Abstract
Plastid terminal oxidase (PTOX) accepts electrons from plastoquinol to reduce molecular oxygen to water. We introduced the gene encoding Chlamydomonas reinhardtii (Cr)PTOX2 into the Arabidopsis (Arabidopsis thaliana) wild-type (WT) and proton gradient regulation5 (pgr5) mutant defective in cyclic electron transport around photosystem I (PSI). The accumulation of CrPTOX2 only mildly affected photosynthetic electron transport in the WT background during steady-state photosynthesis but partly complemented the induction of nonphotochemical quenching (NPQ) in the pgr5 background. During the induction of photosynthesis by actinic light (AL) of 130 µmol photons m−2 s−1, the high level of PSII yield (Y(II)) was induced immediately after the onset of AL in WT plants accumulating CrPTOX2. NPQ was more rapidly induced in the transgenic plants than in WT plants. P700 was also oxidized immediately after the onset of AL. Although CrPTOX2 does not directly induce a proton concentration gradient (ΔpH) across the thylakoid membrane, the coupled reaction of PSII generated ΔpH to induce NPQ and the downregulation of the cytochrome b6f complex. Rapid induction of Y(II) and NPQ was also observed in the pgr5 plants accumulating CrPTOX2. In contrast to the WT background, P700 was not oxidized in the pgr5 background. Although the thylakoid lumen was acidified by CrPTOX2, PGR5 was essential for oxidizing P700. In addition to acidification of the thylakoid lumen to downregulate the cytochrome b6f complex (donor-side regulation), PGR5 may be required for draining electrons from PSI by transferring them to the plastoquinone pool. We propose a reevaluation of the contribution of this acceptor-side regulation by PGR5 in the photoprotection of PSI.
A safety valve for electrons originating from Chlamydomonas efficiently functions in Arabidopsis but the PGR5 protein is still necessary to oxidize photosystem I during induction of photosynthesis.
Introduction
Light reactions of photosynthesis convert solar energy into chemical energy in the form of ATP and NADPH. The linear electron transport from water to NADP+ is driven by a series of photochemical reactions catalyzed by PSII and PSI, coupled with proton translocation across the thylakoid membrane to generate proton motive force. The proton motive force consists of the proton concentration gradient (ΔpH) and membrane potential (ΔΨ) and is utilized in ATP synthesis (Kramer et al., 2003). However, this linear electron transport does not satisfy the ATP/NADPH production ratio of 1.5–1.67, which is required by the Calvin–Benson cycle plus photorespiration (Allen, 2002; Shikanai, 2007). Additional ATP is considered to be generated by cyclic electron transport (CET) around PSI recycling electrons from ferredoxin (Fd) to plastoquinone (PQ; Yamori and Shikanai, 2016). In angiosperms including Arabidopsis thaliana, CET consists of two pathways. The main pathway is sensitive to antimycin A and depends on PROTON-GRADIENT REGULATION 5 (PGR5) and PGR5-like Photosynthetic Phenotype 1 (PGRL1) proteins (Tagawa et al., 1963; Munekage et al., 2002; DalCorso et al., 2008; Sugimoto et al., 2013), whereas the minor pathway is mediated by the NADH dehydrogenase-like (NDH) complex (Shikanai et al., 1998; Munekage et al., 2004). CET generates proton motive force without net production of NADPH, consequently increasing the ATP/NADPH production ratio.
In addition to the contribution to ATP synthesis, PGR5/PGRL1-dependent CET downregulates electron transport under excessive light conditions via lumenal acidification (Munekage et al., 2002). Low lumenal pH induces the structural alteration of the PSII supercomplex to dissipate excessively absorbed light energy from PSII antennae safely as heat, the process that is monitored as nonphotochemical quenching (NPQ) of chlorophyll fluorescence (Ruban, 2018). Lumenal acidification also downregulates the activity of the cytochrome (Cyt) b6f complex. This process is known as photosynthetic control (Rumberg and Siggel, 1969; Tikhonov et al., 1981; Nishio and Whitmarsh, 1993) and is essential for protecting PSI under fluctuating light intensity (Joliot and Johnson, 2011; Suorsa et al., 2012). In addition to this regulation at the donor-side of PSI, PGR5/PGRL1-dependent CET is also important to oxidize a PSI reaction center chlorophyll pair (P700) by accepting electrons from PSI (Munekage et al., 2002; Yamamoto and Shikanai, 2019). Flavodiiron proteins function as a safety valve for electrons accumulating in PSI and are excellent machinery for the acceptor-side regulation but angiosperms have lost the genes (Yamamoto et al., 2016).
Chlororespiration is respiration-like electron transport from the stromal reducing power (NADPH or Fd) to O2 and its high activity is observed in Chlamydomonas reinhardtii (Peltier and Cournac, 2002). Chlamydomonas does not have the NDH complex (type-I NDH) and a single subunit-type NADPH dehydrogenase (type-II NDH, Nda2) reduces the PQ pool in chlororespiration (Desplats et al., 2009). The resulting plastoquinol (PQH2) is oxidized by plastid terminal oxidase (PTOX), which reduces molecular oxygen to water (Joët et al., 2002), like mitochondrial alternative oxidase. PTOX was discovered in the Arabidopsis immutans (im) mutant displaying the variegated leaf phenotype (Carol et al., 1999; Wu et al., 1999). PTOX is required for carotenoid biosynthesis by providing oxidized PQ to phytoene desaturase (Shahbazi et al., 2007). Once chloroplasts are fully developed, however, the contribution of PTOX to photosynthetic electron transport is almost negligible in the light and is unlikely to function as a safety valve for electrons (Rosso et al., 2006; Okegawa et al., 2010). However, PTOX from a salt-tolerant brassica species, Eutrema salsugineum translocated from the stromal lamellae to the grana by salinity stress in Arabidopsis, suggesting that the activation step is necessary for PTOX functioning as a safety valve in angiosperms (Stepien and Johnson, 2018).
In Chlamydomonas, two genes encode PTOXs: CrPTOX1 (49 kDa) and CrPTOX2 (44 kDa). The maximum rate of light-independent PQH2 oxidation is faster in CrPTOX2 (4.5–5 e− s−1 PSII−1) than in CrPTOX1 (0.4 e− s−1 PSII−1), suggesting PTOX2 is the main player of chlororespiration in Chlamydomonas (Houille-Vernes et al., 2011; Nawrocki et al., 2019). Despite this fact, focus has been placed on CrPTOX1 in an attempt to improve the stress tolerance of photosynthetic machinery in angiosperms by introducing a strong algal machinery of chlororespiration (Heyno et al., 2009; Ahmad et al., 2012, 2020; Feilke et al., 2016).
In this study, we constructed transgenic Arabidopsis plants, which overexpressed CrPTOX2 to study the impact of the strong PTOX activity of Chlamydomonas on the regulatory network of photosynthetic electron transport. We also introduced the gene into the Arabidopsis pgr5 mutant defective in the main pathway of CET to assess how CrPTOX2 complemented the function of PGR5. CrPTOX2 behaved as a safety valve for electrons and complemented the function of PGR5 except for the oxidation of P700 in the pgr5 mutant background.
Results
Introduction of CrPTOX2 into the wild-type and pgr5 Arabidopsis plants
To express the CrPTOX2 gene in Arabidopsis, its coding sequence for the mature protein without the transit peptide was codon-optimized and synthesized (Supplemental Table S1). The genomic sequence of the Arabidopsis rbcS1A gene was cloned and its coding sequence for the mature protein was replaced by the synthesized sequence of CrPTOX2. The chimeric gene was introduced into both Arabidopsis wild-type (WT) and pgr5 mutant plants. The lines accumulating CrPTOX2 were screened by the immunoblot analysis using the antibody raised against CrPTOX2 in the T1 generation and the phenotype in photosynthetic electron transport was preliminary characterized in the T2 generation in multiple transgenic lines. Finally, two representative lines accumulating a high level of CrPTOX2 and homozygous for the transgene were selected in the T3 generation for WT (#1 and #2) and pgr5 (#1 and #2), respectively. The transgenic lines grew like WT plants under the growth chamber condition (Figure 1A). The pgr5-1 allele was reported to include slightly less chlorophylls than the WT (Munekage et al., 2002). The PTOX lines also contained slightly less chlorophylls and the reduction was statistically significant in the CrPTOX2-pgr5 #1 line (Table 1).
Figure 1.

Growth and protein accumulation of plants expressing CrPTOX2. A, Arabidopsis plants were cultured at 50 µmol photons m−2 s−1 for 28 d under the long-day condition (16-h light/8-h dark cycles at 23°C). B, Soluble and insoluble fractions of leaf proteins were separated by SDS–PAGE and probed with the antibody raised against CrPTOX2. Cyt f was also detected as a loading control. Each fraction corresponding to 0.25-µg chlorophyll was loaded per lane.
Table 1.
Chlorophyll contents in leaves
| CrPTOX2 |
||||||
|---|---|---|---|---|---|---|
| Genotype | WT | pgr5 | WT #1 | WT #2 | pgr5 #1 | pgr5 #2 |
| Chlorophyll contenta | 19.8 ± 2.1 | 18.6 ± 1.7 | 18.5 ± 1.4 | 17.4 ± 0.99 | 14.6 ± 0.99 | 17.6 ± 1.1 |
| Statistical analysis | a | a | a | ab | b | ab |
aSPAD values (means ± sd). Different letters mean statistically significant differences by the Tukey–Kramer test (P < 0.05, n = 4 biological replicates).
The levels of CrPTOX2 were analyzed by immunoblot analysis in the T4 generation (Figure 1B). The antibody did not cross-react with the endogenous PTOX since no signal was detected in WT and pgr5 plants. On the basis of the size of proteins detected in the transgenic lines, chimeric CrPTOX2 precursor proteins were imported into chloroplasts and correctly processed to the mature protein. The accumulation level varied in the order of pgr5 #1 ≫ WT #1 > pgr5 #2 > WT #2. In the transgenic lines, more than half of CrPTOX2 was detached from the thylakoid membrane.
Post-illumination reduction of the PQ pool was suppressed in the transgenic plants accumulating CrPTOX2
To monitor CrPTOX2 activity, we focused on the transient increase in chlorophyll fluorescence after the illumination of AL of 50 µmol photons m−2 s−1 (Figure 2). This fluorescence change is due to the reduction of the PQ pool depending on the chloroplast NDH complex (Shikanai et al., 1998; Hashimoto et al., 2003) and was enhanced in the tomato (Solanum lycopersicum) ghost mutant defective in PTOX (Trouillard et al., 2012). In WT plants, this post-illumination transient increase in chlorophyll fluorescence was observed but was absent in the chlororespiratory reduction 2-2 (crr2-2) mutant (Hashimoto et al., 2003) defective in the accumulation of the NDH complex (Figure 2). Consistent with the previous report (Munekage et al., 2004), the PQ reduction was not affected in the pgr5 mutant. In the transgenic plants accumulating CrPTOX2, this post-illumination transient increase in chlorophyll fluorescence was not observed in both WT and pgr5 backgrounds, similar to the crr2-2 mutant (Figure 2). This observation is consistent with the phenotype of tobacco (Nicotiana tabacum) plants accumulating CrPTOX1 in chloroplasts (Ahmad et al., 2012). It is unlikely that the accumulation of CrPTOX2 inhibited NDH activity. Instead, CrPTOX2 likely oxidized the PQ pool after the AL illumination even in the presence of NDH activity. CrPTOX2 is active in Arabidopsis chloroplasts. On the other hand, the very low flux of electrons is sufficient for this fluorescence rise and we could not estimate CrPTOX2 activity quantitatively.
Figure 2.
Post-illumination transient increase in chlorophyll fluorescence was suppressed by the introduction of CrPTOX2. After the AL illumination (50 µmol photons m−2 s−1 for 5 min), the transient increase in chlorophyll fluorescence depending on NDH activity was monitored. A typical pattern of a WT plant is shown. The boxed area is magnified in the upper right panel. The fluorescence levels were normalized by the Fm levels. SP, saturating pulse of white light; Fo, the minimum fluorescence level from open PSII in the dark; Fm, the maximum fluorescence level from closed PSII in the dark; a.u., arbitrary unit.
Accumulation of CrPTOX2 mildly affects electron transport during steady-state photosynthesis in the WT background
Light intensity-dependence of chlorophyll fluorescence parameters were analyzed in WT plants accumulating CrPTOX2 using a light curve program of the Dual-PAM (pulse amplitude modulation) system (Figure 3). At 11 and 24 µmol photons m−2 s−1, the level of NPQ was slightly lower in the CrPTOX2 lines (Figure 3A). CrPTOX2 may oxidize the PQ pool at the low light intensities, keeping light harvesting complex II proteins dephosphorylated (state 1) in state transitions. At 24–50 µmol photons m−2 s−1, Y(II) was slightly reduced in the CrPTOX2 lines (Figure 3B). The 1-qL parameter estimates the reduction level of the PQ pool (Kramer et al., 2004) and was slightly but significantly higher at 24–50 µmol photons m−2 s−1 in the transgenic lines than in WT plants (Figure 3C). All the mild phenotypes observed at the low light intensities are likely explained by the overexcitation of PSII by the attenuated induction of state transitions. This phenotype might be a sign of PTOX2 activity in the WT background. At light intensities higher than 100 µmol photons m−2 s−1, the level of NPQ was mildly elevated in the CrPTOX2-WT #1 line accumulating the higher level of CrPTOX2 than the CrPTOX2-WT #2 line (Figure 3A). The mild impact of CrPTOX2 in the WT background is consistent with the WT-like growth of the transgenic plants (Figure 1A) but is in contrast to the impaired growth of N. tabacum plants accumulating CrPTOX1 (Feilke et al., 2016).
Figure 3.

Light-intensity dependence of chlorophyll fluorescence parameters during steady-state photosynthesis in the WT background. NPQ (A), Y(II) (B), and 1-qL (C) were analyzed in detached leaves from WT and CrPTOX2 lines under the WT background (#1 and #2). Results at low light intensities are closed up in insets. Data represent means ± sd (n = 5 biological replicates). Different letters indicate statistical differences confirmed by the Tukey–Kramer test (P < 0.05). Asterisks indicate a statistically significant difference from the WT (*P < 0.05, **P < 0.01), confirmed by the Dunnett test. PPFD; photosynthetic photon flux density.
The status of PSI during steady-state photosynthesis was also analyzed by monitoring the absorbance changes of P700, a reaction center chlorophyll pair of PSI (Supplemental Figure S1). Y(ND) represents the ratio of oxidized P700 and is used to monitor the ΔpH-dependent downregulation of the Cyt b6f complex (photosynthetic control). If CrPTOX2 functions as a strong safety valve for electrons, the activity may be monitored as an increase in Y(ND). On the contrary, Y(NA) represents the ratio of reduced P700, which cannot be oxidized by a saturating flash. Y(NA) is indicative of the acceptor limitation from PSI. Y(I) also represents the ratio of reduced P700 in AL but is oxidized by a saturating flash. Y(I) is often used to estimate the yield of photochemical energy conversion in PSI. Because P700 takes one of three states, Y(I) + Y(ND) + Y(NA) = 1 (Klughammer and Schreiber, 1994).
Consistent with the rather normal activity of PSII, no P700 parameters were drastically affected in the CrPTOX2 lines in the WT background. At low light intensities less than 50 µmol photons m−2 s−1, Y(I) was slightly but significantly lower in the transgenic lines than in WT plants (Supplemental Figure S1A). This was accompanied by the slight but significant increase in Y(NA) (Supplemental Figure S1C). Because the deviation of P700 parameters has been reported from the results of dark-interval relaxation kinetics measurements (Theune et al., 2021), we may have to be careful on the interpretation of these parameters at low light intensity. However, the statistically significant differences suggest something unusual in electron transport. Y(II) was also slightly lowered at 50 µmol photons m−2 s−1 (Figure 3B). The CrPTOX2-dependent oxidation of the PQ pool may have disturbed state transitions, resulting in the overexcitation of PSII. It is also possible that the alteration in state transitions caused the acceptor limitation via altering the thylakoid membrane structure (Hepworth et al., 2021).
At higher light intensities, Y(ND) was slightly lower in CrPTOX2-WT #1 than in WT plants but this was not the case in CrPTOX2-WT #2 (Supplemental Figure S1B). This is in contrast to the high Y(ND) observed in N. tabacum plants accumulating CrPTOX1 (Feilke et al., 2016). This low Y(ND) phenotype is not simply explained by the slightly higher lumenal pH, because the level of NPQ was higher in CrPTOX2-WT #1 than in WT plants (Figure 3A). In summary, the accumulation of CrPTOX2 mildly affected electron transport during steady-state photosynthesis, and the phenotype was more evident in PSI. However, its impact was too mild to affect the plant growth (Figure 1A).
NPQ induction is partly complemented by CrPTOX2 during the steady-state photosynthesis in the pgr5 mutant
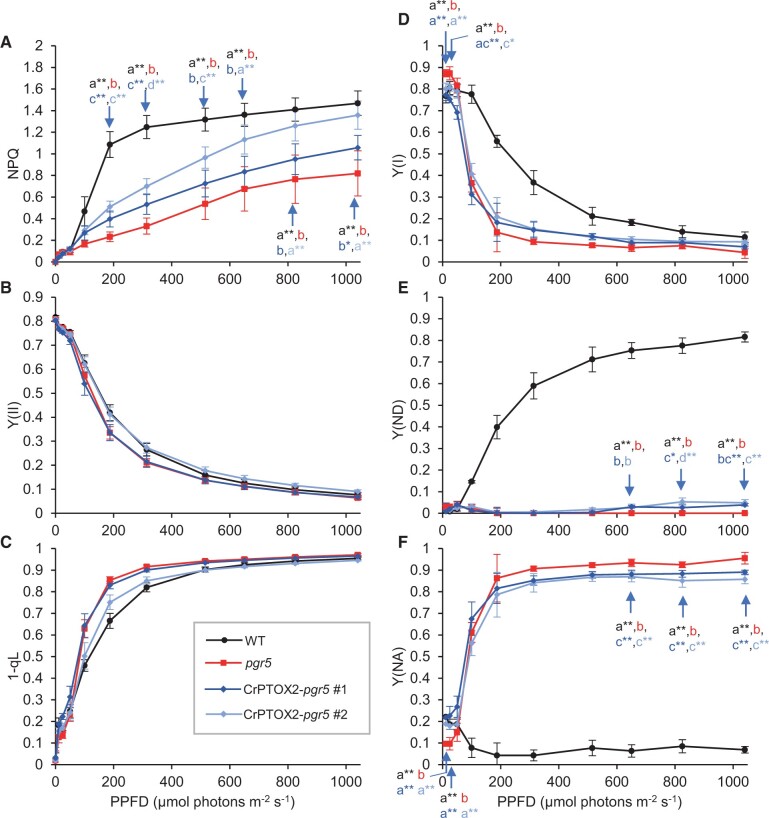
The pgr5 mutant was originally isolated on the basis of its reduced NPQ level at high light intensity (Munekage et al., 2002). In the pgr5 plants accumulating CrPTOX2, NPQ was restored to the level between that of WT and pgr5 plants (Figure 4A). Although PTOX2 activity does not directly generate ΔpH, it activates the PSII-dependent ΔpH formation via water oxidation at the lumenal side and the PQ reduction at the stromal side of PSII. Consistent with this idea, Y(II) was restored to the WT level in the CrPTOX2 line #2, although this was not the case in the CrPTOX2 line #1 under the pgr5 background (Figure 4B).
Figure 4.
Light-intensity dependence of chlorophyll fluorescence and P700 parameters during steady-state photosynthesis in the pgr5 background. NPQ (A), Y(II) (B), 1-qL (C), Y(I) (D), Y(ND) (E), and Y(NA) (F) were analyzed in detached leaves from the WT, pgr5, and CrPTOX2 lines under the pgr5 background (#1 and #2). Data represent means ± sd (n = 5 biological replicates). Different letters indicate the statistical differences confirmed by the Tukey–Kramer test (P < 0.05). Asterisks indicate a statistically significant difference from pgr5 (*P < 0.05, **P < 0.01), confirmed by the Dunnett’s test. PPFD; photosynthetic photon flux density.
In contrast to the partial restoration of NPQ, the level of Y(ND) was only slightly upregulated in the CrPTOX2 lines in the pgr5 background at higher light intensities than 825 µmol photons m−2 s−1 (Figure 4E). The restoration was slightly more evident in the Y(NA) parameter (Figure 4F). Consequently, Y(I) was also mildly restored in the lines (Figure 4D). Although the reason is unclear, Y(I) was higher in pgr5 plants than in WT plants at low light intensities less than 24 µmol photons m−2 s−1, which was accompanied by smaller Y(NA) (Figure 4, D and F). This mutant phenotype was also restored by the accumulation of CrPTOX2.
CrPTOX2 efficiently operates as a safety valve for electrons during the induction of photosynthesis in the WT background
Although it is unlikely that CrPTOX2 operates efficiently during steady-state photosynthesis in the WT background, it may function during the induction of photosynthesis. After the 30-min dark adaptation (in room light), photosynthesis was induced by AL of 130 µmol photons m−2 s−1 (Figure 5). We selected this light intensity because we can monitor the induction and subsequent relaxation of ΔpH-dependent downregulation of electron transport within 5 min (Wang et al., 2017; Wang and Shikanai, 2019; Basso et al., 2021). NPQ was more rapidly induced in the CrPTOX2 lines than in WT plants (Figure 5A). In WT plants, Y(II) was transiently reduced to ∼0.1 at 10 s after the onset of AL and then gradually recovered to the steady-state level (Figure 5B). In contrast, the high level of Y(II) (∼0.35) was induced immediately after the onset of AL in the WT plants accumulating CrPTOX2 (Figure 5B). Most likely, the CrPTOX2-dependent safety valve for electrons efficiently induced Y(II). This idea was supported by the higher oxidation level of the PQ pool monitored by the 1-qL parameter in the transgenic lines than in WT plants (Figure 5C). The rapid induction of NPQ is consistent with the rapid induction of the CrPTOX2-dependent PSII activity (Figure 5A). Rapid induction of NPQ was also observed in N. tabacum plants accumulating CrPTOX1 (Feilke et al., 2016).
Figure 5.
Chlorophyll fluorescence and P700 parameters during the induction of photosynthesis by AL (130 µmol photons m−2 s−1) in the WT background. NPQ (A), Y(II) (B), 1-qL (C), Y(I) (D), Y(ND) (E), and Y(NA) (F) were analyzed in detached leaves from the WT and CrPTOX2 lines under the WT background (#1 and #2). Data represent means ± sd (n = 3–4 biological replicates).
In WT plants, Y(ND) started to increase at 30 s and reached the maximum level at 80 s after the onset of AL (Figure 5E). Because the induction of ΔpH-dependent downregulation of the Cyt b6f complex requires a lower lumenal pH than NPQ does (Takizawa et al., 2007), a longer time is needed for its induction. Consequently, the level of Y(NA) was extremely high in the initial 20 s after the onset of AL (Figure 5F). In the CrPTOX2 lines in the WT background, however, the high level of Y(ND) was induced immediately after the onset of AL, and the level gradually declined to the WT level for 80 s. In addition to the lumenal acidification depending on CrPTOX2, CrPTOX2 also contributed to oxidize P700 as a safety valve for electrons. Y(I) was also higher in the CrPTOX2 lines in the initial 30 s during the induction of photosynthesis than in WT plants (Figure 5D).
Supplemental Figure S2 (two upper panels) shows the representative original traces of P700 analysis in the WT background. Prior to the exposure of AL, a saturating pulse was applied under the background of far-red (FR) light. Although the reason is unclear, the oxidation of P700 by FR light formed an additional first peak in the presence of CrPTOX2.
CrPTOX2 induces NPQ in the absence of PGR5 during the induction of photosynthesis but PGR5 is essential for oxidizing P700
We also analyzed the induction of photosynthesis in the pgr5 plants accumulating CrPTOX2. As in the WT background, NPQ was more rapidly induced in the transgenic lines than in WT plants (Figure 6A). However, the NPQ level was lower than the WT level after 90 s, indicating that PGR5 is essential for maintaining the NPQ level in the subsequent phase. Also as in the CrPTOX2 lines in the WT background, the high level of Y(II) was induced immediately after the onset of AL (Figure 6B), although the Y(II) levels were lower (0.2–0.25) than those in the WT background (∼0.35). The reduction level of the PQ pool was lower in the transgenic lines than in the WT and pgr5 mutant plants within 1 min after the onset of AL, but the level was between that of the WT and pgr5 plants after 1 min (Figure 6C). The analysis of chlorophyll fluorescence indicates the efficient operation of CrPTOX2-dependent electron flow during the induction of photosynthesis in the pgr5 mutant background, as in the WT background.
Figure 6.
Chlorophyll fluorescence and P700 parameters during the induction of photosynthesis by AL (130 µmol photons m−2 s−1) in the pgr5 background. NPQ (A), Y(II) (B), 1-qL (C), Y(I) (D), Y(ND) (E), and Y(NA) (F) were analyzed in detached leaves from the WT, pgr5, and CrPTOX2 lines under the pgr5 background (#1 and #2). Data represent means ± sd (n = 3–4 biological replicates).
In contrast to the chlorophyll fluorescence parameters, P700 parameters were not restored by the accumulation of CrPTOX2 in the pgr5 mutant background (Figure 6, D–F). Y(ND) was not induced within 3 min of the onset of AL in the pgr5 plants accumulating CrPTOX2, similar to the pgr5 mutant (Figure 6E). Consequently, the level of Y(NA) was extremely high, although it was slightly lower in the transgenic lines than that in pgr5 plants in the initial 2 min (Figure 6F). Y(I) was also slightly higher in the transgenic lines than in the pgr5 mutant in the same period but was much lower than in WT plants (Figure 6D). On the basis of the NPQ induction, CrPTOX2 is likely sufficient for acidifying the thylakoid lumen but PGR5 is essential for the oxidation of P700. This observation is consistent with the results in the steady state (Figure 4).
To assess the possibility that the reduction of P700 in the CrPTOX2-pgr5 lines was due to the photodamage of PSI during the induction of photosynthesis at 130 µmol photons m−2 s−1, the maximum P700+ levels (Pm) were compared between before and after the AL illumination (Supplemental Figure S3). At 30 s after turning off the AL, a saturating pulse was applied to record the Pm level under the background of FR light. The Pm levels were slightly reduced after the AL illumination in the pgr5 mutant background, although the difference was significant only in the CrPTOX2-pgr5 #1 and #2 plants from WT plants. However, it is unlikely that this minor reduction in the Pm levels fully explains the strong reduction of P700+ levels observed during the AL illumination (Figure 6, E and F; see “Discussion”).
In the representative trace of P700+ (Supplemental Figure S2), the formation of an additional first peak by FR light was enhanced in the CrPTOX2-pgr5 #1 line. In the WT or transgenic plants in the WT background, P700 was transiently reduced by electrons from PSII and returned to the original oxidation level (Supplemental Figure S2), but this was not the case in the pgr5 mutant background, because P700 was already severely reduced prior to the exposure to a saturating pulse. Instead P700 was transiently and partly oxidized after a lag of ∼1.5 s, although the reason is unclear.
Discussion
Chlamydomonas has strong activity of chlororespiration depending on type-II NDH and PTOX (Peltier et al., 2016). In this study, we focused on CrPTOX2 because this is a major isoform functioning in chlororespiration in Chlamydomonas (Houille-Vernes et al., 2011). In angiosperms, PTOX is unlikely to function as a safety valve for electrons because of its negligible activity in the light (Rosso et al., 2006; Trouillard et al., 2012) and a further activation process may be necessary for it to become machinery for the stress tolerance (Stepien and Johnson, 2018). The catalytic rate of CrPTOX1 is similar to that of PTOX in tomato and is much slower than that of CrPTOX2, and this second PTOX would be necessary to counterbalance the nonphotochemical reduction of PQ by type-II NDH in Chlamydomonas (Trouillard et al., 2012). This explains why we observed the clear contribution of CrPTOX2 as a safety valve for electrons in Arabidopsis WT and pgr5 plants. A substantial amount of CrPTOX2 was detected in the soluble fraction, suggesting that some protein was localized in the stroma (Figure 1B). It is unclear whether this is the nature of the overaccumulating exogenous CrPTOX2 in Arabidopsis or suggests some regulatory mechanism by controlling the localization in chloroplasts (Bolte et al., 2020). We do not eliminate the possibility that the CrPTOX2 weakly associated with the thylakoid membrane in vivo was detached during the protein preparation, as recently reported in Fd:NADP(H) oxidoreductase (Kramer et al., 2021).
In the WT background, only the mild effect of CrPTOX2 accumulation was observed during steady-state photosynthesis (Figure 3;Supplemental Figure S1). This observation is consistent with the lack of the mutant phenotype in the Chlamydomonas ptox2 mutant in continuous light (Nawrocki et al., 2019). This is in contrast to its high impact observed during the induction of photosynthesis (Figure 5). We reported a similar case in the Arabidopsis plants accumulating flavodiiron proteins originated from Physcomitrium patens (PpFlv) in chloroplasts (Yamamoto et al., 2016). The PpFlv-dependent O2 reduction was not observed during steady-state photosynthesis in the WT background, but it was active immediately after the shift from low light to high light under fluctuating light intensity. PpFlv-dependent O2 reduction was also evident in the pgr5 mutant background even during steady-state photosynthesis (Yamamoto et al., 2016). PpFlv is likely active only when the stroma is highly reduced and does not compete with endogenous enzymes functioning in the Calvin–Benson cycle or CET during steady-state photosynthesis probably because of the lower affinity to the electron donor. PTOX2 may be active during the induction of photosynthesis, because Arabidopsis lacks another safety valve for electrons, Flv. Like PpFlv, the level of NPQ was partially recovered by CrPTOX2 in the pgr5 mutant background during steady-state photosynthesis (Figure 4). It is unlikely that CrPTOX2 competes with the Cyt b6f complex for PQ oxidation and may be active only when the PQ pool is highly reduced. This would be an ideal character for the safety valve for electrons.
In this study, we found a clear phenotype of CrPTOX2 accumulation during the induction of photosynthesis, in which the PQ pool was highly reduced transiently. The high level of Y(II) was induced immediately after the onset of AL in the WT background, suggesting the efficient operation of CrPTOX2-dependent O2 reduction (Figure 5B). Unexpectedly, Y(I) was also elevated in the initial 30 s during the induction of photosynthesis (Figure 5D). If the mode of electron transport was predominately from water to O2 via PSII and CrPTOX2, the electron transport would not be accompanied by the upregulation of Y(I). Y(I) may not represent the actual quantum yield of PSI photochemistry because the acceptor side of PSI was unusually oxidized, as observed in low Y(NA) (Figure 5F).
During the induction of photosynthesis, NPQ was more rapidly induced even in the pgr5 mutant background than in WT plants (Figure 6A). This result is consistent with partial restoration of NPQ during steady-state photosynthesis (Figure 4A). CrPTOX2 does not directly induce the ΔpH formation but contributes to the acidification of the thylakoid lumen by coupling with water oxidation at the lumenal side and the PQ reduction at the stromal side of PSII (CrPTOX2-dependent water–water cycle). Rapid induction of NPQ was also observed in the pgr5 mutant background, suggesting that the CrPTOX2-dependent water–water cycle could substitute PGR5-dependent CET during the induction of NPQ.
In contrast to the transgenic lines in the WT background, Y(ND) was not induced by the introduction of CrPTOX2 in the pgr5 mutant background (Figure 6E). To oxidize P700 during the induction of photosynthesis, PGR5-dependent CET is essential to downregulate the Cyt b6f complex via the acidification of the thylakoid lumen. However, this donor-side regulation may not be able to explain the reduced P700 because NPQ was induced to an even higher level in the pgr5 plants accumulating CrPTOX2 than in WT plants: at 30 s after the onset of AL, the pgr5 plants accumulating CrPTOX2 induced a higher level of NPQ (0.8–0.9) than in WT plants (0.6) (Figures 5, A and 6, A), suggesting that the thylakoid lumen was more acidified in the transgenic lines than in WT plants. At the same time point, Y(ND) started to increase in WT plants (Figure 6E). However, Y(ND) was not induced in the pgr5 plants accumulating CrPTOX2 at all, similar to the pgr5 mutant. PGR5 is required for oxidizing P700 during the induction of photosynthesis independently of the acidification of the thylakoid lumen.
To explain the stronger impact of the pgr5 defect in Chlamydomonas than in Arabidopsis, Johnson et al. (2014) discussed the funneling effect of electron transport by the reduced level of PSI by photodamage. We do not eliminate the possibility that the reduction of P700 in the CrPTOX2 lines under the pgr5 mutant background (Figure 5) is due to the reduced level of active PSI to some extent. However, the level of redox active PSI is not affected in the Arabidopsis pgr5 mutant under the growth chamber conditions (Tiwari et al., 2016) and PSI is not photodamaged significantly by our experimental conditions (induction of photosynthesis at 130 µmol photons m−2 s−1; Supplemental Figure S3). Shimakawa and Miyake (2019) reported that the Y(NA) level was only mildly elevated to 0.2–0.4 at 820 µmol photons m−2 s−1 even after the 70% loss of the PSI activity. The strong funneling effect is represented by the elevated Y(I) rather than Y(NA) in Arabidopsis. This is contrasting to the extremely high level of Y(NA) (0.8–0.9) observed in the pgr5 mutant background (Figure 4F).
ΔpH-dependent downregulation of the Cyt b6f complex plays a critical role in the process (photosynthetic control; Malone et al., 2021). Even when the thylakoid lumen is acidified, PGR5 may be required to downregulate the activity of the Cyt b6f complex. The story would be plausible, if PGR5 directly interacted with the Cyt b6f complex. In Chlamydomonas, PGR5 and the Cyt b6f complex are in the same supercomplex involved in CET (Johnson et al., 2014). It is also suggested that PGR5 is required for the Q cycle of the Cyt b6f complex (Buchert et al., 2020). We do not eliminate the possibility that PGR5 directly affects the activity of the Cyt b6f complex in Arabidopsis but the following observations do not support this idea. (1) Although the supercomplex including PSI, the Cyt b6f complex and the NDH complex was suggested (Yadav et al., 2017), PGR5 was detected in a PSI preparation in Arabidopsis (DalCorso et al., 2008). Recently, PGR5 was reported to interact with the lumenal side of the Rieske subunit of the Cyt b6f complex (Wu et al., 2021). However, PGR5 attaches to the stromal side of the thylakoid membrane (DalCorso et al., 2008). (2) The amino acid alteration in the Rieske subunit in the Arabidopsis pgr1 mutant did not affect PGR5-dependent electron transfer from Fd to PQ in ruptured chloroplasts, suggesting that PGR5 function is not directly linked to the Q cycle (Okegawa et al., 2005). (3) The thylakoid lumen was similarly acidified by linear electron transport in ruptured chloroplasts isolated from WT and pgr5 plants, suggesting that photosynthetic control was similarly operating between two genotypes in Arabidopsis (Yamamoto and Shikanai, 2020).
In the absence of PGR5, P700 may not be oxidized even when the lumen is sufficiently acidified and as a result the Cyt b6f complex is downregulated. PGR5 protects PSI from photodamage through two mechanisms at the donor and acceptor sides of PSI (Yamamoto and Shikanai, 2019). As discussed above, the impaired donor-side regulation (photosynthetic control) is unlikely to fully explain the reduction of P700 in the pgr5 plants accumulating CrPTOX2. It is probably necessary to reconsider the contribution of PGR5 to the acceptor-side regulation. In contrast to CrPTOX2, the introduction of PpFlv into the pgr5 mutant largely complemented the pgr5 defect including the oxidation of P700 (Yamamoto et al., 2016). Both PpFlv and CrPTOX2 function as a safety valve for electrons but PpFlv accepts electrons at the acceptor side of PSI from Fd or NADPH. To oxidize P700, it is probably necessary to drain electrons accumulating in electron carriers in PSI in addition to the induction of the donor-side regulation. Unlike PpFlv, PGR5 is not a safety valve for electrons but recycles them from the acceptor side of PSI to the PQ pool. Consequently, electrons are recycled to PSI but often stagnate to make pools. PGR5 is probably necessary to move this pool of electrons from the PSI acceptor side including electron carriers present in PSI to the PQ pool to oxidize P700. It is difficult to conclude this idea simply based on the results in this study because the peak of the 1-qL level was higher in the CrPTOX2-pgr5 lines (∼0.9) than in the CrPTOX2-WT lines (∼0.8; Figures 5, C and 6, C). This is probably because of much higher Y(NA) in the CrPTOX2-pgr5 lines (0.8–0.9) than in the CrPTOX2-WT lines (∼0.1; Figures 5, F and 6, F). However, the proposal is supported by our previous observation that the PQ pool was more reduced by electrons in ruptured chloroplasts isolated from WT plants than in those isolated from pgr5 plants when the acceptors from PSI were limited (Okegawa et al., 2008). It is also supported by the fact that the PQ pool was more reduced by electrons in Arabidopsis plants overaccumulating PGR5 (Okegawa et al., 2007).
In our original idea of the acceptor-side regulation, PGR5-dependent CET is required for supplying sufficient acceptors from PSI by balancing the ATP/NADPH production ratio (Munekage et al., 2002). We propose a modification of this model of the acceptor-side regulation for regulating the distribution of electron pools. It would also be necessary to assign more contribution of the acceptor-side regulation to the protective mechanism of PSI under fluctuating light intensity (Yamamoto and Shikanai, 2019).
Materials and methods
Plant materials and growth conditions
To provide the promoter, plastid targeting signal, and terminator, the Arabidopsis (A. thaliana) rbcs1A gene was amplified and cloned into a pENTER/D-TOPO plasmid. Its coding region for the mature protein lacking the plastid targeting signal was replaced by the codon optimized CrPTOX2 sequence (Supplemental Table S1). The sequence of the chimeric gene was confirmed and then transferred to the binary vector pGWB1 (Nakagawa et al., 2007) by an LR Clonase reaction. The plasmid was introduced into Agrobacterium tumefaciens by electroporation and Arabidopsis plants were transformed by the floral dip method. The original strong allele of pgr5-1 (Munekage et al., 2002) and its corresponding WT (Columbia gl1) were used in this study. Transgenic plants were selected on Murashige and Skoog medium containing 50 μg·mL–1 kanamycin. The T3 and T4 generations were used for the physiological analyses. The plants were grown in soil in a growth chamber (50–60 μmol photons m−2 s−1, 16-h/8-h light–dark cycle, 23°C, 55% humidity) for 24–28 d.
Sodium dodecyl sulphate–polyacrylamide gel electrophoresis and immunoblot analyses
Leaves were ground in liquid nitrogen with a mortar and pestle and suspended in a buffer (20-mM Tricine/KOH [pH 8.4], 5-mM EGTA, 2.5-mM EDTA, and 10-mM NaHCO3). The suspension was filtered through a layer of Miracloth (Millipore) and centrifuged at 18,000g for 10 min at 4°C. The chlorophyll concentration was determined as described in Porra et al. (1989). Proteins were solubilized by adding 2× sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS–PAGE) sample buffer and separated by 12.5% (w/v) SDS–PAGE and then electrotransferred onto a polyvinylidene fluoride membrane. The sample corresponding to 0.25-μg chlorophyll was applied per lane. The antibody was added and the protein antibody complexes were labeled using the ECL Prime western-blotting detection system (GE Healthcare). Chemiluminescence was detected with a lumino-image analyzer LAS4000 (GE Healthcare).
Analysis of chlorophyll contents in leaves
Chlorophyll content per leaf area was determined as a SPAD (Soil and Plant Analyzer Development) value using a chlorophyll meter (SPAD-502Plus, Konica Minolta, Japan).
In vivo measurements of chlorophyll fluorescence and P700 absorption changes
The activity of the NDH complex was monitored using a MINI-PAM portable chlorophyll fluorometer (Walz), as described previously (Shikanai et al., 1998). Chlorophyll fluorescence parameters and P700 parameters were determined by Dual-PAM 100 (Walz). The plants were adapted to room light (2–3 µmol photons m−2 s−1) for 30 min before the analysis. The quantum yield of PSII, Y(II) was calculated as (Fm′ – Fs)/Fm′. Fs is the steady-state fluorescence. Fm and Fm′ are the maximum fluorescence levels in the dark and the light, respectively. The proportion of a closed PSII center that reflects the reduced level of the PQ pool (1-qL) was calculated as 1 − (Fm′ − F)/(Fm′ − Fo′) × Fo′/Fs (Kramer et al., 2004). Fo′ was calculated as Fo/(Fv/Fm + Fo/Fm′) (Oxborough and Baker, 1997). The redox change of P700 was analyzed by monitoring the absorbance changes of transmission light of 830 and 875 nm. Pm was determined by the application of a saturation pulse (SP) in the background of FR light (720 nm). The maximal level of oxidized P700 during AL illumination (Pm′) was determined by an SP application. At the steady-state P700+ level, P was recorded just before applying an SP. The quantum yield of PSI, Y(I) was calculated as (Pm′ – P)/Pm. The acceptor side limitation of PSI {Y(NA)} was calculated as (Pm – Pm′)/Pm, whereas the donor side limitation of PSI {Y(ND)} was calculated as P/Pm (Yamamoto et al., 2016; Wang and Shikanai, 2019). Y(I) was calculated from the complementary PSI quantum yields of nonphotochemical energy dissipation Y(ND) and Y(NA). Y(I) = 1 – Y(ND) – Y(NA) (Klughammer and Schreiber, 1994). The light-intensity dependence of fluorescence and P700 parameters was determined using a light-curve program of the Dual-PAM system. The induction of photosynthesis was also monitored by AL of 130 µmol photons m−2 s−1. After the AL illumination, leaves were kept in the dark for 30 s and an SP was applied again in the background of FR light to evaluate the photodamage of PSI.
Statistical analyses
Tukey–Kramer test and the Dunnett’s test were performed for the statistical analyses.
Accession numbers
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession numbers A8IEF7 (CrPTOX2) and Q9SL05 (AtPGR5).
Supplemental data
The following materials are available in the online version of this article.
Supplemental Figure S1. Light-intensity dependence of P700 parameters during steady-state photosynthesis in the WT background.
Supplemental Figure S2. Representative trances of P700+ absorbance during the induction of photosynthesis by AL of 130-µmol photons m−2 s−1.
Supplemental Figure S3. Evaluation of PSI photodamage during the induction of photosynthesis.
Supplemental Table S1. Sequences used for transformation.
Supplemental Table S2. Statistical analyses of Supplemental Figure S1.
Supplemental Table S3. Statistical analyses of Figure 4.
Supplementary Material
Acknowledgments
We thank Drs Francis-André Wollman (CNRS, France) and Amane Makino (Tohoku University, Japan) for kindly providing us with the anti-CrPTOX2 and anti-Cyt f antibodies, respectively. We also thank Dr Nakagawa (Shimane University, Japan) for providing the pGWB1 vector.
Funding
This work was supported by the Japanese Society for the Promotion of Science KAKENHI (16H06555 and 19H00992).
Conflict of interest statement. None declared.
All the authors designed the research; Q.Z. performed the experiments; C.W. experimentally guided Q.Z.; Q.Z., H.Y., and T.S. analyzed the data; Q.Z. and T.S. wrote the article.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (https://academic.oup.com/plphys/pages/general-instructions) is: Toshiharu Shikanai (shikanai@pmg.bot.kyoto-u.ac.jp).
References
- Ahmad N, Michoux F, Nixon PJ (2012) Investigating the production of foreign membrane proteins in tobacco chloroplasts: expression of an algal plastid terminal oxidase. PLoS One 7:e41722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad N, Khan MO, Islam E, Wei ZY, McAusland L, Lawson T, Johnson GN, Nixon PJ (2020) Contrasting responses to stress displayed by tobacco overexpressing an algal plastid terminal oxidase in the chloroplast. Front Plant Sci 11:501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen J (2002) Photosynthesis of ATP-electrons, proton pumps, rotors, and poise. Cell 110:273–276 [DOI] [PubMed] [Google Scholar]
- Basso L, Yamori W, Szabo I, Shikanai T (2021) Collaboration between NDH and KEA3 allows maximally efficient photosynthesis after a long dark adaptation. Plant Physiol 184:2078–2090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolte S, Marcon E, Jaunario M, Moyet L, Paternostre M, Kuntz M, Krieger-Liszkay A (2020) Dynamics of the localization of the plastid terminal oxidase inside the chloroplast. J Exp Bot 71:2661–2669 [DOI] [PubMed] [Google Scholar]
- Buchert F, Mosebach L, Gäbelein P, Hippler M (2020) PGR5 is required for efficient Q cycle in the cytochrome b6f complex during cyclic electron flow. Biochem J 477:1631–1650 [DOI] [PubMed] [Google Scholar]
- Carol P, Stevenson D, Bisanz C, Breitenbach J, Sandmann G, Mache R, Coupland G, Kuntz M (1999) Mutations in the Arabidopsis gene IMMUTANS cause a variegated phenotype by inactivating a chloroplast terminal oxidase associated with phytoene desaturation. Plant Cell 11:57–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DalCorso G, Pesaresi P, Masiero S, Aseeva E, Schünemann D, Finazzi G, Joliot P, Barbato R, Leister D (2008) A complex containing PGRL1 and PGR5 is involved in the switch between linear and cyclic electron flow in Arabidopsis. Cell 132:273–285 [DOI] [PubMed] [Google Scholar]
- Desplats C, Mus F, Cuiné S, Billon E, Cournac L, Peltier G (2009) Characterization of Nda2, a plastoquinone-reducing type II NAD(P)H dehydrogenase in Chlamydomonas chloroplasts. J Biol Chem 84:4148–4157 [DOI] [PubMed] [Google Scholar]
- Feilke K, Streb P, Cornic G, Perreau F, Kruk J, Krieger-Liszkay A (2016) Effect of Chlamydomonas plastid terminal oxidase 1 expressed in tobacco on photosynthetic electron transfer. Plant J 85:219–228 [DOI] [PubMed] [Google Scholar]
- Hashimoto M, Endo T, Peltier G, Tasaka M, Shikanai T (2003) A nucleus-encoded factor, CRR2, is essential for the expression of chloroplast ndhB in Arabidopsis. Plant J 36:541–549 [DOI] [PubMed] [Google Scholar]
- Heyno E, Gross CM, Laureau C, Culcasi M, Pietri S, Krieger-Liszkay A (2009) Plastid alternative oxidase (PTOX) promotes oxidative stress when overexpressed in tobacco. J Biol Chem 284:31174–831180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hepworth C, Wood WHJ, Emrich-Mills TZ, Proctor MS, Casson S, Johnson MP (2021) Dynamic thylakoid stacking and state transitions work synergistically to avoid acceptor-side limitation of photosystem I. Nat Plants 7:87–98 [DOI] [PubMed] [Google Scholar]
- Houille-Vernes L, Rappaport F, Wollman F-A, Alric J, Johnson X (2011) Plastid terminal oxidase 2 (PTOX2) is the major oxidase involved in chlororespiration in Chlamydomonas. Proc Natl Acad Sci USA 108:20820–20825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joët T, Genty B, Josse E-M, Kuntz M, Cournac L, Peltier G (2002) Involvement of a plastid terminal oxidase in plastoquinone oxidation as evidenced by expression of the Arabidopsis thaliana enzyme in tobacco. J Biol Chem 277:31623–31630 [DOI] [PubMed] [Google Scholar]
- Johnson X, Steinbeck J, Dent RM, Takahashi H, Richaud P, Ozawa S, Houille-Vernes L, Petroutsos D, Rappaport F, Grossman AR, et al. (2014) Proton gradient regulation 5-mediated cyclic electron flow under ATP- or redox-limited conditions: a study of ΔATPase pgr5 and ΔrbcL pgr5 mutants in the green alga Chlamydomonas reinhardtii. Plant Physiol 165:438–452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joliot P, Johnson GN (2011) Regulation of cyclic and linear electron flow in higher plants. Proc Natl Acad Sci USA 108:13317–13322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klughammer C, Schreiber U (1994) An improved method, using saturating light pulses, for the determination of photosystem I quantum yield via P7001-absorbance changes at 830 nm. Planta 192:261–268 [Google Scholar]
- Kramer DM, Cruz JA, Kanazawa A (2003) Balancing the central roles of the thylakoid proton gradient. Trends Plant Sci 8:27–32 [DOI] [PubMed] [Google Scholar]
- Kramer DM, Johnson G, Kiirats O, Edwards GE (2004) New fluorescence parameters for the determination of QA redox state and excitation energy fluxes. Photosynth Res 79:209–218 [DOI] [PubMed] [Google Scholar]
- Kramer M, Rodriguez-Heredia M, Saccon F, Mosebach L, Twachtmann M, Krieger-Liszkay A, Duffy C, Knell RJ, Finazzi G, Hanke GT (2021) Regulation of photosynthetic electron flow on dark to light transition by ferredoxin:NADP(H) oxidoreductase interactions. eLife 10:e56088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malone LA, Proctor MS, Hitchcock A, Hunter CN, Johnson MP (2021) Cytochrome b6f - Orchestrator of photosynthetic electron transfer. Biochim Biophys Acta 1862:148380. [DOI] [PubMed] [Google Scholar]
- Munekage Y, Hojo M, Meurer J, Endo T, Tasaka M, Shikanai T (2002) PGR5 is involved in cyclic electron flow around photosystem I and is essential for photoprotection in Arabidopsis. Cell 110:361–371 [DOI] [PubMed] [Google Scholar]
- Munekage Y, Hashimoto M, Miyake C, Tomizawa K, Endo T, Tasaka M, Shikanai T (2004) Cyclic electron flow around photosystem I is essential for photosynthesis. Nature 429:579–582 [DOI] [PubMed] [Google Scholar]
- Nakagawa T, Kurose T, Hino T, Tanaka K, Kawamukai M, Niwa Y, Toyooka K, Matsuoka K, Jinbo T, Kimura T (2007) Development of series of gateway binary vectors, pGWBs, for realizing efficient construction of fusion genes for plant transformation. J Biosci Bioeng 104:34–41. [DOI] [PubMed] [Google Scholar]
- Nawrocki WJ, Buchert F, Joliot P, Rappaport F, Bailleul B, Wollman F-A (2019) Chlororespiration controls growth under intermittent light. Plant Physiol 179:630–639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishio JN, Whitmarsh J (1993) Dissipation of the proton electrochemical potential in intact chloroplasts (II. The pH gradient monitored by cytochrome f reduction kinetics). Plant Physiol 101:89–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okegawa Y, Tsuyama M, Kobayashi Y, Shikanai T (2005) The pgr1 mutation in the Rieske subunit of the cytochrome b6f complex does not affect PGR5-dependent cyclic electron transport around photosystem I. J Biol Chem 280:28332–28336 [DOI] [PubMed] [Google Scholar]
- Okegawa Y, Long TA, Iwano M, Takayama S, Kobayashi Y, Covert SF, Shikanai T (2007) Balanced PGR5 level is required for chloroplast development and optimum operation of cyclic electron transport around photosystem I. Plant Cell Physiol 48:1462–1471 [DOI] [PubMed] [Google Scholar]
- Okegawa Y, Kagawa Y, Kobayashi Y, Shikanai T (2008) Characterization of factors affecting the activity of photosystem I cyclic electron transport in chloroplasts. Plant Cell Physiol 49:825–834 [DOI] [PubMed] [Google Scholar]
- Okegawa Y, Kobayashi Y, Shikanai T (2010) Physiological links among alternative electron transport pathways reducing and oxidizing plastoquinone in Arabidopsis. Plant J 63:458–468 [DOI] [PubMed] [Google Scholar]
- Oxborough K, Baker NR (1997) Resolving chlorophyll a fluorescence images of photosynthetic efficiency into photochemical and non-photochemical components – calculation of qP and Fv′/Fm′ without measuring Fo′. Photosynth Res 54:135–142 [Google Scholar]
- Peltier G, Cournac L (2002) Chlororespiration. Annu Rev Plant Biol 53:523–550 [DOI] [PubMed] [Google Scholar]
- Peltier G, Aro E-M, Shikanai T (2016) NDH-1 and NDH-2 plastoquinone reductases in oxygenic photosynthesis. Annu Rev Plant Biol 67:55–80 [DOI] [PubMed] [Google Scholar]
- Porra R, Thompson W, Kriedemann P (1989) Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophylls a and b extracted with four different solvents: verification of the concentration of chlorophyll standards by atomic absorption spectroscopy. Biochim Biophys Acta 975:384–394 [Google Scholar]
- Rosso D, Ivanov AG, Fu A, Geisler-Lee J, Hendrickson L, Geisler M, Stewart G, Krol M, Hurry V, Rodermel SR, et al. (2006) IMMUTANS does not act as a stress-induced safety valve in the protection of the photosynthetic apparatus of Arabidopsis during steady-state photosynthesis. Plant Physiol 142:574–585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruban AV (2018) Light harvesting control in plants. FEBS Lett 592:3030–3039 [DOI] [PubMed] [Google Scholar]
- Rumberg B, Siggel U (1969) pH changes in the inner phase of the thylakoids during photosynthesis. Naturwissenschaften 56:130–132 [DOI] [PubMed] [Google Scholar]
- Shahbazi M, Gilbert M, Labouré AM, Kuntz M (2007) Dual role of the plastid terminal oxidase in tomato. Plant Physiol 145:691–702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shikanai T (2007) Cyclic electron transport around photosystem I: genetic approaches. Annu Rev Plant Biol 58:199–217 [DOI] [PubMed] [Google Scholar]
- Shikanai T, Endo T, Hashimoto T, Yamada Y, Asada K, Yokota A (1998) Directed disruption of the tobacco ndhB gene impairs cyclic electron flow around photosystem I. Proc Natl Acad Sci USA 95:9705–9709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimakawa G, Miyake C (2019) What quantity of photosystem I is optimum for safe photosynthesis? Plant Physiol 179:1479–1485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepien P, Johnson GN (2018) Plastid terminal oxidase requires translocation to the grana stacks to act as a sink for electron transport. Proc Natl Acad Sci USA 115:9634–9639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimoto K, Okegawa Y, Tohri A, Long TA, Sarah FS, Hisabori T, Shikanai T (2013) A single amino acid alteration in PGR5 confers resistance to antimycin A in cyclic electron transport around PSI. Plant Cell Physiol 54:1525–1534 [DOI] [PubMed] [Google Scholar]
- Suorsa M, Järvi S, Grieco M, Nurmi M, Pietrzykowska M, Rantala M, Kangasjärvi S, Paakkarinen V, Tikkanen M, Jansson S, et al. (2012) PROTON GRADIENT REGULATION5 is essential for proper acclimation of Arabidopsis photosystem I to naturally and artificially fluctuating light conditions. Plant Cell 24:2934–2948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagawa K, Tsujimoto HY, Arnon DI (1963) Role of chloroplast ferredoxin in the energy conversion process of photosynthesis. Proc Natl Acad Sci USA 49:567–572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takizawa K, Cruz JA, Kanazawa A, Kramer DM (2007) The thylakoid proton motive force in vivo. Quantitative, non-invasive probes, energetics, and regulatory consequences of light-induced pmf. Biochim Biophys Acta 1767:1233–1244 [DOI] [PubMed] [Google Scholar]
- Theune ML, Hildebrandt S, Steffen-Heins A, Bilger W, Gutekunst K, Appel J. ( 2021) In-vivo quantification of electron flow through photosystem I – cyclic electron transport makes up about 35% in a cyanobacterium. Biochim Biophys Acta – Bioenergetics 1862:148353. [DOI] [PubMed] [Google Scholar]
- Tikhonov AN, Khomutov GB, Ruuge EK, Blumenfeld LA (1981) Electron transport control in chloroplasts. Effects of photosynthetic control monitored by the intrathylakoid pH. Biochim Biophys Acta 637:321–333 [Google Scholar]
- Tiwari A, Mamedov F, Grieco M, Suorsa M, Jajoo A, Styring S, Tikkanen M, Aro E-M (2016) Photodamage of iron–sulphur clusters in photosystem I induces non-photochemical energy dissipation. Nat Plants 2: 16035. [DOI] [PubMed] [Google Scholar]
- Trouillard M, Shahbazi M, Moyet L, Rappaport F, Joliot P, Kuntz M, Finazzi G (2012) Kinetic properties and physiological role of the plastoquinone terminal oxidase (PTOX) in a vascular plant. Biochim Biophys Acta 1817:2140–2148 [DOI] [PubMed] [Google Scholar]
- Yadav KNS, Semchonok DA, Nosek L, Kouřil R, Fucile G, Boekema EJ, Eichacker LA (2017) Supercomplexes of plant photosystem I with cytochrome b6f, light-harvesting complex II and NDH. Biochim Biophys Acta – Bioenerg 1858:12–20 [DOI] [PubMed] [Google Scholar]
- Yamamoto H, Shikanai T (2019) PGR5-dependent cyclic electron flow protects PSI under fluctuating light at donor and acceptor sides. Plant Physiol 179:588–600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto H, Shikanai T (2020) Does the Arabidopsis proton gradient regulation 5 mutant leak protons from the thylakoid membrane? Plant Physiol 184:421–427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto H, Takahashi S, Badger MR, Shikanai T (2016) Artificial remodeling of alternative electron flow by flavodiiron proteins in Arabidopsis. Nature Plants 2:16012. [DOI] [PubMed] [Google Scholar]
- Yamori W, Shikanai T (2016) Physiological functions of cyclic electron transport around photosystem I in sustaining photosynthesis and plant growth. Annu Rev Plant Biol 67:81–106 [DOI] [PubMed] [Google Scholar]
- Wang C, Yamamoto H, Narumiya F, Munekage YN, Finazzi G, Szabo I, Shikanai T (2017) Fine-tuned regulation of the K+/H+ antiporter KEA3 is required to optimize photosynthesis during induction. Plant J 89:540–553 [DOI] [PubMed] [Google Scholar]
- Wang C, , Shikanai T (2019) Modification of activity of the thylakoid H+/K+ antiporter KEA3 disturbs ΔpH-dependent regulation of photosynthesis. Plant Physiol 181: 762–773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu D, Wright DA, Wetzel C, Voytas DF, Rodermel S (1999) The IMMUTANS variegation locus of Arabidopsis defines a mitochondrial alternative oxidase homolog that functions during early chloroplast biogenesis. Plant Cell 11:43–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Wu J, Wang Y, He M, He M, Liu W, Shu S, Sun J, Guo S (2021) The key cyclic electron flow protein PGR5 associates with cytochrome b6f, and its function is partially influenced by the LHCII state transition. Hortic Res 2021 8:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.