Abstract
Background
The 2-dose recombinant zoster vaccine (RZV) series is recommended for prevention of herpes zoster (HZ) in adults aged ≥50 years, but data are limited on the impact of concomitant administration with other vaccines on subsequent HZ risk.
Methods
This cohort study included Kaiser Permanente Southern California members aged ≥50 years who received 2 doses of RZV 4 weeks to ≤6 months apart during 1 April 2018–30 September 2019. RZV recipients with and without same-day concomitant vaccination for either RZV dose were followed up for incident HZ beginning 31 days after the second RZV dose until 30 September 2020. The hazard ratio (HR) for HZ comparing RZV recipients with and without concomitant vaccination was estimated using Cox proportional hazards regression, adjusting for confounders.
Results
RZV with and without concomitant vaccination was received by 12 898 and 28 353 individuals, respectively. HZ occurred among 41 individuals with concomitant vaccination (incidence rate, 2.2 [95% confidence interval {CI}, 1.6–3.0] per 1000 person-years) and 136 without concomitant vaccination (3.4 [95% CI, 2.9–4.0] per 1000 person-years). The adjusted HR for HZ comparing RZV recipients with and without concomitant vaccination was 0.75 (95% CI, .53–1.08).
Conclusions
HZ risk was not significantly different between RZV recipients with and without concomitant vaccination, supporting recommendations allowing for concomitant administration of RZV with other vaccines.
Keywords: concomitant vaccination, herpes zoster, real-world data, recombinant zoster vaccine, simultaneous vaccination
Recombinant zoster vaccine (RZV) is a 2-dose subunit vaccine containing recombinant glycoprotein E in combination with AS01B adjuvant. In 2017, RZV was preferentially recommended over zoster vaccine live (ZVL) by the Advisory Committee on Immunization Practices (ACIP) for prevention of herpes zoster (HZ) and associated complications in immunocompetent adults aged ≥50 years [1]. In clinical trials, efficacy of RZV was 97.2% (95% confidence interval [CI], 93.7%–99.0%) overall and remained high (89.8% [95% CI, 84.2%–93.7%]) in adults aged ≥70 years [2, 3]. A postlicensure study among Medicare beneficiaries aged >65 years found vaccine effectiveness of 70.1% (95% CI, 68.6%–71.5%) [4]. No serious safety concerns have been identified [5].
Concomitant administration of vaccines is recommended for many vaccines, including RZV, to minimize individual and health system barriers to vaccination and to improve vaccine uptake [6]. Also referred to as simultaneous vaccination or same-day vaccination, concomitant vaccination is the administration of 2 or more vaccines at the same health care encounter [7]. Concomitant vaccination decreases the number of visits needed, reduces the potential for missed doses, and enables earlier protection. For health systems, proactive concomitant vaccination when not contraindicated is an efficient strategy to increase vaccination coverage.
In the United States, almost all recommended vaccines can be safely administered concomitantly at different anatomic sites to immunocompetent adults, without compromising immunogenicity or safety [8]. An exception is 13-valent pneumococcal conjugate vaccine (PCV13) and 23-valent pneumococcal polysaccharide vaccine (PPSV23), which can elicit lower antibody responses if received together [9]. In addition, a study of ZVL (no longer used in the United States) found that concomitant administration with PPSV23 resulted in a lower varicella zoster virus antibody response than receipt of the vaccines ≥4 weeks apart [10]. However, real-world observational studies reported no difference in the risk of HZ among individuals who received PPSV23 and ZVL concomitantly and those who received the vaccines ≥4 weeks apart [11, 12], and ACIP continued to recommend concomitant vaccination of ZVL with other vaccines for eligible persons [13]. In randomized controlled trials, coadministration of RZV with other vaccines (ie, tetanus, diphtheria, and acellular pertussis vaccine [Tdap], quadrivalent inactivated influenza vaccine [IIV4], and PPSV23) did not interfere with humoral immune responses, and no safety concerns were identified [14–16].
Because the relationship between humoral immune responses and HZ risk is unclear, and HZ risk following concomitant vaccination with RZV has not been evaluated, we conducted a large retrospective cohort study at Kaiser Permanente Southern California (KPSC) to directly evaluate HZ risk among individuals who received RZV with and without concomitant vaccination.
METHODS
Study Setting
KPSC is an integrated health care system serving 7 counties in Southern California. The diverse sociodemographic characteristics of the >4.7 million members are generally representative of the underlying population [17]. KPSC is organized in 15 medical centers, each with a hospital and associated office buildings. Members enroll in health plans through commercial plans or through federally or state-funded programs (eg, Medicare, MediCal). KPSC proactively offers all recommended vaccines free of charge at walk-in clinics and at other care visits. All aspects of patient care, including vaccinations, diagnoses, laboratory tests, procedures, and medications are recorded in a comprehensive electronic health record (EHR). Vaccinations and claims for care received outside the KPSC system are entered into the EHR with appropriate documentation.
Patient Consent Statement
The study received approval from the KPSC Institutional Review Board, which waived the requirement for written informed consent due to minimal risk to patients.
Participants, Exposure, and Outcome
KPSC members were eligible for inclusion in the study if they had received 2 doses of RZV 4 weeks to ≤6 months apart during the period 1 April 2018 to 30 September 2019 and were aged ≥50 years on the date of the second dose (defined as the index date). We required individuals to have had ≥1 year of KPSC membership prior to the index date (allowing for a 31-day gap in membership) to ensure accurate capture of prior comorbidities and health care utilization. Furthermore, we required receipt of ≥1 other vaccine in the year prior to or on the index date to minimize potential care-seeking bias. We excluded individuals with HZ in the ≤6 months before the index date to avoid carryover of prevalent cases into the follow-up period. We also excluded individuals with HZ ≤30 days after the index date to remove HZ cases that may have begun prior to the index date and to allow sufficient time to mount an immune response following vaccination.
We defined the main exposure in this study as receipt of either or both RZV doses on the same day as receipt of another vaccine (ie, with concomitant vaccination), compared to receipt of RZV without another vaccine on the same day (ie, without concomitant vaccination). We excluded individuals who received another vaccine ≤30 days prior to the index date from the RZV without concomitant vaccination group in order to clearly distinguish the comparison group from the RZV concomitant group.
We defined the main outcome as HZ identified by International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM) diagnosis codes B02.xx with a nontopical antiviral prescription within ±7 days of the diagnosis date but not within –183 to –8 days. RZV recipients with and without concomitant vaccination were followed up for occurrence of incident HZ beginning 31 days after the index date until HZ occurrence, disenrollment, death, or end of study (30 September 2020).
Statistical Analyses
We described the characteristics of individuals who received RZV with and without concomitant vaccination. Characteristics included age group (50–59, 60–69, 70–79, and ≥80 years), sex, race/ethnicity, prior receipt of ZVL (ever), prior history of HZ (ever), season and year of RZV index date, immunocompromised status (human immunodeficiency virus, leukemia, lymphoma, congenital immunodeficiencies, asplenia/hyposplenia, and transplant [including heart, kidney, liver, lung, pancreas, and bone marrow] prior to index date, or receipt of nonsteroidal immunosuppressing medications overlapping with index date), comorbidities in the year prior to index date (kidney disease, heart disease, lung disease, liver disease, and diabetes), and health care utilization in the year prior to index date (number of hospitalization, emergency department visits, outpatient visits, and virtual contacts). We reported frequencies and percentages and used the χ2 test or Fisher exact test to compare distributions of categorical variables.
Incidence was calculated by dividing the number of incident HZ cases by the total number of person-years, estimating CIs by assuming that occurrence of HZ follows a Poisson distribution. We used Kaplan-Meier analysis to plot the cumulative incidence of HZ and the log-rank test to compare the cumulative incidence curves. We then used Cox proportional hazards regression to estimate the hazard ratio (HR) and 95% CI for HZ comparing RZV recipients with and without concomitant vaccination, adjusting for covariates described above, overall and stratified by age, sex, and race/ethnicity.
RESULTS
We included 41 251 RZV recipients, of whom there were 12 898 RZV recipients with concomitant vaccination and 28 353 RZV recipients without concomitant vaccination (Table 1, Supplementary Figure 1). Overall, 12.5% of RZV recipients were aged ≥80 years, 58.5% were female, 60.8% were non-Hispanic White, 47.1% had not previously received ZVL, and 11.7% had a prior history of HZ (Table 1). RZV recipients with concomitant vaccination were generally younger and healthier than RZV recipients without concomitant vaccine; they were more often male and Hispanic, had received ZVL less often, had lower prevalence of history of HZ, had lower prevalence of prior kidney, heart, and lung disease, and had lower health care utilization in the year prior to index date.
Table 1.
Characteristics of Individuals Who Received 2 Doses of Recombinant Zoster Vaccine With and Without Concomitant Vaccine
| Characteristic | RZV With Concomitant Vaccinea | RZV Without Concomitant Vaccine | Total (N = 41 251) | P Value |
|---|---|---|---|---|
| (n = 12 898) | (n = 28 353) | |||
| Age at index dateb | <.001 | |||
| 50–59 y | 2134 (16.5) | 3782 (13.3) | 5916 (14.3) | |
| 60–69 y | 5676 (44.0) | 9528 (33.6) | 15 204 (36.9) | |
| 70–79 y | 3920 (30.4) | 11 043 (38.9) | 14 963 (36.3) | |
| ≥80 y | 1168 (9.1) | 4000 (14.1) | 5168 (12.5) | |
| Sex | <.001 | |||
| Female | 7359 (57.1) | 16 767 (59.1) | 24 126 (58.5) | |
| Male | 5539 (42.9) | 11 586 (40.9) | 17 125 (41.5) | |
| Race/Ethnicity | <.001 | |||
| Non-Hispanic White | 7788 (60.4) | 17 279 (60.9) | 25 067 (60.8) | |
| Non-Hispanic Black | 498 (3.9) | 1109 (3.9) | 1607 (3.9) | |
| Hispanic | 2171 (16.8) | 4194 (14.8) | 6365 (15.4) | |
| Non-Hispanic Asian | 1991 (15.4) | 4909 (17.3) | 6900 (16.7) | |
| Otherc | 450 (3.5) | 862 (3.0) | 1312 (3.2) | |
| Receipt of ZVLd | <.001 | |||
| ≤5 y ago | 1086 (8.4) | 2291 (8.1) | 3377 (8.2) | |
| >5 y ago | 4956 (38.4) | 13 479 (47.5) | 18 435 (44.7) | |
| No ZVL | 6856 (53.2) | 12 583 (44.4) | 19 439 (47.1) | |
| History of HZd | 1420 (11.0) | 3401 (12.0) | 4821 (11.7) | .004 |
| Season/year of index date | <.001 | |||
| Apr 2018–Aug 2018 | 587 (4.6) | 2087 (7.4) | 2674 (6.5) | |
| Sep 2018–Mar 2019 | 5573 (43.2) | 9397 (33.1) | 14 970 (36.3) | |
| Apr 2019–Sep 2019 | 6738 (52.2) | 16 869 (59.5) | 23 607 (57.2) | |
| Immunocompromisede | 557 (4.3) | 1320 (4.7) | 1877 (4.6) | .128 |
| Comorbiditiesf | ||||
| Kidney disease | 1453 (11.3) | 3752 (13.2) | 5205 (12.6) | <.001 |
| Heart disease | 606 (4.7) | 1770 (6.2) | 2376 (5.8) | <.001 |
| Lung disease | 1818 (14.1) | 4446 (15.7) | 6264 (15.2) | <.001 |
| Liver disease | 492 (3.8) | 1188 (4.2) | 1680 (4.1) | .074 |
| Diabetes | 2681 (20.8) | 5857 (20.7) | 8538 (20.7) | .765 |
| No. of hospitalizationsf | <.001 | |||
| 0 | 12 334 (95.6) | 26 871 (94.8) | 39 205 (95.0) | |
| 1 | 464 (3.6) | 1166 (4.1) | 1630 (4.0) | |
| ≥2 | 100 (0.8) | 316 (1.1) | 416 (1.0) | |
| No. of ED visitsf | <.001 | |||
| 0 | 10 988 (85.2) | 23 603 (83.2) | 34 591 (83.9) | |
| 1 | 1388 (10.8) | 3329 (11.7) | 4717 (11.4) | |
| ≥2 | 522 (4.0) | 1421 (5.0) | 1943 (4.7) | |
| No. of outpatient visitsf | <.001 | |||
| 0–4 | 3008 (23.3) | 4249 (15.0) | 7257 (17.6) | |
| 5–10 | 4777 (37.0) | 9902 (34.9) | 14 679 (35.6) | |
| ≥11 | 5113 (39.6) | 14 202 (50.1) | 19 315 (46.8) | |
| No. of virtual contactsf,g | <.001 | |||
| 0–10 | 10 763 (83.4) | 22 647 (79.9) | 33 410 (81.0) | |
| 11–20 | 1507 (11.7) | 3843 (13.6) | 5350 (13.0) | |
| ≥21 | 628 (4.9) | 1863 (6.6) | 2491 (6.0) |
Data are presented as No. (%) unless otherwise indicated.
Abbreviations: ED, emergency department; HZ, herpes zoster; RZV, recombinant zoster vaccine; ZVL, zoster vaccine live.
Defined as another vaccine received on the same day as either the first or second RZV dose.
The index date was the date of the second RZV dose.
Includes other, multiple, or unknown race/ethnicity.
Defined in any year prior to index date.
Defined as human immunodeficiency virus, leukemia, lymphoma, congenital immunodeficiencies, asplenia/hyposplenia, and transplant (including heart, kidney, liver, lung, pancreas, and bone marrow) prior to index date, or receipt of nonsteroidal immunosuppressing medications overlapping with index date.
Defined in 1 year prior to index date.
Virtual contacts included email, e-visit, telephone appointment visit, and video.
Of RZV recipients with concomitant vaccination, 6427 individuals received a concomitant vaccine with the first RZV dose, 5349 individuals received a concomitant vaccine with the second RZV dose, and 1122 individuals received a concomitant vaccine with both RZV doses (Supplementary Table 1). Among individuals who received RZV with concomitant vaccination, the most common concomitant vaccines were influenza vaccines (65.9%), pneumococcal vaccines (20.2%), Td/Tdap (12.3%), and hepatitis vaccines (10.2%) (Supplementary Table 2). Few concomitant vaccines (1.9%) had a novel adjuvant (ie, adjuvanted influenza vaccine or adjuvanted hepatitis B vaccine).
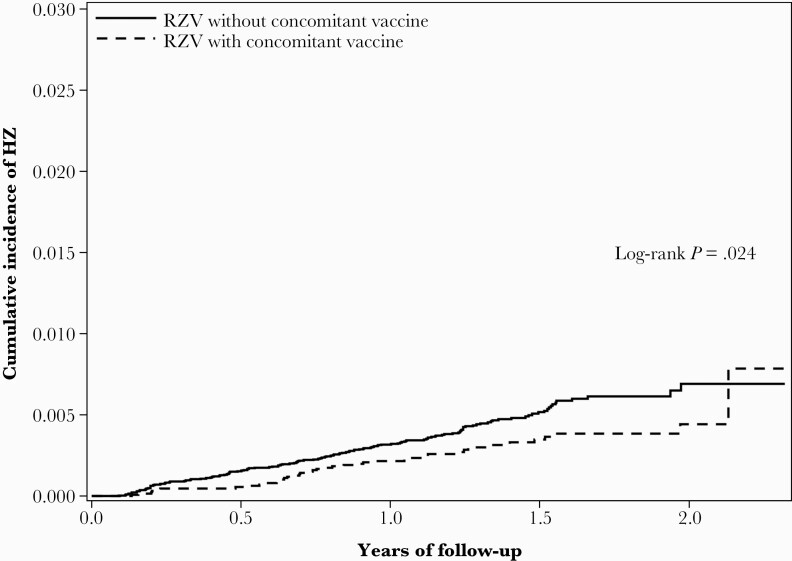
During the follow-up period, HZ occurred among 41 RZV recipients with concomitant vaccination (at either or both doses) and 136 RZV recipients without concomitant vaccination (Table 2). Incidence rates per 1000 person-years were 2.2 (95% CI, 1.6–3.0) and 3.4 (95% CI, 2.9–4.0), respectively. Kaplan-Meier plots showed slightly lower cumulative incidence of HZ for RZV recipients with concomitant vaccination vs RZV recipients without concomitant vaccination (P value for log-rank test = .024) (Figure 1). HZ incidence rates per 1000 person-years for RZV recipients who received a concomitant vaccine at the first dose, second dose, or both doses were 2.3 (95% CI, 1.5–3.4), 2.0 (95% CI, 1.2–3.4), and 3.2 (95% CI, 1.3–7.8), respectively (Supplementary Table 3).
Table 2.
Incidence of Herpes Zoster Among Individuals Who Received Recombinant Zoster Vaccine With and Without Concomitant Vaccine
| No. of Individuals | No. of HZa Cases | Total No. of Person-Years | Incidence Rate per 1000 Person-Years (95% CI) | |
|---|---|---|---|---|
| With concomitant vaccine | 12 898 | 41 | 18 327.7 | 2.2 (1.6–3.0) |
| Without concomitant vaccine | 28 353 | 136 | 40 297.4 | 3.4 (2.9–4.0) |
Abbreviations: CI, confidence interval; HZ, herpes zoster.
HZ was defined as an HZ diagnosis code plus prescription of an antiviral. Individuals were followed from index date until occurrence of HZ, disenrollment from Kaiser Permanente Southern California, death, or end of follow-up (30 September 2020).
Figure 1.
Cumulative incidence of herpes zoster (HZ) among individuals who received recombinant zoster vaccine (RZV) with and without concomitant vaccine.
After adjusting for demographic and clinical covariates, individuals who received RZV with concomitant vaccination did not have a significantly higher rate of HZ compared to individuals who received RZV without concomitant vaccination (adjusted HR [aHR], 0.75 [95% CI, .53–1.08]) (Table 3). Analyses stratified by age group, sex, and race/ethnicity consistently found aHR <1 with 95% CI overlapping 1, except among non-Hispanic Black RZV recipients. In this group, the aHR was 3.57 (95% CI, .26–48.24), but there were only 2 HZ events among RZV recipients with concomitant vaccines and 4 HZ events among RZV recipients without concomitant vaccines.
Table 3.
Unadjusted and Adjusted Hazard Ratios for Herpes Zoster Comparing Individuals Who Received Recombinant Zoster Vaccine With and Without Concomitant Vaccine
| Characteristic | Unadjusted HR (95% CI) | Adjusted HRa (95% CI) |
|---|---|---|
| Overall | 0.67 (.47–.95) | 0.75 (.53–1.08) |
| Age at index dateb | ||
| 50–59 y | 0.79 (.28–2.26) | 0.74 (.25–2.21) |
| 60–69 y | 0.72 (.43–1.22) | 0.82 (.48–1.40) |
| 70–79 y | 0.57 (.31–1.06) | 0.61 (.33–1.14) |
| ≥80 y | 0.76 (.26–2.25) | 0.86 (.28–2.58) |
| Sex | ||
| Female | 0.66 (.44–1.01) | 0.75 (.49–1.15) |
| Male | 0.71 (.38–1.33) | 0.82 (.43–1.55) |
| Race/Ethnicityc | ||
| Non-Hispanic White | 0.76 (.49–1.16) | 0.84 (.54–1.30) |
| Non-Hispanic Black | 1.07 (.19–5.86) | 3.57 (.26–48.24) |
| Hispanic | 0.61 (.26–1.41) | 0.70 (.29–1.66) |
| Non-Hispanic Asian | 0.42 (.14–1.20) | 0.45 (.15–1.33) |
Abbreviations: CI, confidence interval; HR, hazard ratio.
Cox proportional hazards model for herpes zoster (HZ) comparing individuals who received recombinant zoster vaccine (RZV) with and without concomitant vaccine, adjusted for all other variables in Table 1.
The index date was the date of the second RZV dose.
There were no HZ events among individuals of other race/ethnicity who received RZV with concomitant vaccine.
DISCUSSION
This large observational study found that there was no significant difference in the rates of HZ among individuals who received RZV with and without concomitant vaccination, after adjusting for demographic and clinical characteristics. Our study provides real-world evidence that individuals who receive RZV concomitantly with other vaccines do not have reduced protection against HZ. These findings are consistent with immunogenicity data from randomized clinical trials, which found no evidence of immunologic interference when RZV was administered concomitantly with Tdap, IIV4, or PPSV23 [14–16]. Together, these data support recommendations allowing for concomitant vaccination of RZV with other vaccines in eligible adults.
RZV is a highly efficacious vaccine, although data are limited in real-world settings, where individuals are more heterogeneous and may have more underlying conditions. A recent study found vaccine effectiveness of 70% among Medicare beneficiaries aged ≥65 years [4], compared to 91% efficacy among adults aged ≥70 years in pooled clinical trial data [3]; potential reasons for the difference included a more frail Medicare population and different methods for HZ case ascertainment. In the Medicare study, the HZ incidence rate among recipients of 2 doses of RZV was 3.09 per 1000 person-years, similar to the incidence rate observed in our study in RZV recipients without concomitant vaccination. Additional studies are needed to evaluate the long-term effectiveness of RZV in different population subgroups, including among concomitant vaccine recipients.
Concomitant vaccination is a recommended strategy for increasing vaccination coverage [8]. In the United States, few adults have received all recommended vaccines, and in National Health Interview Survey data from 2018, 24.1% of adults aged ≥50 years and 34.5% of adults aged ≥60 years had received a dose of HZ vaccine [18]. In another study at KPSC, completion of the 2-dose RZV series among those who initiated was observed to be suboptimal [19]. Interventions to increase concomitant vaccination, when recommended and when patients are eligible for multiple vaccines, may help increase adult vaccination coverage and series completion. For example, strategies could include provider and patient education on concomitant vaccination safety and effectiveness, and the expansion of electronic clinical decision support tools to proactively support concomitant vaccination.
Our study had several strengths and limitations. We included a large, diverse cohort of RZV recipients, leveraging comprehensive EHRs with detailed data on potential confounders, with follow-up for a minimum of 1 year. Misclassification of the concomitant vaccine exposure status was possible, although this study only included RZV-vaccinated individuals who received another vaccine in the prior year, making such misclassification less likely. Misclassification of the HZ outcome was possible if individuals did not seek care for HZ, but because the study population comprised individuals who had received another vaccine in the prior year, care-seeking bias was likely nondifferential for RZV recipients with and without concomitant vaccine. Misclassification of the HZ outcome was also possible if HZ was not accurately captured in the EHR; however, we required a new antiviral prescription along with ICD-10 codes for HZ to improve the specificity of the case definition [20]. In addition, our study used an observational design and could be subject to residual confounding despite the comprehensive KPSC data, but such confounding was unlikely to substantially change the results.
In conclusion, receipt of RZV with concomitant vaccination was not associated with a higher risk of HZ compared to receipt of RZV without concomitant vaccination. These findings support current ACIP guidelines for RZV allowing for concomitant vaccination, which should be encouraged to increase RZV uptake and series completion.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. We thank Kaiser Permanente Southern California members for contribution of their electronic health record information to this study.
Financial support. This work was supported by internal funds from Kaiser Permanente Southern California.
Potential conflicts of interest. K. J. B. has received research support outside the scope of this study from Dynavax, Gilead, GlaxoSmithKline, Moderna, Pfizer, and Seqirus. L. Q. has received research support outside the scope of this study from Dynavax, GlaxoSmithKline, and Moderna. J. W. has received research support outside the scope of this study from funding from GlaxoSmithKline and Genentech. A. F. has received research support outside the scope of this study from Gilead, GlaxoSmithKline, Moderna, and Pfizer. B. A. received funding from GlaxoSmithKline, Dynavax, Seqirus, Moderna, and Pfizer unrelated to this manuscript. L. S. S. has received research support outside the scope of this study from Dynavax, GlaxoSmithKline, Moderna, and Seqirus. L. V. D. has received research support outside the scope of this study from Bayer, GlaxoSmithKline, and Lundbeck. H. T. has received research support outside the scope of this study from ALK, GlaxoSmithKline, Moderna, Pfizer, and Wellcome. H. F. T. has received research support outside the scope of this study from GlaxoSmithKline, Moderna, and Seqirus.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Presented in part: Annual Conference of Vaccinology Research Virtual Meeting, 26–27 April 2021.
References
- 1. Dooling KL, Guo A, Patel M, et al. . Recommendations of the Advisory Committee on Immunization Practices for use of herpes zoster vaccines. MMWR Morb Mortal Wkly Rep 2018; 67:103–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lal H, Cunningham AL, Godeaux O, et al. . Efficacy of an adjuvanted herpes zoster subunit vaccine in older adults. N Engl J Med 2015; 372:2087–96. [DOI] [PubMed] [Google Scholar]
- 3. Cunningham AL, Lal H, Kovac M, et al. . Efficacy of the herpes zoster subunit vaccine in adults 70 years of age or older. N Engl J Med 2016; 375:1019–32. [DOI] [PubMed] [Google Scholar]
- 4. Izurieta HS, Wu X, Forshee R, et al. . Recombinant zoster vaccine (Shingrix) real-world effectiveness in the first two years post-licensure. Clin Infect Dis 2021; 73:941–8. [DOI] [PubMed] [Google Scholar]
- 5. Hesse EM, Shimabukuro TT, Su JR, et al. . Postlicensure safety surveillance of recombinant zoster vaccine (Shingrix)—United States, October 2017-June 2018. MMWR Morb Mortal Wkly Rep 2019; 68:91–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. National Center for Immunization and Respiratory Diseases. General recommendations on immunization—recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2011; 60:1–64. [PubMed] [Google Scholar]
- 7. Moss JL, Reiter PL, Brewer NT.. Concomitant adolescent vaccination in the U.S., 2007-2012. Am J Prev Med 2016; 51:693–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kroger S, Bahta L, Hunter P.. General best pratice guidelines for immunization. Best practices guidance of the Advisory Committee on Immunization Practices (ACIP). 2021. https://www.cdc.gov/vaccines/hcp/acip-recs/general-recs/downloads/general-recs.pdf. Accessed 4 May 2021. [Google Scholar]
- 9. Tomczyk S, Bennett NM, Stoecker C, et al. . Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine among adults aged ≥65 years: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2014; 63:822–5. [PMC free article] [PubMed] [Google Scholar]
- 10. MacIntyre CR, Egerton T, McCaughey M, et al. . Concomitant administration of zoster and pneumococcal vaccines in adults ≥60 years old. Hum Vaccin 2010; 6:894–902. [DOI] [PubMed] [Google Scholar]
- 11. Tseng HF, Smith N, Sy LS, Jacobsen SJ.. Evaluation of the incidence of herpes zoster after concomitant administration of zoster vaccine and polysaccharide pneumococcal vaccine. Vaccine 2011; 29:3628–32. [DOI] [PubMed] [Google Scholar]
- 12. Bruxvoort K, Sy LS, Luo Y, Tseng HF.. Real-world evidence for regulatory decisions: concomitant administration of zoster vaccine live and pneumococcal polysaccharide vaccine. Am J Epidemiol 2018; 187:1856–62. [DOI] [PubMed] [Google Scholar]
- 13. Harpaz R, Ortega-Sanchez IR, Seward JF.. Advisory Committee on Immunization Practices, Centers for Disease Control and Prevention. Prevention of herpes zoster: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2008; 57:1–30. [PubMed] [Google Scholar]
- 14. Strezova A, Lal H, Enweonye I, et al. The adjuvanted recombinant zoster vaccine co-administered with a tetanus, diphtheria and pertussis vaccine in adults aged ≥50 years: a randomized trial. Vaccine 2019; 37:5877–85. [DOI] [PubMed] [Google Scholar]
- 15. Schwarz TF, Aggarwal N, Moeckesch B, et al. . Immunogenicity and safety of an adjuvanted herpes zoster subunit vaccine coadministered with seasonal influenza vaccine in adults aged 50 years or older. J Infect Dis 2017; 216:1352–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Marechal C, Lal H, Poder A, et al. . Immunogenicity and safety of the adjuvanted recombinant zoster vaccine co-administered with the 23-valent pneumococcal polysaccharide vaccine in adults ≥50 years of age: a randomized trial. Vaccine 2018; 36:4278–86. [DOI] [PubMed] [Google Scholar]
- 17. Koebnick C, Langer-Gould AM, Gould MK, et al. . Sociodemographic characteristics of members of a large, integrated health care system: comparison with US Census Bureau data. Perm J Summer 2012; 16:37–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lu PJ, Hung MC, Srivastav A, et al. . Surveillance of vaccination coverage among adult populations—United States, 2018. MMWR Surveill Summ 2021; 70:1–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ackerson B, Qian L, Sy LS, et al. . Completion of the two-dose recombinant zoster vaccine series in adults 50 years and older. Vaccine 2021; 39:926–32. [DOI] [PubMed] [Google Scholar]
- 20. Tseng HF, Bruxvoort K, Ackerson B, et al. . The epidemiology of herpes zoster in immunocompetent, unvaccinated adults ≥50 years old: incidence, complications, hospitalization, mortality, and recurrence. J Infect Dis 2020; 222:798–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.