inverse probability weighting to create pseudo-populations in which the exposure is not associated with the covariates. Using robust methods to evaluate the role of cumulative stress exposures in racial disparities in cognitive aging can help suggest potential targets for interventions that might reduce disparities and improve cognitive outcomes for all.
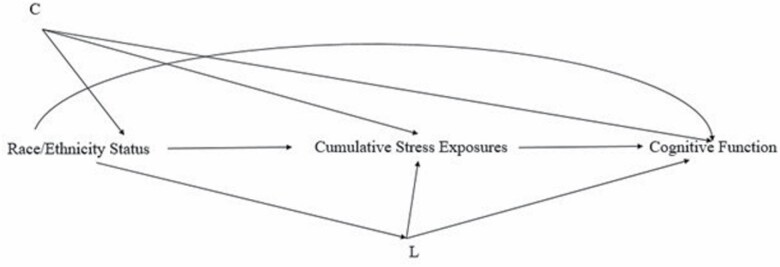
The present study aimed to determine the extent to which cumulative stress exposures across the life course account for Black–White disparities in cognitive function among middle-aged to older adults. We defined disparities as systematic and plausibly avoidable differences in cognition that adversely affect Black people (32). Pooling data from 2 separate large cohorts of the MIDUS study, following prior work (33–35), we created a composite score for cumulative stress by combining 10 domains of acute and chronic stress exposures occurring from childhood to adulthood. We hypothesized that cumulative stress exposures across the life course would partially explain Black–White disparities in both executive function and episodic memory (see Figure 1 for the directed acyclic graph).
Figure 1.
Directed acyclic graph showing the hypothesized relationships between the exposure, mediator, outcome, and confounders. “C” denotes exposure–outcome confounders, including age, sex, and sample status; “L” denotes mediator–outcome confounders, including education, income, spousal status, parental status, working status, and chronic conditions.
Method
Sample
We pooled cross-sectional data from the second wave of the MIDUS study and the MIDUS Refresher sample. MIDUS is a longitudinal survey of noninstitutionalized adults in the United States. The first wave of MIDUS (MIDUS1) was collected in 1995 and 1996 from a national random-digit-dial sample of adults aged 25–74 years (N = 7,108). Of the MIDUS1 respondents, 4,963 were reinterviewed approximately 9 years later. For this second wave (MIDUS2), a supplemental sample of Black participants (N = 592) was recruited from Milwaukee, WI to improve the representation of Black people. Sociodemographic (eg, race/ethnicity status), psychosocial (eg, perceived discrimination), and health (eg, hypertension) variables were assessed in the MIDUS2 Project 1 Survey. Starting with MIDUS2, the MIDUS team initiated the cognitive project (Project 3), which administered cognitive assessments in separate phone interviews after the completion of Project 1.
From 2011 to 2014, the MIDUS team initiated the Refresher study, which recruited a national probability sample of an additional 3,577 adults aged 25–74 to replenish and parallel the original MIDUS 1 baseline survey (36). Additionally, to replenish the MIDUS Milwaukee Black American sample, they also recruited a new sample of 508 Milwaukee Black American adults aged 25–64 (37). Although MIDUS2 and the MIDUS Refresher studies were collected at different time points, their measures and response formats are almost identical. This allows researchers to pool data and maximize their sample size to test hypotheses about health differences across gender, race/ethnicity groups, and socioeconomic status while taking account of potential period effects (36).
The eligible sample comprised 9,640 individuals who participated in MIDUS2 (N = 5,555) and the Refresher study (N = 4,085). We excluded respondents who did not complete the self-administered questionnaire in both samples, which contained the stressor questions (N = 2,290). We further excluded those who had missing data on race/ethnicity status or did not identify as “Black” or “White” as well as those who had missing information on either executive function or episodic memory scores (N = 1,383), leaving an analytic sample of 5,947 participants. Of these 5,947 individuals, 3,809 were from the MIDUS2 study and 2,138 were from the Refresher sample (see Supplementary Figure 1 for a flow chart of sample selection and exclusion). Compared with the MIDUS sample, the Refresher sample was slightly younger, had a larger proportion of male participants, had better average executive function and episodic memory scores, and had a higher level of cumulative stress exposures (see Supplementary Table 1 for the demographic characteristics in each sample).
Measures
Race/ethnicity status
Participants were asked to self-identify their racial origins as White, Black and/or African American, Native American or Alaska Native/Eskimo, Asian, Native Hawaiian or Pacific Islander, and other. For this study, we only included Black and White participants.
Cumulative stress exposures across the life course
Following previous research in MIDUS (33), 10 stressor domains assessed at MIDUS2 and in the Refresher sample were included: childhood stress, stressful life events in adulthood, financial stress, neighborhood stress, work psychological stress, work physical stress, work–family conflict, relationship stress, perceived inequality, and perceived discrimination. Validated multi-item measures were used to assess each stressor domain. Childhood stress was assessed by reporting whether any given event occurred before age 18, resulting in a sum score across 16 items (for each item, yes = 1 and no = 0): 7 were from the childhood event checklist and 9 were from the stressful life event inventory. Stressful life events in adulthood were assessed by reporting whether any given event occurred after age 18, resulting in a sum score across 20 items (for each item, yes = 1 and no = 0) from the stressful life event inventory (38). Financial stress was assessed with 2 items asking participants (i) if they currently have enough money for their needs and (ii) how difficult it is for them to pay their monthly bills (response range: 2–7; α = .78). Neighborhood stress was derived from summing 4 items assessing neighborhood safety, perceived neighbor support, and perceived neighborhood trust (range: 4–16; α = .66). Work psychological stress consisted of 5 separate measures combined, including skill discretion (3 items; range: 3–15; α = .71), decision authority (6 items; range: 6–30; α = .87), job demand (4 items; range: 4–20; α = .74), coworker support (2 items; range: 2–10; α = .89), and supervisor support (3 items; range: 2–10; α = .87). Work physical stress included a measure of risk of injury or accident on the job (1 item; range: 1–4) and frequency of job strain (9 items; range: 9–45; α = .90). Work–family conflict measured negative work-to-family spillover (4 items; range: 4–20; α = .82) and family-to-work spillover (4 items; range: 4–20; α = .79). Relationship stress consisted of 4 measures assessing family strain (4 items; range: 4–16; α = .79), friend strain (4 items; range: 4–16; α = .80), perceived troubles in marriage (5 items; range: 5–21; α = .72), and spouse/partner strain (6 items; range: 6–24; α = .87). Perceived inequality was derived from separate measures assessing people’s perceptions of inequality across 3 domains including (i) child rearing (6 items; range: 6–24; α = .71; eg, I believe that I have been able to do as much for my children as most other people), (ii) housing and neighborhood conditions (6 items; range: 6–24; α = .79), and (iii) work (6 items; range: 6–24; α = .76). Perceived discrimination included a sum of scores across the lifetime discrimination inventory (11 items; range: 0–11) and the everyday discrimination scale (9 items, range: 9–26; α = .91). A detailed description of the stressor measures has been published elsewhere (33).
To facilitate comparisons across all stressor domains, we standardized each stressor domain to have a standard normal distribution. We assigned the lowest value of the scale if a given stressor domain was not applicable (eg, work stress did not apply to those who were unemployed) and adjusted for each respondent’s status on relevant domains (eg, employed vs unemployed) (18). Following previous research (33), we created a continuous cumulative stressor measure by adding the z scores of all stressors and then transformed the sum score to have a standard normal distribution.
Cognitive function was measured by the Brief Test of Adult Cognition by Telephone (39), which assessed 7 domains: (i) immediate recall, (ii) delayed recall, (iii) working memory, (iv) verbal fluency, (v) inductive reasoning, (vi) processing speed, and (vii) attention-switching tasks. Based on prior confirmatory factor analyses (39), we created 2 summary cognitive scores: executive function and episodic memory. Executive function score was calculated by averaging the standardized scores for the 5 subtests of verbal fluency, inductive reasoning, processing speed, working memory, and attention-switching tasks. Episodic memory score was calculated by averaging the standardized scores for immediate and delayed recall. In order to make comparisons more interpretable, the final executive function and episodic memory scores were standardized.
Covariates were assessed in 2004–2006 for MIDUS2 and 2011–2014 for the Refresher data and were selected based on a review of the previous literature and their hypothetical relationships with the exposure, mediator, and outcomes (16,18,40). Potential exposure–outcome confounders included age (years), sex (female vs male), and sample (MIDUS2 vs Refresher). Potential mediator–outcome confounders included education level (less than high school, high school or GED, some college, and college or more), annual household income (<$25,000, $25,000–$44,999, $45,000–$69,999, and >$70,000), whether working at the time of the interview (yes vs no), whether married or not (yes vs no), whether had any child or not (yes vs no), and whether had experienced or been treated for any of 31 chronic conditions (eg, diabetes, stroke) in the past 12 months (yes vs no).
Analyses
We conducted t tests and chi-squared tests to calculate means (SD) for continuous variables and percentages for categorical variables in the pooled sample and by race/ethnicity status. Before investigating the mediating role of cumulative stress exposures, we evaluated the relationship between exposure (ie, race/ethnicity status) and mediator (ie, cumulative stress exposures) as well as relationships between mediator (ie, cumulative stress exposures) and outcomes (ie, executive function and episodic memory). We fitted generalized estimating equation regression models with identity link and normal distribution to evaluate the relationship between race/ethnicity status and cumulative stress exposures as well as the relationship between cumulative stress exposures and cognitive function (separately for executive function and episodic memory), accounting for twin and sibling clusters. We included an interaction term between race/ethnicity status and cumulative stress exposures to determine whether the relationship between cumulative stress exposures and cognitive function differed by race/ethnicity status. Results suggested no evidence of interactions between race/ethnicity status and cumulative stress exposures on executive function scores (Race/ethnicity × Stress: β = −0.02, 95% CI: −0.09, 0.04) and episodic memory scores (Race/ethnicity × Stress: β = 0.02, 95% CI: −0.05, 0.09), and thus we did not include the interaction terms in the analysis.
Next, we used marginal structural models to estimate the controlled direct effect (CDE) of race/ethnicity status on cognitive function (ie, not through measured cumulative stress exposures). Marginal structural models handle mediator–outcome covariates through weighting, accurately estimating the CDE of race/ethnicity status on cognitive function even in settings where the stress–cognition confounders differ by race/ethnicity status (41). Specifically, we ran stabilized inverse-probability-weighted marginal structural models with the final weight trimmed at the 99th and first percentiles (42). Detailed descriptions of the inverse probability weights are included in Supplementary Methods.
Following the marginal structural models, we calculated proportion eliminated (PE), which captures the proportion of the total effect (TE) of race/ethnicity on cognitive function that would be eliminated by setting cumulative stress exposures across the life course (mediator M) at the same level between Black and White participants using the following equation as described in VanderWeele (43):
The proportion of missing covariates and mediators (ie, cumulative stressor exposure) ranged from 0.1% for education to 10.6% for childhood adversity, with most variables missing less than 3% of the observations. We performed chained equations imputation (also known as fully conditional specification) to handle missing data. We generated 20 imputations and used Proc Mianalyze to combine the results of multiple imputations. Note that for the marginal structural models, following the recommendation of recent research (44), we estimated the inverse probability weights for each imputed dataset and then combined results of the imputed datasets to produce an overall estimate of the total effect and the CDE. All analyses were performed in SAS, version 9.4. Figures were created in R.
Sensitivity Analyses
Given the large age range in the study sample and because declines in cognitive function tend to be more evident among older adults, we median-split age (younger than 55 years old vs 55 years and older) to evaluate if relations between race/ethnicity, cumulative stress exposures, and cognitive function differed by age group. Due to concerns about sample size and power to detect differences, we divided the groups based on a median-split (younger than 55 years old vs 55 years and older) instead of the traditional age cutoff (ie, 65 years old). Additionally, given some research suggesting discontinuous effects of stress (ie, effects of stress on cognition primarily occur only when stress levels are high) (45), we categorized cumulative stress into a high stress dichotomous variable (top quartile vs bottom 3 quartiles) and re-ran the models replacing the continuous cumulative stress variable from the primary analyses. Furthermore, as education has been identified as a particularly important mediator for racial disparities in cognitive function (6,46), we calculated the proportion of disparities in cognitive function explained by education to compare it with the proportion explained by cumulative stress exposures.
Results
Sample Characteristics
The mean age was 55 years (SD =13; range: 23–84), and more than half (55.19%; N = 3,282) were women. Table 1 details the distribution of the variables in the pooled sample and by race/ethnicity status. A total of 5,262 (88.48%) were White participants, and 685 (11.52%) were Black participants. Compared with White participants, Black participants were more likely to be younger, female, married, have lower levels of education, have an annual income that was lower than $45,000, and not be working at the time of the interview. On average, levels of cumulative stress exposures were higher among Black participants (mean = 0.61, SE = 0.04) than White participants (mean = −0.08, SE = 0.01). For individual stressor domains, Black participants reported higher levels for all stressors except for relationship stress and work–family conflict.
Table 1.
Sample Characteristics, Midlife Development in the United States Study (N = 5,947)
| Sample | Race/Ethnicity Status | ||
|---|---|---|---|
| White Participants | Black Participants | ||
| Age, mean (SD) (range: 23–84) | 55 (13) | 56 (13) | 52 (12) |
| Sex, N (%) | |||
| Female | 3,282 (55.19) | 2,820 (53.59) | 462 (67.25) |
| Male | 2,665 (44.81) | 2,442 (46.41) | 223 (32.75) |
| Race/ethnicity status, N (%) | |||
| White participants | 5,262 (88.48) | — | — |
| Black participants | 685 (11.52) | — | — |
| Education | |||
| Less than high school | 345 (5.80) | 251 (4.77) | 94 (13.72) |
| High school or GED | 1,435 (24.12) | 1,248 (23.72) | 187 (27.30) |
| Some college | 1,206 (20.28) | 1,040 (19.76) | 166 (24.23) |
| College or more | 2,961 (49.79) | 2,723 (51.75) | 238 (34.74) |
| Annual household income, N (%) | |||
| <$25,000 | 1,245 (20.94) | 954 (18.12) | 291 (42.48) |
| $25,000–$44,999 | 1,003 (16.87) | 880 (16.71) | 123 (17.96) |
| $45,000–$69,999 | 1,116 (18.76) | 997 (18.94) | 119 (17.37) |
| >$70,000 | 2,583 (43.44) | 2,431 (46.17) | 152 (22.19) |
| Have a child, N (%) | |||
| Yes | 5,069 (85.24) | 4,484 (85.21) | 585 (85.40) |
| No | 878 (14.76) | 778 (14.79) | 100 (14.60) |
| Have a spouse, N (%) | |||
| Yes | 4,211 (70.81) | 3,936 (74.80) | 275 (40.15) |
| No | 1,736 (29.19) | 1,326 (25.20) | 410 (59.85) |
| Currently working, N (%) | |||
| Yes | 3,853 (64.79) | 3,428 (65.14) | 425 (62.04) |
| No | 2,094 (35.21) | 1,834 (34.85) | 260 (37.96) |
| Have any chronic conditions, N (%) | |||
| Yes | 4,672 (78.55) | 4,108 (78.07) | 564 (82.34) |
| No | 1,275 (21.45) | 1,154 (21.93) | 121(17.66) |
| Sample, N (%) | |||
| MIDUS2 | 3,809 (64.05) | 3,413 (64.86) | 396 (57.81) |
| Refresher | 2,138 (35.95) | 1,849 (35.14) | 289 (42.19) |
| Stressor scores, mean (SE) | |||
| Financial stress | — | −0.08 (0.01) | 0.60 (0.04) |
| Work psychological stress | — | −0.01 (0.01) | 0.06 (0.04) |
| Work physical stress | — | −0.002 (0.01) | 0.02 (0.04) |
| Work–family conflict | — | 0.02 (0.01) | −0.12 (0.04) |
| Neighborhood stress | — | −0.06 (0.01) | 0.43 (0.05) |
| Perceived discrimination | — | −0.11 (0.01) | 0.82 (0.05) |
| Perceived inequality | — | −0.04 (0.01) | 0.32 (0.04) |
| Relationship stress | — | 0.01 (0.01) | −0.06 (0.04) |
| Adult stressful life events | — | −0.05 (0.01) | 0.42 (0.05) |
| Childhood stress | — | −0.07 (0.01) | 0.54 (0.06) |
| Cumulative stress exposures | — | −0.08 (0.01) | 0.61 (0.04) |
Notes: Percentages refer to the proportion of individuals within each category with that characteristic. Standard errors are reported because standard deviations are not available for multiply imputed data. The means and SEs of stressors in the full sample were not reported because all were standardized to have a mean of 0 and an SD of 1.
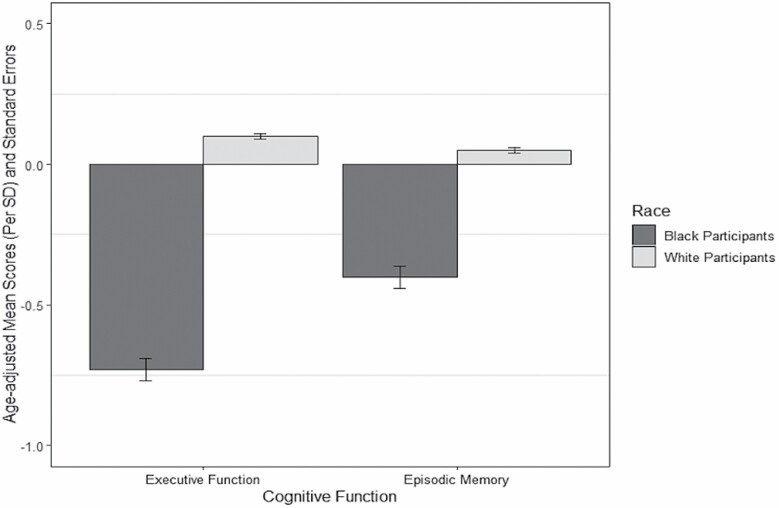
With respect to racial differences in executive function and episodic memory, on average, Black participants had lower age-adjusted scores than White participants on standardized executive function (Black participants: mean = −0.73, SE = 0.04; White participants: mean = 0.10, SE = 0.01) and episodic memory (Black participants: mean = −0.40, SE = 0.04; White participants: mean = 0.05, SE = 0.01) (Figure 2).
Figure 2.
Age-adjusted mean and standard errors of executive function and episodic memory in Black and White participants in the Midlife Development in the United States Study.
Relationship Between Race/Ethnicity Status, Cumulative Stress Exposures, and Executive Function
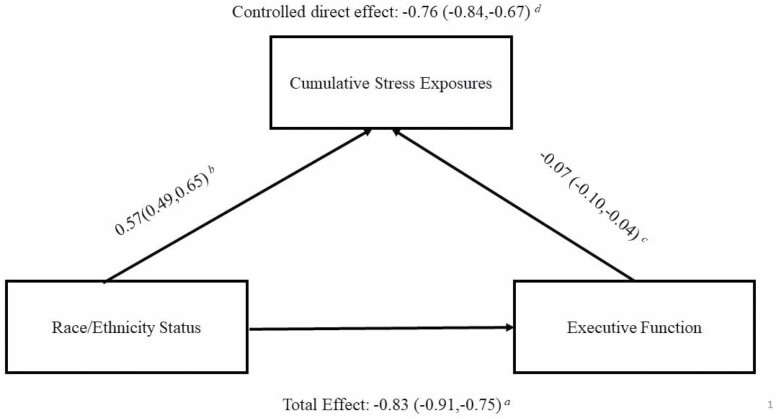
As expected, being Black was associated with 0.57 SD higher (95% CI: 0.49, 0.65) in cumulative stress exposures, after adjusting for age, sex, and sample. Additionally, a 1-SD increase in cumulative stress exposure corresponded to an executive function score that was 0.07 SD lower (95% CI: −0.10, −0.04), after adjusting for age, sex, race/ethnicity status, sample, education, income, spousal status, parental status, working status, and chronic conditions. Figure 3 depicts the relationship between race/ethnicity status, cumulative stress exposures, and executive function.
Figure 3.
Relationships between race/ethnicity status, cumulative stress exposures, and executive function.Notes: aTotal effects of race/ethnicity status on executive function, adjusting for age, sex, and sample. bAssociations between race/ethnicity status on cumulative stress exposures, adjusting for age, sex, and sample. cAssociations between cumulative stress exposures and executive function, adjusting for age, sex, race/ethnicity status, sample, education, income, spousal status, parental status, working status, and chronic conditions. dMarginal structural model evaluating the controlled direct effect of race/ethnicity status on executive function. eGeneralized estimating equations with identity link and normal distribution were used in all models to adjust for clustering by family and were calculated using SAS PROC GENMOD. fResults were generated from 20 multiply imputed datasets.
Under the hypothetical scenario in which each Black participant’s stress exposure level was the level it would have been had the participant been White, all covariates being equal, Black participants’ executive function score was an average of 0.76 SD lower (95% CI: −0.84, −0.67) than White participants’ executive function score. Setting cumulative stress exposures at the same level for all participants would eliminate 8.4% of the racial disparity in executive function.
Relationship Between Race/Ethnicity Status, Cumulative Stress Exposures, and Episodic Memory
A 1-SD increase in cumulative stress exposure corresponded to an episodic memory score that was 0.04 SD lower (95% CI: −0.06, −0.002), after adjusting for age, sex, race/ethnicity status, sample, education, income, spousal status, parental status, working status, and chronic conditions. Figure 4 depicts the relationship between race/ethnicity status, cumulative stress exposures, and episodic memory.
Figure 4.
Relationships between race/ethnicity status, cumulative stress exposures, and episodic memory.Notes: aTotal effects of race/ethnicity status on episodic memory, adjusting for age, sex, and sample. bAssociations between race/ethnicity status on cumulative stress exposures, adjusting for age, sex, and sample. cAssociations between cumulative stress exposures and episodic memory, adjusting for age, sex, race/ethnicity status, sample, education, income, spousal status, parental status, working status, and chronic conditions. dMarginal structural model evaluating the controlled direct effect of race/ethnicity status on episodic memory. eGeneralized estimating equations with identity link and normal distribution were used in all models to adjust for clustering by family and were calculated using SAS PROC GENMOD. fResults were generated from 20 multiply imputed datasets.
Under the hypothetical scenario in which each Black participant’s stress exposure level was the level it would have been had the participant been White, all covariates being equal, Black participants’ episodic memory score was an average of 0.46 SD lower (95% CI: −0.55, −0.37) than White participants’ episodic memory score. Setting cumulative stress exposures at the same level for all participants would eliminate 13.2% of the racial disparity in episodic memory.
Sensitivity Analyses
When evaluating disparities in cognitive function and the potential mediating role of cumulative stress exposures by age groups, the results showed some differences with cumulative stress exposures accounting for more disparities in executive function in older versus younger groups (eg, in the older group, cumulative stress exposures explained 11.7% of disparities in executive function; in the younger group, they explained 3.4% of disparities in executive function) (see Supplementary Table 2). When considering cumulative stress exposures as a dichotomous variable, the proportion of racial disparities explained was smaller (6.0% for disparities in executive function and 9.4% for disparities in episodic memory) than that explained by the continuous stressor variables (see Supplementary Table 3). For comparative purposes, we calculated the proportion of disparities explained by education. Results show that education explained 18.1% of the disparities in executive function scores and 26.4% of the disparities in episodic memory scores (see Supplementary Table 4).
Discussion
With combined data from 2 large samples of the MIDUS study, we found Black participants experienced higher exposure in most stressor domains and in overall cumulative stress exposure and had lower executive function and episodic memory scores compared with White participants. Using marginal structural models, we found that approximately 8.4% of the Black–White disparities in executive function and 13.2% of the Black–White disparities in episodic memory could be eliminated if we could reduce the differential exposure to cumulative stress between Black and White participants.
Our finding that Black participants reported lower levels of executive function and episodic memory than White participants is consistent with previous research on racial differences in levels of cognitive function (6,32). Additionally, our finding that cumulative stress exposures were more common among Black participants than White participants is in line with findings of prior research using data from larger population-based cohorts such as the Health and Retirement Study (21), the Chicago Community Adult Health Study (16), and the Women’s Health Study (35). Taken together with previous research, these findings provide strong empirical support for the notion that stress exposures are socially patterned and that Black Americans are disproportionately exposed to stress across their life course.
In the present study, cumulative stress exposures appeared to explain a modest amount of racial/ethnic disparities in executive function and episodic memory scores, and the findings were robust to whether cumulative stress exposure was operationalized as a continuous or a dichotomous variable (12). Compared with education, which is a key mediator of racial disparities in cognitive function identified from prior research (6), we found cumulative stress exposures explained about half as much of the Black–White disparities in cognitive function. Our findings suggest that cumulative stress exposure is an important pathway to consider, even if it does not have as large an effect as education. Notably, cumulative stress exposures explained more disparities in executive function in the older age group than the younger age group. Perhaps with aging, the amount of time that Black adults have been disproportionately exposed to adverse environmental conditions also increases, leading to more salient effects of cumulative stressors on cognition in the older age group. Older populations may also be more likely to experience social isolation and lack resources to buffer against the negative effects of stress (47), which may explain why stress exposures appeared to take a bigger toll on the older group compared to the younger group.
Our study should be considered in light of several limitations. Due to the cross-sectional nature of the study design, we cannot ensure that stress exposures precede poor cognition. While it is possible that poorer cognitive function leads to a greater level of stress exposures, our hypothesized direction is theoretically more plausible and has been widely supported by evidence from several previous studies (23,25,48,49). Future research should replicate our study with longitudinal designs to better understand the causal relationship between cumulative stress exposures and racial disparities in cognitive function. While we have attempted to minimize confounding bias through conditioning on several exposure–outcome and mediator–outcome confounders, it is plausible that unmeasured confounders may still be present. For example, we did not have information about early childhood cognition which may lead both to high stress (eg, high childhood stress) and poor cognitive function simultaneously. Another limitation of our study relates to our stress exposure assessments, which relied on self-report scales including both more objective events and individual perceptions of stress; such reports can be subject to faulty recall. For example, it has been suggested that racial/ethnic minorities may ignore evidence of discrimination in order to avoid false alarms that may affect life satisfaction and personal control (50). If that is the case, then our results on the roles of stress exposures on disparities in cognitive function might have been underestimated (50). Lastly, most of the Black participants in the study were from Milwaukee, WI, whereas the White participants were selected from a much wider geographic distribution. Hence, the generalizability of the findings to other geographic areas may be limited. Nevertheless, the racial composition of Milwaukee is similar to other big cities in the United States, and thus studying stress and cognitive function among Black individuals who live there will have informative implications.
Despite these limitations, our study has numerous strengths, including data from 2 large population-based cohorts with nearly identical measurements, the utilization of a broad range of stressors to construct the cumulative stressor variable, and the application of the novel marginal structural models to account for mediator–outcome confounders. As our aging population continues to increase and becomes more racially diverse, addressing racial disparities in cognitive aging is imperative. Findings of this study, if replicable, would suggest that reducing stress exposures among Black Americans could help eliminate some racial disparities in executive function and episodic memory at the population level. Special attention may need to be given to target factors (eg, structural racism and discrimination) that lead to the high prevalence of stress exposures among Black Americans in the first place.
Supplementary Material
Acknowledgments
We thank the staff and participants of the Midlife Development in the United States Study (MIDUS) for their important contributions.
Funding
R.C. was supported by the National Institute of Aging (NIA) under award number F99AG068431-01 and the Harvard Lee Kum Sheung Center for Health and Happiness Dissertation Award. D.R.W. was supported by the NIA under award number U19 AG051426.
Conflict of Interest
None declared.
Author Contributions
R.C., J.W., L.D.K., and D.R.W. contributed to the conception and design of the study. R.C. conducted the statistical analysis. All authors contributed to interpretation of the results. All the authors critically revised the manuscript for important intellectual content and approved the final version for publications.
References
- 1. Alzheimer’s Association. 2018 Alzheimer’s disease facts and figures. Alzheimer Dement. 2018;14(3):367–429. doi: 10.1016/j.jalz.2018.02.001 [DOI] [Google Scholar]
- 2. Matthews KA, Xu W, Gaglioti AH, et al. . Racial and ethnic estimates of Alzheimer’s disease and related dementias in the United States (2015–2060) in adults aged ≥65 years. Alzheimer Dement. 2019;15(1):17–24. doi: 10.1016/j.jalz.2018.06.3063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mayeda ER, Glymour MM, Quesenberry CP, Whitmer RA. Inequalities in dementia incidence between six racial and ethnic groups over 14 years. Alzheimer Dement. 2016;12(3):216–224. doi: 10.1016/j.jalz.2015.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hill NL, McDermott C, Mogle J, et al. . Subjective cognitive impairment and quality of life: a systematic review. Int Psychogeriatr. 2017;29(12):1965–1977. doi: 10.1017/S1041610217001636 [DOI] [PubMed] [Google Scholar]
- 5. Shimada H, Makizako H, Lee S, et al. . Impact of cognitive frailty on daily activities in older persons. J Nutr Health Aging. 2016;20(7):729–735. doi: 10.1007/s12603-016-0685-2 [DOI] [PubMed] [Google Scholar]
- 6. Weuve J, Barnes LL, Mendes de Leon CF, et al. . Cognitive aging in black and white Americans: cognition, cognitive decline, and incidence of Alzheimer disease dementia. Epidemiology (Cambridge, Mass). 2018;29(1):151–159. doi: 10.1097/EDE.0000000000000747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wilson RS, Capuano AW, Sytsma J, Bennett DA, Barnes LL. Cognitive aging in older Black and White persons. Psychol Aging. 2015;30(2):279–285. doi: 10.1037/pag0000024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zahodne LB, Manly JJ, Azar M, Brickman AM, Glymour MM. Racial disparities in cognitive performance in mid- and late adulthood: analyses of two cohort studies. J Am Geriatr Soc. 2016;64(5):959–964. doi: 10.1111/jgs.14113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhang Z, Hayward MD, Yu YL. Life course pathways to racial disparities in cognitive impairment among older Americans. J Health Soc Behav. 2016;57(2):184–199. doi: 10.1177/0022146516645925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Peterson RL, Fain MJ, A Butler E, Ehiri JE, Carvajal SC. The role of social and behavioral risk factors in explaining racial disparities in age-related cognitive impairment: a structured narrative review. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn. 2020;27(2):173–196. doi: 10.1080/13825585.2019.1598539 [DOI] [PubMed] [Google Scholar]
- 11. Sisco S, Gross AL, Shih RA, et al. . The role of early-life educational quality and literacy in explaining racial disparities in cognition in late life. J Gerontol B Psychol Sci Soc Sci. 2015;70(4):557–567. doi: 10.1093/geronb/gbt133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Glymour MM, Manly JJ. Lifecourse social conditions and racial and ethnic patterns of cognitive aging. Neuropsychol Rev. 2008;18(3):223–254. doi: 10.1007/s11065-008-9064-z [DOI] [PubMed] [Google Scholar]
- 13. Zahodne LB, Manly JJ, Smith J, Seeman T, Lachman ME. Socioeconomic, health, and psychosocial mediators of racial disparities in cognition in early, middle, and late adulthood. Psychol Aging. 2017;32(2):118–130. doi: 10.1037/pag0000154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zuelsdorff M, Okonkwo OC, Norton D, et al. . Stressful life events and racial disparities in cognition among middle-aged and older adults. J Alzheimers Dis. 2020;73(2):671–682. PMID: 31815690;PMCID: PMC7481054. doi: 10.3233/JAD-190439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Forrester SN, Gallo JJ, Whitfield KE, Thorpe RJ. A framework of minority stress: from physiological manifestations to cognitive outcomes. Gerontologist. 2019;59(6):1017–1023. doi: 10.1093/geront/gny104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sternthal MJ, Slopen N, Williams DR. Racial disparities in health: how much does stress really matter? Du Bois Rev. 2011;8(1):95–113. doi: 10.1017/S1742058X11000087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Williams DR, Mohammed SA. Discrimination and racial disparities in health: evidence and needed research. J Behav Med. 2009;32(1):20–47. doi: 10.1007/s10865-008-9185-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lantz PM, House JS, Mero RP, Williams DR. Stress, life events, and socioeconomic disparities in health: results from the Americans’ Changing Lives Study. J Health Soc Behav. 2005;46(3):274–288. doi: 10.1177/002214650504600305 [DOI] [PubMed] [Google Scholar]
- 19. Geronimus AT. The weathering hypothesis and the health of African-American women and infants: evidence and speculations. Ethn Dis. 1992;2(3):207–221. [PubMed] [Google Scholar]
- 20. Boehm JK, Chen Y, Williams DR, Ryff C, Kubzansky LD. Unequally distributed psychological assets: are there social disparities in optimism, life satisfaction, and positive affect? PLoS ONE 2015;10(2):e0118066. doi: 10.1371/journal.pone.0118066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Boen C. Death by a thousand cuts: stress exposure and black–white disparities in physiological functioning in late life. J Gerontol B Psychol Sci Soc Sci. 2020;75(9):1937–1950. doi: 10.1093/geronb/gbz068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Brondolo E, Byer K, Gianaros PJ, et al. . Stress and Health Disparities: Contexts, Mechanisms, and Interventions Among Racial/Ethnic Minority and Low-Socioeconomic Status Populations. American Psychological Association (APA) Working Group Report; 2017. [Google Scholar]
- 23. Aggarwal NT, Wilson RS, Beck TL, et al. . Perceived stress and change in cognitive function among adults 65 years and older. Psychosom Med. 2014;76(1):80–85. doi: 10.1097/PSY.0000000000000016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Barnes LL, Lewis TT, Begeny CT, Yu L, Bennett DA, Wilson RS. Perceived discrimination and cognition in older African Americans. J Int Neuropsychol Soc. 2012;18(5):856–865. doi: 10.1017/S1355617712000628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Munoz E, Sliwinski MJ, Scott SB, Hofer S. Global perceived stress predicts cognitive change among older adults. Psychol Aging. 2015;30(3):487–499. doi: 10.1037/pag0000036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lewis TT, Aiello AE, Leurgans S, Kelly J, Barnes LL. Self-reported experiences of everyday discrimination are associated with elevated C-reactive protein levels in older African-American adults. Brain Behav Immun. 2010;24(3):438–443. doi: 10.1016/j.bbi.2009.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Beatty Moody DL, Brown C, Matthews KA, Bromberger JT. Everyday discrimination prospectively predicts inflammation across 7-years in racially diverse midlife women: study of women’s health across the nation. J Soc Issue. 2014;70(2):298–314. doi: 10.1111/josi.12061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sartori AC, Vance DE, Slater LZ, Crowe M. The impact of inflammation on cognitive function in older adults: implications for health care practice and research. J Neurosci Nurs. 2012;44(4):206. doi: 10.1097/JNN.0b013e3182527690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cipriani G, Lucetti C, Danti S, Nuti A. Sleep disturbances and dementia. Psychogeriatrics. 2015;15(1):65–74. doi: 10.1111/psyg.12069 [DOI] [PubMed] [Google Scholar]
- 30. McGonagle KA, Kessler RC. Chronic stress, acute stress, and depressive symptoms. Am J Community Psychol. 1990;18(5):681–706. doi: 10.1007/BF00931237 [DOI] [PubMed] [Google Scholar]
- 31. Naimi AI, Schnitzer ME, Moodie EE, Bodnar LM. Mediation analysis for health disparities research. Am J Epidemiol. 2016;184(4):315–324. doi: 10.1093/aje/kwv329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Braveman PA, Kumanyika S, Fielding J, et al. . Health disparities and health equity: the issue is justice. Am J Public Health. 2011;101(suppl. 1):S149–S155. doi: 10.2105/AJPH.2010.300062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Slopen N, Kontos EZ, Ryff CD, Ayanian JZ, Albert MA, Williams DR. Psychosocial stress and cigarette smoking persistence, cessation, and relapse over 9-10 years: a prospective study of middle-aged adults in the United States. Cancer Causes Control. 2013;24(10):1849–1863. doi: 10.1007/s10552-013-0262-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cuevas AG, Chen R, Slopen N, et al. . Assessing the role of health behaviors, socioeconomic status, and cumulative stress for racial/ethnic disparities in obesity. Obesity (Silver Spring). 2020;28(1):161–170. doi: 10.1002/oby.22648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Burroughs Pena MS, Mbassa RS, Slopen NB, Williams DR, Buring JE, Albert MA. Cumulative psychosocial stress and ideal cardiovascular health in older women: data by race/ethnicity. Circulation. 2019;139(17):2012–2021. doi: 10.1161/CIRCULATIONAHA.118.033915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ryff C, Almeida D, Ayanian J, et al. . Midlife in the United States (MIDUS Refresher), 2011–2014. ICPSR36532-v2. Inter-university Consortium for Political and Social Research; 2016. [Google Scholar]
- 37. Midlife in the United States (MIDUS Refresher): Milwaukee African American Sample, 2012-2013 (ICPSR 36722). Published October 22, 2018. Accessed October 1, 2019. https://www.icpsr.umich.edu/web/NACDA/studies/36722 [Google Scholar]
- 38. Turner RJ, Wheaton B. Checklist measurement of stressful life events. In: Cohen S, Kessler RC, Gordon LU, eds. Measuring Stress: A Guide for Health and Social Scientists. New York, NY: Oxford University Press Inc; 1995:29–58. [Google Scholar]
- 39. Lachman ME, Agrigoroaei S, Tun PA, Weaver SL. Monitoring cognitive functioning: psychometric properties of the brief test of adult cognition by telephone. Assessment. 2014;21(4):404–417. doi: 10.1177/1073191113508807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Duncan GJ, Magnuson K. Socioeconomic status and cognitive functioning: moving from correlation to causation. Wiley Interdiscip Rev Cogn Sci. 2012;3(3):377–386. doi: 10.1002/wcs.1176 [DOI] [PubMed] [Google Scholar]
- 41. Robins JM, Hernán MA, Brumback B. Marginal structural models and causal inference in epidemiology. Epidemiology. 2000;11(5):550–560. doi: 10.1097/00001648-200009000-00011 [DOI] [PubMed] [Google Scholar]
- 42. Cole SR, Hernán MA, Robins JM, et al. . Effect of highly active antiretroviral therapy on time to acquired immunodeficiency syndrome or death using marginal structural models. Am J Epidemiol. 2003;158(7):687–694. doi: 10.1093/aje/kwg206 [DOI] [PubMed] [Google Scholar]
- 43. VanderWeele TJ. Policy-relevant proportions for direct effects. Epidemiology (Cambridge, Mass). 2013;24(1):175–6. doi: 10.1097/EDE.0b013e3182781410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Granger E, Sergeant JC, Lunt M. Avoiding pitfalls when combining multiple imputation and propensity scores. Stat Med. 2019;38(26):5120–5132. doi: 10.1002/sim.8355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sandi C. Stress and cognition. Wiley Interdiscip Rev Cogn Sci. 2013;4(3):245–261. doi: 10.1002/wcs.1222 [DOI] [PubMed] [Google Scholar]
- 46. Masel MC, Raji M, Peek MK. Education and physical activity mediate the relationship between ethnicity and cognitive function in late middle-aged adults. Ethn Health. 2010;15(3):283–302. doi: 10.1080/13557851003681273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Courtin E, Knapp M. Social isolation, loneliness and health in old age: a scoping review. Health Soc Care Community. 2017;25(3):799–812. doi: 10.1111/hsc.12311 [DOI] [PubMed] [Google Scholar]
- 48. Scott SB, Graham-Engeland JE, Engeland CG, et al. . The Effects of Stress on Cognitive Aging, Physiology and Emotion (ESCAPE) project. BMC Psychiatry. 2015;15(1):146. doi: 10.1186/s12888-015-0497-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Turner AD, James BD, Capuano AW, Aggarwal NT, Barnes LL. Perceived stress and cognitive decline in different cognitive domains in a cohort of older African Americans. Am J Geriatr Psychiatry. 2017;25(1):25–34. doi: 10.1016/j.jagp.2016.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Contrada RJ, Ashmore RD, Gary ML, et al. . Ethnicity-related sources of stress and their effects on well-being. Curr Direct Psychol Sci. 2000;9(4):136–139. doi: 10.1111/1467-8721.00078 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.