Abstract
Background
The anti-tumor activity and acceptable tolerability of pyrotinib plus chemotherapy have been demonstrated in phase III trials in human epidermal growth factor receptor 2-positive metastatic breast cancer (BC). In this study, we assessed the efficacy and safety of neoadjuvant pyrotinib plus trastuzumab and albumin-bound paclitaxel in women with human epidermal growth factor receptor 2-positive early or locally advanced BC.
Methods
In this single-arm exploratory phase II trial, patients with untreated human epidermal growth factor receptor 2-positive BC (stage IIA–IIIC) received pyrotinib 400 mg once daily, trastuzumab 4 mg/kg loading dose, followed by 2 mg/kg once a week, and albumin-bound paclitaxel 125 mg/m2 once a week for four 21-day cycles before surgery. The primary endpoint of the study was total pathological complete response (pCR) rate, defined as no microscopic invasive tumor remnants in the breast and axillary lymph nodes. The secondary endpoints were investigator-assessed objective response rate (ORR) and adverse event profiles.
Results
Between May 17, 2019 and November 26, 2019, a total of 21 patients were enrolled. The total pCR rate was 57.1% (12/21), whereas 23.8% (5/21) and 19.0% (4/21) of patients had minimal and moderate residual disease (RD), respectively. The ORR reached 100% (21/21) at the end of the neoadjuvant therapy. Grade ≥3 treatment-related adverse events were observed in 42.9% (9/21) of patients, including decreased neutrophil count [7 (33.3%)], diarrhoea [6 (28.6%)], decreased white blood cell count [5 (23.8%)], and vomiting [2 (9.5%)]. Adverse event-related dose reduction and interruption of pyrotinib occurred in 6 (28.6%) and 11 (52.4%) patients, respectively.
Conclusions
In women with human epidermal growth factor receptor 2-positive early or locally advanced BC, neoadjuvant pyrotinib plus trastuzumab and albumin-bound paclitaxel effectively promoted total pCR rate with an acceptable safety profile (ClinicalTrials.gov, NCT04152057).
Keywords: Neoadjuvant therapy, pyrotinib, trastuzumab, albumin-bound paclitaxel, human epidermal growth factor receptor 2-positive breast cancer
Introduction
Anti-human epidermal growth factor receptor 2 (HER2)-targeted therapy is an important treatment strategy to improve the efficacy and prognosis of HER2-positive (HER2+) breast cancer (BC) patients (1,2). According to NCCN (National Comprehensive Cancer Network) Clinical Practice Guidelines in Oncology Breast Cancer, neoadjuvant therapy is recommended for patients with HER2+ non-metastatic BC if cT ≥2 or cN ≥1 (3). For locally advanced HER2+ BC patients, residual tumor after neoadjuvant chemotherapy results in a higher risk of recurrence and death; therefore, it is particularly important to tailor the preoperative systematic treatment (4,5). Trastuzumab is a recombinant humanized monoclonal antibody targeting the HER2, which was approved by the FDA for HER2-positive metastatic BC in 1998 (6,7). The NOAH study confirmed, for the first time, that for HER2+ early BC patients, the addition of trastuzumab on the basis of neoadjuvant chemotherapy can significantly improve the pathological complete response (pCR) rate, and the risk of postoperative recurrence in patients with pCR is significantly lower than that in patients without pCR (8,9).
The NeoSphere (10) and PEONY (11) studies showed that chemotherapy combined with trastuzumab and pertuzumab further improves the pCR rate of HER2+ BC patients. Both NCCN guidelines and CSCO (Chinese Society Of Clinical Oncology) guidelines recommend taxane in combination with trastuzumab and pertuzumab as standard treatment for HER2+ BC (3,12).
The NeoALTTO study confirmed that neoadjuvant therapy with the small molecule tyrosine kinase inhibitor (TKI), lapatinib, combined with trastuzumab can induce a higher pCR rate than that with trastuzumab alone (13,14). With the emergence of novel anti-HER2-targeted drugs, better combinations need to be explored as neoadjuvant therapies for HER2+ BC.
Pyrotinib is an oral, irreversible pan-ErbB receptor TKI that targets HER1, HER2, and HER4 (15,16). In the phase III PHENIX (17) and phase III PHOEBE (18) studies, pyrotinib combined with capecitabine showed significantly improved efficacy in HER2+ metastatic BC patients. Whether pyrotinib has the potential to exhibit improved efficacy in the neoadjuvant setting is unclear. In 2020, Xuhong et al. (19) firstly reported that in patients with HER2+ locally advanced BC, the adoption of a novel neoadjuvant regimen of eight 3-week cycles of pyrotinib in combination with four cycles of epirubicin and cyclophosphamide, followed by four cycles of docetaxel and trastuzumab (Py + EC-TH) resulted in a total pCR (tpCR) rate of 73.7% (14/19), and its safety was controllable.
For combination chemotherapy, a new generation albumin-bound paclitaxel formulation, which is devoid of any additional excipients, has solved the problem of paclitaxel solubility (20). The GBG69 study results suggested that for early BC patients who received neoadjuvant chemotherapy, nanoparticle albumin-bound paclitaxel significantly improved the pCR rate and reduced the recurrence risk compared with solvent-based paclitaxel (21,22).
This phase II trial aimed to preliminarily explore the efficacy and safety of a new dual-targeted neoadjuvant regimen of pyrotinib plus trastuzumab and albumin-bound paclitaxel in the treatment of HER2+ early or locally advanced BC. The value of therapeutic markers in the proportion of primary tumor infiltrating lymphocytes (TILs) was preliminarily analyzed to provide evidence and a basis for further phase III clinical trial. Compared with the published report, this study was innovative in neoadjuvant regimen design, using albumin-bound paclitaxel as combined chemotherapy and only 4 cycles, which may achieve a quick and better effect. We present the following article in accordance with the TREND reporting checklist (available at https://gs.amegroups.com/article/view/10.21037/gs-21-911/rc).
Methods
Study design
This was a single-arm, open-label phase II trial conducted at the West China Hospital of Sichuan University. The trial was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Clinical Trial Ethics Committee of West China Hospital [approval number: 2019(No.2)] and was registered with ClinicalTrials.gov (NCT04152057). All patients provided written informed consent for study participation.
Patient population
The key inclusion criteria were as follows: (I) patients aged 18–70 years at initial treatment; (II) those with clinical BC stage of II–III according to the criteria of American Joint Committee on Cancer; (III) HER2 immunohistochemical staining intensity of 3+, or 2+ with HER2 gene amplification confirmed by fluorescence in situ hybridization; (IV) Eastern Cooperative Oncology Group (ECOG) performance status of 0–1; and (V) at least one measurable lesion according to the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1.
The key exclusion criteria were as follows: (I) patients with inflammatory BC; (II) those with metastatic BC (stage IV); (III) patients who had undergone previous radiotherapy, chemotherapy, or major surgery for BC, molecular targeted therapy within 4 weeks before enrolment, or previous endocrine therapy within 7 days before enrolment; and (IV) those who had received current or previous use of HER2 targeted monoclonal antibodies or TKIs (including trastuzumab, pertuzumab, lapatinib, neratinib, and pyrotinib).
Study treatment and procedures
The patients received oral pyrotinib 400 mg once daily, intravenous trastuzumab 4 mg/kg loading dose, followed by 2 mg/kg on days 1, 8, and 15, and intravenous albumin-bound paclitaxel 125 mg/m2 on days 1, 8, and 15 for four 21-day cycles before surgery. The dose of drugs was adjusted according to the adverse events (AEs). The dose of pyrotinib was adjusted according a gradient of 400, 320, and 240 mg. The cumulative interruption time of pyrotinib in each cycle was expected not to exceed 14 days to ensure the drug intensity of the treatment.
After surgery, patients received adjuvant therapy with epirubicin 90–100 mg/m2 plus cyclophosphamide 600 mg/m2 once every 3 weeks for four cycles combined with an anti-HER2 targeted drug selected by the investigator. Endocrine therapy was ideally started at the end of adjuvant chemotherapy for patients with hormone receptor (HR)-positive BC. Patients with clinical indications at the end of adjuvant chemotherapy were administered with radiotherapy.
The efficacy of the neoadjuvant therapy was evaluated once every two cycles (6 weeks). After four cycles of the neoadjuvant therapy, a comprehensive review was conducted, and the AEs were evaluated once a week until at least 28 days after the end of the neoadjuvant therapy.
Study endpoints and assessments
The primary endpoint was the tpCR rate, defined as no microscopic invasive tumor remnants in the breast and axillary lymph nodes, although ductal carcinoma in situ may exist (ypT0/Tis ypN0). The secondary endpoints were investigator-assessed objective response rate (ORR), which referred to the proportion of patients with the best response of complete response (CR) or partial response (PR) to the neoadjuvant therapy, and safety. The RECIST 1.1 criteria were used to assess tumor response, which was divided into CR, PR, stable disease (SD), and progressive disease (PD). The residual cancer burden (RCB) was simultaneously measured as a continuous variable derived from the primary tumor dimensions, cellularity of the tumor bed, and axillary nodal burden. The RCB score was divided into the following four levels according to standard of the University of Texas MD Anderson Cancer Center: RCB-0 (pCR): 0, RCB-I [minimal residual disease (RD)]: >0 to 1.36, RCB-II (moderate RD): >1.36 to 3.28, RCB-III (extensive RD): >3.28 (23). AEs were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.0.
TILs
TILs are heterogeneous lymphocyte populations dominated by lymphocytes in the tumor nests and stroma, which are considered to be a marker of anti-tumor immunity. Based on the recommendations of the International TILs Working Group 2014 (24), the percentage of TILs in primary tumor puncture tissues was detected using hematoxylin and eosin (H&E) staining, and the score was determined.
Statistical analysis
The analysis population of this study consisted of participants in the intent-to-treat (ITT) population. The results of this study were analyzed using descriptive statistical methods. The continuous data were presented as means ± standard deviation or medians (range), and the categorical data were presented as frequency, percentage, and 95% confidence intervals (CIs). The rank-sum test was used to compare the difference in TIL levels between patients with different outcomes. All statistical analyses were conducted using SPSS 22.0 (IBM Company, Armonk, NY, USA). All statistical tests were performed using two-sided tests. A P value ≤0.05 was considered statistically significant.
Results
Patient characteristics
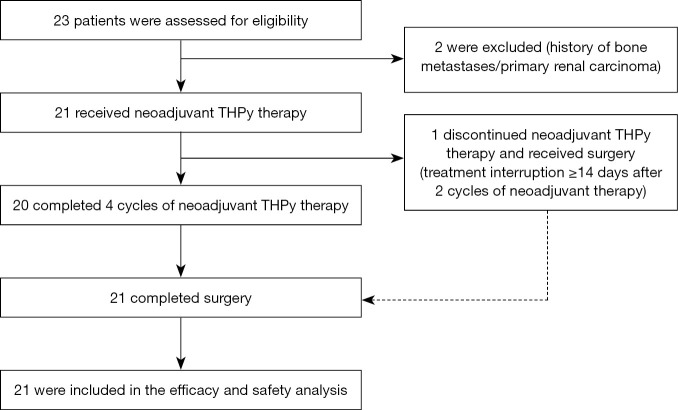
From May 17, 2019 to November 26, 2019, a total of 23 patients were screened and 21 were subsequently enrolled (Figure 1). The median follow-up time was 14.9 (range, 11.9–17.9) months and 20 (95.2%) patients completed the entire neoadjuvant therapy. After two cycles of neoadjuvant therapy, one patient withdrew from the trial more than 14 days after discontinuing the drug in cycle 3. After leaving the trial, the patient continued pyrotinib plus trastuzumab treatment and surgery. All 21 patients were included in the data analysis of efficacy and safety.
Figure 1.
Study flowchart. THPy, pyrotinib plus trastuzumab and albumin-bound paclitaxel.
The baseline characteristics of the participants are shown in Table 1. The median age was 48 years (range, 28–57 years). The proportion of patients with T4 disease was 52.4% (11/21), the proportion of patients with N1–3 disease was 90.5% (19/21), and the proportion of patients with clinical stage III disease was 81.0% (17/21). The proportion of HR-negative patients was 61.9% (13/21), and 71.4% (15/21) of patients had high proliferative index Ki-67 ≥30%.
Table 1. Clinical and pathological characteristics of the included patients at baseline.
| Characteristic | Patients (n=21) |
|---|---|
| Age (years), median [range] | 48 [28–57] |
| Clinical tumor stage, n (%) | |
| T1 | 2 (9.5) |
| T2 | 6 (28.6) |
| T3 | 2 (9.5) |
| T4 | 11 (52.4) |
| Clinical lymph node status, n (%) | |
| N0 | 2 (9.5) |
| N1 | 6 (28.6) |
| N2 | 8 (38.1) |
| N3 | 5 (23.8) |
| Clinical stage, n (%) | |
| II | 4 (19.0) |
| III | 17 (81.0) |
| ECOG performance status, n (%) | |
| 0 | 21 (100.0) |
| 1 | 0 |
| Histological type, n (%) | |
| Invasive ductal carcinoma | 20 (95.2) |
| Paget’s disease | 1 (4.8) |
| Hormone receptor status, n (%) | |
| ER and/or PgR positive | 8 (38.1) |
| ER and PgR negative | 13 (61.9) |
| Ki-67 level, n (%) | |
| <30% | 5 (23.8) |
| ≥30% | 15 (71.4) |
| Unknown | 1 (4.8) |
ER, estrogen receptor; PgR, progestogen receptor.
Efficacy
Postoperative pathological assessment showed that 57.1% (12/21) of the total population achieved tpCR (Table 2). The tpCR rate of the HR-negative subgroup was higher than that of the HR-positive subgroup (69.2% vs. 37.5%), but the difference was not statistically significant (P=0.203). There were 12 (57.1%), 5 (23.8%), and 4 (19.0%) patients with RCB-0, -I, and -II, whereas no patient had RCB-III (Table S1).
Table 2. Pathological response in the ITT population, and by HR status at surgery.
| ITT (n=21) | HR positive (n=8) | HR negative (n=13) | ||||||
|---|---|---|---|---|---|---|---|---|
| n | % (95% CI) | n | % (95% CI) | n | % (95% CI) | |||
| tpCR | 12 | 57.1 (36.0–78.3) | 3 | 37.5 (4.0–71.1) | 9 | 69.2 (44.1–94.3) | ||
ITT, intent-to-treat; HR, hormone receptor; tpCR, total pathological complete response; CI, confidence interval.
The image evaluation (Figure 2) showed that after four cycles of neoadjuvant therapy, the ORR was 100%, including 1 (4.8%) patient with CR and 20 (95.2%) patients with PR. The median regression rate of target lesions in the first two cycles, last two cycles, and in total was 47.9% (range, 33.3–87.5%), 5.6% (range, 0–33.3%), 57.9% (range, 42.3–95.7%), respectively. It is worth noting that 100% of the patients achieved PR after two cycles of neoadjuvant therapy, and the median optimal regression rate of target lesions was 47.9% (range, 33.3–87.5%).
Figure 2.
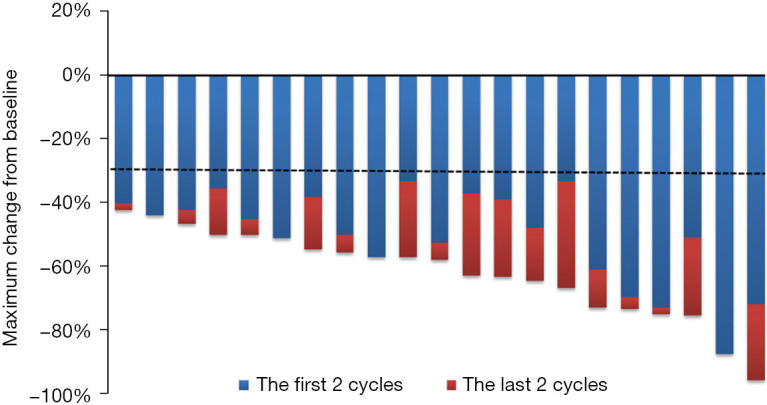
Maximum reduction of target lesions from baseline in the intent-to-treat population.
Safety
Twenty patients completed four cycles of the neoadjuvant chemotherapy with a median duration of 2.7 months (range, 2.6–3.1 months). The safety of the neoadjuvant regimen was analyzed in all 21 patients. Treatment-related AEs (TRAEs) with an incidence of ≥10% are listed in Table 3. All patients who received the neoadjuvant therapy had some level of TRAEs, with the most common being diarrhoea (100%), decreased neutrophil count (81.0%), decreased white blood cell count (81.0%), numbness of hands and feet (81.0%), fatigue (71.4%), vomiting (66.7%), rash (57.1%), and nausea (52.4%).
Table 3. Treatment-related adverse events occurred in ≥10% of patients who received neoadjuvant therapy.
| Adverse event | Patients (n=21), n (%) | ||
|---|---|---|---|
| Any grade | Grade 3 | Grade 4 | |
| Diarrhea | 21 (100.0) | 6 (28.6) | 0 |
| Neutrophil count decreased | 17 (81.0) | 6 (28.6) | 1 (4.8) |
| WBC count decreased | 17 (81.0) | 5 (23.8) | 0 |
| Hand and foot numbness | 17 (81.0) | 0 | 0 |
| Fatigue | 15 (71.4) | 0 | 0 |
| Vomiting | 14 (66.7) | 2 (9.5) | 0 |
| Rash | 12 (57.1) | 0 | 0 |
| Nausea | 11 (52.4) | 0 | 0 |
| Positive fecal occult blood test | 10 (47.6) | 0 | 0 |
| ALT increased | 9 (42.9) | 0 | 0 |
| AST increased | 9 (42.9) | 0 | 0 |
| Oral mucositis | 9 (42.9) | 0 | 0 |
| Anemia | 8 (38.1) | 0 | 0 |
| Hand-foot syndrome | 6 (28.6) | 0 | 0 |
| Loss of appetite | 5 (23.8) | 0 | 0 |
| Blood bilirubin increased | 4 (19.0) | 0 | 0 |
| Creatinine increased | 4 (19.0) | 0 | 0 |
| Flatulence | 4 (19.0) | 0 | 0 |
| Nasal mucosa bleeding | 4 (19.0) | 0 | 0 |
| Skin pruritus | 3 (14.3) | 0 | 0 |
| Stomachache | 3 (14.3) | 0 | 0 |
WBC, white blood cell; ALT, alanine transaminase; AST, aspartate transaminase.
Nine (42.9%) patients had ≥ grade 3 TRAEs, including diarrhoea (28.6%), decreased neutrophil count (33.3%), decreased white blood cell count (23.8%), and vomiting (9.5%). Only one patient had grade 4 decreased neutrophil count, and no agranulocytic fever or cardiotoxic events were observed. Figure 3 lists the occurrence of common AEs according to the number of cycles of neoadjuvant therapy. Diarrhoea and decreased neutrophil count at any level occurred in cycles 1–4. It should be noted that grade ≥3 diarrhoea and grade ≥3 decreased neutrophil count all occurred in cycles 1–2.
Figure 3.
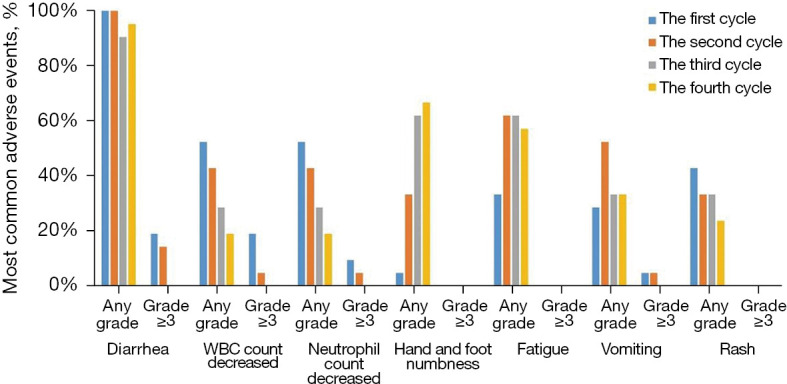
Most common adverse events presented by treatment cycles. The incidence of different grades of adverse events was assessed in each neoadjuvant therapy cycle. WBC, white blood cell.
Table 4 shows that 6 (28.6%) cases of pyrotinib dose adjustment and 11 (52.4%) cases of pyrotinib interruption were caused by AEs, and the median cumulative interruption time was 7 days (ranges, 1–24 days). There were 7 (33.3%) and 8 (38.1%) patients with delayed treatment with trastuzumab and albumin-bound paclitaxel due to AEs, respectively.
Table 4. Influence of AEs on the completion of neoadjuvant therapy.
| Treatment plan adjustment | Patients (n=21) |
|---|---|
| Dose reduction due to AEs, n (%) | |
| Pyrotinib | 6 (28.6) |
| Trastuzumab | 0 |
| Albumin-bound paclitaxel | 0 |
| Treatment interruption due to AEs, n (%) | |
| Pyrotinib† | 11 (52.4) |
| Treatment delay due to AEs‡, n (%) | |
| Trastuzumab | 7 (33.3) |
| Albumin-bound paclitaxel | 8 (38.1) |
†, the median cumulative interruption time of pyrotinib was 7 days (range, 1–24 days); ‡, these delays were considered to be related to AEs caused by weekly albumin-bound paclitaxel treatment. AEs, adverse events.
Correlation between TILs and pCR or RCB
We evaluated the TIL proportion of puncture samples of the primary tumor lesions in 20 patients, while one patient with Paget’s disease was not evaluated. The rank-sum test showed that there was no significant difference in the proportion of primary tumor TILs in the pCR and non-pCR subgroups after neoadjuvant therapy (14.3% vs. 11.1%, P=0.305) (Figure S1A). Similarly, no difference in the TIL proportion was found among the three subgroups at the RCB-0, -I, and -II levels (14.3% vs. 4.3% vs. 18.0%, P=0.442) (Figure S1B). The HE staining images of TILs in representative cases were showed as Figure S2.
Discussion
This study provides preliminary evidence for the usefulness of neoadjuvant therapy with pyrotinib in HER2+ early or locally advanced BC. The data showed that the new dual-targeted regimen of pyrotinib and trastuzumab combined with albumin-bound paclitaxel chemotherapy, exhibited pCR with controllable safety and good tolerance in more than half of the patients (57.1%). The mechanisms of pyrotinib and trastuzumab are different. Trastuzumab binds to the extracellular domain of the transmembrane HER2 receptor to induce its internalization and degradation. This effect inhibits the associated downstream signalling via the RAS/mitogen-activated protein kinase (MAPK) and phosphoinositide 3-kinase (PI3K)/AKT pathways, ultimately suppressing cellular growth and proliferation signalling. The binding of trastuzumab to the HER2 receptor also induces antibody-dependent cellular cytotoxicity (25). In contrast, pyrotinib competes with tyrosine kinase to bind to the HER2 intracellular kinase domain, where it inhibits the phosphorylation of tyrosine residues and blocks downstream signalling pathways (26). Both agents may have synergistic anti-HER2 effects in the extracellular and intracellular space.
Given that this was a single-arm study, we horizontally compared the trial design and efficacy of the dual-targeted neoadjuvant therapy. The NeoSphere (10) and PEONY (11) research group co-administered docetaxel with trastuzumab and pertuzumab for a four-cycle regimen, and the tpCR rates were both 39.3%. The WSG TP-II study was reported during the 2020 congress of the American Society of Clinical Oncology (ASCO); paclitaxel combined with trastuzumab and pertuzumab as a dual-targeted therapy was infused continuously for 12 weeks, and the pCR rate was high (56.9%). Regarding neoadjuvant studies of pyrotinib, a phase I study included 31 patients with HER2+ locally advanced BC (stage III disease was 35.5%, with a 74.2% rate of positive lymph nodes). After treatment with six cycles of dual-targeted therapy with pyrotinib plus trastuzumab combined with docetaxel plus carboplatin (three-week regimen), the tpCR rate was 51.6%. Similarly, a phase II study reported the results of 20 patients with HER2+ locally advanced BC, and the proportion of patients with stage III disease was 10%, with a 65% rate of positive lymph nodes (19). Nineteen patients were evaluated after eight cycles of pyrotinib combined with EC-TH (3-week regimen), and the tpCR rate was 73.7% (19).
In contrast to the abovementioned study, patients in the present study had the characteristics of late stage disease and a high tumor burden, and the proportion of those in stage III was as high as 81.0%, with a 90.5% rate of positive lymph nodes. Furthermore, fewer cycles of neoadjuvant therapy were administered in this study than in the above two studies. However, weekly treatment with pyrotinib plus trastuzumab and albumin-bound paclitaxel for 12 consecutive weeks still achieved a tpCR rate of 57.1%. It is worth noting that the regimen had a rapid onset, and all patients reached a PR after two cycles, which is very important to increase the confidence of patients and improve compliance.
Previous clinical studies have shown that the main AE of pyrotinib is diarrhoea, and its safety and tolerability are good (27,28). In the two aforementioned neoadjuvant studies of pyrotinib, the incidence of diarrhea, as the most common AE, was approximately 90%, whereas grade ≥3 diarrhea occurred in 64.5% and 45% of patients, respectively. The incidence of grade ≥3 diarrhoea in our study was 28.6%, which was lower than that of studies mentioned above and was consistent with the data of the PHENIX (30.8%) (17) and PHOEBE (30.6%) (18) studies of advanced patients. Also, the incidence of grade ≥3 decreased neutrophil count was 33.3%, which was higher than that of the previous two studies of pyrotinib, but slightly lower than that of the NeoSphere (45%) (10) and PEONY (38.1%) (11) studies. Decreased neutrophil count may be related to albumin-bound paclitaxel weekly chemotherapy (29). In this study, diarrhea was alleviated after treatment with montmorillonite powder or Imodium, whereas the decreased neutrophil count returned to normal levels after granulocyte colony stimulating factor therapy. Therefore, the AEs of this novel neoadjuvant regimen can be adequately controlled.
Presently, there is a lack of reliable markers to predict the pCR of neoadjuvant therapy. Different molecular types of BC may have different predictive values for the efficacy of TILs (30). Several studies have confirmed that HER2+ BC patients who were co-administered with neoadjuvant chemotherapy and HER2-targeted therapy (trastuzumab combined with pertuzumab or lapatinib) and had a high TIL baseline level before treatment exhibited an increased pCR rate (31,32). In the neoadjuvant study of pyrotinib by Xuhong et al. (19), a trend of a higher tpCR rate was observed in the group with high levels of TILs. However, this study did not observe a correlation between the proportion of TILs in primary BC and neoadjuvant efficacy. There were some limitations of this study that are worth mentioning. Firstly, this study included a limited sample size from a single center. Also, the correlation between TILs phenotype and the tpCR rate was not analyzed.
Our study suggested pyrotinib plus trastuzumab and nab-paclitaxel as a promising neoadjuvant regimen for HER2+ early or locally advanced BC. Its advantages were as follows: relatively shorter treatment period, dual-target therapy throughout the treatment period, rapid objective remission and tolerable adverse reactions with no Grage IV adverse events.
Conclusions
The results of this phase II trial suggest that pyrotinib and trastuzumab combined with albumin-bound paclitaxel chemotherapy can lead to a higher tpCR rate and ORR for patients with HER2+ early or locally advanced BC following a relatively short administration of four cycles. Furthermore, the AEs were tolerable and the safety was controllable. This study provides evidence to support the co-administration of pyrotinib with neoadjuvant therapy in BC patients, and this investigation is worthy of further expansion to clinical phase III studies.
Acknowledgments
We thank the patients who participated in this study and the contributions of research staff. Jiangsu Hengrui Pharmaceuticals Co., Ltd. provided pyrotinib, trastuzumab, and albumin-bound paclitaxel for the study.
Funding: This work was supported by the 135 Projects for Disciplines of Excellence, West China Hospital, Sichuan University (grant number ZYGD18012).
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The trial was conducted in accordance with the Declaration of Helsinki (as revised in 2013), and was approved by Clinical Trial Ethics Committee of West China Hospital [approval number: 2019(No.2)]. Informed consent was obtained from all individual participants.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
Reporting Checklist: The authors have completed the TREND reporting checklist. Available at https://gs.amegroups.com/article/view/10.21037/gs-21-911/rc
Data Sharing Statement: Available at https://gs.amegroups.com/article/view/10.21037/gs-21-911/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://gs.amegroups.com/article/view/10.21037/gs-21-911/coif). All authors report that Jiangsu Hengrui Pharmaceuticals Co., Ltd. provided pyrotinib, trastuzumab, and albumin-bound paclitaxel for the study, and the study received funding from the 135 Projects for Disciplines of Excellence, West China Hospital, Sichuan University (grant number ZYGD18012). The authors have no other conflicts of interest to declare.
(English Language Editor: A. Kassem)
References
- 1.Pernas S, Barroso-Sousa R, Tolaney SM. Optimal treatment of early stage HER2-positive breast cancer. Cancer 2018;124:4455-66. 10.1002/cncr.31657 [DOI] [PubMed] [Google Scholar]
- 2.Pondé N, Brandão M, El-Hachem G, et al. Treatment of advanced HER2-positive breast cancer: 2018 and beyond. Cancer Treat Rev 2018;67:10-20. 10.1016/j.ctrv.2018.04.016 [DOI] [PubMed] [Google Scholar]
- 3.NCCN Clinical Practice Guidelines in Oncology-Invasive Breast Cancer (2022 Version II). Available online: http://www.nccn.org
- 4.Choong GM, Cullen GD, O'Sullivan CC. Evolving standards of care and new challenges in the management of HER2-positive breast cancer. CA Cancer J Clin 2020;70:355-74. 10.3322/caac.21634 [DOI] [PubMed] [Google Scholar]
- 5.Pusztai L, Foldi J, Dhawan A, et al. Changing frameworks in treatment sequencing of triple-negative and HER2-positive, early-stage breast cancers. Lancet Oncol 2019;20:e390-6. 10.1016/S1470-2045(19)30158-5 [DOI] [PubMed] [Google Scholar]
- 6.Baselga J, Tripathy D, Mendelsohn J, et al. Phase II study of weekly intravenous recombinant humanized anti-p185HER2 monoclonal antibody in patients with HER2/neu-overexpressing metastatic breast cancer. J Clin Oncol 1996;14:737-44. 10.1200/JCO.1996.14.3.737 [DOI] [PubMed] [Google Scholar]
- 7.Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med 2001;344:783-92. 10.1056/NEJM200103153441101 [DOI] [PubMed] [Google Scholar]
- 8.Gianni L, Eiermann W, Semiglazov V, et al. Neoadjuvant chemotherapy with trastuzumab followed by adjuvant trastuzumab versus neoadjuvant chemotherapy alone, in patients with HER2-positive locally advanced breast cancer (the NOAH trial): a randomised controlled superiority trial with a parallel HER2-negative cohort. Lancet 2010;375:377-84. 10.1016/S0140-6736(09)61964-4 [DOI] [PubMed] [Google Scholar]
- 9.Gianni L, Eiermann W, Semiglazov V, et al. Neoadjuvant and adjuvant trastuzumab in patients with HER2-positive locally advanced breast cancer (NOAH): follow-up of a randomised controlled superiority trial with a parallel HER2-negative cohort. Lancet Oncol 2014;15:640-7. 10.1016/S1470-2045(14)70080-4 [DOI] [PubMed] [Google Scholar]
- 10.Gianni L, Pienkowski T, Im YH, et al. 5-year analysis of neoadjuvant pertuzumab and trastuzumab in patients with locally advanced, inflammatory, or early-stage HER2-positive breast cancer (NeoSphere): a multicentre, open-label, phase 2 randomised trial. Lancet Oncol 2016;17:791-800. 10.1016/S1470-2045(16)00163-7 [DOI] [PubMed] [Google Scholar]
- 11.Shao Z, Pang D, Yang H, et al. Efficacy, Safety, and Tolerability of Pertuzumab, Trastuzumab, and Docetaxel for Patients With Early or Locally Advanced ERBB2-Positive Breast Cancer in Asia: The PEONY Phase 3 Randomized Clinical Trial. JAMA Oncol 2020;6:e193692. 10.1001/jamaoncol.2019.3692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Breast Cancer Guidelines of Chinese Society of Clinical Oncology (CSCO) (2021). Available online: http://www.csco.org.cn/.
- 13.Baselga J, Bradbury I, Eidtmann H, et al. Lapatinib with trastuzumab for HER2-positive early breast cancer (NeoALTTO): a randomised, open-label, multicentre, phase 3 trial. Lancet 2012;379:633-40. 10.1016/S0140-6736(11)61847-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Azambuja E, Holmes AP, Piccart-Gebhart M, et al. Lapatinib with trastuzumab for HER2-positive early breast cancer (NeoALTTO): survival outcomes of a randomised, open-label, multicentre, phase 3 trial and their association with pathological complete response. Lancet Oncol 2014;15:1137-46. 10.1016/S1470-2045(14)70320-1 [DOI] [PubMed] [Google Scholar]
- 15.Li X, Yang C, Wan H, et al. Discovery and development of pyrotinib: A novel irreversible EGFR/HER2 dual tyrosine kinase inhibitor with favorable safety profiles for the treatment of breast cancer. Eur J Pharm Sci 2017;110:51-61. 10.1016/j.ejps.2017.01.021 [DOI] [PubMed] [Google Scholar]
- 16.Blair HA. Pyrotinib: First Global Approval. Drugs 2018;78:1751-5. 10.1007/s40265-018-0997-0 [DOI] [PubMed] [Google Scholar]
- 17.Yan M, Bian L, Hu X, et al. Pyrotinib plus capecitabine for human epidermal growth factor receptor 2-positive metastatic breast cancer after trastuzumab and taxanes (PHENIX): a randomized, double-blind, placebo-controlled phase 3 study. Transl Breast Cancer Res 2020;1:13. 10.21037/tbcr-20-25 [DOI] [Google Scholar]
- 18.Xu B, Yan M, Ma F, et al. Pyrotinib plus capecitabine versus lapatinib plus capecitabine for the treatment of HER2-positive metastatic breast cancer (PHOEBE): a multicentre, open-label, randomised, controlled, phase 3 trial. Lancet Oncol 2021;22:351-60. 10.1016/S1470-2045(20)30702-6 [DOI] [PubMed] [Google Scholar]
- 19.Xuhong J, Qi X, Tang P, et al. Neoadjuvant Pyrotinib plus Trastuzumab and Chemotherapy for Stage I-III HER2-Positive Breast Cancer: A Phase II Clinical Trial. Oncologist 2020;25:e1909-20. 10.1002/onco.13546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gardner ER, Dahut WL, Scripture CD, et al. Randomized crossover pharmacokinetic study of solvent-based paclitaxel and nab-paclitaxel. Clin Cancer Res 2008;14:4200-5. 10.1158/1078-0432.CCR-07-4592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Untch M, Jackisch C, Schneeweiss A, et al. Nab-paclitaxel versus solvent-based paclitaxel in neoadjuvant chemotherapy for early breast cancer (GeparSepto-GBG 69): a randomised, phase 3 trial. Lancet Oncol 2016;17:345-56. 10.1016/S1470-2045(15)00542-2 [DOI] [PubMed] [Google Scholar]
- 22.Untch M, Jackisch C, Schneeweiss A, et al. NAB-Paclitaxel Improves Disease-Free Survival in Early Breast Cancer: GBG 69-GeparSepto. J Clin Oncol 2019;37:2226-34. 10.1200/JCO.18.01842 [DOI] [PubMed] [Google Scholar]
- 23.Symmans WF, Peintinger F, Hatzis C, et al. Measurement of residual breast cancer burden to predict survival after neoadjuvant chemotherapy. J Clin Oncol 2007;25:4414-22. 10.1200/JCO.2007.10.6823 [DOI] [PubMed] [Google Scholar]
- 24.Kojima YA, Wang X, Sun H, et al. Reproducible evaluation of tumor-infiltrating lymphocytes (TILs) using the recommendations of International TILs Working Group 2014. Ann Diagn Pathol 2018;35:77-9. 10.1016/j.anndiagpath.2018.05.007 [DOI] [PubMed] [Google Scholar]
- 25.Kreutzfeldt J, Rozeboom B, Dey N, et al. The trastuzumab era: current and upcoming targeted HER2+ breast cancer therapies. Am J Cancer Res 2020;10:1045-67. [PMC free article] [PubMed] [Google Scholar]
- 26.Xuhong JC, Qi XW, Zhang Y, et al. Mechanism, safety and efficacy of three tyrosine kinase inhibitors lapatinib, neratinib and pyrotinib in HER2-positive breast cancer. Am J Cancer Res 2019;9:2103-19. [PMC free article] [PubMed] [Google Scholar]
- 27.Li Q, Guan X, Chen S, et al. Safety, Efficacy, and Biomarker Analysis of Pyrotinib in Combination with Capecitabine in HER2-Positive Metastatic Breast Cancer Patients: A Phase I Clinical Trial. Clin Cancer Res 2019;25:5212-20. 10.1158/1078-0432.CCR-18-4173 [DOI] [PubMed] [Google Scholar]
- 28.Ma F, Li Q, Chen S, et al. Phase I Study and Biomarker Analysis of Pyrotinib, a Novel Irreversible Pan-ErbB Receptor Tyrosine Kinase Inhibitor, in Patients With Human Epidermal Growth Factor Receptor 2-Positive Metastatic Breast Cancer. J Clin Oncol 2017;35:3105-12. 10.1200/JCO.2016.69.6179 [DOI] [PubMed] [Google Scholar]
- 29.Kuwayama T, Nakamura S, Hayashi N, et al. Randomized Multicenter Phase II Trial of Neoadjuvant Therapy Comparing Weekly Nab-paclitaxel Followed by FEC With Docetaxel Followed by FEC in HER2- Early-stage Breast Cancer. Clin Breast Cancer 2018;18:474-80. 10.1016/j.clbc.2018.06.012 [DOI] [PubMed] [Google Scholar]
- 30.Stanton SE, Disis ML. Clinical significance of tumor-infiltrating lymphocytes in breast cancer. J Immunother Cancer 2016;4:59. 10.1186/s40425-016-0165-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hwang HW, Jung H, Hyeon J, et al. A nomogram to predict pathologic complete response (pCR) and the value of tumor-infiltrating lymphocytes (TILs) for prediction of response to neoadjuvant chemotherapy (NAC) in breast cancer patients. Breast Cancer Res Treat 2019;173:255-66. 10.1007/s10549-018-4981-x [DOI] [PubMed] [Google Scholar]
- 32.Solinas C, Ceppi M, Lambertini M, et al. Tumor-infiltrating lymphocytes in patients with HER2-positive breast cancer treated with neoadjuvant chemotherapy plus trastuzumab, lapatinib or their combination: A meta-analysis of randomized controlled trials. Cancer Treat Rev 2017;57:8-15. 10.1016/j.ctrv.2017.04.005 [DOI] [PubMed] [Google Scholar]