Abstract
Background:
Posttraumatic Stress Disorder (PTSD) is a debilitating disorder and there is no current accurate prediction of who develops it after trauma. Neurobiologically, individuals with chronic PTSD exhibit aberrant resting-state functional connectivity (rsFC) between the hippocampus and other brain regions (e.g., amygdala, prefrontal cortex, posterior cingulate), and these aberrations correlate with severity of illness. Prior small-scale research (n < 25) has also shown that hippocampal-rsFC measured acutely after trauma is predictive of future severity using an ROI-based approach. While a promising biomarker, to-date no study has employed a data-driven approach to test whole-brain hippocampal-FC patterns in forecasting the development of PTSD symptoms.
Methods:
Ninety-eight adults at risk of PTSD were recruited from the emergency department following traumatic injury and completed resting functional magnetic resonance imaging (rsfMRI; 8min) within 1-month; 6-months later they completed the Clinician-Administered PTSD Scale (CAPS-5) for assessment of PTSD symptom severity. Whole-brain rsFC values with bilateral hippocampi were extracted (CONN) and used in a machine learning kernel ridge regression analysis (PRoNTo); both a k-folds (k=10) and 70/30 testing vs. training split approach were used for cross-validation (1,000 iterations to bootstrap confidence intervals for significance values).
Results:
Acute hippocampal-rsFC significantly predicted CAPS-5 scores at 6-months (r=0.30, p=0.006; MSE=120.58, p=0.006; R2=0.09, p=0.025). In post-hoc analyses, hippocampal-rsFC remained significant after controlling for demographics, PTSD symptoms at baseline, and depression, anxiety, and stress severity at 6-months (B=0.59, SE=0.20, p=0.003).
Conclusions:
Findings suggest functional connectivity of the hippocampus across the brain acutely after traumatic injury is associated with prospective PTSD symptom severity.
Keywords: trauma, PTSD, hippocampus, resting state, machine learning, MPVA
1. Introduction
Approximately 8–10% of American adults who experience a traumatic event will develop symptoms of post-traumatic stress disorder (PTSD), including hyperarousal, unwanted thoughts (e.g., flashbacks), and altered cognitive states(1). Among the most prevalent types of trauma is physical injury(2), with adults at heighted risk of developing symptoms (e.g., ~20% of survivors admitted to an emergency room meet criteria for PTSD diagnosis within one year).(3,4) While the overall understanding of PTSD etiology and its treatment continues to improve, implementation of therapeutic interventions early (e.g., in the weeks after trauma) yields the greatest benefits.(2,5,6) In order to provide early treatment, however, clinicians must be able to identify which individuals are at risk of developing PTSD.
Prior research demonstrates that pre-trauma risk factors such as sleep quality and presence of anxiety and depression increases the incidence of PTSD(7), while a number of pre- and peritraumatic factors, including those that are clinical (e.g., symptoms of distress) and biological (i.e., heart rate/blood pressure), can significantly add to the development of posttraumatic stress symptoms(8,9). The study of neural abnormalities qualified in the acute aftermath of trauma may also help identify those most at risk.(10–14) Much of this work has centered on the amygdala, involved in generation of negative affect.(10,15–18) However, the hippocampus, which is densely functionally and structurally connected to the amygdala, is responsible for the consolidation of fear memories(19) and is strongly implicated in PTSD.(20) Indeed, a fundamental feature of PTSD is atypical memory encoding and retrieval, particularly in the context of emotional memory(19,21–23), functions that are hippocampal-dependent(24). This is particularly true in the context of fear and extinction learning, whereby alterations in the hippocampus are often found in the context of trauma-related stimuli or general negative affect.(22,25) Notably, hippocampal aberrations frequently coincide with altered amygdala functioning.(22,25) Despite alterations in both regions(25), hippocampal functional discrepancies – but not always amygdala – are distinctly correlated with PTSD symptoms.(25) This suggests utility in explicitly studying hippocampal functioning as it relates to PTSD outcomes after trauma.
Prior theoretical models postulate altered stress hormone release via cortisol in individuals with PTSD has deleterious effects on the hippocampus, either by inducing cytotoxic effects(26) or impeding neuroplasticity.(27) Indeed, trauma-induced structural changes to the hippocampus may be associated with altered function.(28) Though prior research shows instances of both hypo-(25,29) and hyper-engagement(30) of the hippocampus in response to negative and neutral stimuli as well trauma-specific reminders, these aberrations are associated with poor memory performance. Specifically, greater engagement of the hippocampus in response to negative words is associated with more false positives (e.g., misremembering novel stimuli).(30) Likewise, reduced activity in the hippocampus in response to trauma-specific stimuli is also associated with the presence of false alarms for trauma-related images.(25) Neurobiologically, this supports what has been demonstrated in behavioral studies of memory functioning for some time, namely that individuals with PTSD are less accurate compared to controls when recalling neutral(31), emotional(21), and episodic autobiographical information.(32) Such memory deficits have been posited to underlie the overgeneralization of fear as a cardinal symptom of PTSD(33), as the hippocampus contributes to both the extinction and/or regulation of fear in inappropriate contexts, by providing context-dependent processing.(22,34)
In addition, hippocampal aberrations in those with PTSD appear across various task-probes and in both affective(20) and cognitive domains(19), adding to its prevalence in this disorder. To this point, one of the most widely used techniques in studying the relationship between the hippocampus and PTSD is to quantify its functioning and associated connectivity during rest (e.g., when participants are not engaged in a task) and, indeed, individuals with chronic PTSD exhibit altered hippocampal functional connectivity at rest (hippocampal-rsFC). First, hippocampal-rsFC connectivity is altered with hubs of the default mode network (DMN), implicated in self-referential processing in the absence of task-demand. Specifically, those with PTSD show reduced connectivity of the hippocampus with the ventromedial prefrontal cortex (vmPFC)(35), medial prefrontal cortex(36), and PCC(37) compared to trauma exposed-controls. Other work has found evidence of greater integration of the hippocampus with the DMN(38) and greater integration of the hippocampus with regions of the salience network (SN), which is involved in the detection of salient stimuli.(39) This suggests that altered processing of learned fear (subserved by the hippocampus) may be related to differences in internally focused (e.g., DMN) and externally focused thought (e.g., SN)(39) in those with PTSD. Second, although hippocampal-rsFC with nodes of the DMN is atypical in individuals with PTSD and generalized anxiety disorder (GAD) compared to healthy controls, this effect is driven by those with PTSD as the primary diagnosis.(40) Finally, several studies demonstrate that hippocampal-rsFC correlates with individual variability of PTSD symptoms.(41,42) Decreased hippocampal-rsFC with the amygdala(43–45), mPFC(35), and PCC(37,46), while hippocampal-rsFC with the vmPFC and dorsolateral prefrontal cortex (dlPFC)(47) are all significantly related to PTSD severity. Combined, this research demonstrates aberrant hippocampal-rsFC in those with PTSD compared to controls and that this characteristic distinguishes PTSD from other internalizing disorders(40) while meaningfully correlating with severity of the disorder.
Although cross-sectional associations with symptoms is informative, the hippocampus may also be a critical brain region important for disease onset and trajectory. A consistent, though not flawless(48–50) biomarker of the development of PTSD after trauma is smaller hippocampal volume pre-trauma.(12,17,51–53) An increasing body of work also suggests the hippocampus may undergo early changes in response to trauma (e.g., within days and up to one year following trauma exposure) that can be measured via hippocampal-rsFC and directly related to PTSD symptom progression.(46,54,55) Greater acute post-trauma hippocampal-rsFC with the amygdala(54), PCC(55), and between hippocampal subfields(46) is a significant predictor of less PTSD symptoms up to four(46) and six months after trauma exposure.(54,55) Other work demonstrates greater acute post-trauma PFC connectivity with an “arousal network” – defined in part by the hippocampus – predicts less PTSD severity three months after trauma.(56) Importantly, PTSD is frequently co-morbid with major depressive depression (MDD)(57), with multiple overlapping symptoms characterizing both disorders.(58) Yet, to our knowledge, the above studies did not test whether hippocampal connectivity measured acutely post-trauma forecasts future PTSD severity while accounting for co-morbid symptoms of depression.
The above work implies value of hippocampal-rsFC for the prediction of PTSD and that this relationship is not dependent on connectivity with a single brain region. Thus, in contrast to singular region approaches, machine-learning methods offer an opportunity to explore the most useful disorder-specific neural patterns across the entire brain without the constraints of traditional univariate schemes.(59) Multivariate variate pattern analysis (MVPA) offers such an innovative approach towards forecasting mental health outcomes. In recent years, MVPA has been applied to understanding the neural correlates of PTSD(15,60,61), MDD(62–64), and other disorders.(65) Briefly, this machine learning approach tests whether whole-brain distributed rsFC patterns are useful in predicting individual symptoms.(66) By analyzing neural spatially-distributed activation, MVPA can be used to “decode” the brain and identify information (i.e., future PTSD symptom severity) that is represented in voxels throughout the whole brain, with voxels representing either activation during task-based activities, or connectivity with another part of the brain (e.g., hippocampus).(66–69) Past MVPA approaches have used hippocampus whole-brain connectivity to discriminate when individuals with PTSD are engaged in trauma recall versus neutral imagery.(60) Other machine learning techniques have shown that mean volume reduction in the hippocampus contributes to accurate classification of those with PTSD from controls (accuracy: 69%, specificity: 81%).(70) Additionally, a machine learning classifier investigation found that amygdala-hippocampal structure via tract strength contributed to accurate prediction of trauma-exposed versus trauma-naive individuals.(71) Thus, patterns of hippocampal-based activation(60) and hippocampal structure(70) can significantly predict trauma history, PTSD symptoms, and unique features of the disorder. However, to our knowledge, MVPA has never been applied to examine the utility of hippocampal-rsFC to forecast individual PTSD symptom severity.
In this study, we employed MVPA to test whether acute (i.e., within one-month post-injury) hippocampal-rsFC patterns forecasted participants’ future (i.e., six-months post-injury) clinician-assessed PTSD symptom severity in a large, heterogenous sample of PTSD at-risk participants. We assessed PTSD symptoms using the Clinician-Administered PTSD Scale for DSM-5(CAPS-5), considered to be the “gold-standard” assessment of PTSD.(72,73) Previous MVPA work in PTSD(15,61) has employed less reliable measures (i.e., self-report measures) of PTSD, such as the PTSD Checklist. Based on findings from previous studies, we hypothesized that post-injury hippocampal-rsFC would significantly forecast individual PTSD total symptom severity at six-months post-injury and, importantly, that the prediction would still be significant in a regression model adjusting for six-month general depression, anxiety, stress scores and baseline PTSD symptoms.
2. Material and Methods
2.1. Participants
Traumatically-injured adults were recruited from a Level 1 Trauma Center either directly from the Emergency Department (ED) or by phone following ED discharge. Participants were eligible if they: a) were between the ages of 18–60, b) were English speaking, c) met the Diagnostic and Statistical Manual-5th edition (DSM-5) Criterion A for a PTSD diagnosis, and d) exhibited a greater risk of developing PTSD based on a minimum score of 3 (out of 5) on the Predicting PTSD Questionnaire.(74) Participants were excluded if they: a) experienced a moderate or severe head injury as the result of their trauma based on a score of > 13 on the Glasgow Coma Scale(75,76), b) suffered a spinal cord injury with neurological deficit, c) were admitted to the ER as the result of intentional self-inflicted injury, d) exhibited severe vision or hearing impairments, e) had a history of psychotic or manic symptoms or were currently taking antipsychotic medications, f) a history of clear substance abuse, g) on police hold following their traumatic injury, or h) were MRI incompatible based on the following: presence of ferromagnetic material in the body, claustrophobia, inability to lie still for two hours, or either currently pregnant or trying to become pregnant. Exclusion criteria was assessed via self-report during the screening process and additionally via a review of medical records for the presence of diagnostic codes. All participants provided written consent and all study procedures were approved by the local Institutional Review Board. Participants were compensated for their time and all procedures complied with the Helsinki Declaration.
2.2. Procedure
Upon enrollment, participants completed an 8-minute rsfMRI scan within one month of their traumatic injury. At that visit (henceforth referred to as ‘baseline’) they also completed a number of demographic and clinical assessments, including the PTSD Checklist for DSM-5 (PCL-5).(77) The PCL-5 is a 20-item self-report measure of post-traumatic stress symptoms with good internal consistency (Cronbach’s alpha = 0.94), convergent validity (r > 0.75), and test-retest reliability (r = 0.92).(78) Six months later, participants returned for a follow-up visit, at which time they completed the Clinician-Administered PTSD Scale for DSM-5 (CAPS-5)(79) with a trained research staff member. The CAPS-5 is considered a “gold-standard” assessment of PTSD, exhibits high internal consistency (α = .88) and good test-retest reliability (ICC = .78).(80) An internal reliability check on the CAPS-5 was completed for this study across two separate raters for 20% of CAPS-5 completed at six months. Results demonstrated excellent agreement among raters (kappa = 0.83, p < 0.001) and excellent reliability between total symptom severity scores (ICC = 0.96 [95% CI: 0.93–0.98]).
In addition to the CAPS-5, participants completed the Depression Anxiety and Stress Scales (DASS-21) at the six month visit for self-reported assessment of general depression, anxiety, and stress severity(81). Each of the depression (α = .81), anxiety (α = .89), and stress (α = .78) scales of the DASS-21 have been found to have excellent internal consistency.(82)
2.2.1. Resting State fMRI Acquisition
During the rsfMRI scan participants viewed a white crosshair displayed on a black background and were instructed to keep their eyes open. Scanning was performed on a 3.0 Tesla short bore GE Signa Excite MRI system at the Medical College of Wisconsin. Functional T2*-weighted echoplanar images (EPI) were collected in a sagittal orientation with the following parameters: repetition time (TR)/echo time (TE)=2000/25ms; FOV=22.4mm; matrix=64×64; flip angle=77°; slice thickness=3.5mm; voxel size=3.5 × 3.5 × 3.5mm; # slices = 41; volumes = 192. A high-resolution T1-weighted anatomical image was also acquired for co-registration with the following parameters: TR/TE=8.2/3.2ms; FOV=240mm; matrix=256×224; flip angle=12°; voxel size=0.9375 × 1.071 × 1mm, # slices = 150.
2.3. Data Analysis
2.3.1. Image Preprocessing
Individual functional images were analyzed using the CONN functional connectivity toolbox(83) and preprocessed according to standard procedures. Briefly, images underwent spatial realignment using the SPM12 realign and unwarp procedure(84) with all scans referenced to the first image and estimated motion parameters calculated across six variables representing three translation (displacement) parameters and three rotation parameters. Temporal misalignment was corrected using slice-time correction.(85) As small head movements can cause spurious noise and distance-dependent changes in signal correlations(86,87), frame-wise displacement (FD) was computed to rule out confounding effects of motion. Volumes with FD > 0.2 mm (plus 1-back and 2-forward neighboring volumes) were ‘scrubbed’ (e.g., removed from analysis). Participants were excluded if more than 25% of the frames were scrubbed. In addition, subjects with cumulative movement > 3 mm or 3 degrees of rotation were identified for removal from analysis. Structural segmentation and normalization were done to classify data into grey matter, white matter, and cerebrospinal fluid (CSF) through the estimation of the posterior tissue probability maps (TPMs) in SPM12.(88) Images were then normalized to the Montreal Neurological Institute (MNI) template and smoothed with a 4 mm3 Gaussian kernel.(89) To isolate rsfMRI signal, resulting data were bandpass filtered at 0.01– 0.09 Hz, while signal from cerebrospinal fluid, white matter, and motion realignment parameters were entered as regressors of no-interest to control for these effects during scanning.
2.3.2. Pattern Recognition Analysis
Two whole-brain hippocampal-rsFC maps were computed for each subject at the first-level using CONN, one representing connectivity with the right hippocampus and one with the left hippocampus. Each map’s voxels represented a Fisher-transformed bivariate correlation coefficient between the respective seeds’ (e.g., right and left hippocampi) BOLD timeseries and every other voxel’s BOLD timeseries. The right and left hippocampi were defined using the Anatomical Automatic Labeling (AAL)-defined mask from the SPM toolbox.(90,91)
Both maps were subsequently used as features in a multivariate kernel ridge regression (KRR) using the PRoNTo toolbox (http://www.mlnl.cs.ucl.ac.uk/pronto/).(92) KRR is a machine learning technique and a form of linear ridge regression (sum of squares) with the addition of a kernel function. Ridge regression introduces bias in order to improve model fit and accuracy of forecasted predictions. Extending this approach, KRR adds a function based on the “kernel trick”, whereby a kernel is used to improve model fit by operating in feature space. KRR is often considered an improvement on regression-based models for prediction as it offers a more efficient way to transform the data without the need to compute coordinates in a higher dimensional space.(93)
Each hippocampal-rsFC map served as its own feature (features: n = 2) and was provided for each individual subject while feature selection was constrained to voxels inside the brain through the use of a standard binary mask(92). In the calculation of features, a linear kernel was used with a square matrix of dimensions N x N, where the kernel reflected a similarity measure between each participant, called the dot product. We did not use a second-level mask to constrain feature selection by a subset of voxels; instead, all voxels within the brain (representing connectivity with the respective hippocampal seed region) were used for model prediction. Model prediction using the KRR approach was then computed and generalizability estimated using two different approaches. First, to utilize the entire sample, we used a k-folds (k = 10) approach for cross-validation. The k-folds approach for cross-validation has been used previously in machine learning investigations involving those with PTSD(94) and may be superior to the use of training versus test datasets for this purpose when sample sizes are considered small by machine learning standards. Importantly, cross-validation ensures that the model is generalizable and prevents overfitting. Identical to past studies(15), features (i.e., L and R hippocampal-rsFC maps) were first mean centered using the training data (9-folds; 90% of dataset). In addition to this approach, we also split our dataset into a training set (~70% of sample) and a testing set (~30%) and used a k-folds approach where k = 1 to train on the 70% sub-sample and subsequently test the model performance on the 30% sub-sample. In both approaches, the performance of the model was characterized using several metrics, including the (cross-validated) Pearson correlation coefficient (r), mean squared error (MSE) and the coefficient of determination (R2) between model-estimated CAPS-5 and the true CAPS-5 scores. Significance values for prediction scores were obtained using permutation testing across 1,000 iterations, a necessary step when dealing with large neuroimaging datasets that violate the assumption that data is independently and identically distributed. The choice for 1,000 permutations was based on current recommendations(95) and identical to prior machine learning MVPA publications using neuroimaging data.(15,61)
Results of the model were also viewed through the calculation of weights for each voxel as a colormap, whereby warmer colors reflected voxels that increased model prediction by a value of the features (e.g., hippocampal-rsFC) and cooler colors reflected voxels that decreased model prediction by a value of the features, assuming all other voxels are fixed. That is, each voxel’s contribution to the model performance was visualized. Post-hoc averaging of weight values by individual brain regions was also done during this step(96), although we did not constrain weight contribution to its average within brain regions for the calculation of the model. Here, post-hoc averaging of weight values was done only for illustrative purposes, similar to other published accounts(15,61,97), as all voxels contributed to model performance and it is inaccurate to single out the predictive utility of one region.(98) For averaging of weight values by brain region, we used the AAL atlas, resulting in the averaged weight values for N = 117 brain regions.
3. Results
3.1. Participants
A total of N = 139 participants was initially recruited for this study. Of this, 31 participants were excluded from analysis for the following reasons: a) lost to follow-up (n = 12), b) excess motion during rsfMRI defined as > 25% volumes lost in scrubbing and/or ≥ 3 mm movement in any one direction (n = 28), or c) alignment problems in reconstruction of imaging data (n = 1). This left a final sample of N = 98.
Participants completed their baseline appointment between 6–33 days after injury (M = 18.57 days, SD = 5.51 days) and their follow-up appointment between 5–8 months after injury (M = 6.07 months, SD = 0.43 months). Mechanism of injury varied across the sample but consisted primarily of survivors of motor vehicle crashes (67%). The remaining injuries were classified as: assault (16%), crush injuries (<5%), pedestrian injuries (<5%), dog bites (<5%), falls (<5%), gunshot (<5%), domestic violence (<5%), sexual assault (<5%), and bicycle accident (<5%; exact percentage is not included to ensure participant confidentiality). Complete participant demographics are reported in Table 1.
Table 1.
Sample Demographics (N = 98)
|
M (SD)
|
|
| Age | 33.52 (10.30) |
| Education (years) | 14.92 (2.39) |
| PCL-5 at baseline | 25.76 (17.41) |
| DASS-21: Depression at six months | 7.38 (9.06) |
| DASS-21: Anxiety at six months | 7.53 (8.07) |
| DASS-21: Stress at six months | 10.70 (9.07) |
| CAPS-5 at six months | 11.98 (11.53) |
|
n (%)
|
|
| Gender (Female) | 53 (54%) |
| Ethnicity (Hispanic or Latino) | 9 (9%) |
| Race | |
| Asian | < 5 (< 5%) |
| Black or African American | 54 (55%) |
| White | 32 (33%) |
| More than one race | 5 (5%) |
| Unknown or not reported | 6 (6%) |
Note. PCL-5, PTSD Checklist for DSM-5; DASS-21, Depression Anxiety Stress Scales; CAPS-5, Clinician-Administered PTSD Scale. Small sample sizes for select racial groups are reported as < 5% to avoid participant identification; thus, cumulative percentage surpasses 100% as reported here.
3.2. PTSD Symptoms
At baseline, PTSD severity measured by the PCL-5 ranged from 0–73 (M = 25.76, SD = 17.41). At six months, PTSD severity as measured by the CAPS-5 ranged from 0–63 (M = 11.98, SD = 11.53), indicating that six months after injury participants ranged from asymptomatic to severe PTSD symptomatology(99).
3.3. MVPA Results
Using the full-sample in cross-validation, model results demonstrated that baseline whole-brain hippocampal-rsFC significantly predicted CAPS-5 scores at six months (r = 0.30, p = 0.006; mean squared error = 120.58, p = 0.006; R2 = 0.09, p = 0.025). Results were the same when using a training (n = 68) vs. testing (n = 30) set for model validation (r = 0.46, p = 0.002; mean squared error = 217.38, p = 0.003; R2 = 0.21, p = 0.007). As results of model fit did not change based on which cross-validation method was used, the remaining results reflect when the full sample (N = 98) was used in cross-validation. Together, this suggests that the model prediction was an accurate fit (based on significant MSE) and that actual CAPS-5 scores (i.e., the targets in our regression) were well correlated with our predicted values based on model fit (given a significant R2). Spatial distribution of color-coded model weights for each voxel are depicted in Figure 1. Similar to other published MVPA studies(15,61,97), KRR-derived weights constrained by brain region for the top 10% of regions that contributed to model prediction are reported in Table 2.
Figure 1.
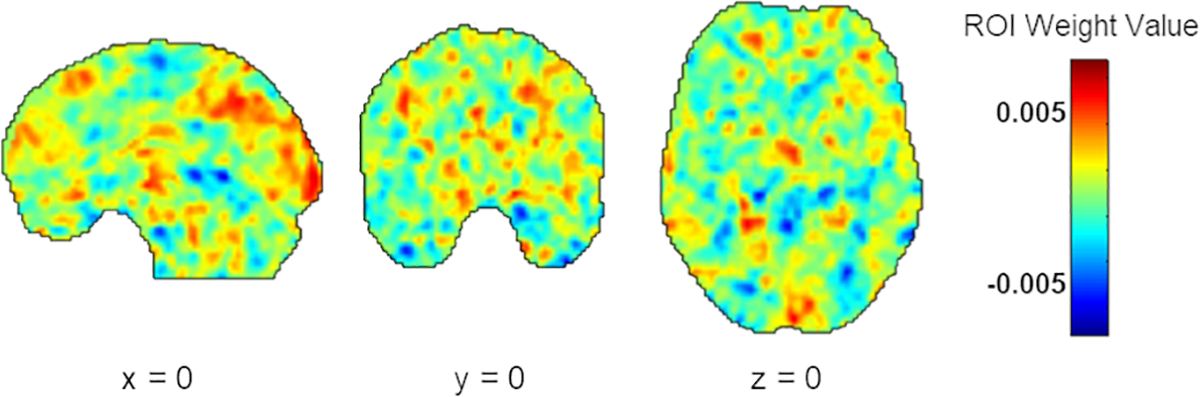
Results of the KRR analysis depicting computed weight values in arbitrary units for each voxel across the entire brain. Warmer colors indicate that these regions positively contributed to model performance. In contrast, voxels with low weight values, represented by cooler colors, indicate weight values that negatively contributed to model performance (e.g., push it toward decreased prediction). Note: KRR, kernel ridge regression.
Table 2.
Top 10% of Model Weights per Regions-of-Interest
| MNI Coordinates | ||||||||
|---|---|---|---|---|---|---|---|---|
| Region-of-Interest | Laterality | Direction | Weight (%) | Size (voxels) | Expected Ranking | x | y | z |
| Cerebellum | L | Negative | 1.39 | 1140 | 116.81 | −14 | −44 | −28 |
| Cerebellum Crus | R | Negative | 1.37 | 2026 | 116.12 | 36 | −70 | −30 |
| Amygdala | R | Positive | 1.28 | 240 | 114.70 | 26 | 0 | −16 |
| Cerebellum Crus | L | Negative | 1.21 | 2239 | 113.39 | −36 | −70 | −28 |
| Hippocampus | R | Negative | 1.20 | 951 | 112.82 | 28 | −18 | −12 |
| Cerebellum | R | Negative | 1.18 | 316 | 111.33 | 32 | −72 | −46 |
| Cerebellar Vermis | Midline | Negative (L) | 1.16 | 669 | 110.50 | 2 | −52 | −6 |
| Parahippocampal Gyrus | L | Positive (R), Negative (L) | 1.16 | 985 | 110.24 | −22 | −16 | −22 |
| Cerebellum Crus | R | Negative | 1.15 | 1668 | 109.51 | 28 | −78 | −40 |
| dmPFC | R | Positive | 1.09 | 1904 | 107.27 | 8 | 52 | 32 |
| Heschl’s Gyrus | L | Positive (L), Negative (R) | 1.08 | 224 | 106.04 | −42 | 54 | 10 |
Note. MNI, Montreal Neurological Institute; dmPFC, dorsomedial prefrontal cortex; L: left; R: right. Reported regions represent top 10% of regions based on weight. Direction indicates whether the connectivity pattern was positive (greater connectivity) or negative (reduced connectivity). When differential connectivity patterns were observed, the laterality of the seed hippocampus is included in parentheses. Weight is determined by the contribution of that region divided by the total contribution of all regions and displayed as a percentage. Expected ranking reflects how stable the ranking of each region is across folds. Although only the top 10% of model weights per regions-of-interest are displayed here, all voxels contributed to model fit and weights were averaged by brain region in a post-hoc fashion for interpretability of model outcome.
Predicted targets based on model fit were subsequently extracted for use in post-hoc analyses to examine this relationship further while controlling for select covariates(100). We controlled for covariates in this fashion given that the addition of covariates within the model prediction is applied to the linear kernel, which is limited in removing the linear confound for each effect for each voxel without assessing the effect of the covariate on the pattern of voxels (e.g., at the multivariate level). Extraction of the predicted model values alternatively allowed us to examine the significance of the multivariate model fit controlling for univariate factors. Here, predicted targets were used in a hierarchical linear regression using SPSS (Version 26) to examine the strength of the relationship between predicted and actual targets controlling for gender (dichotomous variable; reference = 0 [male]), mean-centered age, mean-centered time since injury at baseline, mean-centered time since injury at six months, mean-centered PCL-5 scores at baseline, and mean-centered DASS-21 depression, anxiety, and stress ratings at six months entered into Step 2 of the model. In addition, for controlling for differences in demographics and timing in the administration of measures, this allowed for the control for the presence of PTSD stress symptoms at baseline (i.e., the time of rsfMRI data collection), and to test for specificity in the relationship between hippocampal-rsFC and PTSD symptoms. Assumptions of the linear model were met, such that residuals were homoscedastic and there were no issues of multicollinearity (VIF < 4.5).
Results demonstrated that the relationship between predicted (based on model fit) and actual CAPS-5 scores remained significant controlling for these factors (B = 0.59, SE = 0.20, p = 0.003). Results of the post-hoc hierarchical linear regression are reported in Table 3; Figure 2 depicts the partial regression relationship between actual CAPS-5 scores (y-axis) plotted against predicted CAPS-5 scores based on the MVPA algorithm (x-axis) controlling for covariates.
Table 3.
Post-hoc Hierarchical Linear Regression
| Actual CAPS-5 Scores at Six Months | ||||||
|---|---|---|---|---|---|---|
|
| ||||||
| Variable | B | SE | β | t | p-value | |
|
| ||||||
|
Step 1
| ||||||
| Intercept | 12.03 | 1.108 | 10.862 | < 0.001 | *** | |
| Target CAPS-5 based on model prediction | 0.81 | 0.23 | 0.34 | 3.51 | 0.001 | ** |
|
Step 2 | ||||||
| Intercept | 10.59 | 1.41 | 7.53 | 0.000 | ||
| Target CAPS-5 based on model prediction | 0.59 | 0.20 | 0.25 | 3.02 | 0.003 | ** |
| Gender | 2.61 | 1.98 | 0.11 | 1.32 | 0.192 | |
| Age | 0.05 | 0.10 | 0.05 | 0.55 | 0.586 | |
| Time since injury at baseline | 2.35 | 5.56 | 0.04 | 0.42 | 0.674 | |
| Time since injury at six months | −2.45 | 2.22 | −0.09 | −1.10 | 0.273 | |
| PCL-5 at baseline | 0.08 | 0.06 | 0.12 | 1.26 | 0.212 | |
| DASS-Depression at six months | 0.10 | 0.20 | 0.08 | 0.50 | 0.621 | |
| DASS-Anxiety at six months | 0.69 | 0.23 | 0.48 | 2.97 | 0.004 | ** |
| DASS-Stress at six months | −0.01 | 0.20 | −0.01 | −0.05 | 0.957 | |
Note. CAPS-5=Clinician-Administered PTSD Scale; PCL-5=PTSD Checklist-Civilian Version; DASS=Depression Anxiety Stress Scales
p < 0.001
p < 0.01; N = 98.
Figure 2.

Significant relationship between actual and predicted CAPS scores based on the MVPA algorithm controlling for all covariates (B = 0.59, SE = 0.20, p = 0.003).
4. Discussion
To assess the utility of whole-brain hippocampal-rsFC to forecast future PTSD symptom severity, adult survivors of a traumatic injury completed a rsfMRI scan acutely post-injury (within one month) and a structured clinical interview evaluating PTSD symptoms approximately six-months post-injury. Results demonstrated that hippocampus-rsFC across the whole brain was a significant predictor of future PTSD severity, even after controlling for gender, PTSD self-reported symptoms at baseline, and general depression, anxiety, and stress symptoms as they were reported at follow-up. That is, findings suggest that functional integration of the hippocampus across the brain acutely after traumatic injury is a promising biomarker for prospective PTSD severity, and that this relationship is specific to PTSD when compared to general depression, anxiety, and stress symptom development.
Results support the use of data-driven, MVPA approaches for the prediction of psychiatric illness(62–65), including PTSD symptom severity(15,61,101) or dichotomous PTSD diagnosis.(102) Further, the strength of the relationship we discovered between predicted and actual symptom severity based on the model performance (r = 0.30) was similar to previous MVPA approaches that have predicted PTSD outcomes using other seed regions (e.g., r’s range from 0.28(103) to 0.46(15)). Thus, akin to other studies investigating brain-based biomarkers using machine learning(15,61,101,102), hippocampal-rsFC has a moderate effect size in forecasting individual PTSD psychopathology.
Importantly, our findings support the recommendation to explore hippocampal-rsFC across the entire brain, rather than narrowing the focus on a priori selection of its connectivity with a select number of brain regions or networks. Increasingly, studies demonstrate the value of studying patterns of voxel-to-voxel activation in those with PTSD. For instance, Cisler and colleagues found voxel-to-voxel patterns of activation during trauma memory recall was a better predictor of PTSD diagnosis than the traditional use of ROI-to-ROI differences in activation(60), while other research shows that whole-brain connectivity is a better predictor of PTSD in combat veterans than 32 non-imaging markers (e.g., behavior, clinical symptoms).(104) Similarly, Suo and colleagues recently demonstrated whole-brain connectivity across 268 ROIs within the brain was able to significantly “predict” cross-sectional PTSD symptom severity in survivors of an earthquake(101). In this latter study, connections between the occipital lobe and cerebellum as well as connections of limbic regions (including hippocampus) with the occipital lobe and cerebellum were the primary connections that successfully predicted PTSD severity.(101) Notably, connections between these brain regions, with exception of the traditional “limbic structures,” are rarely studied in the context of PTSD. Finally, model-fits determined by whole-brain resting-state average amplitude of low frequency fluctuations have been shown to be better predictors of PTSD symptom severity than constraining the feature-selection with a mask encompassing the bilateral PFC, amygdalae, and hippocampi.(61) Indeed, in addition to hippocampal connectivity with regions involved in fear generation (i.e., amygdala) as well intra-hippocampal connectivity (including with the parahippocampal gyrus), we also found that hippocampal-cerebellum rsFC contributed greatly to model fit based on our post-hoc review of the top 10% of regions that contributed to model performance. Structural abnormalities of the cerebellum, a region traditionally associated with motor coordination and movement-related learning(105), have recently been indicated as a risk factor for common cognitive and affective disorders across categorial diagnoses(106), and recent research highlights that cortico-cerebellum circuitry may be important for integration and coordination of affective functioning that is related to psychiatric illness.(107) Taken together, this suggests a need for revisiting the traditional view of PTSD as a disorder specific to fronto-limbic aberrations.(108)
The present findings have important treatment implications. First, our study assessed hippocampal-rsFC within weeks of trauma exposure as a predictor of severity of symptoms months later. This research demonstrates that early screening for risk for PTSD diagnosis may benefit by examining neurobiological features such as hippocampal-rsFC early in disease progression. Second, unlike other studies, we explored the prediction of PTSD symptoms controlling for general depression, anxiety, and stress severity. Thus, results suggest that although PTSD is comorbid with depression and anxiety disorders(109) and prior studies have questioned the utility of hippocampal volume as a unique biomarker of PTSD(49), PTSD may still be qualified by unique neurobiological features, such as altered whole-brain hippocampal-rsFC. Finally, given the use of a continuous PTSD measurement, findings demonstrate that the prediction of continuous PTSD severity that includes sub-threshold presentation remains crucial, as it also causes clinically significant impairments(110) and represents a significant subset of trauma-exposed adults.(111)
The present study is not without limitations. First, despite the fact that this sample was comprised of individuals who experienced varied mechanisms of injury, the majority of our sample (67%) were admitted to the emergency department following motor vehicle crash. Thus, findings may not be generalizable to individuals who have developed PTSD resulting from other trauma types. In addition, the use of a Glasgow Coma Scale score of > 13 during screening means that some individuals may have had a mild traumatic brain injury (mTBI). Although this score suggests that the mTBI injury is minor(75,76), future research on the impact of mTBI on rsFC should be examined. Although our sample is moderately-large for neuroimaging studies and keeping with other machine learning investigations in PTSD using fMRI data (which used sample sizes ranging from N = 40(112) to N = 186(113)), our sample is considered small (though still acceptable based on n = 80 cut-off to reduce error below 0.01) for machine learning approaches that utilize biomedical data.(114) As depression prognosis was a secondary interest in the present study, we relied on a self-reported measure of depression symptoms at six months through the DASS-21. Thus, it will be helpful to re-investigate these findings with the use of more robust measures of depression (e.g., CESD-R(115)). Finally, although significance of the model was retained after controlling for univariate confounds in post-hoc regression, we were unable to adequately account for multivariate confounding, or the effect of a confound on the pattern of voxels, which is a known limitation of the present analysis.
Despite these limitations, several important conclusions can be drawn from our findings. First, this is one of the only published studies to-date that has examined hippocampal-rsFC in the acute aftermath of traumatic injury as a prospective predictor of PTSD symptom development and using a large sample size (prior accounts have used samples of n < 25(46,55) or reported preliminary data in conference proceedings(54)). In addition, this is the only study to our knowledge that employs a multivariate, machine-learning analytic approach to the question of hippocampal-rsFC and PTSD prediction. Results provide further rationale for not restricting the study of the biological underpinnings of PTSD to limbic structures. Given the multitudinous role of the hippocampus in both memory formation and fear regulation, in addition to the constellation of PTSD symptoms spanning domains of memory alterations and altered arousal, results support the conclusion that hippocampal distributed connectivity across the brain may be consequential for understanding PTSD prognosis in trauma survivors.
Acknowledgments
The authors would like to acknowledge the participants for their time and participation in this research study.
Disclosures
This research was supported by a National Institute of Mental Health grant (R01 MH106574; PI: Larson). E.K.W was supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant Numbers 2UL1TR001436 and 2TL1TR001437. All authors report no biomedical financial interests or potential conflicts of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.American Psychiatric Association; (2013): Diagnostic and Statistical Manual of Mental Disorders, 5th ed. Arlington, VA. [Google Scholar]
- 2.Center for Substance Abuse Treatment (US) (2014): Trauma-Informed Care in Behavioral Health Services. Rockville (MD): Substance Abuse and Mental Health Services Administration (US). Retrieved February 19, 2021, from http://www.ncbi.nlm.nih.gov/books/NBK207201/ [PubMed] [Google Scholar]
- 3.deRoon-Cassini TA, Hunt JC, Geier TJ, Warren AM, Ruggiero KJ, Scott K, et al. (2019): Screening and treating hospitalized trauma survivors for posttraumatic stress disorder and depression. Journal of Trauma and Acute Care Surgery 87: 440–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O’Donnell ML, Creamer M, Holmes ACN, Ellen S, McFarlane AC, Judson R, et al. (2010): Posttraumatic Stress Disorder After Injury: Does Admission to Intensive Care Unit Increase Risk? Journal of Trauma and Acute Care Surgery 69: 627–632. [DOI] [PubMed] [Google Scholar]
- 5.Rothbaum BO, Kearns MC, Price M, Malcoun E, Davis M, Ressler KJ, et al. (2012): Early Intervention May Prevent the Development of PTSD: A Randomized Pilot Civilian Study with Modified Prolonged Exposure. Biol Psychiatry 72: 957–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rothbaum BO, Kearns MC, Reiser E, Davis JS, Kerley KA, Rothbaum AO, et al. (2014): Early Intervention Following Trauma May Mitigate Genetic Risk for PTSD in Civilians: A Pilot Prospective Emergency Department Study. J Clin Psychiatry 75: 1380–1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schultebraucks K, Qian M, Abu-Amara D, Dean K, Laska E, Siegel C, et al. (2020): Pre-deployment risk factors for PTSD in active-duty personnel deployed to Afghanistan: a machine-learning approach for analyzing multivariate predictors. Mol Psychiatry. 10.1038/s41380-020-0789-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schultebraucks K, Shalev AY, Michopoulos V, Grudzen CR, Shin S-M, Stevens JS, et al. (2020): A validated predictive algorithm of post-traumatic stress course following emergency department admission after a traumatic stressor. Nat Med 26: 1084–1088. [DOI] [PubMed] [Google Scholar]
- 9.Saxe GN, Ma S, Morales LJ, Galatzer-Levy IR, Aliferis C, Marmar CR (2020): Computational causal discovery for post-traumatic stress in police officers. Transl Psychiatry 10: 233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Belleau EL, Ehret LE, Hanson JL, Brasel KJ, Larson CL, deRoon-Cassini TA (2020): Amygdala functional connectivity in the acute aftermath of trauma prospectively predicts severity of posttraumatic stress symptoms. Neurobiology of Stress 12: 100217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fitzgerald JM, Gorka SM, Kujawa A, DiGangi JA, Proescher E, Greenstein JE, et al. (2018): Neural indices of emotional reactivity and regulation predict course of PTSD symptoms in combat-exposed veterans. Progress in Neuro-Psychopharmacology and Biological Psychiatry 82: 255–262. [DOI] [PubMed] [Google Scholar]
- 12.Gilbertson MW, Shenton ME, Ciszewski A, Kasai K, Lasko NB, Orr SP, Pitman RK (2002): Smaller hippocampal volume predicts pathologic vulnerability to psychological trauma. Nat Neurosci 5: 1242–1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lanius RA, Bluhm RL, Coupland NJ, Hegadoren KM, Rowe B, Théberge J, et al. (2010): Default mode network connectivity as a predictor of post-traumatic stress disorder symptom severity in acutely traumatized subjects. Acta Psychiatr Scand 121: 33–40. [DOI] [PubMed] [Google Scholar]
- 14.Weis CN, Belleau EL, Pedersen WS, Miskovich TA, Larson CL (2018): Structural Connectivity of the Posterior Cingulum Is Related to Reexperiencing Symptoms in Posttraumatic Stress Disorder. Chronic Stress 2: 2470547018807134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fitzgerald JM, Belleau EL, Miskovich TA, Pedersen WS, Larson CL (2020): Multi- voxel pattern analysis of amygdala functional connectivity at rest predicts variability in posttraumatic stress severity. Brain Behav 10. 10.1002/brb3.1707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McLaughlin KA, Busso DS, Duys A, Green JG, Alves S, Way M, Sheridan MA (2014): AMYGDALA RESPONSE TO NEGATIVE STIMULI PREDICTS PTSD SYMPTOM ONSET FOLLOWING A TERRORIST ATTACK. Depression and Anxiety 31: 834–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morey RA, Garrett ME, Stevens JS, Clarke EK, Haswell CC, van Rooij SJH, et al. (2020): Genetic predictors of hippocampal subfield volume in PTSD cases and trauma-exposed controls. European Journal of Psychotraumatology 11: 1785994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brown VM, LaBar KS, Haswell CC, Gold AL, McCarthy G, Morey RA (2014): Altered Resting-State Functional Connectivity of Basolateral and Centromedial Amygdala Complexes in Posttraumatic Stress Disorder [no. 2]. Neuropsychopharmacology 39: 351–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Joshi SA, Duval ER, Kubat B, Liberzon I (2020): A review of hippocampal activation in post-traumatic stress disorder. Psychophysiology 57: e13357. [DOI] [PubMed] [Google Scholar]
- 20.Fitzgerald JM, DiGangi JA, Phan KL (2018): Functional Neuroanatomy of Emotion and Its Regulation in PTSD. Harvard Review of Psychiatry 26: 116–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ehlers A, Clark DM (2000): A cognitive model of posttraumatic stress disorder. Behaviour research and therapy 38: 319–345. [DOI] [PubMed] [Google Scholar]
- 22.Milad MR, Pitman RK, Ellis CB, Gold AL, Shin LM, Lasko NB, et al. (2009): Neurobiological Basis of Failure to Recall Extinction Memory in Posttraumatic Stress Disorder. Biological Psychiatry 66: 1075–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shin LM (2006): Amygdala, Medial Prefrontal Cortex, and Hippocampal Function in PTSD. Annals of the New York Academy of Sciences 1071: 67–79. [DOI] [PubMed] [Google Scholar]
- 24.Dere E, Pause BM, Pietrowsky R (2010): Emotion and episodic memory in neuropsychiatric disorders. Behavioural Brain Research 215: 162–171. [DOI] [PubMed] [Google Scholar]
- 25.Hayes JP, LaBar KS, McCarthy G, Selgrade E, Nasser J, Dolcos F, Morey RA (2011): Reduced hippocampal and amygdala activity predicts memory distortions for trauma reminders in combat-related PTSD. Journal of Psychiatric Research 45: 660–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bremner JD (2006): Traumatic stress: effects on the brain. Dialogues in clinical neuroscience 8: 445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Niibori Y, Yu T-S, Epp JR, Akers KG, Josselyn SA, Frankland PW (2012): Suppression of adult neurogenesis impairs population coding of similar contexts in hippocampal CA3 region. Nat Commun 3: 1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bremner JD (2005): Effects of Traumatic Stress on Brain Structure and Function: Relevance to Early Responses to Trauma. Journal of Trauma & Dissociation 6: 51–68. [DOI] [PubMed] [Google Scholar]
- 29.Werner NS, Meindl T, Engel RR, Rosner R, Riedel M, Reiser M, Fast K (2009): Hippocampal function during associative learning in patients with posttraumatic stress disorder. Journal of Psychiatric Research 43: 309–318. [DOI] [PubMed] [Google Scholar]
- 30.Thomaes K, Dorrepaal E, Draijer NPJ, de Ruiter MB, Elzinga BM, van Balkom AJ, et al. (2009): Increased activation of the left hippocampus region in Complex PTSD during encoding and recognition of emotional words: A pilot study. Psychiatry Research: Neuroimaging 171: 44–53. [DOI] [PubMed] [Google Scholar]
- 31.Samuelson KW (2011): Post-traumatic stress disorder and declarative memory functioning: a review. Dialogues Clin Neurosci 13: 346–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brown AD, Addis DR, Romano TA, Marmar CR, Bryant RA, Hirst W, Schacter DL (2014): Episodic and semantic components of autobiographical memories and imagined future events in post-traumatic stress disorder. Memory 22: 595–604. [DOI] [PubMed] [Google Scholar]
- 33.Kaczkurkin AN, Burton PC, Chazin SM, Manbeck AB, Espensen-Sturges T, Cooper SE, et al. (2017): Neural Substrates of Overgeneralized Conditioned Fear in PTSD. American Journal of Psychiatry 174: 125–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Garfinkel SN, Abelson JL, King AP, Sripada RK, Wang X, Gaines LM, Liberzon I (2014): Impaired Contextual Modulation of Memories in PTSD: An fMRI and Psychophysiological Study of Extinction Retention and Fear Renewal. Journal of Neuroscience 34: 13435–13443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhu X, Suarez-Jimenez B, Lazarov A, Helpman L, Papini S, Lowell A, et al. (2018): Exposure-based therapy changes amygdala and hippocampus resting-state functional connectivity in patients with posttraumatic stress disorder. Depress Anxiety 35: 974–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jin C, Qi R, Yin Y, Hu X, Duan L, Xu Q, et al. (2014): Abnormalities in whole-brain functional connectivity observed in treatment-naive post-traumatic stress disorder patients following an earthquake. Psychological Medicine 44: 1927–1936. [DOI] [PubMed] [Google Scholar]
- 37.Miller DR, Hayes SM, Hayes JP, Spielberg JM, Lafleche G, Verfaellie M (2017): Default Mode Network Subsystems Are Differentially Disrupted in Posttraumatic Stress Disorder. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging 2: 363–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang X-D, Yin Y, Hu X-L, Duan L, Qi R, Xu Q, et al. (2017): Altered default mode network configuration in posttraumatic stress disorder after earthquake: A resting-stage functional magnetic resonance imaging study. Medicine 96: e7826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sripada RK, King AP, Welsh RC, Garfinkel SN, Wang X, Sripada CS, Liberzon I (2012): Neural Dysregulation in Posttraumatic Stress Disorder: Evidence for Disrupted Equilibrium Between Salience and Default Mode Brain Networks. Psychosomatic Medicine 74: 904–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen AC, Etkin A (2013): Hippocampal network connectivity and activation differentiates post-traumatic stress disorder from generalized anxiety disorder. Neuropsychopharmacology 38: 1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kunimatsu A, Yasaka K, Akai H, Kunimatsu N, Abe O (2020): MRI findings in posttraumatic stress disorder. J Magn Reson Imaging 52: 380–396. [DOI] [PubMed] [Google Scholar]
- 42.Liberzon I, Sripada CS (2008): The functional neuroanatomy of PTSD: a critical review. Prog Brain Res 167: 151–169. [DOI] [PubMed] [Google Scholar]
- 43.Etkin A, Wager TD (2007): Functional Neuroimaging of Anxiety: A Meta-Analysis of Emotional Processing in PTSD, Social Anxiety Disorder, and Specific Phobia. American Journal of Psychiatry 164: 1476–1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liberzon I, Abelson JL (2016): Context Processing and the Neurobiology of Post-Traumatic Stress Disorder. Neuron 92: 14–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Spielberg JM, McGlinchey RE, Milberg WP, Salat DH (2015): Brain network disturbance related to posttraumatic stress and traumatic brain injury in veterans. Biol Psychiatry 78: 210–216. [DOI] [PubMed] [Google Scholar]
- 46.Malivoire BL, Girard TA, Patel R, Monson CM (2018): Functional connectivity of hippocampal subregions in PTSD: relations with symptoms. BMC Psychiatry 18. 10.1186/s12888-018-1716-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Abdallah CG, Wrocklage KM, Averill CL, Akiki T, Schweinsburg B, Roy A, et al. (2017): Anterior hippocampal dysconnectivity in posttraumatic stress disorder: a dimensional and multimodal approach. Translational Psychiatry 7: e1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bonne O, Brandes D, Gilboa A, Gomori JM, Shenton ME, Pitman RK, Shalev AY (2001): Longitudinal MRI Study of Hippocampal Volume in Trauma Survivors With PTSD. AJP 158: 1248–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gosnell SN, Meyer MJ, Jennings C, Ramirez D, Schmidt J, Oldham J, Salas R (2020): Hippocampal Volume in Psychiatric Diagnoses: Should Psychiatry Biomarker Research Account for Comorbidities? Chronic Stress 4: 2470547020906799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Weis CN, Webb EK, Huggins AA, Kallenbach M, Miskovich TA, Fitzgerald JM, et al. (2021): Stability of hippocampal subfield volumes after trauma and relationship to development of PTSD symptoms. NeuroImage 236: 118076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bae S, Sheth C, Legarreta M, McGlade E, Lyoo I, Yurgelun-Todd D (2020): Volume and shape analysis of the Hippocampus and amygdala in veterans with traumatic brain injury and posttraumatic stress disorder. Brain Imaging and Behavior 14. 10.1007/s11682-019-00127-2 [DOI] [PubMed] [Google Scholar]
- 52.Bremner JD, Vythilingam M, Vermetten E, Southwick SM, McGlashan T, Nazeer A, et al. (2003): MRI and PET study of deficits in hippocampal structure and function in women with childhood sexual abuse and posttraumatic stress disorder. Am J Psychiatry 160: 924–932. [DOI] [PubMed] [Google Scholar]
- 53.McEwen BS, Nasca C, Gray JD (2016): Stress Effects on Neuronal Structure: Hippocampus, Amygdala, and Prefrontal Cortex. Neuropsychopharmacol 41: 3–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ben-Zion Z, Keynan NJ, Admon R, Sharon H, Halpern P, Liberzon I, et al. (2020): Hippocampal-Amygdala Resting State Functional Connectivity Serves as Resilience Factor for Short- and Long-Term Stress Exposure. Biological Psychiatry 87: S88–S89. [Google Scholar]
- 55.Zhou Y, Wang Z, Qin L, Wan J, Sun Y, Su S, et al. (2012): Early Altered Resting-State Functional Connectivity Predicts the Severity of Post-Traumatic Stress Disorder Symptoms in Acutely Traumatized Subjects ((Dickey CA, editor)). PLoS ONE 7: e46833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Harnett NG, van Rooij SJH, Ely TD, Lebois LAM, Murty VP, Jovanovic T, et al. (2021): Prognostic neuroimaging biomarkers of trauma-related psychopathology: resting-state fMRI shortly after trauma predicts future PTSD and depression symptoms in the AURORA study. Neuropsychopharmacol. 10.1038/s41386-020-00946-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nichter B, Norman S, Haller M, Pietrzak RH (2019): Psychological burden of PTSD, depression, and their comorbidity in the U.S. veteran population: Suicidality, functioning, and service utilization. Journal of Affective Disorders 256: 633–640. [DOI] [PubMed] [Google Scholar]
- 58.Flory JD, Yehuda R (2015): Comorbidity between post-traumatic stress disorder and major depressive disorder: alternative explanations and treatment considerations. Clinical research 17: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ramos-Lima LF, Waikamp V, Antonelli-Salgado T, Passos IC, Freitas LHM (2020): The use of machine learning techniques in trauma-related disorders: a systematic review. Journal of Psychiatric Research 121: 159–172. [DOI] [PubMed] [Google Scholar]
- 60.Cisler JM, Bush K, James GA, Smitherman S, Kilts CD (2015): Decoding the Traumatic Memory among Women with PTSD: Implications for Neurocircuitry Models of PTSD and Real-Time fMRI Neurofeedback ((Elhai JD, editor)). PLoS ONE 10: e0134717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gong Q, Li L, Du M, Pettersson-Yeo W, Crossley N, An X, et al. (2014): Quantitative Prediction of Individual Psychopathology in Trauma Survivors Using Resting-State fMRI. Neuropsychopharmacology 39: 681–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mwangi B, Matthews K, Steele JD (2012): Prediction of illness severity in patients with major depression using structural MR brain scans. Journal of Magnetic Resonance Imaging 35: 64–71. [DOI] [PubMed] [Google Scholar]
- 63.Yang W, Chen Q, Liu P, Cheng H, Cui Q, Wei D, et al. (2015): Abnormal brain activation during directed forgetting of negative memory in depressed patients. Journal of affective disorders 190. 10.1016/j.jad.2015.05.034 [DOI] [PubMed] [Google Scholar]
- 64.Zhong X, Shi H, Ming Q, Dong D, Zhang X, Zeng L-L, Yao S (2017): Whole-brain resting-state functional connectivity identified major depressive disorder: A multivariate pattern analysis in two independent samples. Journal of Affective Disorders 218: 346–352. [DOI] [PubMed] [Google Scholar]
- 65.Hou Y, Luo C, Yang J, Ou R, Song W, Wei Q, et al. (2016): Prediction of individual clinical scores in patients with Parkinson’s disease using resting-state functional magnetic resonance imaging. Journal of the Neurological Sciences 366: 27–32. [DOI] [PubMed] [Google Scholar]
- 66.Davis T, LaRocque KF, Mumford JA, Norman KA, Wagner AD, Poldrack RA (2014): What do differences between multi-voxel and univariate analysis mean? How subject-, voxel-, and trial-level variance impact fMRI analysis. NeuroImage 97: 271–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kambeitz J, Cabral C, Sacchet MD, Gotlib IH, Zahn R, Serpa MH, et al. (2017): Detecting Neuroimaging Biomarkers for Depression: A Meta-analysis of Multivariate Pattern Recognition Studies. Biological Psychiatry 82: 330–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ramos-Lima LF, Waikamp V, Antonelli-Salgado T, Passos IC, Freitas LHM (2020): The use of machine learning techniques in trauma-related disorders: a systematic review. Journal of Psychiatric Research 121: 159–172. [DOI] [PubMed] [Google Scholar]
- 69.Ritchie JB, Carlson TA (2016): Neural Decoding and “Inner” Psychophysics: A Distance-to-Bound Approach for Linking Mind, Brain, and Behavior. Front Neurosci 10. 10.3389/fnins.2016.00190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Li L, Pan N, Zhang L, Lui S u, Huang X, Xu X, et al. (2020): Hippocampal subfield alterations in pediatric patients with post-traumatic stress disorder. Social Cognitive and Affective Neuroscience nsaa162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Im JJ, Kim B, Hwang J, Kim JE, Kim JY, Rhie SJ, et al. (2017): Diagnostic potential of multimodal neuroimaging in posttraumatic stress disorder ((Harpaz-Rotem I, editor)). PLoS ONE 12: e0177847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Forbes D, Creamer M, Biddle D (2001): The validity of the PTSD checklist as a measure of symptomatic change in combat-related PTSD. Behav Res Ther 39: 977–986. [DOI] [PubMed] [Google Scholar]
- 73.Parker-Guilbert KS, Leifker FR, Sippel LM, Marshall AD (2014): The differential diagnostic accuracy of the PTSD Checklist among men versus women in a community sample. Psychiatry Res 220: 679–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rothbaum BO, Kearns MC, Reiser E, Davis JS, Kerley KA, Rothbaum AO, et al. (2014): Early intervention following trauma may mitigate genetic risk for PTSD in civilians: a pilot prospective emergency department study. J Clin Psychiatry 75: 1380–1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sternbach GL (2000): The Glasgow coma scale. J Emerg Med 19: 67–71. [DOI] [PubMed] [Google Scholar]
- 76.Teasdale G, Maas A, Lecky F, Manley G, Stocchetti N, Murray G (2014): The Glasgow Coma Scale at 40 years: standing the test of time. Lancet Neurol 13: 844–854. [DOI] [PubMed] [Google Scholar]
- 77.Weathers FW, Litz BT, Huska JA, Keane TM (1994): PTSD Checklist-Civilian Version. Boston: National Center for PTSD, Behavioral Science Division. [Google Scholar]
- 78.Ruggiero KJ, Ben KD, Scotti JR, Rabalais AE (2003): Psychometric properties of the PTSD checklist—civilian version. Journal of Traumatic Stress 16: 495–502. [DOI] [PubMed] [Google Scholar]
- 79.Weathers FW, Litz BT, Keane TM, Palmieri PA, Marx BP, Schnurr PP (2013): The PTSD Checklist for DSM-5 (PCL-5) – Standard [Measurement instrument]. Retrieved from http://www.ptsd.va.gov/
- 80.Weathers FW, Bovin MJ, Lee DJ, Sloan DM, Schnurr PP, Kaloupek DG, et al. (2018): The Clinician-Administered PTSD Scale for DSM–5 (CAPS-5): Development and initial psychometric evaluation in military veterans. Psychological Assessment 30: 383–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lovibond SH, Lovibond PF (1995): Manual for the Depression Anxiety Stress Scales, 2nd ed. Sydney: Psychology Foundation. [Google Scholar]
- 82.Coker AO, Coker OO, Sanni D (2018): Psychometric properties of the 21-item Depression Anxiety Stress Scale (DASS-21). Afr Res Rev 12: 135. [Google Scholar]
- 83.Whitfield-Gabrieli S, Nieto-Castanon A (2012): Conn: A Functional Connectivity Toolbox for Correlated and Anticorrelated Brain Networks. Brain Connectivity 2: 125–141. [DOI] [PubMed] [Google Scholar]
- 84.Andersson JLR, Hutton C, Ashburner J, Turner R, Friston K (2001): Modeling Geometric Deformations in EPI Time Series. NeuroImage 13: 903–919. [DOI] [PubMed] [Google Scholar]
- 85.Henson R, Büchel C, Josephs O, Friston K (1999): The slice-timing problem in event-related fMRI. 2. [Google Scholar]
- 86.Power JD, Mitra A, Laumann TO, Snyder AZ, Schlaggar BL, Petersen SE (2014): Methods to detect, characterize, and remove motion artifact in resting state fMRI. NeuroImage 84: 320–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Power JD, Schlaggar BL, Petersen SE (2015): Recent progress and outstanding issues in motion correction in resting state fMRI. NeuroImage 105: 536–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ashburner J, Friston KJ (2005): Unified segmentation. NeuroImage 26: 839–851. [DOI] [PubMed] [Google Scholar]
- 89.Hagler DJ, Saygin AP, Sereno MI (2006): Smoothing and cluster thresholding for cortical surface-based group analysis of fMRI data. NeuroImage 33: 1093–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH (2003): An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. 7. [DOI] [PubMed] [Google Scholar]
- 91.Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, et al. (2002): Automated Anatomical Labeling of Activations in SPM Using a Macroscopic Anatomical Parcellation of the MNI MRI Single-Subject Brain. NeuroImage 15: 273–289. [DOI] [PubMed] [Google Scholar]
- 92.Schrouff J, Rosa MJ, Rondina JM, Marquand AF, Chu C, Ashburner J, et al. (2013): PRoNTo: Pattern Recognition for Neuroimaging Toolbox. Neuroinform 11: 319–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Stock M, Pahikkala T, Airola A, De Baets B, Waegeman W (2018): A Comparative Study of Pairwise Learning Methods Based on Kernel Ridge Regression. Neural Computation 30: 2245–2283. [DOI] [PubMed] [Google Scholar]
- 94.Salminen LE, Morey RA, Riedel BC, Jahanshad N, Dennis EL, Thompson PM (2019): Adaptive Identification of Cortical and Subcortical Imaging Markers of Early Life Stress and Posttraumatic Stress Disorder. Journal of Neuroimaging 29: 335–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ashburner J, Chu C, Marquand A, Mourao-Miranda J, Monteiro JM, Phillips C, et al. (2018): PRoNTo Manual. London: University of College London. [Google Scholar]
- 96.Schrouff J, Cremers J, Garraux G, Baldassare L, Mourao-Miranda J, Phillips C (2013): Localizing and Comparing Weight Maps Generated from Linear Kernel Machine Learning Models. presented at the International Workshop on Pattern Recognition in Neuroimaging (PRNI). Retrieved from 10.1109/PRNI.2013.40 [DOI] [Google Scholar]
- 97.Harricharan S, Nicholson AA, Thome J, Densmore M, McKinnon MC, Théberge J, et al. (2020): PTSD and its dissociative subtype through the lens of the insula: Anterior and posterior insula resting- state functional connectivity and its predictive validity using machine learning. Psychophysiology 57. 10.1111/psyp.13472 [DOI] [PubMed] [Google Scholar]
- 98.Haufe S, Meinecke F, Görgen K, Dähne S, Haynes J-D, Blankertz B, Bießmann F (2014): On the interpretation of weight vectors of linear models in multivariate neuroimaging. NeuroImage 87: 96–110. [DOI] [PubMed] [Google Scholar]
- 99.Weathers FW, Ruscio AM, Keane TM (1999): Psychometric properties of nine scoring rules for the Clinician-Administered Posttraumatic Stress Disorder Scale. Psychological Assessment 11: 124–133. [Google Scholar]
- 100.Rao A, Mourao-Miranda J (n.d.): Feature Adjustment in Kernel Space when using Cross-Validation. 6. [Google Scholar]
- 101.Suo X, Lei D, Li W, Yang J, Li L, Sweeney JA, Gong Q (2020): Individualized Prediction of PTSD Symptom Severity in Trauma Survivors From Whole-Brain Resting-State Functional Connectivity. Front Behav Neurosci 14. 10.3389/fnbeh.2020.563152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gong Q, Li L, Tognin S, Wu Q, Pettersson-Yeo W, Lui S, et al. (2014): Using structural neuroanatomy to identify trauma survivors with and without post-traumatic stress disorder at the individual level. Psychological Medicine 44: 195–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Li Y, Zhu H, Ren Z, Lui S, Yuan M, Gong Q, et al. (2020): Exploring memory function in earthquake trauma survivors with resting-state fMRI and machine learning. BMC Psychiatry 20: 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Rangaprakash D, Dretsch MN, Venkataraman A, Katz JS, Denney TS, Deshpande G (2018): Identifying disease foci from static and dynamic effective connectivity networks: Illustration in soldiers with trauma: Identifying disease foci from fMRI connectivity. Human Brain Mapping 39: 264–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Reeber SL, Otis TS, Sillitoe RV (2013): New roles for the cerebellum in health and disease. Front Syst Neurosci 7. 10.3389/fnsys.2013.00083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Romer AL, Knodt AR, Houts R, Brigidi BD, Moffitt TE, Caspi A, Hariri AR (2018): Structural alterations within cerebellar circuitry are associated with general liability for common mental disorders. Mol Psychiatry 23: 1084–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hariri AR (2019): The Emerging Importance of the Cerebellum in Broad Risk for Psychopathology. Neuron 102: 17–20. [DOI] [PubMed] [Google Scholar]
- 108.Ross MC, Cisler JM (2020): Altered large-scale functional brain organization in posttraumatic stress disorder: A comprehensive review of univariate and network-level neurocircuitry models of PTSD. NeuroImage: Clinical 27: 102319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kessler RC (1995): Posttraumatic Stress Disorder in the National Comorbidity Survey. Arch Gen Psychiatry 52: 1048. [DOI] [PubMed] [Google Scholar]
- 110.Cukor J, Wyka K, Jayasinghe N, Difede J (2010): The nature and course of subthreshold PTSD. Journal of Anxiety Disorders 24: 918–923. [DOI] [PubMed] [Google Scholar]
- 111.Bergman HE, Przeworski A, Feeny NC (2017): Rates of Subthreshold PTSD Among U.S. Military Veterans and Service Members: A Literature Review. Mil Psychol 29: 117–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Liu F, Xie B, Wang Y, Guo W, Fouche J-P, Long Z, et al. (2015): Characterization of Post-traumatic Stress Disorder Using Resting-State fMRI with a Multi-level Parametric Classification Approach. Brain Topogr 28: 221–237. [DOI] [PubMed] [Google Scholar]
- 113.Nicholson AA, Harricharan S, Densmore M, Neufeld RWJ, Ros T, McKinnon MC, et al. (2020): Classifying heterogeneous presentations of PTSD via the default mode, central executive, and salience networks with machine learning. NeuroImage: Clinical 27: 102262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Figueroa RL, Zeng-Treitler Q, Kandula S, Ngo LH (2012): Predicting sample size required for classification performance. BMC Med Inform Decis Mak 12: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Eaton WW, Smith C, Ybarra M, Muntaner C, Tien A (2004): Center for Epidemiologic Studies Depression Scale: review and revision (CESD and CESD-R). The Use of Psychological Testing for Treatment Planning and Outcomes Assessment, 3rd ed. Mahwah, NJ: Lawrence Erlbaum, pp 363–377. [Google Scholar]


