Abstract
Calciphylaxis has high mortality. Vitamin K deficiency is common in haemodialysis patients and may be a trigger for calciphylaxis due to its role in activating a tissue inhibitor of calcification, matrix Gla protein. We report a second case of a female haemodialysis patient who developed calciphylaxis twice and was successfully treated with vitamin K supplementation on both occasions. She did not receive sodium thiosulphate or bisphosphonates nor was there a change made to her dialysis time or prescription. This case highlights how supplementation with vitamin K may improve the outcome of this condition.
Keywords: calciphylaxis, haemodialysis, phylloquinone, renal dialysis, vitamin K
BACKGROUND
Calciphylaxis is a disorder of vascular calcification resulting in painful subcutaneous nodules that ulcerate. It has high mortality and no proven treatment, although sodium thiosulphate, parathyroidectomy and bisphosphonates have been suggested [1]. Matrix Gla protein (MGP) requires vitamin K-dependent γ-carboxylation to function as an inhibitor of tissue calcification. Vitamin K antagonism with warfarin is associated with more than three times the odds of developing calciphylaxis [2], and vitamin K deficiency, in the absence of warfarin use, is common in dialysis patients whether or not they have calciphylaxis [3]. Even at ‘normal’ vitamin K levels, MGP carboxylation is incomplete [4] and so calcification inhibition may be improved with pharmacological doses of phylloquinone. We have previously successfully treated calciphylaxis with vitamin K [5] and here report a second case.
CASE REPORT
An 82-year-old female who had been on thrice weekly haemodialysis for 7 years reported a several week history of painful, dark lesions on her calves. The cause of her kidney failure was unknown and, in addition to kidney failure, she had a history of systolic heart failure (ejection fraction 20%) and ischaemic heart disease with myocardial infarction and coronary bypass surgery 5 years prior.
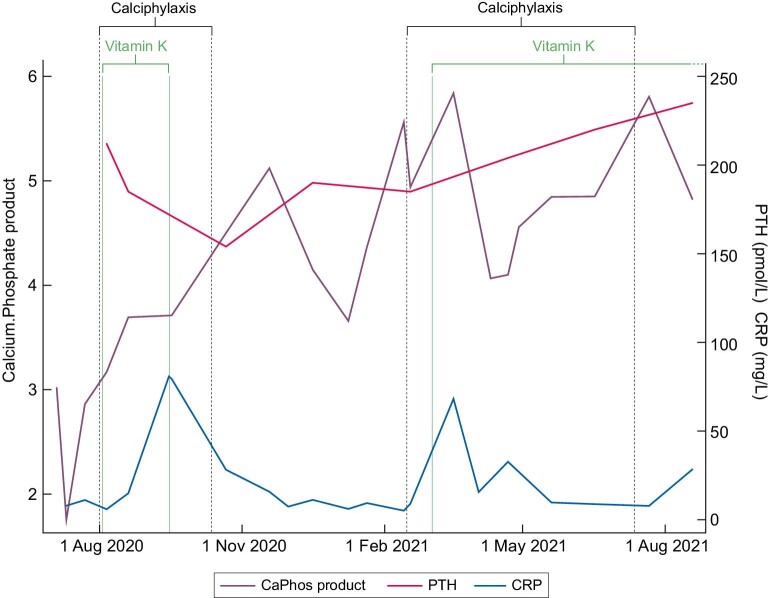
Examination revealed bilateral leg ulcers with black necrotic eschar and a surrounding violaceous border. There were also several dark, tender subcutaneous nodules in the calves that had not ulcerated. At presentation, her body mass index was 25.6 kg/m2. X-rays of her legs revealed extensive vascular calcification of the popliteal, anterior tibial, posterior tibial and peroneal arteries. Based on her clinical presentation, she was diagnosed with calciphylaxis. At diagnosis, her parathyroid hormone (PTH) was ∼150 pmol/L (reference range 1.6–7.2 pmol/L) and had been at a similar level for several years, with the patient previously declining cinacalcet or surgical parathyroidectomy (see Figure 1 for the pattern of mineral bone disease markers). She was commenced on 10 mg phylloquinone orally thrice weekly and also on cinacalcet; however, the cinacalcet caused severe nausea and was stopped within a few weeks. At presentation, her other medications were darbepoetin and aspirin. In addition to phylloquinone, she received standard wound care but did not undergo surgical debridement. She was reviewed by a vascular surgeon who felt, given the location of the ulcers, that these were not atherosclerotic, but nevertheless she underwent percutaneous angioplasty to arterial stenoses of the lower limbs 8 weeks after her initial presentation.
Figure 1:
Time course of plasma calcium phosphate product (given in mmol2/L2), PTH, C-reactive protein, calciphylaxis and vitamin K administration.
Two months after starting phylloquinone, her pain and the ulcers had significantly improved and by 3 months they had resolved. Against our advice, she decided to cease phylloquinone. Seven months after her initial presentation (4 months after ceasing phylloquinone), the calciphylaxis recurred (see Supplementary Data). At this time, her plasma vitamin K was 0.7 nmol/L (reference range 0.3–2.6), and her PTH was over 200 pmol/L. She agreed to recommence 10 mg phylloquinone thrice weekly, this time given intravenously. She did not undergo further angioplasty. Her ulcers began to improve 2 months after resuming phylloquinone treatment and, within 3 months, there was near-complete healing of her ulcers and significant improvement of her pain (see Supplementary Data).
DISCUSSION
Calciphylaxis is a life-threatening disorder with an incidence in dialysis patients of 3.5/1000 patient-years and a 6-month mortality of ∼27% [2]. Secondary infection of ulcers is common, often leading to sepsis and death. Optimal therapy is unclear and although sodium thiosulphate is commonly used, it is expensive. The cost of 1-month treatment with sodium thiosulphate in Australia is US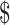 18 000, compared with US
18 000, compared with US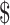 29 for phylloquinone.
29 for phylloquinone.
Our patient had classic risk factors for calciphylaxis, including female sex, end-stage kidney disease and severe hyperparathyroidism. In addition to wound care, the main additional intervention our patient received was high-dose phylloquinone therapy and, at both her first and second presentations, this resulted in rapid improvement. Phylloquinone appears promising as cheap and non-toxic therapy for calciphylaxis. Controlled trials, such as the Beat-Cali placebo-controlled trial (ClinicalTrials.gov registration number NCT05018221), which is not yet recruiting, are required to help determine its place in the treatment of this devastating condition.
PATIENT CONSENT
The patient provided written consent for publication.
Supplementary Material
Contributor Information
Zainab Wajih, Canberra Health Services, Garran, ACT, Australia.
Richard Singer, Canberra Health Services, Garran, ACT, Australia; Australian National University School of Medicine, Acton, ACT, Australia.
CONFLICT OF INTEREST STATEMENT
The results presented in this paper have not been published previously in whole or part, except in abstract format. The authors have no conflicts of interest to disclose.
REFERENCES
- 1. Nigwekar SU, Thadhani R, Brandenburg VM. Calciphylaxis. N Engl J Med 2018; 378: 1704–1714 [DOI] [PubMed] [Google Scholar]
- 2. Nigwekar SU, Zhao S, Wenger J et al. A nationally representative study of calcific uremic arteriolopathy risk factors. J Am Soc Nephrol 2016; 27: 3421–3429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nigwekar SU, Bloch DB, Nazarian RM et al. Vitamin K-dependent carboxylation of matrix Gla protein influences the risk of calciphylaxis. J Am Soc Nephrol 2017; 28: 1717–1722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Binkley NC, Krueger DC, Kawahara TN et al. A high phylloquinone intake is required to achieve maximal osteocalcin γ-carboxylation. Am J Clin Nutr 2002; 76: 1055–1060 [DOI] [PubMed] [Google Scholar]
- 5. Christiadi D, Singer RF. Calciphylaxis in a dialysis patient successfully treated with high-dose vitamin K supplementation. Clin Kidney J 2018; 11: 528–529 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.