Abstract
Background
Optimizing recombinant antibody production is important for cost-effective therapeutics and diagnostics. With impact on commercialization, higher productivity beyond laboratory scales is highly sought, where efficient production can also accelerate antibody characterizations and investigations.
Methods
Investigating HEK293E cells for mammalian antibody production, various transfection and culture parameters were systematically analyzed for antibody light chain production before evaluating them for whole antibody production. Transfection parameters investigated include seeding cell density, the concentration of the transfection reagent and DNA, complexation time, temperature, and volume, as well as culture parameters such as medium replacement, serum deprivation, use of cell maintenance antibiotic, incubation temperature, medium volume, post-transfection harvest day, and common nutrient supplements.
Results
Using 2 mL adherent HEK293E cell culture transfections with 25 kDa linear polyethylenimine in the most optimized parameters, we demonstrated a ~2-fold production increase for light chain alone and for whole antibody production reaching 536 and 49 μg, respectively, in a cost-effective manner. With the addition of peptone, κ light chain increased by ~4-fold to 1032 μg, whereas whole antibody increased to a lesser extent by ~2.5-fold to 51 μg, with benefits potentially for antibodies limited by their light chains in production.
Conclusions
Our optimized findings show promise for a more efficient and convenient antibody production method through transfection and culture optimizations that can be incorporated to scale-up processes and with potential transferability to other mammalian-based recombinant protein production using HEK293E.
Keywords: recombinant antibody production, antibody therapeutics manufacturing, transient transfection, transfection parameters, HEK293E
Statement of Significance
Recombinant antibody production is crucial for antibody research and development. Systematically investigating transfection and culture parameters such as PEI/DNA concentrations, complexation time, volume, temperature, supplements, etc., we demonstrated a ~4-fold light chain alone production increase to 1032 μg and a 2.5-fold whole antibody production increase to 51 μg from 2 mL transfections.
INTRODUCTION
The efficient production of recombinant monoclonal antibodies underpins their functional and structural investigations [1] in toxicology and drug formulation studies [2] and thereby, success of both therapeutic and diagnostic antibodies development. With a direct impact on production costs during development, optimizations of the already expensive mammalian production of recombinant antibodies are highly desired.
Currently, whole recombinant antibodies are predominantly produced by mammalian cells, given the need for complex folding, assembly and post-translational modifications to generate functionally active proteins [3, 4]. Typically, the protein genes of interest are first transfected into mammalian cells, followed by the selection and generation of stable cell lines expressing the gene to produce the protein of interest [2]. As this process is often tedious and laborious, the transient gene expression method on easily transfected cell lines is typically used for research scale investigations. One of the most widely used is the Human Embryonic Kidney 293 (HEK293) family of cell lines with several genetic variants developed to enhance protein expressions such as the HEK293E expressing the Epstein–Barr Virus Nuclear Antigen-1 [5], utilized in this study, and HEK293FT and HEK293T expressing the SV40 T-antigen [6]. Given the scalability of HEK293 [3, 5, 7], they are suitable for producing a wide range of recombinant proteins including whole viral particles [8].
Given that transient recombinant protein production depends on a myriad of transfection and culture parameters, optimizing these parameters directly influences the scalability and efficiency of recombinant protein production. Transfection parameters include seeding cell density [9], transfection reagent concentration [10], DNA concentration [11], complexation time, temperature, and volume [11], together with culture parameters such as medium replacements [5, 12], serum deprivation [13], use of cell line maintenance antibiotic [14], incubation temperature [15, 16], culture volume, harvesting time [17–19], and nutrient supplementation [20–22]. Three main strategies are commonly used to optimize these parameters: the reductionist “one factor at a time” approach [23], stepwise optimization from one condition to another [24], and the design of experiment approach [9], of which we took the single isolated factor approach.
In this study, the key drivers were determined by the “one factor at a time” approach and subsequently combined to maximize antibody production [23]. We optimized individual parameters using Pertuzumab (Perjeta®) antibody light chains (Variable κ-1 or Vκ1), given that light chains are prerequisites for whole antibody production [25, 26] and to mitigate the lopsided effect of dual plasmid transfections. This was followed by validating the combined parameters using recombinant whole Pertuzumab antibodies produced using two separate variable kappa (Vκ)-1 and variable heavy (VH)-3 plasmids. Based on our previous study where we generated Pertuzumab variants of VH family 1–7 and Vκ1–6 frameworks via complementarity determining regions (CDRs) grafting [27, 28], we analyzed light chains known to limit antibody production i.e. the Vκ5 grafted Pertuzumab light chain as a pairing partner.
The final combination of optimized parameters for whole antibody production would complement our previous findings for production that discusses the role of heavy chain constant [29], Vκ–VH pairings [28], amino acid usage with leader selection [27] in a holistic manner [1, 30] to significantly boost antibody production for antibody-based therapeutics and diagnostics.
MATERIALS & METHODS
Plasmid DNA
Pertuzumab light chain of Vκ1 region and heavy chain IgG1 of the VH3 region genes, including their CDR-grafted V-region variants, designated Vκ2–6 and VH1 2, 4–7 were ligated into pTT5 plasmid (YouBio, Cat. No. VT2202) and prepared as previously described [27–29, 31, 32]. Trastuzumab plasmid variants in Supplementary Fig. 1B were prepared similarly. Optimization of light chain production was performed by single plasmid transfection of Pertuzumab light chain Vκ1 before optimizing whole Vκ1|VH3 antibody production by dual plasmid transfection with its corresponding heavy chain VH3. To investigate if the optimized parameters can boost production of antibodies limited by light chains, Pertuzumab Vκ5 was paired with VH3 for whole Vκ5|VH3 antibody production. Comparisons of culture incubation temperatures performed using other antibody variants in large flasks are shown in Supplementary Fig. 1.
Cell culture conditions
HEK293E adherent cells were routinely cultured in Dulbecco's Modified Eagle Medium (DMEM) medium (Gibco, Cat. No. 11965–084), supplemented with 10% Fetal Bovine Serum (FBS) (Gibco, Cat. No. 10270–106) or 10% Ultra Low Immunoglobulin (IgG) FBS (Gibco, Cat. No. 16250078), 1 mM sodium pyruvate (Gibco, Cat. No. 11360–070), 100 units/mL penicillin, 100 μg/mL streptomycin (Nacalai Tesque, Cat. No. 09367–34), G418 (Roche, Cat. No. 04727894001) and incubated at 37 °C in 5% CO2.
Transfection parameters
Linear 25 kDa polyethylenimine (PEI) (Polysciences, Cat. No. 23966–1) at pH 7.0 was used in the transfections. 2 mL and 40 mL scale transfections of HEK293E cells were performed in six-well plates (Costar, Cat. No. 3516) and T175 flasks (Greiner Bio-one, Cat. No. 660175), respectively, with at least three independent replicates. The conditions tested for the transient gene expression in six-well plate format included: Cell seeding densities from 1 to 10 × 105 cells/mL; PEI concentrations from 1 to 10 μg/mL; DNA concentrations from 0.25 to 2 μg/mL; PEI–DNA complexation time from 5 to 120 min; PEI–DNA complexation temperatures of 18 to 45 °C; PEI–DNA complexation in 1 to 25% serum-free medium (SFM) of total 2 mL volume; PEI–DNA complexation in light or dark environments; culture medium replacements; serum deprivation before transfection; culture with or without G418 antibiotic; culture incubation temperature at 32 or 37 °C; culture volume from 2 to 5 mL; post-transfection harvest of 3 to 21 days. Experimental conditions were compared against control parameters of: Seeding density at 2 × 105 cells/mL; DNA concentration at 1 μg/mL; PEI concentration at 2 μg/mL; PEI–DNA complexation time at 30 min in 18 °C; with 10% SFM of total 2 mL volume, performed in the dark and added into cultures without G418; with the cultures grown at 37 °C and harvesting made on day 14 post-transfection, as were performed routinely in previous work [27–29, 33].
For cell culture conditions, 10% FBS DMEM was used for light chain production, whereas 10% Ultra Low IgG FBS DMEM was used for whole antibody IgG production. The culture conditions used for Supplementary Fig. 1 were scaled up proportionately from the six-well cultures for use at T175 flask scale transfections.
Supplement preparation and usage
The nutrient supplements tested were: OmniPur® casamino acids (CA) (EMD Chemicals, Cat. No. 65072–00-6), casein (CS) from bovine milk (Sigma-Aldrich, Cat. No. C3400), Bacto™ peptone (PT) (Becton, Dickinson and Company, Cat. No. 21167), skim milk (SM) (Millipore, Cat. No. 70166), tryptone (TT) [Bio basic, Cat. No. TG217 (G211)], Bacto™ yeast extract (YE) (Becton, Dickinson and Company, Cat. No. 212750), and impact essential amino (EA) acids (MYPROTEIN™) [27]. Supplements were dissolved in water or phosphate buffer saline, filtered using 0.22 μm filters and stored at 4 °C before use.
The supplements were added post-transfection of Pertuzumab light chain cultures on day 7 at 1 mg/mL final concentration. Further optimizations with PT were performed at 1, 2, 4, and 6 mg/mL, added on days 1 to 10. Subsequently, the combined optimized parameters of (1) without nutrient supplementation; (2) PT at 1 mg/mL; (3) EA acids at 3.5 mg/mL [27] or (4) both were tested for Pertuzumab whole antibody production (Vκ1|VH3) and light chain limiting variant (Vκ5|VH3) with the supplementations (conditions 2, 3, and 4) added on day 7 post-transfection.
Recombinant antibody harvest and titre quantification
Cell culture supernatants were harvested on various days stipulated by the experimental conditions and at the end of 14 days, and stored at −20 °C before further testing. In the small-scale 2 mL experiment set-ups where evaporation could be a potential confounding factor, total protein production was calculated from the concentration and remaining volumes measured by a micropipette at the point of harvest. The light chain and whole antibody quantification of the cell culture supernatants were performed using Octet Red 96 instrument as previously described [27–29], with Protein L (PpL) and G (SpG) biosensors (ForteBio, Cat. No. 18–5085 and 18–5082), respectively.
Data analysis
The data were analyzed and plotted using Microsoft Excel 365 and GraphPad Prism Version 9.0.0. Protein titre or total protein production data were normalized against the control and expressed as a percentage with the control set at 100%. One-way Analysis of Variance (ANOVA) with Dunnett’s comparison test against the control was used when examining three or more different parameters, whereas a two-tailed unpaired parametric t-test was performed when comparing two parameters.
RESULTS
Optimizing transfection parameters
Cell seeding densities
Cell seeding densities ranging from 1 to 10 × 105 cells/mL in a six-well plate were evaluated with respect to their impact on Pertuzumab light chain production and normalized against that of the control condition of 2 × 105 cells/mL set at 100% in Fig. 1A. The one-way ANOVA showed no significant differences (p > 0.05) in Pertuzumab light chain production across the seeding densities tested despite a decreasing trend above 6 × 105 cells/mL, where production at 10 × 105 cells/mL was only 41% (70 μg/mL) that of the control (177 μg/mL). The average titre data with accompanying standard error are shown in Supplementary Table 1.
Figure 1.

Pertuzumab light chain production in 2 mL transfections from optimizing the transfection parameters of (A) seeding density; (B) PEI concentration; (C) DNA concentration; (D) PEI–DNA complexation time (E); PEI–DNA complexation temperature; (F) PEI–DNA complexation volume; (G) PEI–DNA complexation in light and dark environments. Black coloured bars represent the controls to which the experimental conditions were compared for statistical analysis. *, **, *** denote significance at p < 0.05, p < 0.01, and p < 0.001, respectively, from one-way ANOVA Dunnett’s post hoc test, whereas + denotes significance at p < 0.05 using t-test analysis. (The experiments were carried out in at least three independent replicates with titres and standard errors provided in Supplementary Table 1.)
PEI and DNA concentrations
PEI concentrations ranging from 1 to 10 μg/mL in six-well plate transfections were tested for Pertuzumab light chain production and normalized against that of the control condition of 2 μg/mL set at 100% in Fig. 1B. The results showed a curvilinear relationship between PEI concentration and light chain production peaking at 145% (206 μg/mL) for 4 μg/mL of PEI that was significant (p < 0.05) with t-test but not the Dunnett’s test against the control (140 μg/mL). PEI concentrations lower or higher than 4 μg/mL negatively impacted light chain production with 1 μg/mL having the lowest production of 17% (22 μg/mL) significant at p < 0.05 when analyzed with Dunnett’s test.
DNA concentrations ranging from 0.25 to 2 μg/mL, at 0.25 μg/mL intervals in six-well plate transfections, were evaluated for Pertuzumab light chain production and normalized against that of the control condition of 1 μg/mL set at 100% in Fig. 1C. An inverse relationship between DNA concentration and light chain production was observed with the lowest and highest DNA concentration (0.25 and 2 μg/mL) corresponding to the best and poorest light chain production at 190% (399 μg/mL) and 21% (64 μg/mL), respectively, that were significant at p < 0.05 when analyzed with Dunnett’s test against the control (208 μg/mL).
PEI and DNA complexation
PEI–DNA complexation time from 5 to 120 min in six-well plate transfections was evaluated with respect to Pertuzumab light chain production and normalized against that of the control condition of 30 min, set at 100% in Fig. 1D. An inverse relationship between complexation time and light chain production showed the shortest and longest incubation time (5 and 120 min) corresponding to the highest and lowest production (166% and 32%, equivalent to a titre of 271 and 54 μg/mL, respectively), which were both significant at p < 0.05 when analyzed with Dunnett’s test against the control (165 μg/mL) production.
PEI–DNA complexation temperature from 18 to 45 °C in six-well plate transfections was evaluated with respect to Pertuzumab light chain production and normalized against that of the control condition of 18 °C, set at 100% in Fig. 1E. An inverse relationship where the decline in light chain production was found with increasing temperatures. Dunnett’s test against the control for 25, 32, and 37 °C showed no significant decrease (p > 0.05) despite slight reductions. However, significant decreases (p < 0.05) were observed for 40 and 45 °C, at 54% (105 μg/mL) and 50% (98 μg/mL) of the control (164 μg/mL) production, respectively.
PEI–DNA complexation volume using SFM from 1 to 25% of the final total culture medium in six-well plate transfections was evaluated with respect to Pertuzumab light chain production and normalized against that of the control condition of 10% final cell culture volume [19] set at 100% in Fig. 1F. PEI–DNA complexation medium volume was found to increase light chain production with the lowest 1% SFM yielding the lowest production of 13% (30 μg/mL, p < 0.05) increasing to the highest production of 131% (273 μg/mL) at 25% SFM (p > 0.05). Nonetheless, production was observed to peak at 15% transfection volume plateauing at higher complexation volumes.
To study photodegradation assumptions of transfection complexes [34], we performed PEI–DNA complexation incubation for light chain using the six-well plate format under the biosafety cabinet light and without (in very dim external room light). There were no significant difference (p > 0.05) in antibody light chain production between the two conditions (Fig. 1G) by t-test.
Optimizing culture conditions
Culture medium replacements
Fresh medium replacement before or after transfection, as well as both before and after, was tested for Pertuzumab light chain using the six-well plates and normalized against that of the control condition of no medium replacement set at 100% in Fig. 2A. Pertuzumab light chain production was found to increase by 36% and 9% (reaching 320 and 250 μg/mL) for before, and both before and after transfection, respectively. A slight reduction of 7% to 235 μg/mL was found for a medium replacement after transfection when compared with the control (226 μg/mL), but the one-way ANOVA showed that these are not significant (p > 0.05). The average titre data with accompanying standard error are shown in Supplementary Table 1.
Figure 2.

Pertuzumab light chain production in 2 mL transfections from optimizing the culture parameters with respect to (A) medium replacement timing; (B) serum deprivation; (C) culturing with G418 antibiotic; (D) culture temperature; (E) culture medium volume; (F) post-transfection harvest day. Black coloured bars in A–D and black coloured dots in E and F represent the controls to which the respective experimental conditions were compared against for statistical analysis. *, **, *** denote significance at p < 0.05, p < 0.01, and p < 0.001, respectively, from one-way ANOVA Dunnett’s post hoc test. +Denotes significance at p < 0.05 using t-test analysis. (The experiments were carried out in at least 3 independent replicates with light chain titres for A–D found in Supplementary Table 1, whereas the total protein for E and F can be found in Supplementary Table 2.)
We next studied serum deprivation by seeding cells in 10% FBS supplemented or SFM a day before transfection in six-well plate format to test for Pertuzumab light chain production. An additional condition of seeding cells in SFM a day before transfection followed by replacement with 10% FBS supplemented medium a day after transfection was included. The serum deprivation condition data was normalized against that of no serum deprivation set at 100% in Fig. 2B. We found the prolonged and short-term serum deprivation to result in a nonsignificant (p > 0.05) 25% and 37% decrease in light chain production using Dunnett’s test against the control.
Culture with G418 antibiotics
Studying the effect of cell culture antibiotics on Pertuzumab light chain production in six-well plates, we did not find G418 to have significant effects (p > 0.05) on the light chain production by t-test (Fig. 2C).
Culture temperature
Culture incubation temperatures at 32 and 37 °C for Pertuzumab light chain six-well transfections were shown with t-tests to reveal a significant production decrease at 32 °C (p < 0.05). 2 mL cultures grown in 32 °C resulted in only 53% (120 μg) of the light chain produced at 37 °C, set at 100% (231 μg) (Fig. 2D). The average total light chain data with standard error are shown in Supplementary Table 2. Further testing with a full panel of whole recombinant Pertuzumab and Trastuzumab variants (VH1–7 and Vκ1–6 combinations) transfected in T175 flasks did not show a significant difference (p > 0.05) between the two temperatures (Supplementary Fig. 1).
Culture medium volumes
Medium volumes varying from 2 to 5 mL in six-well plate transfections were evaluated for Pertuzumab light chain production and normalized against that of the control condition of 2 mL set at 100% in Fig. 2E. The Dunnett’s test showed a significantly lower difference (p < 0.05) when using a culture volume of 1 mL, producing 148 μg of total light chain compared with the control of 300 μg from a 2 mL culture. Although there seems to be a gradual increase in light chain production for higher volumes used, with the highest medium volume of 5 mL showing a rise of 40% (reaching 413 μg), it was not significantly different (p > 0.05) from the control.
Culture supernatant harvest day
Post-transfection harvest of culture supernatants across 3 to 21 days from Pertuzumab light chain six-well plate transfections was normalized against that of the control condition of 14 days set at 100% in Fig. 2F. We found a direct relationship between the day of supernatant harvesting and light chain production where total light chain from 2 mL cultures increased from 81 μg on day 3 to plateau at 522 μg from day 14 (Supplementary Table 2). With analysis by Dunnett’s test against harvest day 14, the control, there was an expected significantly lower (p < 0.05) light chain production on harvest days 3, 6, and 9. No significant increase (p > 0.05) was detected after day 14.
Nutrient supplements
A variety of culture supplements commonly used to improve recombinant protein production were evaluated for Pertuzumab light chains and normalized with respect to the control condition of no supplement set at 100% in Fig. 3A. Adding the supplements to post-transfected cultures on day 7 at a final concentration of 1 mg/mL, the one-way ANOVA showed no significant difference (p < 0.05) across the supplements tested. Given that PT is the most common supplement of mammalian recombinant protein production [24, 35–37], further investigations were performed using a two-tailed unpaired t-test against the nonsupplemented control, revealing that it significantly boosted (p < 0.05) light chain production by 25% (Fig. 3A) to 351 μg in an initial 2 mL culture (Supplementary Table 2). A similar t-test was performed for YE showing no significant (p > 0.05) increases. Further optimizations of concentration and timing of PT addition showed that 1 mg/mL, added on post-transfection days 5, 6, or 7, gave the highest boost to light chain production (Fig. 3B). Single supplementation of PT at 1 mg/mL alone on day 1 or day 7 was found sufficient to enhance light chain production (data not shown), showing that repeated addition of low concentrations of PT was not necessary when a midpoint culture addition on day 7 was sufficient.
Figure 3.
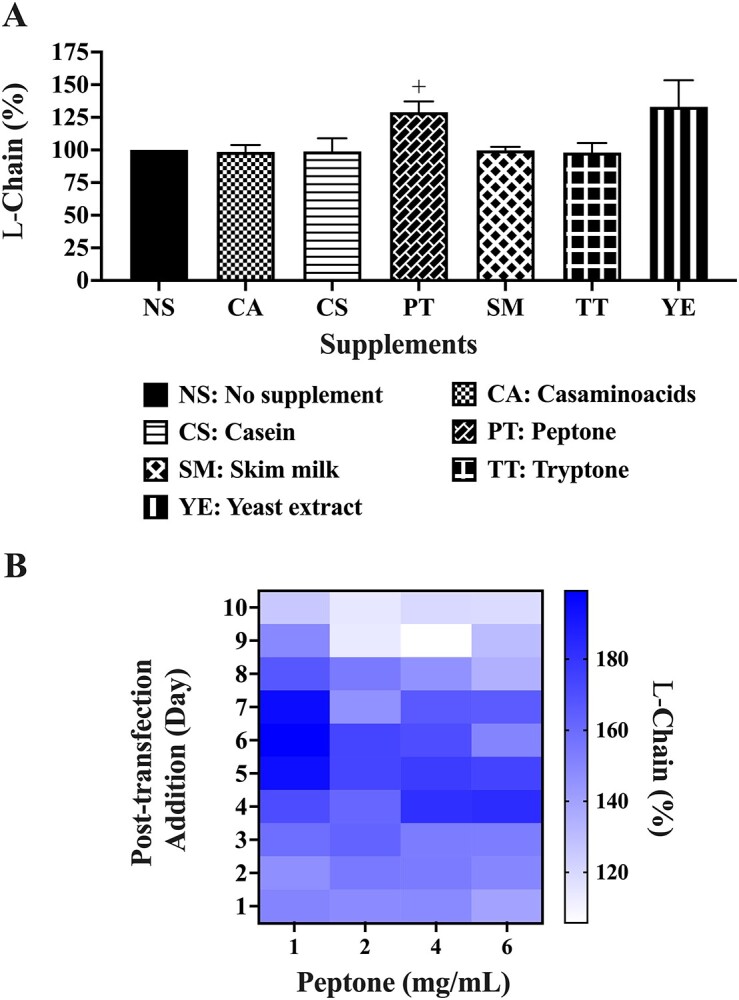
Antibody light chain production in 2 mL transfections with (A) nutrient supplements and (B) heat map of one time additions of different PT concentrations. Black coloured bars in A represent the control to which the respective supplements were compared for statistical analysis. +Denotes significance at p < 0.05 using t-test analysis. (The experiments were carried out in at least 3 independent replicates where the light chain total protein values are provided in Supplementary Table 2).
Combination of transfection and culture parameters
From 2 mL culture optimizations of the individual transfection and culture parameters in Table 1, the combined parameters for Pertuzumab light chain production led to a 217% significant increase (p < 0.05) to 536 μg. This increase was further enhanced ~2-fold to 452% reaching 1032 μg after PT supplementation (Fig. 4A and Supplementary Table 3). Validating this on whole Pertuzumab antibody production in 2 mL cultures, a significant increase (p < 0.05) of 236% to 49 μg was elicited from the control of 22 μg. With PT supplementation, a subtle increase of 252% was observed, reaching 51 μg (Supplementary Table 3).
Table 1.
Control and optimized parameters for transient recombinant production in 2 mL, six-well plate cultures
| Parameters | Control | Optimal |
|---|---|---|
| Cell density (cells/mL) | 2 × 105 | 2 × 105 |
| PEI concentration (μg/mL) | 2 | 4 |
| Plasmid DNA concentration (μg/mL) | 1 | 0.25 |
| Complexation time (min) | 30 | 5 |
| Complexation temperature (°C) | 18 | 18 |
| Complexation in SFM (% of transfection volume) | 10 | 10 |
| Culture medium volume (mL) | 2 | 2 |
| Post-transfection harvest (day) | 14 | 14 |
| Culture incubation temperature (°C) | 37 | 37 |
| Supplements (post-transfection) | – | 1 mg/mL PT on Day 7 |
Figure 4.

Production of recombinant Pertuzumab in 2 mL transfections of (A) light chain (Vκ1) and whole Vκ1|VH3 antibody among standard, optimized and with PT supplementation conditions; (B) whole Vκ1|VH3 Pertuzumab production at optimized parameters with PEI–DNA complex time variations; (C) Vκ1|VH3 and Vκ5|VH3 variants of recombinant Pertuzumab among optimized parameters with PT and/or EA acids supplements. Black coloured bars in A and C represent the control to which the respective experiment parameters were compared by one-way ANOVA Dunnett’s post hoc test. *, **. *** Denote significance at p < 0.05, p < 0.01, and p < 0.001, respectively. (The experiments were carried out in at least 3 independent replicates where the total protein values are provided in Supplementary Table 3).
One-way ANOVA showed no significant difference (p > 0.05) in total whole antibody produced from initial 2 mL cultures with PEI–DNA complexation incubation times of 5, 15, and 30 min in Fig. 4B. Following on from our previous work where 3.5 mg/ml of EA acids showed improvements in Pertuzumab antibody production [27], EA supplementation was added to our evaluation where we found an unexpected significant decrease (p < 0.05) of 13% by Dunnett’s test, from an optimized control of 44 to 38 μg (Fig. 4C and Supplementary Table 3). Dual supplementations of both PT and EA gave a significant increase (p < 0.05) of 23% to 54 μg, with PT alone giving a significantly higher (p < 0.05) increase of 47% to 65 μg (Fig. 4C and Supplementary Table 3). Comparing the single and dual addition of PT and EA to boost light chain limiting antibodies (Pertuzumab Vκ5|VH3), we found PT alone to significantly improve (p < 0.05) production by 28% but not for other supplements. The average total protein data with accompanying standard error are shown in Supplementary Table 3.
DISCUSSION
The cost-effective and efficient scalable production of recombinant monoclonal antibodies is key to the commercial success of both therapeutic and diagnostic antibodies. For this purpose, we set out to optimize transfection and culture parameters with relevance beyond antibody production to that of recombinant proteins using mammalian cell culture. Whole recombinant antibody production is affected by many factors such as the constant region [29] and CDRs of the Vκ and VH chains [28], their pairings [28], deletions in the Vκ [33], the signal peptides and amino acid usage [27]. Given the importance of light chains for whole antibody production [25, 26] and to mitigate the possible lopsided effect of dual plasmid transfection, we performed single parameter optimizations on Pertuzumab light chain production. Thereafter, we applied the combined optimized parameters to produce whole Pertuzumab.
Counter intuitively to the assumption where higher cell seeding density could increase antibody production [38], we did not find significant differences in light chain production levels over the range of tested seeding densities (Fig. 1A), hinting at the influence of other factors. In fact, high seeding cell densities significantly decreased production, which was consistent with previous literature [24], likely due to the reduction in transfection efficiency. It is important to note that the two cited papers here tested higher cell densities as suspension cultures while we used adherent HEK293E. For this, we found our typical usage of adherent cells at 2 × 105 cells/mL in 2 mL transfection within six-well plates to be suitable as a baseline for further optimizations. Notably, there would be expected differences between suspension and adherent cell cultures.
With linear PEI previously reported to be a cost-effective [19], scalable [39] and efficient [40, 41] transfection reagent, we managed to obtain a peak light chain titre at 4 μg/mL of PEI with diminishing returns at higher concentrations. This result supports previous findings that increasing PEI to a certain peak improves protein expression but declines thereafter [24, 42]. Although increasing PEI can improve transfection efficiency, higher levels can be toxic, resulting in cell death, poorer transfection efficiencies and decreased recombinant protein production [11, 24]. Although Dunnett’s test did not show a significant increase in light chain production between our control of 2 μg/mL and 4 μg/mL (Fig. 1B), the t-test showed a significant increase of 45%, pointing 4 μg/mL as optimal. Interestingly, DNA concentration as low as 0.25 μg/mL augmented light chain production (Fig. 1C) and was thus incorporated into the optimized parameters. High amounts of DNA were likely to decrease production due to cellular toxicity associated with higher phosphoric acid levels [11].
Next, we optimized PEI–DNA complexation incubation conditions finding good agreement with other studies [11, 23, 43] that longer complexation incubation decreased protein production (Fig. 1D). This is likely explained by the increase in PEI–DNA complex size with time [44, 45], reducing cell penetration and lowering transfection efficiency. From these findings, we reduced our complexation incubation time to 5 min. With temperature affecting PEI and DNA complexation speed, we also investigated the incubation temperatures, finding 18 °C to be optimal. Higher temperatures reduced light chain production (Fig. 1E) possibly due to larger complex sizes being formed that decreased entry into cells [11]. Although these effects may not be the same for co-transfections of more than one type of plasmid, smaller complexes are likely to improve transfection efficiency for single plasmid transfections.
Further examining PEI–DNA complexation volume where complexation typically occurs in a volume of SFM [46] at 10% of the total medium volume [19]. Our results (Fig. 1F) were in agreement with a previous study finding no significant differences between 5 and 10% [11], and even at higher SFM percentages up to 25%. From this, we retained the recommended 10%. Since the serum free-medium percentage had effects on light chain production, there were hints that reducing complexation volume could increase the proximity of PEI and DNA by enhancing its complexation to form larger complexes that could lead to poorer transfection efficiency [11].
As PEI–DNA complex is critical in the transfection process, we explored certain lab-culture practices with regard to complexation under light to explore if nucleic acid photodegradation [34] may affect transfection efficiency. Given that our results (Fig. 1G) shows no significant difference in the light chain production, we ruled out photodegradation as a possible operational concern.
Turning to optimize culture conditions, we studied the effects of medium replacements, serum deprivation, culture in the presence of G418 antibiotic, culture incubation temperature, culture medium volume, post-transfection harvest time and supplements. Interestingly, we did not find medium replacements to cause any significant effects by one-way ANOVA, even though the t-test showed a significant increase in light chain production with fresh medium replacement before transfection (Fig. 2A). This could be due to the transfection efficiency improving with waste product removal [5, 18]. Although such a practice is reasonable in laboratories, such a need could be costly and laborious [5] for larger scale conditions. Interestingly, we found that medium replacements after transfection, conventionally performed to reduce transfection reagent toxicity unnecessary, for it reduced light chain production (Fig. 2A).
With serum deprivation reported to improve transfection efficiency in some cell lines for purposes other than recombinant antibody production [13], we examined if serum deprivation could improve transfection efficiency and enhance light chain production. We found a nonsignificant decline in light chain production (Fig. 2B) for the transfected HEK293E cells in agreement with a previous study [36]. This could be due to the limited availability of specific EA acids from serum deprivation resulting in reduced antibody production [27].
Since G418 antibiotic is a requirement for HEK293E cells maintenance, we investigated if it could potentially increase metabolic stress and affect recombinant protein production after transient transfection [14]. Since our results (Fig. 2C) showed no significant difference in the light chain production with its addition, large-scale protein production can be performed with or without G418.
Based on the assumption that growth rate retardation would retain higher cell viability, lower nutrient consumption rate and reduce waste accumulation, amongst better mRNA stability, improved protein folding, and fidelity of post-translational events [47], cell culture incubation temperature was investigated. Notably, the literature in this area was inconclusive. Some were reportedly better [48], having no effect [49] or decreasing production [50] at lower temperatures such as 32 °C, with only one study on HEK293S suspension cells finding better production of nonantibody recombinant proteins at lower temperatures [12]. Controlling for different evaporation rates by performing normalizations using total protein data from 2 mL transfections, we found lower temperatures detrimental to light chain production (Fig. 2D). An extended experiment using larger 40 mL T175 transfections with a panel of Pertuzumab and Trastuzumab whole antibody variants showed no clear trends for production (Supplementary Fig. 1A and B). Thus, we continued using the optimal growth temperature of 37 °C.
On the reasonable assumption that the culture medium would provide the necessary nutrients for protein production, we investigated if increasing the culture medium would overcome nutrition bottlenecks and improve light chain production. Since different culture volumes were used, total protein production data from experimental conditions were normalized to the control. Dunnett’s test showed significantly lower light chain production with the usage of 1 mL cultures, but there were no significant increases despite a 40% increase with the use of 5 mL cultures (Fig. 2F). Apart from the significantly lower light chain production, culture volumes below 2 mL are generally not recommended as evaporation becomes an issue. On the contrary, volume increases in six-well plates with possible spillage and culture medium cost is not practical in larger scale productions.
Following this, we explored post-transfection harvest, finding a direct relationship between post-transfection harvest time and light chain production which plateaued on day 14. Depending on the downstream application and the quantity of antibody desired, our results suggest that culture supernatant can be reasonably harvested anytime between day 3 to 21 post-transfection (Fig. 2F), preferably between days 5 to 14 [521, 52]. It should also be noted that longer culture times could lead to the accumulation of host cell proteins, possibly confounding subsequent purification processes. This consideration may also be useful for proteins with poor stability where prolonged culture conditions can cause degradation.
Exploring the addition of supplements, PT [24, 35–37], TT [37], CA [53], YE [54], CS [55], and SM [56] were tested. Given that the high amino acid content in the supplements could lead to toxic accumulation of ammonia culminating in cellular toxicity [35], we tested them at 1 mg/mL. PT was found to significantly boost light chain production in agreement with previous reports [24, 35–37]. However, light chain production was not augmented by other supplements (Fig. 3A). Determining the optimal time of supplementation, addition of 1 mg/mL PT on either post-transfection days 5, 6, or 7 was best in boosting production (Fig. 3B). Repeated additions of supplements did not yield better productions (data not shown), possibly due to toxic accumulation of ammonia [35].
Combining the optimized parameters, we improved Pertuzumab light chain production in 2 mL transfections by 217% to 536 μg, which was further enhanced ~2-fold to 452% reaching 1032 μg with PT supplementation (Fig. 4A and Supplementary Table 3). Applied to whole Pertuzumab antibody in 2 mL transfections, a similar increase of 236% from 22 μg to 49 μg was observed, with PT addition further significantly augmenting production to 252% reaching 51 μg (Fig. 4A and Supplementary Table 3). Hypothesizing that dual plasmid transfection might require a longer PEI–DNA complexation time, we attempted to optimize it for whole antibody production but did not observe significant differences (Fig. 4B). From a possible lead in our previous work [27] where 3.5 mg/mL EA supplementation improved whole antibody production, we explored both single and dual supplementations of EA and PT. We found an unexpected significant whole antibody production decrease upon EA addition which was rescued by the addition of PT (Fig. 4C) for the Pertuzumab antibody.
Considering that some whole antibody productions are limited by the light chain (i.e. Pertuzumab Vκ5) from our previous work [28] and that PT appeared to benefit light chain production in this study, we performed similar experiments using Pertuzumab Vκ5|VH3 whole antibodies for validation. We were able to significantly boost the whole Vκ5|VH3 antibodies production (Fig. 4C) by 28% from 2.4 to 3.0 μg in 2 mL transfections (Supplementary Table 3) supporting the use of PT supplements for antibodies limited by light chains in transient production. Thereby, PT could be added for light chain limiting antibody production given its benefits and lack of detrimental effects.
CONCLUSION
Through a detailed systematic selection of antibodies in a holistic [1, 30] manner, the selection of previously published factors of heavy chain constant [29], Vκ–VH pairings [28], amino acid usage with signal peptide selection [27], antibody production could be more efficient, mitigating commercial failures due to production costs. Here our optimizations of the parameters led to a ~2-fold increase of light chain only and whole antibody production from HEK293E using 25 kDa linear PEI in a cost-effective and operationally convenient manner. With PT supplements, light chain increased by ~4-fold, whereas whole antibody increased to a lesser extent, with most benefits found for antibodies limited by their light chains in production. These findings have potential applications to other mammalian-based recombinant protein production.
DATA AVAILABILITY STATEMENT
The data underlying this article will be shared at reasonable request to the corresponding author.
AUTHOR CONTRIBUTIONS
Conceptualization, W.-L.L. and S.K.-E.G.; methodology, W.-L.L. and Z.S.-L.H.; formal analysis, W.-L.L., Z.S.-L.H.; S.K.-E.G.; investigation, W.-L.L. and Z.S.-L.H.; validation, D.W.-S.K., J.Y.Y., writing—original draft preparation, Z.S.-L.H.; writing—review and editing, D.W.-S.K., J.Y.Y., S.K.-E.G., W.-L.L. and Z.S.-L.H.; visualization, S.K.-E.G., W.-L.L. and Z.S.-L.H.; supervision, S.K.-E.G. and W.-L.L.; funding acquisition, S.K.-E.G. All authors have read and agreed to the published version of the manuscript.
FUNDING STATEMENT
This work was initially supported by the Joint Council Office, Agency for Science, Technology, and Research, Singapore, under Grant number JCO1334i00050 and later by the National Research Foundation (NRF) Singapore grant to Experimental Drug Development Centre (EDDC) for the platform AMDED, acquired by S.K.-E.G.
CONFLICT OF INTEREST STATEMENT
None declared.
ETHICS AND CONSENT STATEMENT
Consent was not required for this study.
ANIMAL RESEARCH STATEMENT
This is not applicable to this study.
Supplementary Material
ACKNOWLEDGEMENTS
The authors would like to thank Jun-Jie Poh for his T175 flasks transfection data contribution.
REFERENCES
- 1. Ling, W-L, Lua, W-H, Gan, SK-E. Sagacity in antibody humanization for therapeutics, diagnostics and research purposes: considerations of antibody elements and their roles. Antib Ther 2020; 3: 71–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pham, PL, Kamen, A, Durocher, Y. Large-scale transfection of mammalian cells for the fast production of recombinant protein. Mol Biotechnol 2006; 34: 225–38. [DOI] [PubMed] [Google Scholar]
- 3. Geisse, S, Henke, M. Large-scale transient transfection of mammalian cells: a newly emerging attractive option for recombinant protein production. J Struct Funct Genomics 2005; 6: 165–70. [DOI] [PubMed] [Google Scholar]
- 4. Wurm, FM. Production of recombinant protein therapeutics in cultivated mammalian cells. Nat Biotechnol 2004; 22: 1393–8. [DOI] [PubMed] [Google Scholar]
- 5. Durocher, Y. High-level and high-throughput recombinant protein production by transient transfection of suspension-growing human 293-EBNA1 cells. Nucleic Acids Res 2002; 30: E9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yuan, J, Xu, WW, Jiang, Set al. The scattered twelve tribes of HEK293. Biomed Pharmacol J 2018; 11: 621–3. [Google Scholar]
- 7. Meissner, P, Pick, H, Kulangara, Aet al. Transient gene expression: recombinant protein production with suspension-adapted HEK293-EBNA cells. Biotechnol Bioeng 2001; 75: 197–203. [DOI] [PubMed] [Google Scholar]
- 8. Ho, YK, Too, HP. Development of a laboratory scalable process for enhancing lentivirus production by transient transfection of HEK293 adherent cultures. Gene Ther 2020; 27: 482–94. [DOI] [PubMed] [Google Scholar]
- 9. Bollin, F, Dechavanne, V, Chevalet, L. Design of Experiment in CHO and HEK transient transfection condition optimization. Protein Expr Purif 2011; 78: 61–8. [DOI] [PubMed] [Google Scholar]
- 10. Sou, SN, Polizzi, KM, Kontoravdi, C. Evaluation of transfection methods for transient gene expression in Chinese hamster ovary cells. Adv Biosci Biotechnol 2013; 04: 1013–9. [Google Scholar]
- 11. Greene, E, Cazacu, D, Tamot, Net al. Optimization of a transient antibody expression platform towards high titer and efficiency. Biotechnol J 2021; 16: e2000251. [DOI] [PubMed] [Google Scholar]
- 12. Lin, C-Y, Huang, Z, Wen, Wet al. Enhancing protein expression in HEK-293 cells by lowering culture temperature. PLoS One 2015; 10: e0123562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wallenstein, EJ, Barminko, J, Schloss, RSet al. Serum starvation improves transient transfection efficiency in differentiating embryonic stem cells. Biotechnol Prog 2010; 26: 1714–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yallop, CA, Svendsen, I. The effects of G418 on the growth and metabolism of recombinant mammalian cell lines. Cytotechnology 2001; 35: 101–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mason, M, Sweeney, B, Cain, Ket al. Reduced culture temperature differentially affects expression and biophysical properties of monoclonal antibody variants. Antibodies 2014; 3: 253–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Xu, J, Tang, P, Yongky, Aet al. Systematic development of temperature shift strategies for Chinese hamster ovary cells based on short duration cultures and kinetic modeling. mAbs 2019; 11: 191–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Baldi, L, Hacker, DL, Adam, Met al. Recombinant protein production by large-scale transient gene expression in mammalian cells: state of the art and future perspectives. Biotechnol. Lett. 2007; 29: 677–84. [DOI] [PubMed] [Google Scholar]
- 18. Fang, XT, Sehlin, D, Lannfelt, Let al. Efficient and inexpensive transient expression of multispecific multivalent antibodies in Expi 293 cells. Biol Proced Online 2017; 19: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Schlaeger, E-J, Christensen, K. Transient gene expression in mammalian cells grown in serum-free suspension culture. Cytotechnology 1999; 30: 71–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Davami, F, Baldi, L, Rajendra, Yet al. Peptone supplementation of culture medium has variable effects on the productivity of CHO cells. Int J Mol Cell Med 2014; 3: 146–56. [PMC free article] [PubMed] [Google Scholar]
- 21. Pérez-Rodriguez, S, Ramírez-Lira, M d J, Trujillo-Roldán, MAet al. Nutrient supplementation strategy improves cell concentration and longevity, monoclonal antibody production and lactate metabolism of Chinese hamster ovary cells. Bioengineered 2020; 11: 463–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. You, M, Liu, Y, Chen, Yet al. Maximizing antibody production in suspension-cultured mammalian cells by the customized transient gene expression method. Biosci Biotechnol Biochem 2013; 77: 1207–13. [DOI] [PubMed] [Google Scholar]
- 23. Liu, C, Dalby, B, Chen, Wet al. Transient transfection factors for high-level recombinant protein production in suspension cultured mammalian cells. Mol Biotechnol 2008; 39: 141–53. [DOI] [PubMed] [Google Scholar]
- 24. Davami, F, Eghbalpour, F, Barkhordari, Fet al. Effect of peptone feeding on transient gene expression process in CHO DG44. Avicenna J Med Biotechnol 2014; 6: 147–55. [PMC free article] [PubMed] [Google Scholar]
- 25. Mains, PE, Sibley, CH. The requirement of light chain for the surface deposition of the heavy chain of immunoglobulin. J Biol Chem 1983; 258: 5027–33. [PubMed] [Google Scholar]
- 26. Schlatter, S, Stansfield, SH, Dinnis, DMet al. On the optimal ratio of heavy to light chain genes for efficient recombinant antibody production by CHO cells. Biotechnol Prog 2005; 21: 122–33. [DOI] [PubMed] [Google Scholar]
- 27. Ling, W-L, Su, CT-T, Lua, W-Het al. Essentially leading antibody production: an investigation of amino acids, myeloma, and natural V-region signal peptides in producing Pertuzumab and Trastuzumab variants. Front Immunol 2020; 11: 604318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ling, W-L, Lua, W-H, Poh, J-Jet al. Effect of VH–VL families in Pertuzumab and Trastuzumab recombinant production, her 2 and Fcγ IIA binding. Front Immunol 2018; 9: 469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lua, W-H, Ling, W-L, Yeo, JYet al. The effects of antibody engineering CH and CL in Trastuzumab and Pertuzumab recombinant models: impact on antibody production and antigen-binding. Sci Rep 2018; 8: 718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Phua, S-X, Chan, K-F, Su, CT-Tet al. Perspective: the promises of a holistic view of proteins—impact on antibody engineering and drug discovery. Biosci Rep 2019; 39: BSR20181958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chan, W-T, Verma, CS, Lane, DPet al. A comparison and optimization of methods and factors affecting the transformation of Escherichia coli. Biosci Rep 2013; 33: e00086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Poh, J-J, Gan, SK-E. The determination of factors involved in column-based nucleic acid extraction and purification. J Bioprocess Biotech 2014; 4: 157. [Google Scholar]
- 33. Su, CT-T, Ling, W-L, Lua, W-Het al. The role of antibody Vκ framework 3 region towards antigen binding: effects on recombinant production and protein L binding. Sci Rep 2017; 7: 3766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Korycka-Dahl, M, Richardson, T. Photodegradation of DNA with fluorescent light in the presence of riboflavin, and photoprotection by flavin triplet-state quenchers. Biochim Biophys Acta 1980; 610: 229–34. [DOI] [PubMed] [Google Scholar]
- 35. Heidemann, R, Zhang, C, Qi, Het al. The use of peptones as medium additives for the production of a recombinant therapeutic protein in high density perfusion cultures of mammalian cells. Cytotechnology 2000; 32: 157–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Pham, PL, Perret, S, Doan, HCet al. Large-scale transient transfection of serum-free suspension-growing HEK293 EBNA1 cells: peptone additives improve cell growth and transfection efficiency. Biotechnol Bioprocess Eng 2003; 84: 332–42. [DOI] [PubMed] [Google Scholar]
- 37. Pham, PL, Perret, S, Cass, Bet al. Transient gene expression in HEK293 cells: peptone addition posttransfection improves recombinant protein synthesis. Biotechnol Bioeng 2005; 90: 332–44. [DOI] [PubMed] [Google Scholar]
- 38. Backliwal, G, Hildinger, M, Hasija, Vet al. High-density transfection with HEK-293 cells allows doubling of transient titers and removes need for a priori DNA complex formation with PEI. Biotechnol Bioeng 2008; 99: 721–7. [DOI] [PubMed] [Google Scholar]
- 39. Nyamay’Antu, A, Kedinger, V, Erbacher, P. Addressing scaling-up limitations: optimized PEI-mediated production of clinical grade viral vectors. Cell Gene Ther Insights 2018; 4: 71–9. [Google Scholar]
- 40. Wiseman, JW, Goddard, CA, McLelland, Det al. A comparison of linear and branched polyethylenimine (PEI) with DCChol/DOPE liposomes for gene delivery to epithelial cells in vitro and in vivo. Gene Ther 2003; 10: 1654–62. [DOI] [PubMed] [Google Scholar]
- 41. Hanzlíková, M, Ruponen, M, Galli, Eet al. Mechanisms of polyethylenimine-mediated DNA delivery: free carrier helps to overcome the barrier of cell-surface glycosaminoglycans: role of free PEI in transfection. J Gene Med 2011; 13: 402–9. [DOI] [PubMed] [Google Scholar]
- 42. Xie, Q, Xinyong, G, Xianjin, Cet al. PEI/DNA formation affects transient gene expression in suspension Chinese hamster ovary cells via a one-step transfection process. Cytotechnology 2013; 65: 263–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bos, AB, Duque, JN, Bhakta, Set al. Development of a semi-automated high throughput transient transfection system. J Biotechnol 2014; 180: 10–16. [DOI] [PubMed] [Google Scholar]
- 44. Han, X, Fang, Q, Yao, Fet al. The heterogeneous nature of polyethylenimine-DNA complex formation affects transient gene expression. Cytotechnology 2009; 60: 63–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sang, Y, Xie, K, Mu, Yet al. Salt ions and related parameters affect PEI–DNA particle size and transfection efficiency in Chinese hamster ovary cells. Cytotechnology 2015; 67: 67–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Yang, J-P, Huang, L. Overcoming the inhibitory effect of serum on lipofection by increasing the charge ratio of cationic liposome to DNA. Gene Ther 1997; 4: 950–60. [DOI] [PubMed] [Google Scholar]
- 47. Masterton, RJ, Roobol, A, Al-Fageeh, MBet al. Post-translational events of a model reporter protein proceed with higher fidelity and accuracy upon mild hypothermic culturing of Chinese hamster ovary cells. Biotechnol Bioeng 2010; 105: 215–20. [DOI] [PubMed] [Google Scholar]
- 48. Yoon, SK, Hwang, SO, Lee, GM. Enhancing effect of low culture temperature on specific antibody productivity of recombinant Chinese hamster ovary cells: clonal variation. Biotechnol Prog 2004; 20: 1683–8. [DOI] [PubMed] [Google Scholar]
- 49. Bloemkolk, J-W, Gray, MR, Merchant, Fet al. Effect of temperature on hybridoma cell cycle and MAb production. Biotechnol Bioeng 1992; 40: 427–31. [DOI] [PubMed] [Google Scholar]
- 50. Barnabé, N, Butler, M. Effect of temperature on nucleotide pools and monoclonal antibody production in a mouse hybridoma. Biotechnol Bioeng 1994; 44: 1235–45. [DOI] [PubMed] [Google Scholar]
- 51. Shatz, W, Ng, D, Dutina, Get al. An efficient route to bispecific antibody production using single-reactor mammalian co-culture. mAbs 2016; 8: 1487–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Li, F, Vijayasankaran, N, Shen, Aet al. Cell culture processes for monoclonal antibody production. mAbs 2010; 2: 466–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kaushik, N, Rohila, D, Arora, Uet al. Casamino acids facilitate the secretion of recombinant dengue virus serotype-3 envelope domain III in Pichia pastoris. BMC Biotechnol 2016; 16: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Li, M, Liao, X, Zhang, Det al. Yeast extract promotes cell growth and induces production of polyvinyl alcohol-degrading enzymes. Enzyme Res 2011; 2011: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Russell, JB, Sniffen, CJ, Van Soest, PJ. Effect of carbohydrate limitation on degradation and utilization of casein by mixed rumen bacteria. J Dairy Sci 1983; 66: 763–75. [DOI] [PubMed] [Google Scholar]
- 56. Khani, M-H, Bagheri, M. Skimmed milk as an alternative for IPTG in induction of recombinant protein expression. Protein Expr Purif 2020; 170: 105593. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared at reasonable request to the corresponding author.


