In 2020, my colleagues and I curated a special issue of The Journals of Gerontology, Biological Science (JoGBS) publishing 10 papers on the subject of “The Microbiome and Aging” (1–10) with a Commentary proceeding characterizing the scientific contribution of the gut to multiple facets of aging and health (11). The impetus for this special issue was based not only upon our own work and interest (2,10,12–15) but also upon growing interest across multiple fields of science generally and the field of the biology of aging/geroscience in particular. The request for papers for the issue stated:
“the human intestinal tract (i.e., “gut”) is inhabited by over 100 trillion microorganisms; including over 1000 species of known bacteria. These organisms have co-evolved with humans over millennia to live together for mutual benefit. Though commonly long overlooked in considerations of human health and disease treatment (hence the nickname the “forgotten organ”), gut microorganisms encode >150 times more genes than the human genome and are highly involved in numerous metabolic reactions which influence normal host physiology. A variety of biologic, medical, and lifestyle factors appear to contribute to gut dysbiosis in late-life, and interventions specifically designed to target these factors may be useful in restoring microbial balance. Indeed, evidence from both clinical and preclinical studies suggests that gut dysbiosis is highly correlated with age-related persistent inflammation, age-related diseases such as Alzheimer’s disease, mobility disability, and perhaps even longevity. Crosstalk between the gut and multiple organ systems (brain, heart, muscle etc.) that subserve the unfolding of aging, in the aging process, including development of age-related diseases and loss of physiological function is not well understood”.
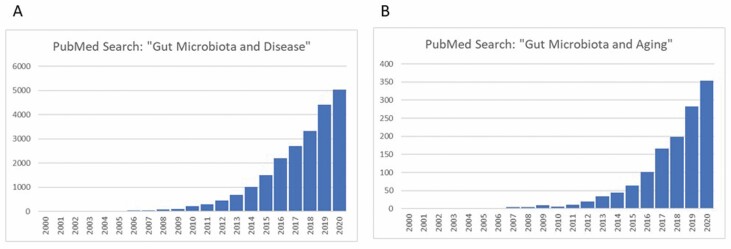
Indeed, over the last 20 years, there has been an exponential increase in this field of study first reported by Buford (11) and updated here. In 2000, there were 3 papers published compared with over 5000 published in 2020 as indexed in PubMed investigating the “gut microbiota and disease” (Figure 1A). A similar trajectory is observed, when searching “gut microbiota and aging” with 0 papers published in 2000 and 356 published in 2020 (Figure 1B). These data clearly attest that this field is growing and can longer be considered as a “fringe” science studying a “forgotten organ” (16), the gut, and must be seriously considered as a fundamental process to aging and age-related disease. Since the publication of the special issue, the JoGBS has received even more submissions related to this subject, inspiring the editors to create an additional collection of articles, published herein, to emphasize the importance of this field of study to aging and age-related disease.
Figure 1.
An investigation of papers related to the gut microbiota and aging. Twenty year trajectory of publications indexed in PubMed investigating the “gut microbiota and disease” (A) and the “gut microbiota and aging” (B).
Although the notion of disturbances in the gut microbiota is touted as a relatively new concept in the context of aging, this is far from true. As early as 1908, Elie Metchnikoff, the father of both cellular immunology and gerontology (he actually coined this word), made the case for the role of, what is now referred to as gut dysbiosis in the “Prolongation of Life” (17). Metchnikoff assumed that aging was likely due to the poisoning of the body by pathogenic micro-organisms in the large intestine and thus proposed to change the microbial population, and so avoid poisoning. He was inspired by Stamen Grigorov’s discovery of “Bacillus bulgaricus,” the lactic acid bacterium responsible for transforming milk into yoghurt and a daily food taken by in Bulgaria’s long-lived rural populations. He opined the local farmers’ long-life expectancy was due to their daily consumption of fresh yoghurt such that the “good” bacteria could offset the impact of the “bad” bacteria that poisoned the gut during the aging process. His views spawned a “sour-milk” craze across Europe and the United States when in a lecture in France in 1908, he touted the life and healthspan enhancing effects of sour milk, espousing that people could live to >150 years (18). The emergence of the use of anti-biotics to combat “bad” bacteria led to a nearly 80-year gap in the study of “good” bacteria as means to combat aging and age-related disease. Since that time, it is now well understood that the use of antibiotics drive the destruction of not only “bad” bacteria but also the healthy gut microbiota environment (19,20) and is often used as an experimental tool to repopulate the gut with “good” bacteria (21).
This evidence lead me to some critical thinking regarding our understanding of the biology of aging through the “Leading Edge Review” published by Lopez-Otin et al. (22) in 2013 defining 9 “Hallmarks of Aging” representing common denominators of aging across a variety of organisms. While this article is certainly not the only approach to studying the biology of aging, it is quite a useful didactic in so far as “Hallmarks” provide a unifying platform from which to approach the very complex multiorgan, multidisease, and multibiomarker nature of the aging process. Since that time, this list of Hallmarks has remained relatively stable. Based upon my own research experience, general scientific evidence, and observation of the field through the Microbiome Special Issue and now this collection, I believe we should consider the contribution of disturbances of the gut microbiota as the “10th Hallmark.” In fact, Guerville et al. (23) made this suggestion recently, with the caveat that there is still some work to do, which I will describe below. And as Lopez-Otin describe there is “extensive interconnectedness between the aging Hallmarks, implying that experimental amelioration of one particular Hallmark may impinge on others” (22). Indeed, the articles in the special issue covered a range of topics and addressed how the gut interacted with several different Hallmarks. Thus, the current collection of articles expands upon these findings and adds evidence to our field that disturbances of the gut fundamentally contributes to aging.
However, to complete the argument that disturbances of the gut microbiota are indeed a true Hallmark of aging, we must assess whether this condition meets the criteria for what defines a Hallmark via Lopez-Otin: (i) the Hallmark should manifest during normal aging; (ii) its experimental aggravation should accelerate aging; and (iii) its experimental amelioration should retard the normal aging process. While it is beyond the scope of this simple perspective to fully review the entire field to support this angle (Figure 1B), I would like to highlight the publications within the new collection to provide this insight, based on the 3 criteria of Hallmarks of aging. This argues that our scientific field of geriatrics/gerontology/geroscience should facilitate/consider publishing those lines of investigation here and perhaps create room in our meetings to make gut research prominent. Conversely, it is imperative that we encourage microbiologists studying the gut to participate in our various annual aging meetings, perhaps by creating specialized integrative symposia in conjunction with calls for papers as our group has participated in over the last few years. The articles published here in this collection use a variety of animal models of aging (both males and females), from rats to mice to non-human primates, furthering the translational strength of this argument to improve the health- and lifespan of older adults, which is the ultimate goal of our biology of aging, gerontology, geriatrics and geroscience fields. We all want to live and age better. Introducing new approaches is fundamental and why I am so excited for this new collection.
Experimental Aggravation Should Accelerate Aging
In line with Guerville’s (23), assessment, none of the articles in the current collection address this aspect of what defines a Hallmark. While gut microbiota dysregulation has been associated with a number of diseases including diabetes, Alzheimer’s and Parkinson’s disease (10,12,13,24–26), there are only a few experimental studies to indicate that aggravating the gut should accelerate aging. A PubMed search using the terms “Microbiota and Longevity” returned a total of 228 articles, although many of these were reviews and in relation to age-related disease states. However, a few stand out. For example, in their recent systematic review of 27 studies, Badal et al. (27) concluded that in special human populations of “healthy” versus “normal aging,” there were distinct differences in the gut microbiota diversity and downstream metabolic functioning indicative of a pro-aging phenotype. Using the turquoise killifish model, Smith et al. demonstrated that transfer of the gut microbiome from young to middle-aged killifish resulted in an increase in lifespan and a delayed behavioral decline, compared to fish that received the microbiota from middle-aged fish (28). Conversely, Kundu et al. found that young germ-free mice receiving a gut microbiota transplant from old donor mice showed increased neurogenesis in the hippocampus of the brain and increased intestinal growth (29). Thus, with regards to this criteria of a Hallmark, I still think we have some work to do.
The Hallmark Should Manifest During Normal Aging
Vemuri et al. (30) in this collection demonstrate, through correlational analyses, that fecal and blood samples from healthy young and old female vervet monkeys reveal microbial co-occurrence patterns relating to inflammation, aging and deleterious shifts in 16s rRNA analysis of microbiome diversity and abundance. Specifically, elevated levels of plasma LBP-1, MCP-1, and C-reactive protein in old monkeys, were indicative of higher microbial translocation into the circulation. These findings provide novel insights into systemic inflammation and gut microbial interactions, highlights the importance of the gut mucosal niche, and the decline in the stability of the microbial community with aging. This study suggests that perhaps the age-related disturbances in the gut may proceed and may even eventually prove to be causative as a source for age-related inflammation and age-related disease. While these data are correlational, this study used an NHP model, in a colony that continues to age and could be related to translational traits of this model to such conditions as metabolic dysfunction, hypertension, and neurodegenerative conditions such as Alzheimer’s disease (31–34).
Rubio et al. (35) show that aged male Wistar rats have increased presence of mucin-degrading and lipopolysaccharide (LPS)-producing bacteria, resulting in a weaker gut barrier in older animals that facilitates LPS leakage. LPS is known to induce leptin resistance of vagal afferents to impact hypothalamic-mediated feeding behavior thus supporting their additional finding that cholecystokinin (CCK) satiating effect is impaired in aged rats. These data provide support for a gut-brain axis contribution to feeding behavior but also raises the question if changes in body composition (increased adiposity and decreased lean mass) are a consequence or cause of this shift in gut barrier function.
Experimental Amelioration Should Retard the Normal Aging Process
The aging field is a bit unusual in that we often find interventions that impact on aging, then use these to understand mechanisms (the opposite to disease-based research, where understanding the biology/mechanism is the first step, and used to develop subsequent interventions). This is why, as stated in the introduction, we must certainly partner with gut microbiologist researchers to integrate approaches of understanding. Below are 2 studies from the new collection which integrate understanding of longevity interventions (senolytics and diet) and their impact on the gut microbiome.
Saccon et al. (15) investigate the senolytic drug combination, Dasatinib plus Quercetin (D+Q), which is known to reduce senescent cell abundance, comparing young and old female BALB/c mice. Their primary outcomes included senescence, and inflammatory markers in small (ileum) and large (caecum and colon) intestine as well as the microbial composition along the intestinal tract in these mice. Overall, aged-treated mice experienced lower senescence and inflammatory markers in the gut, and this correlated with specific amelioration of deleterious microbial signatures. What is particularly interesting about this article is the question that a regional specific approach is an important consideration for targeting the gut during intervention.
There are previous studies to suggest that macronutrient intake may influence longevity in the context of host/gut microbiome interactions (36,37).
Nichenametla et al. (38) utilized male 21-week-old C57BL6/J mice to compare a lifespan enhancing diet, sulfur amino acid restriction (SAAR, restriction of dietary methionine at 0.12%, in the absence of cysteine, also called methionine restriction) with other diets that controlled for levels caloric intake (low calorie diet or LCD with/isocaloric to SAAR) as well as of cysteine and methionine nutrition (control diet or CD with 0.86% methionine). After 10 weeks on the diet, plasma markers of bile acids and fecal microbial profiles were determined. Overall, SAAR and LCD diets induced distinct positive changes in the gut microbiome environment, β-diversity, and bile acid profiles, although the patterns of these changes were different between these groups. This study, controlling for caloric intake, provides evidence that manipulating micronutrients can have both overlapping and distinctive effects, which may lead to a better understanding of how caloric versus nutrient intake may modulate the gut microbiome during aging to impact health and longevity.
Conclusion
So, are we at complete “Hallmark” status regarding disturbance of the gut during aging? To be sure, some of the results presented in this collection are correlational/observational and have not quite reached mechanistic maturity as may be required for establishing a true Hallmark. What else is needed: (i) empirical studies that manipulate the gut at various points in the lifespan to envision the impact on longevity; (ii) sex difference studies using both males and females within the same experiments; (iii) use of multiple aging models to provide convergent evidence; and (iv) use of well-described longevity enhancing interventions. But this type of approach always represents a nascent science, even when steeped in a theoretical yet checkered history. Hopefully, this piece and the articles included in this collection engenders discussion and opens a window to expand our modes of thinking as gerontologists/geoscientists to help find a way to mitigate how, when, and why we age to consider our “gut feelings.”
Funding
This work was supported by National Institute of Aging (R01AG054538 to C.S.C.).
Conflict of Interest
None declared.
References
- 1. Seo DO, Holtzman DM. Gut microbiota: from the forgotten organ to a potential key player in the pathology of Alzheimer’s disease. J Gerontol A Biol Sci Med Sci. 2020;75:1232–1241. doi: 10.1093/gerona/glz262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Carter CS, Morgan D, Verma A, et al. Therapeutic delivery of Ang(1–7) via genetically modified probiotic: a dosing study. J Gerontol A, Biol Sci Med Sci. 2020;75(7):1299–1303. doi: 10.1093/gerona/glz222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Yahfoufi N, Matar C, Ismail N. Adolescence and aging: impact of adolescence inflammatory stress and microbiota alterations on brain development, aging, and neurodegeneration. J Gerontol A Biol Sci Med Sci. 2020;75:1251–1257. doi: 10.1093/gerona/glaa006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Shardell M, Parimi N, Langsetmo L, et al. Comparing analytical methods for the gut microbiome and aging: gut microbial communities and body weight in the Osteoporotic Fractures in Men (MrOS) study. J Gerontol A Biol Sci Med Sci. 2020;75:1267–1275. doi: 10.1093/gerona/glaa034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Adriansjach J, Baum ST, Lefkowitz EJ, Van Der Pol WJ, Buford TW, Colman RJ. Age-related differences in the gut microbiome of rhesus macaques. J Gerontol A Biol Sci Med Sci. 2020;75:1293–1298. doi: 10.1093/gerona/glaa048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wiesenborn DS, Gálvez EJC, Spinel L, et al. The role of Ames dwarfism and calorie restriction on gut microbiota. J Gerontol A Biol Sci Med Sci. 2020;75:e1–e8. doi: 10.1093/gerona/glz236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Liu A, Lv H, Wang H, Yang H, Li Y, Qian J. Aging increases the severity of colitis and the related changes to the gut barrier and gut microbiota in humans and mice. J Gerontol A Biol Sci Med Sci. 2020;75:1284–1292. doi: 10.1093/gerona/glz263 [DOI] [PubMed] [Google Scholar]
- 8. Jaworska K, Konop M, Hutsch T, et al. Trimethylamine but not trimethylamine oxide increases with age in rat plasma and affects smooth muscle cells viability. J Gerontol A Biol Sci Med Sci. 2020;75:1276–1283. doi: 10.1093/gerona/glz181 [DOI] [PubMed] [Google Scholar]
- 9. Ahmadi S, Razazan A, Nagpal R, et al. Metformin reduces aging-related leaky gut and improves cognitive function by beneficially modulating gut microbiome/goblet cell/mucin axis. J Gerontol A Biol Sci Med Sci. 2020;75:e9–e21. doi: 10.1093/gerona/glaa056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sun Y, Baptista LC, Roberts LM, et al. The gut microbiome as a therapeutic target for cognitive impairment. J Gerontol A Biol Sci Med Sci. 2020;75:1242–1250. doi: 10.1093/gerona/glz281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Buford TW. The gut microbiome and aging. J Gerontol A Biol Sci Med Sci. 2020;75:1229–1231. doi: 10.1093/gerona/glaa103 [DOI] [PubMed] [Google Scholar]
- 12. Baptista LC, Sun Y, Carter CS, Buford TW. Crosstalk between the gut microbiome and bioactive lipids: therapeutic targets in cognitive frailty. Front Nutr. 2020;7:17. doi: 10.3389/fnut.2020.00017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Qi Y, Goel R, Kim S, et al. Intestinal permeability biomarker zonulin is elevated in healthy aging. J Am Med Dir Assoc. 2017;18:810.e1–810.e4. doi: 10.1016/j.jamda.2017.05.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Buford TW, Sun Y, Roberts LM, et al. Angiotensin (1-7) delivered orally via probiotic, but not subcutaneously, benefits the gut-brain axis in older rats. Geroscience. 2020;42:1307–1321. doi: 10.1007/s11357-020-00196-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Saccon TD, Nagpal R, Yadav H, et al. Senolytic combination of Dasatinib and Quercetin alleviates intestinal senescence and inflammation and modulates the gut microbiome in aged mice. J Gerontol A Biol Sci Med Sci. 2021;76:1895–1905. doi: 10.1093/gerona/glab002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. O’Hara AM, Shanahan F. The gut flora as a forgotten organ. EMBO Rep. 2006;7:688–693. doi: 10.1038/sj.embor.7400731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Metchnikoff E. The prolongation of life. Nature. 1908;77(1996):289–290. doi: 10.1038/077289b0 [DOI] [Google Scholar]
- 18. A Science Lecture Accidentally Sparked a Global Craze for Yogurt | Science | Smithsonian Magazine. https://www.smithsonianmag.com/science-nature/science-lecture-accidentally-sparked-global-craze-yogurt-180958700/. Accessed April 17, 2021.
- 19. Jeffery IB, Lynch DB, O’Toole PW. Composition and temporal stability of the gut microbiota in older persons. ISME J. 2016;10:170–182. doi: 10.1038/ismej.2015.88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Podolsky SH. Metchnikoff and the microbiome. Lancet. 2012;380:1810–1811. doi: 10.1016/s0140-6736(12)62018-2 [DOI] [PubMed] [Google Scholar]
- 21. Dethlefsen L, Relman DA. Incomplete recovery and individualized responses of the human distal gut microbiota to repeated antibiotic perturbation. Proc Natl Acad Sci USA. 2011;108Suppl 1:4554–4561. doi: 10.1073/pnas.1000087107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153:1194–1217. doi: 10.1016/j.cell.2013.05.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Guerville F, De Souto Barreto P, Ader I, et al. Revisiting the hallmarks of aging to identify markers of biological age. J Prev Alzheimers Dis. 2020;7:56–64. doi: 10.14283/jpad.2019.50 [DOI] [PubMed] [Google Scholar]
- 24. Buford TW. (Dis)Trust your gut: the gut microbiome in age-related inflammation, health, and disease. Microbiome. 2017;5:80. doi: 10.1186/s40168-017-0296-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Buford TW, Carter CS, VanDerPol WJ, et al. Composition and richness of the serum microbiome differ by age and link to systemic inflammation. Geroscience. 2018;40:257–268. doi: 10.1007/s11357-018-0026-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mailing LJ, Allen JM, Buford TW, Fields CJ, Woods JA. Exercise and the gut microbiome: a review of the evidence, potential mechanisms, and implications for human health. Exerc Sport Sci Rev. 2019;47:75–85. doi: 10.1249/JES.0000000000000183 [DOI] [PubMed] [Google Scholar]
- 27. Badal VD, Vaccariello ED, Murray ER, et al. The gut microbiome, aging, and longevity: a systematic review. Nutrients. 2020;12(12):3759. doi: 10.3390/nu12123759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Smith P, Willemsen D, Popkes M, et al. Regulation of life span by the gut microbiota in the short-lived African turquoise killifish. Elife. 2017;6:e27014. doi: 10.7554/eLife.27014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kundu P, Lee HU, Garcia-Perez I, et al. Neurogenesis and prolongevity signaling in young germ-free mice transplanted with the gut microbiota of old mice. Sci Transl Med. 2019;11(518):eaau4760. doi: 10.1126/scitranslmed.aau4760 [DOI] [PubMed] [Google Scholar]
- 30. Vemuri R, Sherrill C, Davis MA, Kavanagh K. Age-Related colonic mucosal microbiome community shifts in monkeys. J Gerontol A Biol Sci Med Sci. 2021;76:1906–1914. doi: 10.1093/gerona/glaa256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kavanagh K, Sherrill C, Ruggiero A, et al. Biomarkers of senescence in non-human primate adipose depots relate to aging. Geroscience. 2021;43:343–352. doi: 10.1007/s11357-020-00230-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Frye BM, Craft S, Latimer CS, et al. Aging-related Alzheimer’s disease-like neuropathology and functional decline in captive vervet monkeys (Chlorocebus aethiops sabaeus ). Am J Primatol. 2021:e23260. doi: 10.1002/ajp.23260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kavanagh K, Brown RN, Davis AT, et al. Microbial translocation and skeletal muscle in young and old vervet monkeys. Age (Dordr). 2016;38:58. doi: 10.1007/s11357-016-9924-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mitchell EL, Davis AT, Brass K, et al. Reduced intestinal motility, mucosal barrier function, and inflammation in aged monkeys. J Nutr Health Aging. 2017;21:354–361. doi: 10.1007/s12603-016-0725-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rubio C, Lizárraga E, Álvarez-Cilleros D, et al. Aging in male Wistar rats associates with changes in intestinal microbiota, gut structure and cholecystokinin-mediated gut-brain axis function. J Gerontol A Biol Sci Med Sci. 2021;76:1915–1921. doi: 10.1093/gerona/glaa313 [DOI] [PubMed] [Google Scholar]
- 36. Solon-Biet SM, McMahon AC, Ballard JW, et al. The ratio of macronutrients, not caloric intake, dictates cardiometabolic health, aging, and longevity in ad libitum-fed mice. Cell Metab. 2014;19:418–430. doi: 10.1016/j.cmet.2014.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Holmes AJ, Chew YV, Colakoglu F, et al. Diet-microbiome interactions in health are controlled by intestinal nitrogen source constraints. Cell Metab. 2017;25:140–151. doi: 10.1016/j.cmet.2016.10.021 [DOI] [PubMed] [Google Scholar]
- 38. Nichenametla SN, Mattocks DAL, Midya V, Shneyder J. Differential effects of sulfur amino acid-restricted and low-calorie diets on gut microbiome profile and bile acid composition in male C57BL6/J mice. J Gerontol A Biol Sci Med Sci. 2021;76:1922–1929. doi: 10.1093/gerona/glaa270 [DOI] [PMC free article] [PubMed] [Google Scholar]