Abstract
Background
Non-immunoglobulin E (IgE)-mediated hypersensitivity reactions (HSRs) to nafcillin are commonly reported, but scarce data are available to guide appropriate antibiotic change following these reactions. Although cefazolin is an attractive therapeutic alternative in methicillin-susceptible Staphylococcus aureus (MSSA) infections when patients experience an HSR to nafcillin, more data are needed to evaluate the tolerability of cefazolin after switching from nafcillin. The purpose of this study was to describe the tolerability of cefazolin in patients who develop a suspected non-IgE-mediated HSR to nafcillin.
Methods
This was a retrospective, descriptive case series of patients who received nafcillin for an MSSA infection, experienced a suspected non-IgE-mediated HSR, and were switched to cefazolin between October 2015 and November 2019 at a single academic medical center. The primary objective was to identify the percentage of patients who completed cefazolin after experiencing a suspected non-IgE-mediated HSR to nafcillin.
Results
There were 80 patients with 87 prespecified non-IgE-mediated HSRs during the study period. Seventy-one (89%) patients completed cefazolin, with 53 (75%) of these patients completing at least 2 weeks of therapy. One patient was ultimately switched from cefazolin to daptomycin due to concern for treatment failure. Eight patients (10%) did not tolerate cefazolin after switching from nafcillin. Of these, 3 patients experienced an unrelated HSR, whereas 5 patients experienced the same non-IgE-mediated HSR that was attributed to nafcillin and discontinued cefazolin within 7 days. The most common HSR cited was immune-mediated nephritis; however, the majority were clinically presumed but did not meet objective diagnostic criteria.
Conclusions
Treatment with cefazolin after experiencing a suspected non-IgE-mediated HSR to nafcillin appears to be safe, even for patients requiring a prolonged duration of cefazolin.
Keywords: cefazolin, nafcillin, hypersensitivity reaction, MSSA
Cefazolin was safe among individuals with a suspected non-immunoglobulin E (IgE)-mediated hypersensitivity reaction (HSR) to nafcillin. Few did not tolerate cefazolin due to an unrelated reaction or persistent effects of the same HSR within 7 days of switching.
Penicillins are the most common reported cause of drug allergies with a worldwide prevalence estimated at approximately 10% [1, 2]. True immunoglobulin E (IgE)-mediated drug allergy, which can result in life-threatening anaphylaxis, is 1 of 4 types of hypersensitivity reactions (HSRs) first classified by Gell and Coombs in 1963 [3]. The remaining categories, types II–IV, describe non-IgE-mediated reactions including hemolytic anemia, thrombocytopenia, serum sickness, maculopapular rash, and organ-specific tissue injury caused by activated T cells [1].
Anti-staphylococcal penicillins (eg, nafcillin) are considered first-line therapy for invasive methicillin-susceptible Staphylococcus aureus (MSSA) infections [4]. However, they have been associated with a variety of type II through IV non-IgE-mediated HSRs including rash, immune-mediated nephritis, and immune-mediated hepatitis [5–7]. Although vancomycin may be considered in these situations, treatment outcomes are inferior when compared to β-lactams for management of MSSA infections, particularly in patients with bacteremia [8–10]. Cefazolin is an effective alternative agent for MSSA infections that is generally well tolerated, which makes it an attractive option for patients who develop an HSR to nafcillin [8].
Although the rate of IgE-mediated cross-reactivity between penicillins and cephalosporins is well known, much less is known about non-IgE-mediated cross-reactivity between nafcillin and cefazolin [11–13]. Published literature supporting tolerance of cefazolin after a non-IgE-mediated HSR to a penicillin is sparse, with only 2 prior studies addressing this issue. These studies are limited by small sample sizes, short duration of cefazolin, and stringent diagnostic criteria that were resource intensive [13, 14]. We describe our real-world institutional experience with cefazolin in patients intolerant of nafcillin due to suspected non-IgE-mediated HSRs.
METHODS
Study Design and Population
This was a retrospective, descriptive case series that was approved by the institutional review board at University of Virginia (UVA) Health. Patients admitted to UVA Health between October 2015 and November 2019 who received nafcillin for an MSSA infection, experienced a suspected, prespecified non-IgE-mediated HSR, and were switched to cefazolin were included in the study. Those switched from nafcillin to cefazolin for all other reasons, including other non-IgE-mediated HSRs, were excluded. An electronic medical record (EMR) report identified patients who received nafcillin with a subsequent cefazolin prescription within 84 days. Patients were reviewed in reverse chronological order to target a convenience sample of 100 patients and were included only once during the study timeframe. Clinical data collected from the EMR included patient demographics, infection diagnosis, HSR classification with associated laboratory values, time to development of an HSR (defined as the day of nafcillin initiation to the day of the HSR), time to cefazolin switch (defined as the day of nafcillin initiation to the day of cefazolin initiation), and duration of therapy.
Objectives and Definitions
The primary objective was to identify the percentage of patients who completed cefazolin after experiencing a suspected non-IgE-mediated HSR to nafcillin. Suspected HSRs evaluated included eosinophilia, immune-mediated hepatitis, immune-mediated nephritis, leukopenia or neutropenia, and maculopapular rash. Patients who did not complete cefazolin were further analyzed to identify whether the discontinuation was related to a primary or a secondary HSR. Primary HSR was defined as recurrence or persistence of the original HSR that led to the switch from nafcillin to cefazolin. Secondary HSR was defined as development of a new HSR that differed from the original HSR attributed to nafcillin. Two investigators determined the rationale for cefazolin discontinuation, and in the event of a discrepancy between the 2 investigators, a third investigator reconciled the assessment.
Secondary objectives included the proportion of HSRs that met preestablished objective diagnostic criteria for each suspected HSR and the proportion of HSRs in each category of the Naranjo Scale. Objective diagnostic criteria utilized for HSR classification were derived from literature definitions and previously reported cases (Table 1) [13, 15, 16, 22]. Baseline value was defined as the minimum value in the last 3 months prior to the hospital encounter. If a value within the last 3 months was unavailable, the last value available prior to the hospital encounter was used. Suspected HSRs also underwent a subjective assessment using the Naranjo Scale to determine the likelihood that the HSR was due to the antibiotic [17]. These were primarily reviewed by 1 investigator, and cases with any uncertainty were discussed with another investigator.
Table 1.
Objective Criteria for Classification of Non-IgE-Mediated HSRs
| Hypersensitivity Reaction | Diagnostic Criteria |
|---|---|
| Eosinophilia | • AEC > 1000/mL without other symptoms |
| Immune-mediated hepatitis | • AEC > 500/μL |
| • Rise in LFTs by 3× upper limit of patient’s baseline | |
| Immune-mediated nephritis | • AEC > 500/μL |
| • Rise in SCr by 1.5× baseline or by > 0.3 mg/dL | |
| Leukopenia or neutropenia | • WBC < 4000 cells/μL or 50% decrease in WBC if baseline < 4000 cells//μL OR • ANC < 1500/mm3 or 50% decrease in ANC if baseline is < 1500/mm3 |
| Maculopapular rash | • Described as “maculopapular rash” in the EMR |
Abbreviations: AEC, absolute eosinophil count; ANC, absolute neutrophil count; EMR, electronic medical record; HSR, hypersensitivity reaction; IgE, immunoglobulin E; LFTs, liver function tests; SCr, serum creatinine; WBC, white blood cell.
Statistical Analysis
A descriptive analysis was performed using IBM SPSS Statistics for Windows, version 26 (IBM Corp, Armonk, NY). Descriptive data were displayed as numbers and frequencies or medians with interquartile ranges, where applicable.
RESULTS
Clinical Characteristics
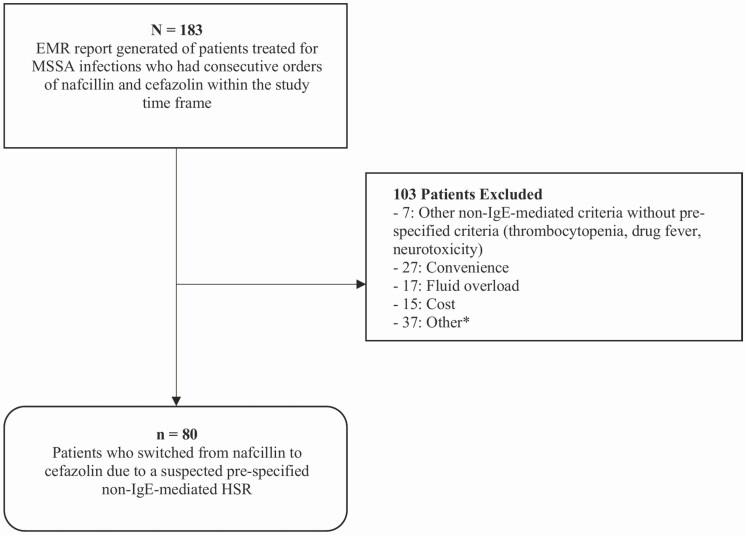
Among 183 patients with MSSA infections who were switched from nafcillin to cefazolin within the study timeframe, 103 patients were excluded for experiencing a non-IgE-mediated HSR that was not prespecified or for reasons other than a reaction to nafcillin, such as dosing inconvenience, fluid overload, and cost (Figure 1). Therefore, there were 80 patients included in the study. Baseline patient characteristics are shown in Table 2. Median age was 60 years, and the majority were White males. Overall, 62 patients (78%) had bacteremia, and the most common identified foci of infection were bone and joint and endovascular (Table 2). The median total duration of therapy was 43 days (24 – 48), whereas the median duration of cefazolin alone was 25 days (14–37).
Figure 1.
Patient population.*Other included drug-drug interactions (n = 13) and inconclusive data (n = 24). Abbreviations: EMR, electronic medical record; HSR, hypersensitivity reaction; MSSA, methicillin-susceptible Staphylococcus aureus.
Table 2.
Baseline Characteristics of Patients
| Characteristic | Overall (N = 80) |
|---|---|
| Age, years | 60 (48–72) |
| Male sex | 57 (71) |
| Race | |
| White | 70 (88) |
| Black | 9 (11) |
| Other | 1 (1) |
| Infection type | |
| Bloodstream | 62 (78) |
| Bone/joint | 27 (34) |
| Central nervous system | 1 (1) |
| Endovascular | 20 (25) |
| Pulmonary | 4 (5) |
| Skin/skin structure | 6 (8) |
| Time to nafcillin HSR, days | 6 (2–14) |
| Time to cefazolin switch, days | 8 (3–15) |
| Total duration of therapy, days | 43 (24–48) |
Data are represented as number (%) or median (IQR) unless otherwise stated.
Abbreviations: HSR, hypersensitivity reaction; IQR, interquartile range.
Among the 80 patients, there were 87 total suspected non-IgE-mediated HSRs, with 7 patients experiencing more than 1 HSR. The most common prespecified HSRs were immune-mediated nephritis (54%), leukopenia or neutropenia (21%), and maculopapular rash (15%) (Table 3). The median time to development of an HSR was 6 days (2–14).
Table 3.
Summary of Objective Criteria Met for Non-IgE-Mediated HSRs
| Objective Classification Criteria | No. of HSRs (n) | HSRs With All Criteria Available in EMR (n) | Criteria Met (n) | Criteria Not Meta (n) |
|---|---|---|---|---|
| Eosinophilia | 4 (4) | 4 | 4 | 0 |
| Immune-mediated hepatitis | 5 (6) | 5 | 0 | 5 |
| Immune-mediated nephritis | 47 (54) | 24 | 3 | 44 |
| Leukopenia or neutropenia | 18 (21) | 18 | 18 | 0 |
| Maculopapular rash | 13 (15) | 13 | 6 | 7 |
| Total | 87 (100) | 64/87 (74) | 31/87 (36) | 56/87 (64) |
Data are represented as no. (%).
Abbreviations: HSR, hypersensitivity reaction; IgE, immunoglobulin E.
aCriteria were not met if they did not concur with the definitions outlined in Table 1 or if data were not available in the EMR.
Outcomes
Of the 80 patients who were switched from nafcillin to cefazolin due to a suspected HSR, 71 patients (89%) tolerated and completed cefazolin therapy. The duration of cefazolin varied among these 71 patients. Eighteen patients (25%) received cefazolin for <2 weeks, 18 (25%) received cefazolin between 2 and 4 weeks, and 35 (50%) received cefazolin for >4 weeks.
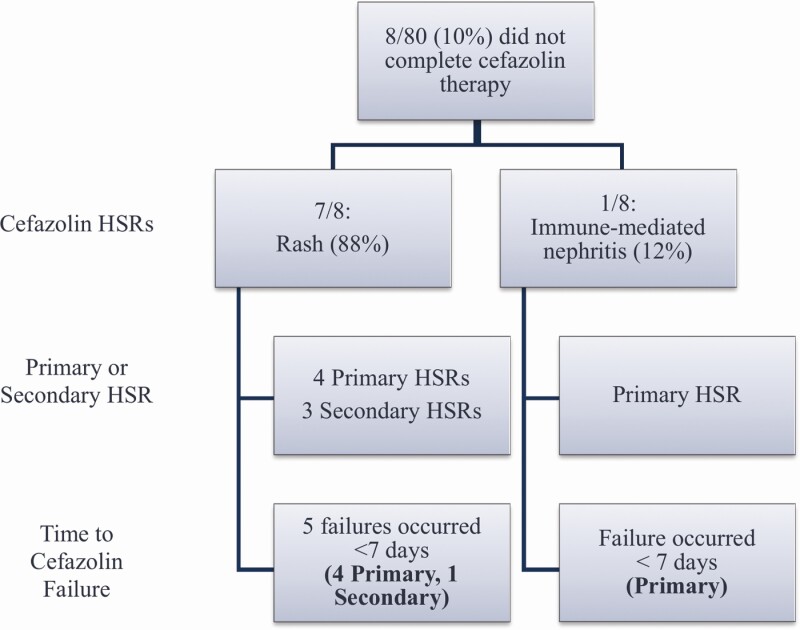
Cefazolin was discontinued in 8 patients (10%) due to rash or immune-mediated nephritis, with 3 instances of emergence of a secondary HSR (Figure 2). All 5 patients with persistence of a primary HSR discontinued cefazolin within 7 days of initiation. Among these patients, the median time to nafcillin HSR was 8 days (5–10.5), which was the same as the median time to cefazolin switch. The median duration of cefazolin was 5.5 days (3–15). Therapy was ultimately changed to daptomycin (n = 2), vancomycin (n = 3), ceftriaxone (n = 1), and doxycycline suppression (n = 1). One patient who was thought to have immune-mediated nephritis from nafcillin was retrialed with nafcillin after experiencing an HSR to cefazolin as the renal biopsy showed infection-associated proliferative glomerulonephritis. One patient was switched from cefazolin to daptomycin due to concern for treatment failure. In all patients who did not tolerate cefazolin, the HSR resolved without lasting abnormalities.
Figure 2.
Description of patients who did not tolerate cefazolin after being switched from nafcillin due to a non-IgE-mediated reaction. Abbreviations: HSR, hypersensitivity reaction; IgE, immunoglobulin E.
Of the 87 evaluable HSRs, full objective diagnostic criteria were available in 64 (74%), with 31 of these HSRs (48%) meeting criteria for classification (Table 3). Immune-mediated nephritis or hepatitis were least likely to meet criteria (3/24 [13%] and 0/5 [0%], respectively). This was primarily due to missing data related to the absolute eosinophil count at the initiation of nafcillin or at the time when therapy was switched to cefazolin or not meeting the prespecified definitions outlined in Table 1. In patients suspected of immune-mediated nephritis who had data available, the median increase in absolute eosinophil count from the day of nafcillin initiation to the day of cefazolin initiation was 115 cells/μL, and the median increase in serum creatinine was 1 mg/dL. Of the HSRs that met criteria, 28/31 (90%) completed cefazolin therapy, whereas 3/31 (10%) were unable to tolerate the switch to cefazolin because of a rash. All 3 were discontinued within 7 days of initiation due to a primary HSR.
When using the Naranjo Scale to determine the likelihood that the suspected HSR was due to nafcillin, none were categorized as definite, 55 (63%) were probable, 31 (36%) were possible, and 1 (1%) was doubtful. In the 8 patients who did not tolerate the switch to cefazolin, the Naranjo Scale to determine the likelihood that the suspected HSR was due to cefazolin showed that 2 (25%) were possible and 6 (75%) were doubtful.
DISCUSSION
Our findings suggest that cefazolin may be safe in patients who develop a suspected non-IgE-mediated HSR to nafcillin, as 89% of patients tolerated the switch to cefazolin. Notably, most patients who tolerated cefazolin completed at least 2 weeks of therapy. Even among those who did not tolerate the change and showed persistent HSRs, they were able to discontinue cefazolin without lasting medical problems.
Anti-staphylococcal penicillins and cefazolin are preferred therapy for the treatment of MSSA infections and are associated with improved outcomes when compared to treatment with vancomycin [4, 8, 18]. Although it is unknown how daptomycin compares to β-lactams for MSSA infections, some may favor it over cephalosporins in cases of severe or suspected non-IgE-mediated HSRs due to concerns of cross-reactivity. However, daptomycin requires additional monitoring of creatine phosphokinase and may pose financial challenges that can impact patient disposition. Cefazolin is generally well tolerated and only requires routine monitoring, thereby making it a more attractive option as compared to vancomycin or daptomycin in patients who develop HSRs to nafcillin [5–10]. Among the 8 patients who did not tolerate the switch to cefazolin, 3 patients developed a different HSR to cefazolin that likely could not have been predicted by the HSR that developed with nafcillin and may possibly have occurred without receiving nafcillin initially. There were 5 patients who had the same HSR that developed with nafcillin. Interestingly, all 5 of these patients discontinued cefazolin within 7 days of initiation. With delayed HSRs, the HSR may temporarily worsen despite discontinuing the offending agent [1]. It is worth noting that in our study, most of these patients were immediately switched to cefazolin without a washout period, as evidenced by the equivalent median time to nafcillin HSR and time to cefazolin switch. Although cefazolin was discontinued in those 5 patients due to concern for persistent or worsening HSR, this could potentially be attributed to lingering effects of nafcillin rather than a true HSR to cefazolin.
A small subset of patients in this study actually met predetermined objective diagnostic criteria for non-IgE-mediated HSR classification. Overall, 64% of HSRs did not meet criteria, and this was even higher in the immune-mediated nephritis (94%) and immune-mediated hepatitis (100%) subsets. As noted previously, 23 (26%) HSRs did not have all necessary information available in the EMR for analysis and were classified as not meeting criteria. Although these data call into question if a closer evaluation of the need to switch is warranted, they also give a realistic view of what occurs inside healthcare facilities when resources cannot be allocated to confirming HSRs via invasive procedures such as biopsies.
There are limited published data evaluating cefazolin tolerance in patients who experienced a non-IgE-mediated HSR to nafcillin. One study evaluated 54 patients presenting for total hip and knee arthroplasty who previously reported a non-IgE-mediated HSR to penicillin [14]. All patients who received cefazolin reported no adverse reactions. However, the study did not specify which penicillin was reported to have caused an allergy and cefazolin was only used for perioperative prophylaxis. Another study evaluated the tolerability of cefazolin after non-IgE-mediated HSRs to nafcillin in an outpatient setting [13]. Although limited by a small sample size, the authors concluded that cefazolin was safe to use, as 16 out of 17 patients who switched from nafcillin to cefazolin completed therapy. Their small sample size was likely due to stringent diagnostic criteria that were required for inclusion after review from 2 independent specialists from the Division of Rheumatology, Allergy, and Immunology to assess HSR types and likely mechanisms. Because these resources were not routinely utilized by or immediately available for primary providers for patients in our study, we included any patient who had switched from nafcillin to cefazolin due to clinical concern for a non-IgE-mediated HSR based on chart review. In the subgroup of patients who met objective criteria in our study, 28 of 31 patients completed cefazolin. Three patients did not complete cefazolin due to a primary HSR of rash, all of whom discontinued cefazolin within 7 days of initiation, which could potentially point to residual effects of nafcillin contributing to cefazolin intolerance.
The incidence of acute kidney injury with nafcillin can be as high as 33% [19–21]. Our study found that over half of the suspected non-IgE-mediated HSRs were immune-mediated nephritis, which is notably greater than what was observed by Blumenthal and colleagues (3/17, 18%), likely due to strict adherence to diagnostic criteria. In our study, there were 47 HSRs due to immune-mediated nephritis; however, only 3 met objective criteria, and almost half of the HSRs did not have all available criteria to evaluate the HSR. These data demonstrate that immune-mediated nephritis is likely attributed to nafcillin more often than necessary and calls into question if a closer evaluation of laboratory values is warranted prior to change in therapy.
This study has several limitations. Given its retrospective nature, this study was subject to selection bias. This study does not include a subset of patients who may have experienced more severe HSRs to nafcillin and were not prescribed cefazolin. Additionally, there were limitations on data availability in the EMR, which led to the inability to completely assess objective criteria and some of the questions in the Naranjo Scale, such as if the reaction reappeared after a rechallenge or placebo. Few HSRs met objective diagnostic criteria in our study, especially for immune-mediated nephritis and hepatitis. Additional objective data are required to make a definitive diagnosis of an HSR, such as skin biopsy for maculopapular rash, renal biopsy, and urinalysis (eg, urine eosinophils and urine sediment) for immune-mediated nephritis, or liver biopsy for immune-mediated hepatitis. Although these data were unavailable in our study, this provides a more realistic perspective of resource allocation for confirming HSRs.
In conclusion, this study adds to the current body of literature as the largest study to date showing cefazolin tolerability in patients with suspected non-IgE-mediated HSRs to nafcillin. It provides a better understanding of non-IgE-mediated HSRs to nafcillin and cross-reactivity between nafcillin and cephalosporins. Not only could this impact the decision making of providers who are concerned about switching patients from nafcillin to cefazolin when a suspected non-IgE-mediated HSR is encountered and might choose suboptimal therapy with vancomycin or daptomycin, but this also further supports those who did switch to cefazolin previously in the setting of limited data. Future research is needed to analyze the tolerability of nafcillin in patients with select comorbidities, such as cirrhosis or chronic kidney disease, to evaluate whether these patients are more prone to HSRs, and to explore the tolerability of cefazolin in patients who experience more severe non-IgE-mediated HSRs to nafcillin.
Note
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Contributor Information
Ankit M Gandhi, Department of Pharmacy, University of Virginia Health, Charlottesville, Virginia, USA; National Institutes of Health, Bethesda, Maryland, USA.
Megan D Shah, Department of Pharmacy, University of Virginia Health, Charlottesville, Virginia, USA.
Lindsay E Donohue, Department of Pharmacy, University of Virginia Health, Charlottesville, Virginia, USA.
Heather L Cox, Department of Pharmacy, University of Virginia Health, Charlottesville, Virginia, USA.
Joshua C Eby, Division of Infectious Diseases and International Health, Department of Medicine, University of Virginia Health, Charlottesville, Virginia, USA.
REFERENCES
- 1. Solensky R, Khan D. Drug allergy: an updated practice parameter. Ann Allergy Asthma Immunol 2010;105:259–73. [DOI] [PubMed] [Google Scholar]
- 2. Patterson RA, Stankewicz HA. Penicillin allergy. [Updated 2019 Apr 23]. In: StatPearls. Treasure Island, FL: StatPearls Publishing; 2020. Available at: https://www.ncbi.nlm.nih.gov/books/NBK459320/. [PubMed] [Google Scholar]
- 3. Gell PGH, Coombs R, eds. The classification of allergic reactions underlying disease. In: Clinical aspects of immunology. 1st ed. Oxford, England: Blackwell, 1963:317–37. [Google Scholar]
- 4. Baddour LM, Wilson WR, Bayer AS, et al. ; American Heart Association Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease of the Council on Cardiovascular Disease in the Young, Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and Stroke Council . Infective endocarditis in adults: diagnosis, antimicrobial therapy, and management of complications: a scientific statement for healthcare professionals from the American Heart Association. Circulation 2015; 132:1435–86. [DOI] [PubMed] [Google Scholar]
- 5. Hoppes T, Prikis M, Segal A. Four cases of nafcillin-associated acute interstitial nephritis in one institution. Nat Clin Pract Nephrol 2007; 3:456–61. [DOI] [PubMed] [Google Scholar]
- 6. Alam MB, Kadoura A, Sathaiah M. A fatal case of nafcillin-induced hepatotoxicity: a case report and the literature review. Case Rep Med 2012; 2012:953714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Baker RJ, Pusey CD. The changing profile of acute tubulointerstitial nephritis. Nephrol Dial Transplant 2004; 19:8–11. [DOI] [PubMed] [Google Scholar]
- 8. Schweizer ML, Furuno JP, Harris AD, et al. Comparative effectiveness of nafcillin or cefazolin versus vancomycin in methicillin-susceptible Staphylococcus aureus bacteremia. BMC Infect Dis 2011; 11:279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Stryjewski ME, Szcech LA, Benjamin DK, et al. Use of vancomycin of first generation cephalosporins for the treatment of hemodialysis dependent patients with methicillin-susceptible Staphylococcus aureus bacteremia. Clin Infect Dis 2007;44:190–6. [DOI] [PubMed] [Google Scholar]
- 10. Chan KE, Warren HS, Thadhani RI, et al. Prevalence and outcomes of antimicrobial treatment for Staphylococcus aureus bacteremia in outpatients with ESRD. J Am Soc Nephrol 2012; 23:1551–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pichichero ME. Cephalosporins can be prescribed safely for penicillin-allergic patients. J Fam Pract 2006; 55:106–12. [PubMed] [Google Scholar]
- 12. Pichichero ME. Use of selected cephalosporins in penicillin-allergic patients: a paradigm shift. Diagn Microbiol Infect Dis 2007; 57:13S–8S. [DOI] [PubMed] [Google Scholar]
- 13. Blumenthal KG, Youngster I, Shenoy ES, Banerji A, Nelson SB. Tolerability of cefazolin after immune-mediated hypersensitivity reactions to nafcillin in the outpatient setting. Antimicrob Agents Chemother 2014; 58:3137–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Haslam S, Yen D, Dvirnik N, Engen D. Cefazolin use in patients who report a non-IgE mediated penicillin allergy: a retrospective look at adverse reactions in arthroplasty. Iowa Orthop J 2012; 32:100–3. [PMC free article] [PubMed] [Google Scholar]
- 15. Kodner CM, Kudrimoti A. Diagnosis and management of acute interstitial nephritis. Am Fam Physician 2003; 67:2527–34. [PubMed] [Google Scholar]
- 16. Kancir LM, Tuazon CU, Cardella TA, Sheagren JN. Adverse reactions to methicillin and nafcillin during treatment of serious Staphylococcus aureus infections. Arch Intern Med 1978; 138:909–11. [PubMed] [Google Scholar]
- 17. Naranjo CA, Busto U, Sellers EM, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther 1981; 30:239–45. [DOI] [PubMed] [Google Scholar]
- 18. Weis S, Kesselmeier M, Davis JS, et al. Cefazolin versus anti-staphylococcal penicillins for the treatment of patients with Staphylococcus aureus bacteraemia. Clin Microbiol Infect 2019; 25:818–27. [DOI] [PubMed] [Google Scholar]
- 19. Chan L, Chan-Tompkins NH, Como J, Guarascio AJ. Retrospective analysis of adverse drug events between nafcillin versus cefazolin for treatment of methicillin-susceptible Staphylococcus aureus infections. Ann Pharmacother 2020; 54:662–8. [DOI] [PubMed] [Google Scholar]
- 20. Flynt LK, Kenney RM, Zervos MJ, Davis SL. The safety and economic impact of cefazolin versus nafcillin for the treatment of methicillin-susceptible Staphylococcus aureus bloodstream infections. Infect Dis Ther 2017; 6:225–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Barriere SL, Conte JE Jr. Absence of nafcillin-associated nephritis: a prospective analysis of 210 patients. West J Med 1980; 133:472–7. [PMC free article] [PubMed] [Google Scholar]
- 22. Lopes JA, Jorge S. The RIFLE and AKIN classifications for acute kidney injury: a critical and comprehensive review. Clin Kidney J 2013; 6:8–14. [DOI] [PMC free article] [PubMed] [Google Scholar]