Abstract
Cytotrophoblasts differentiate in two directions during early placentation: syncytiotrophoblasts (STBs) and extravillous trophoblasts (EVTs). STBs face maternal immune cells in placentas, and EVTs, which invade the decidua and uterine myometrium, face the cells in the uterus. This situation, in which trophoblasts come into contact with maternal immune cells, is known as the maternal-fetal interface. Despite fetuses and fetusderived trophoblast cells being of the semi-allogeneic conceptus, fetuses and placentas are not rejected by the maternal immune system because of maternal-fetal tolerance. The acquired tolerance develops during normal placentation, resulting in normal fetal development in humans. In this review, we introduce placental development from the viewpoint of molecular biology. In addition, we discuss how the disruption of placental development could lead to complications in pregnancy, such as hypertensive disorder of pregnancy, fetal growth restriction, or miscarriage.
Keywords: autophagy, placenta, regulatory T cells, senescence, syncytialization
1. Introduction
Blastocysts are composed of the trophectoderm (TE), the outer layer of the blastocyst, and the inner cell mass, from which the fetus arises. Placental development starts in the uterus after the attachment of the trophectoderm to the decidualized endometrium in a low-nutrient and hypoxic environment [1]. Early placentation is supported by cytokines, including inflammatory cytokines, and growth factors produced by the endometrial glands. The trophectoderm then starts to proliferate and differentiate into proliferative cytotrophoblasts (CTBs). CTBs, which form cell columns at the tips of villi, are able to differentiate in two directions: into syncytiotrophoblasts (STBs) on the fetal side and into extravillous trophoblasts (EVTs) on the uterine side (Figure 1) [2]. Early placentation occurs under hypoxic conditions, with approximately 2% oxygen tension [3]. This protects the fetus from oxidative stress as it does not yet have a redox system. Then, the oxygen concentration increases to 7% oxygen tension by approximately the 13th week of gestation, at which point the trophoblastic plug disintegrates away from the spiral arteries.
Figure 1. Differentiation of trophoblasts.
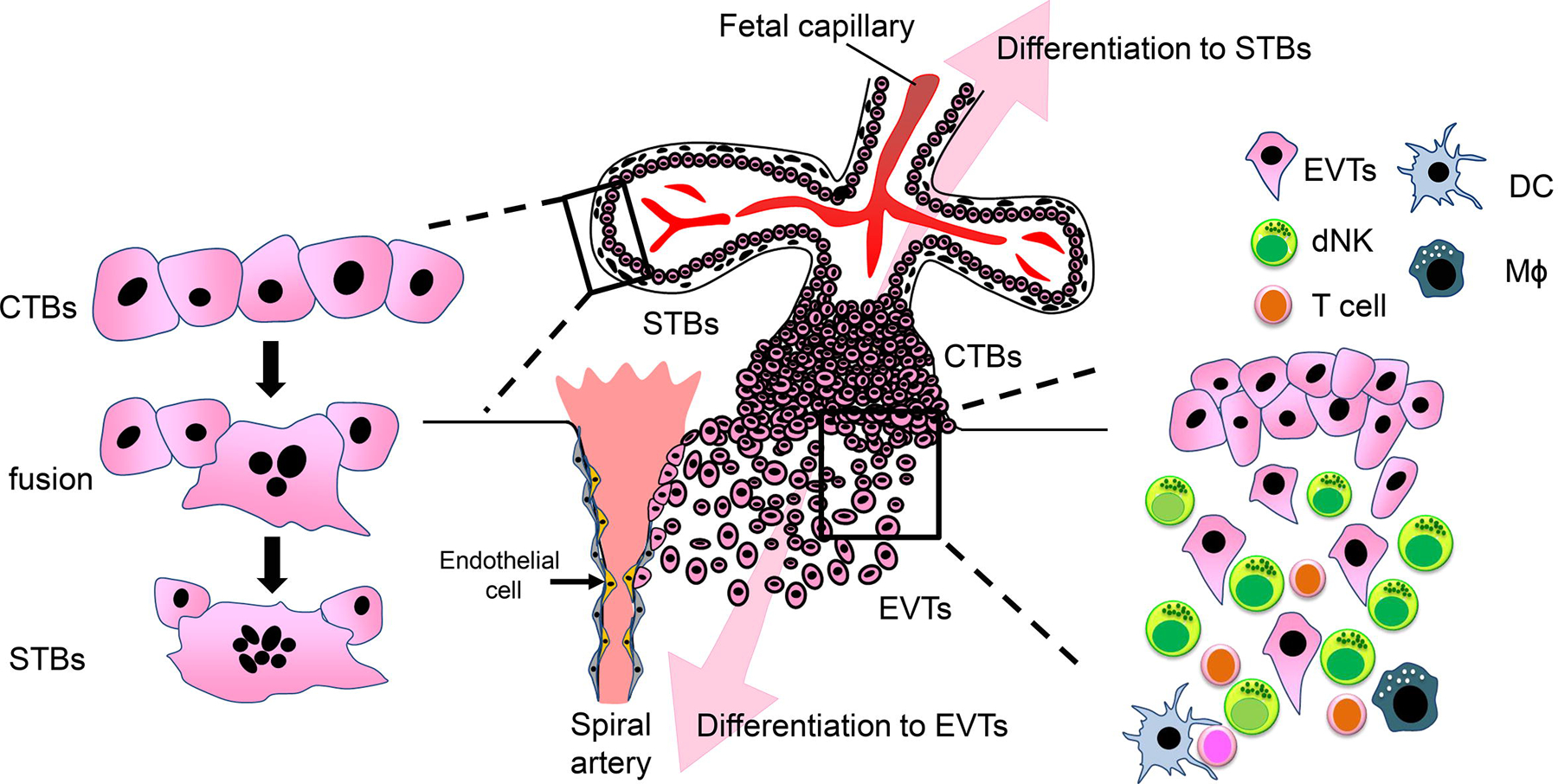
Cytotrophoblasts (CTBs) differentiate in two directions: syncytiotrophoblasts (STBs) and extravillous trophoblasts (EVTs). STBs are formed by fusion of CTBs on the maternal side. On the other hand, EVTs detach from CTBs in the cell column, and invade the maternal decidua and myometrium. At the decidua, EVTs contact with maternal immune cells. Endovascular EVTs, which replace the endothelium and tunica media in the spiral artery, help maintain maternal blood flow into the placenta. dNK; decidual NK cells, DC; dendritic cells, Mϕ; macrophages.
STBs are generated by the fusion of CTBs via syncytialization (Figure 1). Syncytialized STBs form villi, which are involved in exchanging glucose, amino acids, organic ions, and oxygen between maternal and fetal blood. In humans, villi are composed of two layers, which are covered with a multinucleated and fused STB layer underpinned by CTBs. Nutrient exchange in the villi occurs via STBs, preventing direct contact between maternal and fetal blood. Since STBs do not express MHC class I or II, and they are not recognized by CD8+ cytotoxic T cells, they are able to escape from maternal immune cells [4]. The other direction of CTB differentiation involves the generation of EVTs. EVTs invade the endometrium, reaching one-third of the depth of the myometrium, and can be classified into two types according to the direction of differentiation: endovascular EVTs toward the spiral arteries, and interstitial EVTs into the myometrium (Figure 1) [5]. Endovascular EVTs are involved in the vascular remodeling of the spiral arteries by replacing themselves, resulting in an adequate supply of blood and nutrients into the placenta. This process is also supported by uterine natural killer (uNK) cells, by inducing apoptosis in vascular smooth muscle cells [6]. By contrast, the role of interstitial EVTs remains somewhat obscure. It is generally accepted that EVTs anchor the placenta with the uterus. Interstitial EVTs first encounter a variety of maternal immune cells—including uNK cells, macrophages, T cells, and stromal cells—during invasion (Figure 1). Upon cell-to-cell or cell-to-matrix contact, EVTs produce numerous cytokines, chemokines, and matrix metalloproteinases to complete the process. However, it remains to be fully elucidated why the invasion of EVTs is stopped after one-third the depth of the myometrium. Clarifying this mechanism is of great clinical importance since the development of a technique for regulating EVT invasion in the uterus may reduce the occurrence of massive bleeding-related maternal death due to placenta percreta. Inhibition of EVT invasion leads to preeclampsia. In addition, disruption of trophoblast differentiation, either STBs or EVTs, could result in multiple pregnancy complications, including miscarriage, fetal growth restriction (FGR), or preeclampsia.
2. Markers of trophoblast differentiation
2.1. Trophoblast stem cells
Although evidence for early embryogenesis in humans is increasing, placentation research often leverages animal models over human ones because of the difficulties associated with obtaining human samples. However, due to the differences between the placental structures of humans and other species, it is difficult to deduce phenomena in human placentas from data obtained using animal models. An alternative approach for the study of human placental development involves the use of trophoblast cell lines and primary human trophoblasts [7]. However, the molecular mechanisms in the former, especially those underlying cellular survival, are often modified or disrupted compared with normal trophoblasts. In the latter, culturing cells for a long time is often not possible. During the development of trophoblast stem cell research, mouse trophoblast stem (mTS) cells were first established from blastocysts and extra-embryonic ectoderm [8]. Human trophoblastic stem (hTS) cells were constructed 20 years later [9]. hTS cells stemness is maintained by culturing with activated wingless/integrated and epidermal growth factor, combined with the inhibition of transforming growth factor-β, histone deacetylase, and Rho-associated protein kinase. In hTS cells fibroblast growth factor receptor 2B (FGFR2B), but not FGFR2C, is highly expressed. In mTS cells on the other hand, caudal type homeobox 2 (CDX2), eomesodermin, estrogen-related receptor β, and SRY-box transcription factor 2—which maintain stemness—are highly expressed; these are very low in hTS cells. Research on human placental differentiation is expected to advance new uses of hTS cells.
2.2. Differentiation to STBs
CDX2 is a highly specific TE marker. CDX2 is temporally expressed in human and mouse TEs [10]. The expression of CDX2 was observed in most cells in TE at 5 days post-fertilization, but only a few cells were observed after 6 days. In spite of the differential stages, trophoblasts commonly express GATA-binding protein (GATA) 2, GATA3, transcription factor AP-2 gamma (TFAP2C), keratin 7, and keratin 19, but not the major histocompatibility complex HLA-A, HLA-B, Thy-1 cell surface antigen (also known as CD90), vimentin [9, 11–13]; the reverse is true in the stromal cells of placentas (Figure 2). Previously, STBs were found to lack GATA3 and TFAP2C expression [14]. Proliferative CTBs express tumor protein 63 (TP63)—a DNA-binding transcription factor that regulates proliferation, differentiation, cell adhesion and apoptosis, E74-like ETS transcription factor 5, and TEA domain transcription factor 4 [9, 14]. TP63 in the third trimester of CTBs was found to be lower than in the first trimester, consistent with the lower proliferative capacity of the third trimester CTBs. For the differentiation of hTS cells into STBs, forskolin, a cyclin AMP activator, enhances cellular fusion, resulting in the formation of multinucleated STBs [9]. These cells express cell surface markers specific to STBs, including syndecan 1 (also known as CD138), human chorionic gonadotropin (hCG), and human placental lactogen (hPL). In pregnant women, hCG expression rises, peaks at 10 weeks of gestation, and gradually decreases. Meanwhile, the serum hPL concentration, which increases linearly during pregnancy, had been used to evaluate placental growth in clinical settings. Syncytialization in primary trophoblasts is induced by a Rho-associated protein kinase inhibitor, which is required for maintaining stemness in hTS cells; whereas in BeWo cells a choriocarcinoma cell line—frequently used as an in vitro syncytialization model—is induced by forskolin [7, 15]. Although hCG and hPL are often used to detect STBs, EVTs also express these hormones [16, 17]. Therefore, these placental hormones are not specific to STBs.
Figure 2. Molecular markers of trophoblast-differentiation.
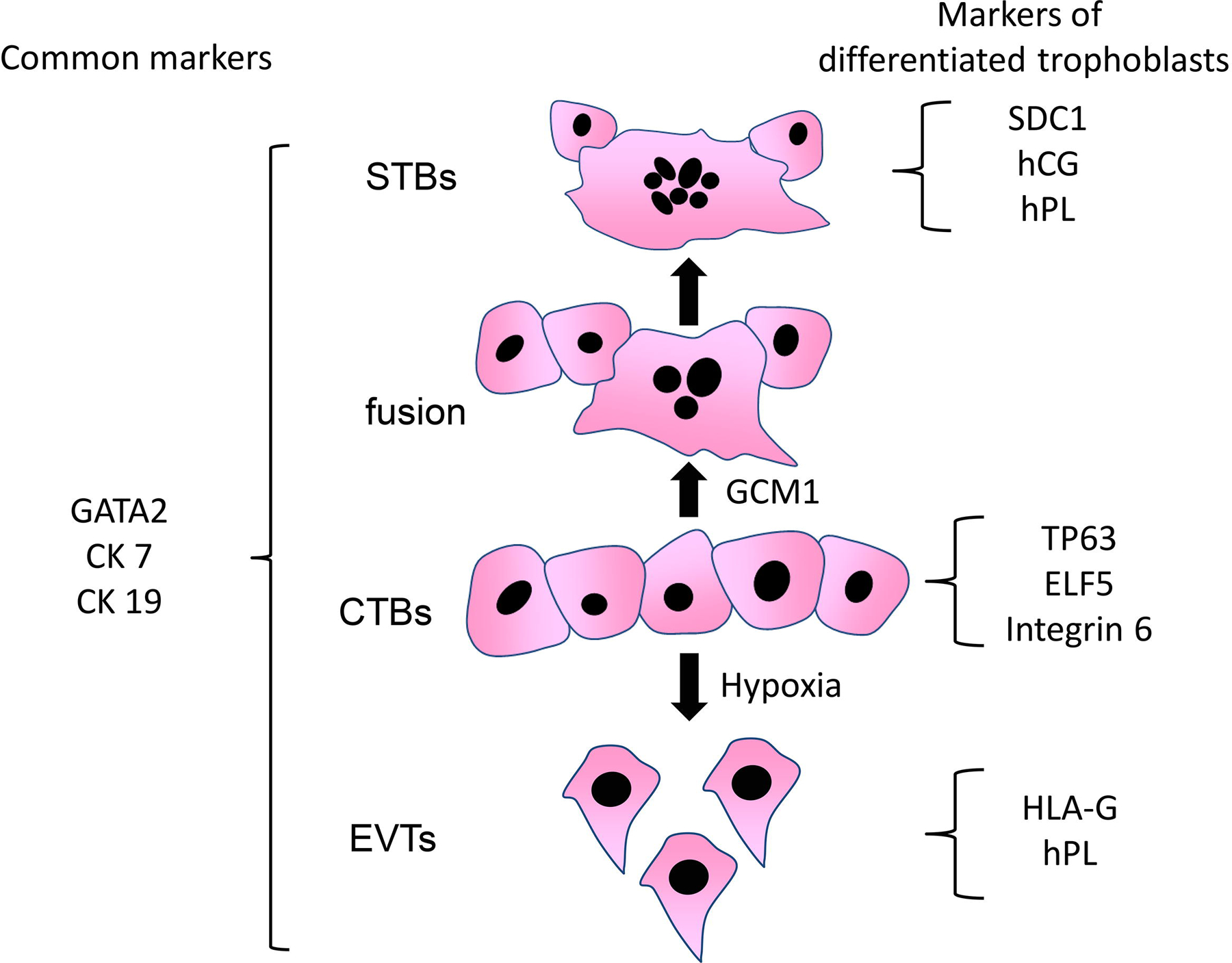
Cytotrophoblasts (CTBs) differentiate into syncytiotrophoblasts (STBs) and extravillous trophoblasts (EVTs). Common markers and differentiation markers are shown. GCM1 (glial cells missing transcription factor 1) is involved in differentiation to STBs, and hypoxia enhances differentiation to EVTs. CK7: cytokeratin 7, CK19; cytokeratin 19, ELF5: E74-like ETS transcription factor 5, hCG; human chorionic gonadotropin, hPL; human placental lactogen, TEAD4; TEA domain transcription factor 4, TP63; tumor protein 63, SDC1; syndecan 1.
2.3. Differentiation to EVTs
Neuregulin-1 and A83–01, an anaplastic lymphoma kinase inhibitor, are required for the differentiation of hTS cells into EVTs on Matrigel (extracellular matrix-based hydrogel). These differentiated EVTs express high levels of HLA-G, but not integrin α6 or cadherin-1 (also known as E-cadherin), which are markers of CTBs; syndecan-1, a marker of STBs; or vimentin, a marker of stromal cells (Figure 2). Inducible EVTs from hTS cells showed similar expression patterns to EVTs isolated from human placental tissues. Combined analysis with gene editing in these cells is expected to enhance the study of human placentation. Induced trophoblast stem cells in mice were established by introducing TFAP2C, GATA3, eomesodermin, and ETS proto-oncogene 2 [18, 19]. Human trophoblast organoids are generated; STBs form a villous structure, and HLA-G-positive EVTs invade the Matrigel three-dimensionally [20].
3. Functions and differentiation in trophoblasts
3.1. Human endogenous retrovirus and STB differentiation
The surface area of expanded and fused STBs amounts to 12–14 m2 in the normal term placenta, contributing to nutrient exchange, pathogen prevention, and hormonal production [21]. Placental growth restriction, which is commonly complicated in severe preeclampsia, largely connects to FGR due to inadequate nutrient exchange. Hypomethylation in human and mouse placentas, which are compared to other somatic cells, is thought to contribute to STB differentiation through the expression of human endogenous retroviruses (HERV) in placentas [22]. The HERV families are important for placental development, including ERV-W and ERVFRD, which correspond to syncytin-1 and syncytin-2, respectively, which are predominantly taken into the placenta from retroviruses during evolution [23]. As the syncytialization of STBs from CTBs proceeds unidirectionally and continuously during pregnancy, the differentiation of STBs is stringently regulated by HERV. During development, syncytin-1 and syncytin-2 are differentially regulated by their methylation status, depending on the developmental stage [24]. Cellular fusion defects in CTBs have been previously found to result in embryonic lethality between 11.5 and 13.5 days of gestation in syncytin-A, corresponding to syncytin-1 knockout mice [25]. This syncytin-A-deficient placenta showed disruptions in the labyrinth layer, with decreased vascularization. Some studies have reported downregulated syncytin-1 mRNA levels in the placenta of humans with preeclampsia, accompanied by hypermethylation of syncytin-1 promoter lesions [26, 27]. Syncytin-1 is transcriptionally regulated by glial cells missing transcription factor 1 (GCM1), a master regulator of STB differentiation, in trophoblast cells [28]. In mice, GCM1 mutations led to failure of the labyrinth layer to develop, resulting in embryonic lethality by embryonic day E10 [29]. GCM1 is upregulated by acute hypoxia [30] but is inhibited by chronic hypoxia via hypoxia-inducible factor 2α [31]. Humans with preeclamptic placenta suffer from chronic hypoxia, which may induce downregulation of GCM1 [32]. Syncytin-2, specifically expressed in CTBs, binds to the major facilitator superfamily domain-containing protein 2A (MFSD2A), which is expressed in STBs [33]. The placenta of syncytin-B knockout mice indicate that syncytin-B is essential for the formation of STB layer-II, resulting in FGR and a reduction in fetal number [34]. In addition, an anti-syncytin protein, suppressyn, has also been discovered [35]. Suppressyn binds to the syncytin-1 receptor and inhibits syncytin-mediated trophoblast fusion. However, the precise mechanism for this suppression during STB differentiation is yet to be elucidated.
3.2. Cellular senescence and STB differentiation
Cellular senescence is a characteristic of aging, recognized as a negative change in cellular functions, inducing a senescence-associated secretory phenotype (SASP) in cancers or aging [36, 37]. Non-trophoblast cells, syncytin-1-induced fusion IMR-90 cells, and normal human diploid fibroblasts all show features of senescent cells, confirmed by SA-β-gal, as well as the cyclin kinase inhibitors p16, p21, and p53, accompanied by SASP phenotypes [38]. This response is due to DNA damage induced by an increase in reactive oxygen species. In the STBs of term placentas, p16, p21, and p53 are considered senescence markers. The expression of p21 in STBs in third trimester placentas has been reported by a research group [39]. However, p21 in STBs is required for the function and differentiation of STBs. p21 forms a complex with GCM1 and transactivates syncytin-2 in G0-phase CTBs, preventing pathogen invasion and reduction of progesterone production [40]. STBs induced to fuse by syncytin-2 overexpression (not at the G0-phase) exhibited compromised functions. Defects in the progesterone production of STBs are consistent with defects in mitochondrial remodeling during STB differentiation, confirmed by the stability of cytochrome P450 family 11 subfamily A member 1, and an increase in small and dense mitochondria [41, 42]. The need for cellular senescence has also been confirmed in p16−/−/p53−/− mice [43], where p16−/−/p53−/− placentas exhibited trophoblast hyperplasia and collapsed vasculature in the labyrinth layer because of continuous cellular proliferation. Differentiated primary STBs also show a tendency toward senescence, hormonal production, and SASP, accompanied by a reduction in gelatinase activity by matrix metallopeptidase (MMP) 2 and MMP-9. Reduced activity is observed in human placentas with FGR, and MMP-9 knockout mice exhibit characteristics associated with preeclampsia and FGR [44]. Thus, senescence in STBs is thought to be functionally required for two reasons: (i) for resistance against apoptosis via B-cell lymphoma 2 expression, and (ii) to enlarge cells for efficient nutrient exchange [45, 46]. In addition, senescence seems to reflect trophoblast invasion in EVTs, as well as STB differentiation.
3.3. Hypoxic conditions and EVT differentiation and functions
Although hypoxia inhibits the differentiation of STBs and reduces hCG secretion and hPL expression [47], hypoxia augments CTBs isolated with high levels of epidermal growth factor receptor expression to differentiate into EVTs rather than STBs [48]. This is also shown by the trophoblast differentiation model of human pluripotent stem cells, in which the aryl hydrocarbon receptor nuclear translocator (also known as HIF-1 β) plays a central role in EVT differentiation [49]. An oxygen tension of 2% transcriptionally enhances HLA-G, an EVT marker; but not hCG, an STB marker, in CTBs. As a mechanism by which EVTs outgrow the cell column, the expression of lysyl oxidase—which is induced by 1% oxygen but not 20% oxygen concentration—is required in villous explant cultures [50]. An in vitro study found side-population trophoblasts to potentially differentiate STBs or EVTs [51]. Fewer side-population trophoblasts were detected in the FGR placenta compared with normal term placentas, suggesting that trophoblast stem cells maintain placental growth until term. Continuous EVT differentiation, even in the third trimester, may be related to continuous placental growth.
Gene ontology analysis has shown that EVT cells are divided into three subtypes at 8 weeks of gestation and two types at 24 weeks of gestation [52]. EVTs in the cell column express ribonucleotide reductase regulatory subunit M2 which is involved in DNA replication; EVTs in the distal site are identified by the expression of plasminogen activator inhibitor-1, suggestive of SERPINE1-mediated invasiveness in distal EVTs. In other methods for distinguishing distal EVTs, interstitial EVTs and endovascular EVTs are differentially detected by placenta-specific protein 8 (PLAC8) expression. PLAC8, which is induced by hypoxia, is involved in the differentiation of interstitial EVTs. Since PLAC8 is highly expressed in preeclamptic placentas compared to normal placentas [53], the balance of differentiation toward interstitial or endovascular EVTs may contribute to normal placentation. At 24 weeks of gestation, tachykinin-3—which plays roles in gonadotropin-releasing hormone and luteinizing hormone secretion and normal follicular development in healthy women [54]—is divided into two types: one showing the characteristics of wounding, digestion, and the negative regulation of the immune system, and the other associated with growth regulation and gonadotropin secretion. Notch Receptor 1 (Notch1) maintains the stemness of the progenitor cells of EVTs via two regulators controlling self-renewal of villous CTBs, namely TP63 and TEAD4 [55]. The activation of Notch1 has also been confirmed in integrin α2-positive cells and proliferative CTBs in the cell column in the first trimester [56]. On the other hand, YAP, which controls organ size and inhibits cancer cell proliferation, is responsible for stemness maintenance and repression of syncytialization of STBs in combination with TEAD4 [57].
Since hypoxic effects can vary temporally and spatially in the placenta, hypoxia has a bifocal effect on pregnancy. A typical pathological placenta, such as preeclampsia or FGR, arises from a maternal-fetal interface with poor perfusion and chronic hypoxia [58, 59]. HLA-G, which not only is a marker of EVTs but also is involved in fetal tolerance [60], is downregulated by hypoxia via miR-365 [61, 62]. Meanwhile, aberrant transcriptional regulation of CTBs in the smooth chorion of the fetal membrane has been reported in severe preeclamptic placentas. Smooth chorion CTBs in the third trimester, which express cadherin-1 and HLA-G-like CTBs in the early second trimester, retain their proliferative and invasive characteristics [63]. These findings suggest that the undisrupted progress of trophoblast differentiation is required for normal placentation. Intact trophoblast differentiation is also sustained by endometrial and decidual tissues before and during placentation. Since decidual cells secrete pro-invasive cytokines and chemokines, including IL-1β, IL-5, IL-6, IL-8, and CCL11, anti-invasive cytokines IL-10, IL-12, and vascular endothelial growth factor (VEGF), these cytokines are involved in balancing the invasiveness of EVTs in a manner dependent on the gestational stage [64]. Normal decidualization is hampered by age; hormonal receptors, including the estrogen receptor α and progesterone receptor, are aberrantly expressed in epithelial and stromal cells in aged mice, resulting in poor placentation due to immature or delayed decidualization [65]. Furthermore, human endometrial stromal cells obtained from non-pregnant women with preeclampsia during the previous pregnancy failed to decidualize in an in vitro differentiation model assay [66]. This indicates that the conditioned medium obtained from the stromal cells did not enhance EVT invasion, due to a reduction in the secretion of prolactin and insulin-like growth factor binding protein 1 (IGFBP)-1.
3.4. The role of autophagy in the differentiation and function of EVTs
Recently, the role of autophagy in oocytogenesis, embryogenesis, implantation, and placentation has been explored [67]. Autophagy is activated by hypoxia in primary trophoblasts in vitro; the activation is observed in interstitial EVTs at 8 weeks of gestation in normal pregnant tissues [68]. The activation of autophagy is observed more frequently in distal EVTs than in proximal EVTs. An in vitro study showed that an autophagy-deficient EVT cell line showed reduced function, invasiveness, and vascular remodeling under hypoxia. Although hypoxia is required for trophoblast differentiation, chronic hypoxia induces inflammation and endoplasmic reticulum stress via pyroptosis in trophoblasts [69]. The autophagy-related proteins Atg16L1 and FIP200 mediate normal decidualization and uterine receptivity [70, 71]. Moreover, autophagy activation in decidual stromal cells improves implantation failure by increasing NK cell residence [72]. However, the role of autophagy in normal placentation remains controversial. Autophagy suppression inhibits trophoblast invasion via decidual NK cells in cases of recurrent miscarriage [73]. On the other hand, a decrease in mitofusin-2 activates autophagy in trophoblasts, resulting in unexplained miscarriage [74]. It is unknown whether the inhibition or overactivation of autophagy contributes to the pathophysiology of preeclampsia [68, 75, 76]. To verify the role of autophagy in placentation, placenta-specific atg7 knockout mice, in which atg7 was deleted in the trophectoderm but not in the inner cell mass, were established. In pseudo-pregnant mice, which possessed intact autophagy function, blastocysts were implanted into the uterus, and the resulting fetuses and placentas were analyzed. During pregnancy, a significant increase in blood pressure was observed in the dams, and placental growth was inhibited due to a reduction in the spongiotrophoblast layer [77]. This reduction could be due to the increase in apoptotic cells in the spongiotrophoblast layer, because atg7 is involved in preventing cell death against metabolic stress via p53 activation [78]. Although the invasion of mouse trophoblasts is shallower than in humans, invasion and vascular remodeling—two necessary characteristics of EVTs for normal placentation—were also impaired in this model. The accumulation of p62 was more frequently observed in spongiotrophoblasts and giant trophoblast cells, corresponding to CTBs and EVTs in humans, than in STBs in the labyrinth layer. Thus, autophagy impairment is likely to affect EVT function and differentiation. Another murine model of autophagy inhibition, labyrinth layer-specific atg7 deficient mice, showed FGR [79]. Thus, placental autophagy is involved not only in placental growth, but also in fetal growth. Moreover, the observed findings in autophagy-deficient placentas are similar to those in the placenta with severe preeclampsia (Figure 3).
Figure 3. Common features between the autophagy-knockout placenta and human placentas with severe preeclampsia.

Invasion failure and vascular remodeling failure, resulting in small placentas, are common features. Though accumulation of p62 and TFEB downregulation are related to autophagy inhibition, these findings are also observed in both the autophagy-knockout placenta and human preeclampsia placentas. However, fetal size is not decreased in the autophagy-knockout placenta, though the blood pressure is significantly increased in dams.
Autophagy impairment, which leads to the deposition of aggregated proteins in the central nervous system, causes neurodegenerative diseases such as Parkinson’s disease, Alzheimer’s disease, and Huntington’s disease [80]. Deposition induced by autophagy impairment has also been observed in preeclamptic placentas [81]. This is related to the inhibition of transcription factor EB (TFEB), a master regulator of autophagy and lysosomal biogenesis, via sera from preeclamptic women [82]. The loss of TFEB in placentas resulted in an aberrant structure in the labyrinth layer due to a reduction in VEGF [83]. The mRNA levels of placental growth factor and the protein levels of TFEB were decreased in placenta-specific atg7 knockout mice and labyrinth-specific atg7 knockout mice, respectively, suggesting that TFEB regulates normal placental development. In addition, excessive endoplasmic reticulum stress, which is observed in preeclamptic placentas [69], also inhibits autophagy in trophoblasts by impairing lysosomes [84]. Consistent with lysosomal impairment in preeclamptic placentas, the concentration of β-galactosidase—a hydrolase in lysosomes, which are detectable in human sera—was found to be significantly lower in the sera of women with preeclampsia than in normal pregnant women, suggesting that it could play a role in placental growth restriction.
4. Immune cells
In the uterus, the growing fetus needs to exercise a degree of immunological tolerance to the mother. Otherwise, the maternal immune cells may attack the fetus, resulting in miscarriage during early pregnancy or preeclampsia in later stages. Various mechanisms in immune cells contribute to the induction of maternal tolerance.
4.1. Natural killer cells
NK cells with fewer granules are dramatically increased during the secretory phase in the endometrium, in response to increased progesterone levels. Further increases in NK cells occur in the uterus upon pregnancy. Peripheral blood lymphocytes are mainly composed of T-cells. However, NK cells constitute the main population of decidual lymphocytes. Uterine immune cells comprise approximately 70% uNK cells, 20% macrophages, and 10% T cells. When comparing peripheral blood NK and uNK cells, peripheral blood NK cells, which express CD16+CD56dim, show high cytotoxicity by introducing cytotoxic granules to target cells. Meanwhile, uNK cells (also called decidual NK cells), which are characterized by CD16-CD56bright, produce a variety of cytokines or angiogenic factors and show low cytotoxicity [85–87]. Although the number of leukocytes, especially macrophages and dendritic cells (DCs), is decreased at the maternal-fetal interface with aging, the maturity and abundance of uNK cells is sustained in the uterus of pregnant women despite the reduction of peripheral NK cells [65]. Although the peripheral blood consists of approximately 10% CD56bright NK cells, these peripheral cells are characteristically different from CD56bright uNK cells [88]. Their numbers gradually decrease after mid-gestation, and only a few can be found in term placentas. Thus, uNK cells are more important for maintaining pregnancy in the early—rather than the mid to late—gestational periods. Although increases in CD16+CD56dim uNK cells, as observed in miscarriages, are thought to induce fetal rejection [89], CD16-CD56bright uNK cells—which possess cytotoxic granules—directly attack trophoblasts and induce apoptosis in vitro [90]. Therefore, CD16-CD56bright uNK cells may exert a two-fold effect during pregnancy.
uNK cells accumulate at the implantation site and are involved in decidualization to induce immune tolerance and vascular remodeling. During placental development, uNK cells are thought to participate in spiral artery remodeling via VEGF during early human pregnancy [6, 91]. An in vitro study showed that IL-6 and CXCL8 produced by endovascular EVTs are involved in the accumulation of uNK in the spiral artery, initiating vascular remodeling via chemokines, CCL14 and CXCL6 [92]. Like trophoblast senescence in STB formation, senescence is also induced in NK cells in response to soluble HLA-G from trophoblasts via CD158d [93]. Despite the SASP phenotype in senescent NK cells, NK cells promote remodeling of the maternal spiral arteries during early pregnancy. Since senescent cells have been reported to express NKG2D ligands, senescent EVTs may attract uNK cells expressing NKG2D receptors to the maternal-fetal interface in humans [94]. In fact, reduced numbers of uNK cells have been observed in placental bed biopsy samples of FGR or pre-eclamptic placentas [95]. In a pregnant mouse model, the administration of IL-11 induces features resembling preeclampsia, accompanied by a reduction in uNK cells and shallow trophoblast invasion [96]. Thus, uNK and trophoblast invasion are required for normal placental development.
4.2. T cells and dendritic cells
As for maternal immune tolerance, regulatory T-cells (Treg cells), which are identified as CD4+CD25+Foxp3+, are increased in the uterus, and preferentially maintain allogenic pregnancy to accept the paternal antigens expressed by the fetus [4]. Treg cells in humans also increase systemically and locally during pregnancy [97]. In a mouse model, uterine CD11c+ antigen-presenting cells in the seminal fluid induce paternal antigen-specific Treg cells [98]. Uterus-derived DCs migrating into the draining lymph nodes highly express programmed cell death ligand-2 (PD-L2), which mediates T-cell suppression via the engagement of its receptor, PD-1 [99]. In humans, clonally expanded CD8+ effector memory T-cells in the decidua—which are assumed to recognize paternal antigens—express PD-1 in normal pregnancies [100]. These results indicate that PD-L2-expressing cells are involved in the inhibition of CD8+ effector memory T cells via PD-L2/PD-1 interaction. In human Treg cells, clonally expanded Tregs—which are also assumed to recognize paternal antigens— were expanded in the decidua, but not in the periphery during early pregnancy [101]. The proportion of clonally expanded decidual Treg cells gradually increased during gestational weeks, and the proportion in normal pregnancy was significantly higher than that in preeclampsia. The same clones of decidual effector Treg cells were maintained between past and subsequent pregnancies in individuals with normal pregnancies. Although preeclampsia is likely to occur in women who are pregnant for the first time, the maintenance or induction of clonal Treg cells, which do recognize paternal antigens, may reduce the occurrence of preeclampsia in the following pregnancy. On the other hand, HLA-G+ EVTs in the first trimester induce Treg cells which express high PD-1 [60]. This ability was also found to be stronger in EVTs obtained from pregnant women with a male fetus than those with a female fetus.
5. Conclusions
Normal placentation depends on the intact development of trophoblasts. In addition, the development and function of trophoblasts are regulated not only by endogenous factors, transcriptional factors, and miRNAs, but also by exogenous factors, growth factors, cytokines, and chemokines. Owing to the development of tools for human trophoblast differentiation in in vitro models by hTS in recent studies, further progress is expected in this field. As discussed in this review, placenta-specific gene manipulation may be more useful than a systemic gene knockout mouse model for the investigation of placental development. This technique has shown that defects in autophagy in placentas affect placental growth rather than fetal growth, as well as inducing hypertension in dams. Future studies should aim to investigate the interconnection between endogenous mechanisms in trophoblasts and local immune reactions at the maternal-fetal interface for the development of new therapies for preeclampsia, FGR, and recurrent miscarriages.
Acknowledgements
This work was supported by grants from the Takahashi Industrial and Economic Research Foundation, Toyama University Hospital Grant (grant numbers 040200–59200003502 and 040200–59200003513), NIH P20GM121298–05, and JSPS KAKENHI (grant numbers JP19K09750 and JP20K09614). We would like to thank Editage (www.editage.com) for English language editing.
Abbreviations:
- CDX2
caudal type homeobox 2
- CTB
cytotrophoblast
- EVT
extravillous trophoblast
- FGR
fetal growth restriction
- GCM1
glial cells missing transcription factor 1
- GATA
GATA binding protein
- hCG
human chorionic gonadotropin
- HERV
human endogenous retrovirus(es)
- HLA
major histocompatibility complex
- hPL
human placental lactogen
- hTS
human trophoblastic stem
- mTS
mouse trophoblast stem
- Notch1
Notch Receptor 1
- PLAC8
placenta-specific protein 8
- SASP
senescence-associated secretory phenotype
- STB
syncytiotrophoblast
- TE
trophectoderm
- TEAD4
TEA domain transcription factor 4
- TFAP2C
transcription factor AP-2 gamma
- TFEB
transcription factor EB
- TP63
tumor protein 63
- uNK
uterine NK
- VEGF
vascular endothelial growth factor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Burton GJ, Jauniaux E, Charnock-Jones DS: The influence of the intrauterine environment on human placental development. Int J Dev Biol 2010;54:303. [DOI] [PubMed] [Google Scholar]
- [2].Saito S, Nakashima A: Review: The role of autophagy in extravillous trophoblast function under hypoxia. Placenta 2013;34 Suppl:S79. [DOI] [PubMed] [Google Scholar]
- [3].Jauniaux E, Watson A, Burton G: Evaluation of respiratory gases and acid-base gradients in human fetal fluids and uteroplacental tissue between 7 and 16 weeks’ gestation. Am J Obstet Gynecol 2001;184:998. [DOI] [PubMed] [Google Scholar]
- [4].Tsuda S, Nakashima A, Shima T, Saito S: New Paradigm in the Role of Regulatory T Cells During Pregnancy. Front Immunol 2019;10:573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Saito S, Nakashima A: A review of the mechanism for poor placentation in early-onset preeclampsia: the role of autophagy in trophoblast invasion and vascular remodeling. J Reprod Immunol 2014;101–102:80. [DOI] [PubMed]
- [6].Smith SD, Dunk CE, Aplin JD, Harris LK, Jones RL: Evidence for immune cell involvement in decidual spiral arteriole remodeling in early human pregnancy. Am J Pathol 2009;174:1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Motomura K, Okada N, Morita H, Hara M, Tamari M, Orimo K et al. : A Rho-associated coiled-coil containing kinases (ROCK) inhibitor, Y-27632, enhances adhesion, viability and differentiation of human term placenta-derived trophoblasts in vitro. PLoS One 2017;12:e0177994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Tanaka S, Kunath T, Hadjantonakis AK, Nagy A, Rossant J: Promotion of trophoblast stem cell proliferation by FGF4. Science 1998;282:2072. [DOI] [PubMed] [Google Scholar]
- [9].Okae H, Toh H, Sato T, Hiura H, Takahashi S, Shirane K et al. : Derivation of Human Trophoblast Stem Cells. Cell Stem Cell 2018;22:50. [DOI] [PubMed] [Google Scholar]
- [10].Niakan KK, Eggan K: Analysis of human embryos from zygote to blastocyst reveals distinct gene expression patterns relative to the mouse. Dev Biol 2013;375:54. [DOI] [PubMed] [Google Scholar]
- [11].Petropoulos S, Edsgard D, Reinius B, Deng Q, Panula SP, Codeluppi S et al. : Single-Cell RNA-Seq Reveals Lineage and X Chromosome Dynamics in Human Preimplantation Embryos. Cell 2016;165:1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Stirparo GG, Boroviak T, Guo G, Nichols J, Smith A, Bertone P: Integrated analysis of single-cell embryo data yields a unified transcriptome signature for the human pre-implantation epiblast. Development 2018;145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Yan L, Yang M, Guo H, Yang L, Wu J, Li R et al. : Single-cell RNA-Seq profiling of human preimplantation embryos and embryonic stem cells. Nat Struct Mol Biol 2013;20:1131. [DOI] [PubMed] [Google Scholar]
- [14].Lee CQ, Gardner L, Turco M, Zhao N, Murray MJ, Coleman N et al. : What Is Trophoblast? A Combination of Criteria Define Human First-Trimester Trophoblast. Stem Cell Reports 2016;6:257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Kudo Y, Boyd CA: Changes in expression and function of syncytin and its receptor, amino acid transport system B(0) (ASCT2), in human placental choriocarcinoma BeWo cells during syncytialization. Placenta 2002;23:536. [DOI] [PubMed] [Google Scholar]
- [16].Fournier T, Guibourdenche J, Evain-Brion D: Review: hCGs: different sources of production, different glycoforms and functions. Placenta 2015;36 Suppl 1:S60. [DOI] [PubMed] [Google Scholar]
- [17].Genbacev O, Jensen KD, Powlin SS, Miller RK: In vitro differentiation and ultrastructure of human extravillous trophoblast (EVT) cells. Placenta 1993;14:463. [DOI] [PubMed] [Google Scholar]
- [18].Benchetrit H, Herman S, van Wietmarschen N, Wu T, Makedonski K, Maoz N et al. : Extensive Nuclear Reprogramming Underlies Lineage Conversion into Functional Trophoblast Stem-like Cells. Cell Stem Cell 2015;17:543. [DOI] [PubMed] [Google Scholar]
- [19].Kubaczka C, Senner CE, Cierlitza M, Arauzo-Bravo MJ, Kuckenberg P, Peitz M et al. : Direct Induction of Trophoblast Stem Cells from Murine Fibroblasts. Cell Stem Cell 2015;17:557. [DOI] [PubMed] [Google Scholar]
- [20].Turco MY, Gardner L, Kay RG, Hamilton RS, Prater M, Hollinshead MS et al. : Trophoblast organoids as a model for maternal-fetal interactions during human placentation. Nature 2018;564:263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Burton GJ, Jauniaux E: Sonographic, stereological and Doppler flow velocimetric assessments of placental maturity. Br J Obstet Gynaecol 1995;102:818. [DOI] [PubMed] [Google Scholar]
- [22].Meyer TJ, Rosenkrantz JL, Carbone L, Chavez SL: Endogenous Retroviruses: With Us and against Us. Front Chem 2017;5:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Aguilar PS, Baylies MK, Fleissner A, Helming L, Inoue N, Podbilewicz B et al. : Genetic basis of cell-cell fusion mechanisms. Trends Genet 2013;29:427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Gimenez J, Montgiraud C, Oriol G, Pichon JP, Ruel K, Tsatsaris V et al. : Comparative methylation of ERVWE1/syncytin-1 and other human endogenous retrovirus LTRs in placenta tissues. DNA Res 2009;16:195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Dupressoir A, Vernochet C, Bawa O, Harper F, Pierron G, Opolon P et al. : Syncytin-A knockout mice demonstrate the critical role in placentation of a fusogenic, endogenous retrovirus-derived, envelope gene. Proc Natl Acad Sci U S A 2009;106:12127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Chen CP, Wang KG, Chen CY, Yu C, Chuang HC, Chen H: Altered placental syncytin and its receptor ASCT2 expression in placental development and pre-eclampsia. BJOG 2006;113:152. [DOI] [PubMed] [Google Scholar]
- [27].Zhuang XW, Li J, Brost BC, Xia XY, Chen HB, Wang CX et al. : Decreased expression and altered methylation of syncytin-1 gene in human placentas associated with preeclampsia. Curr Pharm Des 2014;20:1796. [DOI] [PubMed] [Google Scholar]
- [28].Yu C, Shen K, Lin M, Chen P, Lin C, Chang GD et al. : GCMa regulates the syncytin-mediated trophoblastic fusion. J Biol Chem 2002;277:50062. [DOI] [PubMed] [Google Scholar]
- [29].Anson-Cartwright L, Dawson K, Holmyard D, Fisher SJ, Lazzarini RA, Cross JC: The glial cells missing-1 protein is essential for branching morphogenesis in the chorioallantoic placenta. Nat Genet 2000;25:311. [DOI] [PubMed] [Google Scholar]
- [30].McCaig D, Lyall F: Hypoxia upregulates GCM1 in human placenta explants. Hypertens Pregnancy 2009;28:457. [DOI] [PubMed] [Google Scholar]
- [31].Colson A, Depoix CL, Baldin P, Hubinont C, Sonveaux P, Debieve F: Hypoxia-inducible factor 2 alpha impairs human cytotrophoblast syncytialization: New insights into placental dysfunction and fetal growth restriction. FASEB J 2020. [DOI] [PubMed]
- [32].Chiang MH, Liang FY, Chen CP, Chang CW, Cheong ML, Wang LJ et al. : Mechanism of hypoxia-induced GCM1 degradation: implications for the pathogenesis of preeclampsia. J Biol Chem 2009;284:17411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Esnault C, Priet S, Ribet D, Vernochet C, Bruls T, Lavialle C et al. : A placenta-specific receptor for the fusogenic, endogenous retrovirus-derived, human syncytin-2. Proc Natl Acad Sci U S A 2008;105:17532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Dupressoir A, Vernochet C, Harper F, Guegan J, Dessen P, Pierron G et al. : A pair of co-opted retroviral envelope syncytin genes is required for formation of the two-layered murine placental syncytiotrophoblast. Proc Natl Acad Sci U S A 2011;108:E1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Sugimoto J, Sugimoto M, Bernstein H, Jinno Y, Schust D: A novel human endogenous retroviral protein inhibits cell-cell fusion. Sci Rep 2013;3:1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Kang C, Xu Q, Martin TD, Li MZ, Demaria M, Aron L et al. : The DNA damage response induces inflammation and senescence by inhibiting autophagy of GATA4. Science 2015;349:aaa5612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Kuilman T, Michaloglou C, Vredeveld LC, Douma S, van Doorn R, Desmet CJ et al. : Oncogene-induced senescence relayed by an interleukin-dependent inflammatory network. Cell 2008;133:1019. [DOI] [PubMed] [Google Scholar]
- [38].Chuprin A, Gal H, Biron-Shental T, Biran A, Amiel A, Rozenblatt S et al. : Cell fusion induced by ERVWE1 or measles virus causes cellular senescence. Genes Dev 2013;27:2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Genbacev O, McMaster MT, Fisher SJ: A repertoire of cell cycle regulators whose expression is coordinated with human cytotrophoblast differentiation. Am J Pathol 2000;157:1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Lu X, Wang R, Zhu C, Wang H, Lin HY, Gu Y et al. : Fine-Tuned and Cell-Cycle-Restricted Expression of Fusogenic Protein Syncytin-2 Maintains Functional Placental Syncytia. Cell Rep 2018;23:3979. [DOI] [PubMed] [Google Scholar]
- [41].Martinez F, Kiriakidou M, Strauss JF 3rd: Structural and functional changes in mitochondria associated with trophoblast differentiation: methods to isolate enriched preparations of syncytiotrophoblast mitochondria. Endocrinology 1997;138:2172. [DOI] [PubMed] [Google Scholar]
- [42].Wasilewski M, Semenzato M, Rafelski SM, Robbins J, Bakardjiev AI, Scorrano L: Optic atrophy 1-dependent mitochondrial remodeling controls steroidogenesis in trophoblasts. Curr Biol 2012;22:1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Gal H, Lysenko M, Stroganov S, Vadai E, Youssef SA, Tzadikevitch-Geffen K et al. : Molecular pathways of senescence regulate placental structure and function. EMBO J 2019;38:e100849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Plaks V, Rinkenberger J, Dai J, Flannery M, Sund M, Kanasaki K et al. : Matrix metalloproteinase-9 deficiency phenocopies features of preeclampsia and intrauterine growth restriction. Proc Natl Acad Sci U S A 2013;110:11109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Cox LS, Redman C: The role of cellular senescence in ageing of the placenta. Placenta 2017;52:139. [DOI] [PubMed] [Google Scholar]
- [46].Yosef R, Pilpel N, Papismadov N, Gal H, Ovadya Y, Vadai E et al. : p21 maintains senescent cell viability under persistent DNA damage response by restraining JNK and caspase signaling. EMBO J 2017;36:2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Alsat E, Wyplosz P, Malassine A, Guibourdenche J, Porquet D, Nessmann C et al. : Hypoxia impairs cell fusion and differentiation process in human cytotrophoblast, in vitro. J Cell Physiol 1996;168:346. [DOI] [PubMed] [Google Scholar]
- [48].Wakeland AK, Soncin F, Moretto-Zita M, Chang CW, Horii M, Pizzo D et al. : Hypoxia Directs Human Extravillous Trophoblast Differentiation in a Hypoxia-Inducible Factor-Dependent Manner. Am J Pathol 2017;187:767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Horii M, Li Y, Wakeland AK, Pizzo DP, Nelson KK, Sabatini K et al. : Human pluripotent stem cells as a model of trophoblast differentiation in both normal development and disease. Proc Natl Acad Sci U S A 2016;113:E3882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Treissman J, Yuan V, Baltayeva J, Le HT, Castellana B, Robinson WP et al. : Low oxygen enhances trophoblast column growth by potentiating differentiation of the extravillous lineage and promoting LOX activity. Development 2020;147. [DOI] [PubMed] [Google Scholar]
- [51].Gamage TK, Perry JJ, Fan V, Groom K, Chamley LW, James JL: Side-Population Trophoblasts Exhibit the Differentiation Potential of a Trophoblast Stem Cell Population, Persist to Term, and are Reduced in Fetal Growth Restriction. Stem Cell Rev Rep 2020;16:764. [DOI] [PubMed] [Google Scholar]
- [52].Liu Y, Fan X, Wang R, Lu X, Dang YL, Wang H et al. : Single-cell RNA-seq reveals the diversity of trophoblast subtypes and patterns of differentiation in the human placenta. Cell Res 2018;28:819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Chang WL, Liu YW, Dang YL, Jiang XX, Xu H, Huang X et al. : PLAC8, a new marker for human interstitial extravillous trophoblast cells, promotes their invasion and migration. Development 2018;145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Skorupskaite K, George JT, Veldhuis JD, Anderson RA: Neurokinin B Regulates Gonadotropin Secretion, Ovarian Follicle Growth, and the Timing of Ovulation in Healthy Women. J Clin Endocrinol Metab 2018;103:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Haider S, Meinhardt G, Saleh L, Fiala C, Pollheimer J, Knofler M: Notch1 controls development of the extravillous trophoblast lineage in the human placenta. Proc Natl Acad Sci U S A 2016;113:E7710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Lee CQE, Turco MY, Gardner L, Simons BD, Hemberger M, Moffett A: Integrin alpha2 marks a niche of trophoblast progenitor cells in first trimester human placenta. Development 2018;145. [DOI] [PMC free article] [PubMed]
- [57].Meinhardt G, Haider S, Kunihs V, Saleh L, Pollheimer J, Fiala C et al. : Pivotal role of the transcriptional co-activator YAP in trophoblast stemness of the developing human placenta. Proc Natl Acad Sci U S A 2020;117:13562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Mise H, Sagawa N, Matsumoto T, Yura S, Nanno H, Itoh H et al. : Augmented placental production of leptin in preeclampsia: possible involvement of placental hypoxia. J Clin Endocrinol Metab 1998;83:3225. [DOI] [PubMed] [Google Scholar]
- [59].Soleymanlou N, Jurisica I, Nevo O, Ietta F, Zhang X, Zamudio S et al. : Molecular evidence of placental hypoxia in preeclampsia. J Clin Endocrinol Metab 2005;90:4299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Papuchova H, Kshirsagar S, Xu L, Bougleux Gomes HA, Li Q, Iyer V et al. : Three types of HLA-G+ extravillous trophoblasts that have distinct immune regulatory properties. Proc Natl Acad Sci U S A 2020;117:15772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Kilburn BA, Wang J, Duniec-Dmuchowski ZM, Leach RE, Romero R, Armant DR: Extracellular matrix composition and hypoxia regulate the expression of HLA-G and integrins in a human trophoblast cell line. Biol Reprod 2000;62:739. [DOI] [PubMed] [Google Scholar]
- [62].Mori A, Nishi H, Sasaki T, Nagamitsu Y, Kawaguchi R, Okamoto A et al. : HLA-G expression is regulated by miR-365 in trophoblasts under hypoxic conditions. Placenta 2016;45:37. [DOI] [PubMed] [Google Scholar]
- [63].Garrido-Gomez T, Ona K, Kapidzic M, Gormley M, Simon C, Genbacev O et al. : Severe pre-eclampsia is associated with alterations in cytotrophoblasts of the smooth chorion. Development 2017;144:767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Sharma S, Godbole G, Modi D: Decidual Control of Trophoblast Invasion. Am J Reprod Immunol 2016;75:341. [DOI] [PubMed] [Google Scholar]
- [65].Woods L, Perez-Garcia V, Kieckbusch J, Wang X, DeMayo F, Colucci F et al. : Decidualisation and placentation defects are a major cause of age-related reproductive decline. Nat Commun 2017;8:352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Garrido-Gomez T, Dominguez F, Quinonero A, Diaz-Gimeno P, Kapidzic M, Gormley M et al. : Defective decidualization during and after severe preeclampsia reveals a possible maternal contribution to the etiology. Proc Natl Acad Sci U S A 2017;114:E8468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Nakashima A, Aoki A, Kusabiraki T, Shima T, Yoshino O, Cheng SB et al. : Role of autophagy in oocytogenesis, embryogenesis, implantation, and pathophysiology of pre-eclampsia. J Obstet Gynaecol Res 2017;43:633. [DOI] [PubMed] [Google Scholar]
- [68].Nakashima A, Yamanaka-Tatematsu M, Fujita N, Koizumi K, Shima T, Yoshida T et al. : Impaired autophagy by soluble endoglin, under physiological hypoxia in early pregnant period, is involved in poor placentation in preeclampsia. Autophagy 2013;9:303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Cheng SB, Nakashima A, Huber WJ, Davis S, Banerjee S, Huang Z et al. : Pyroptosis is a critical inflammatory pathway in the placenta from early onset preeclampsia and in human trophoblasts exposed to hypoxia and endoplasmic reticulum stressors. Cell Death Dis 2019;10:927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Oestreich AK, Chadchan SB, Medvedeva A, Lydon JP, Jungheim ES, Moley KH et al. : The autophagy protein, FIP200 (RB1CC1) mediates progesterone responses governing uterine receptivity and decidualizationdagger. Biol Reprod 2020;102:843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Oestreich AK, Chadchan SB, Popli P, Medvedeva A, Rowen MN, Stephens CS et al. : The Autophagy Gene Atg16L1 is Necessary for Endometrial Decidualization. Endocrinology 2020;161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Lu H, Yang HL, Zhou WJ, Lai ZZ, Qiu XM, Fu Q et al. : Rapamycin prevents spontaneous abortion by triggering decidual stromal cell autophagy-mediated NK cell residence. Autophagy 2020. [DOI] [PMC free article] [PubMed]
- [73].Tan HX, Yang SL, Li MQ, Wang HY: Autophagy suppression of trophoblast cells induces pregnancy loss by activating decidual NK cytotoxicity and inhibiting trophoblast invasion. Cell Commun Signal 2020;18:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Cai H, Chen L, Zhang M, Xiang W, Su P: Low expression of MFN2 is associated with early unexplained miscarriage by regulating autophagy of trophoblast cells. Placenta 2018;70:34. [DOI] [PubMed] [Google Scholar]
- [75].Melland-Smith M, Ermini L, Chauvin S, Craig-Barnes H, Tagliaferro A, Todros T et al. : Disruption of sphingolipid metabolism augments ceramide-induced autophagy in preeclampsia. Autophagy 2015;11:653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Kalkat M, Garcia J, Ebrahimi J, Melland-Smith M, Todros T, Post M et al. : Placental autophagy regulation by the BOK-MCL1 rheostat. Autophagy 2013;9:2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Aoki A, Nakashima A, Kusabiraki T, Ono Y, Yoshino O, Muto M et al. : Trophoblast-Specific Conditional Atg7 Knockout Mice Develop Gestational Hypertension. Am J Pathol 2018;188:2474. [DOI] [PubMed] [Google Scholar]
- [78].Lee IH, Kawai Y, Fergusson MM, Rovira II, Bishop AJ, Motoyama N et al. : Atg7 modulates p53 activity to regulate cell cycle and survival during metabolic stress. Science 2012;336:225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Muralimanoharan S, Gao X, Weintraub S, Myatt L, Maloyan A: Sexual dimorphism in activation of placental autophagy in obese women with evidence for fetal programming from a placenta-specific mouse model. Autophagy 2016;12:752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Nixon RA: The role of autophagy in neurodegenerative disease. Nat Med 2013;19:983. [DOI] [PubMed] [Google Scholar]
- [81].Nakashima A, Shima T, Tsuda S, Aoki A, Kawaguchi M, Yoneda S et al. : Disruption of Placental Homeostasis Leads to Preeclampsia. Int J Mol Sci 2020;21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Nakashima A, Cheng SB, Ikawa M, Yoshimori T, Huber WJ, Menon R et al. : Evidence for lysosomal biogenesis proteome defect and impaired autophagy in preeclampsia. Autophagy 2020;16:1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Steingrimsson E, Tessarollo L, Reid SW, Jenkins NA, Copeland NG: The bHLH-Zip transcription factor Tfeb is essential for placental vascularization. Development 1998;125:4607. [DOI] [PubMed] [Google Scholar]
- [84].Nakashima A, Cheng SB, Kusabiraki T, Motomura K, Aoki A, Ushijima A et al. : Endoplasmic reticulum stress disrupts lysosomal homeostasis and induces blockade of autophagic flux in human trophoblasts. Sci Rep 2019;9:11466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Hanna J, Goldman-Wohl D, Hamani Y, Avraham I, Greenfield C, Natanson-Yaron S et al. : Decidual NK cells regulate key developmental processes at the human fetal-maternal interface. Nat Med 2006;12:1065. [DOI] [PubMed] [Google Scholar]
- [86].Lash GE, Schiessl B, Kirkley M, Innes BA, Cooper A, Searle RF et al. : Expression of angiogenic growth factors by uterine natural killer cells during early pregnancy. J Leukoc Biol 2006;80:572. [DOI] [PubMed] [Google Scholar]
- [87].Saito S, Nishikawa K, Morii T, Enomoto M, Narita N, Motoyoshi K et al. : Cytokine production by CD16-CD56bright natural killer cells in the human early pregnancy decidua. Int Immunol 1993;5:559. [DOI] [PubMed] [Google Scholar]
- [88].King A, Loke YW: On the nature and function of human uterine granular lymphocytes. Immunol Today 1991;12:432. [DOI] [PubMed] [Google Scholar]
- [89].Giuliani E, Parkin KL, Lessey BA, Young SL, Fazleabas AT: Characterization of uterine NK cells in women with infertility or recurrent pregnancy loss and associated endometriosis. Am J Reprod Immunol 2014;72:262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Nakashima A, Shiozaki A, Myojo S, Ito M, Tatematsu M, Sakai M et al. : Granulysin produced by uterine natural killer cells induces apoptosis of extravillous trophoblasts in spontaneous abortion. Am J Pathol 2008;173:653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Kalkunte SS, Mselle TF, Norris WE, Wira CR, Sentman CL, Sharma S: Vascular endothelial growth factor C facilitates immune tolerance and endovascular activity of human uterine NK cells at the maternal-fetal interface. J Immunol 2009;182:4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Choudhury RH, Dunk CE, Lye SJ, Aplin JD, Harris LK, Jones RL: Extravillous Trophoblast and Endothelial Cell Crosstalk Mediates Leukocyte Infiltration to the Early Remodeling Decidual Spiral Arteriole Wall. J Immunol 2017;198:4115. [DOI] [PubMed] [Google Scholar]
- [93].Rajagopalan S, Long EO: Cellular senescence induced by CD158d reprograms natural killer cells to promote vascular remodeling. Proc Natl Acad Sci U S A 2012;109:20596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Sagiv A, Burton DG, Moshayev Z, Vadai E, Wensveen F, Ben-Dor S et al. : NKG2D ligands mediate immunosurveillance of senescent cells. Aging (Albany NY) 2016;8:328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Williams PJ, Bulmer JN, Searle RF, Innes BA, Robson SC: Altered decidual leucocyte populations in the placental bed in pre-eclampsia and foetal growth restriction: a comparison with late normal pregnancy. Reproduction 2009;138:177. [DOI] [PubMed] [Google Scholar]
- [96].Winship AL, Koga K, Menkhorst E, Van Sinderen M, Rainczuk K, Nagai M et al. : Interleukin-11 alters placentation and causes preeclampsia features in mice. Proc Natl Acad Sci U S A 2015;112:15928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Sasaki Y, Sakai M, Miyazaki S, Higuma S, Shiozaki A, Saito S: Decidual and peripheral blood CD4+CD25+ regulatory T cells in early pregnancy subjects and spontaneous abortion cases. Mol Hum Reprod 2004;10:347. [DOI] [PubMed] [Google Scholar]
- [98].Shima T, Nakashima A, Yasuda I, Ushijima A, Inada K, Tsuda S et al. : Uterine CD11c+ cells induce the development of paternal antigen-specific Tregs via seminal plasma priming. J Reprod Immunol 2020;141:103165. [DOI] [PubMed] [Google Scholar]
- [99].Yasuda I, Shima T, Moriya T, Ikebuchi R, Kusumoto Y, Ushijima A et al. : Dynamic Changes in the Phenotype of Dendritic Cells in the Uterus and Uterine Draining Lymph Nodes After Coitus. Front Immunol 2020;11:557720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Morita K, Tsuda S, Kobayashi E, Hamana H, Tsuda K, Shima T et al. : Analysis of TCR Repertoire and PD-1 Expression in Decidual and Peripheral CD8(+) T Cells Reveals Distinct Immune Mechanisms in Miscarriage and Preeclampsia. Front Immunol 2020;11:1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Tsuda S, Zhang X, Hamana H, Shima T, Ushijima A, Tsuda K et al. : Clonally Expanded Decidual Effector Regulatory T Cells Increase in Late Gestation of Normal Pregnancy, but Not in Preeclampsia, in Humans. Front Immunol 2018;9:1934. [DOI] [PMC free article] [PubMed] [Google Scholar]


