Abstract
The COVID-19 pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is a global crisis with unprecedented challenges for public health. Vaccinations against SARS-CoV-2 have slowed the incidence of new infections and reduced disease severity. As the time of day of vaccination has been reported to influence host immune responses to multiple pathogens, we quantified the influence of SARS-CoV-2 vaccination time, vaccine type, participant age, sex, and days post-vaccination on anti-Spike antibody responses in health care workers. The magnitude of the anti-Spike antibody response is associated with the time of day of vaccination, vaccine type, participant age, sex, and days post-vaccination. These results may be relevant for optimising SARS-CoV-2 vaccine efficacy.
Keywords: COVID-19, SARS-CoV-2, HCW, Spike antibody, time of day
The circadian clock is an endogenous 24-h clock that regulates many aspects of physiology, including the response to infectious disease and vaccination (Allada and Bass, 2021). A recent report demonstrated significant daytime variation in multiple immune parameters in >300,000 participants in the UK Biobank, highlighting the diurnal nature of innate and adaptive immune responses (Wyse et al., 2021). Human lung diseases frequently show time-of-day variation in symptom severity and respiratory function and the circadian transcriptional activator BMAL1 has been shown to regulate respiratory inflammation (Ehlers et al., 2018; Ince et al., 2019). Influenza A virus infection of circadian-arrhythmic mice is associated with elevated inflammatory responses and a higher viral burden (Edgar et al., 2016; Sengupta et al., 2019). The time of day of influenza vaccination in elderly men affected antibody responses with higher titres noted in the morning (Phillips et al., 2008; Long et al., 2016). An additional influenza vaccination study reported that the time of sample collection rather than vaccination had a more significant effect on antibody responses (Kurupati et al., 2017). We and others have proposed a role for circadian signalling in regulating SARS-CoV-2 host immune responses and COVID-19 severity (Ray and Reddy, 2020; Maidstone et al., 2021; Sengupta et al., 2021). Clearly, it is important to assess whether the time of SARS-CoV-2 vaccination impacts host antibody responses.
In the UK, health care workers were identified as a priority group to receive SARS-CoV-2 vaccine starting in December 2020. At this time, the Alpha B.1.1.7 variant was the dominant circulating strain. As part of this initiative, data were collected on all asymptomatic staff members (Eyre et al., 2021; Lumley et al., 2021) in keeping with enhanced hospital infection prevention and control guidelines issued by the UK Department of Health and Social Care. Anonymised data were obtained from the Infections in Oxfordshire Research Database with Research Ethics Committee approvals (19/SC/0403, ECC5-017 (A)/2009). Peripheral blood samples were collected during December 2020-February 2021 and were tested for anti-Spike (Abbott IgG assay) (Ainsworth et al., 2020) and anti-nucleocapsid (Abbott SARS-CoV-2 IgG anti-nucleocapsid assay) antibody levels. We analysed anti-Spike responses during the 2-10 weeks after vaccination. In this data set, 2190 people contributed one blood sample, 549 contributed two samples, and 45 three or more samples (total of 3425 samples). Participants with evidence of prior SARS-CoV-2 infection (PCR for viral RNA or anti-nucleocapsid antibody), samples with anti-Spike responses < 50 AU, and samples obtained after second vaccination were excluded.
Data from 2784 participants (Table 1) were analysed using linear mixed modelling to investigate the effects of time of vaccination on anti-Spike antibody levels. Variation between participants was modelled with fixed factors of time of day of vaccination (Time 1, 0700-1059 h; Time 2, 1100-1459 h; Time 3, 1500-2159 h) (Suppl. Fig. S1), vaccine type (Pfizer, mRNA bnt162b2 or AstraZeneca, Adenoviral AZD1222), age group (16-29, 30-39, 40-49, or 50-74 years), sex, and the number of days post-vaccination. A B-spline transformation of days post-vaccination was used to model the non-linear pattern of anti-Spike responses (log10 transformed) (Suppl. Fig. S2). This analysis allowed us to estimate the average anti-Spike levels in each participant group at 2 and 6 weeks post-vaccination (Figure 1).
Table 1.
Participant numbers.
| Age (Years) | Pfizer mRNA (Time 1/Time 2/Time 3) |
AstraZeneca Adenoviral (Time 1/Time 2/ Time 3) |
||
|---|---|---|---|---|
| Female | Male | Female | Male | |
| 16-29 | 90/143/163 | 18/26/26 | 39/54/53 | 11/12/10 |
| 30-39 | 100/146/149 | 30/46/40 | 38/44/34 | 10/7/8 |
| 40-49 | 120/160/170 | 17/36/42 | 43/56/43 | 8/11/8 |
| 50-74 | 127/152/199 | 24/26/38 | 68/52/59 | 7/4/7 |
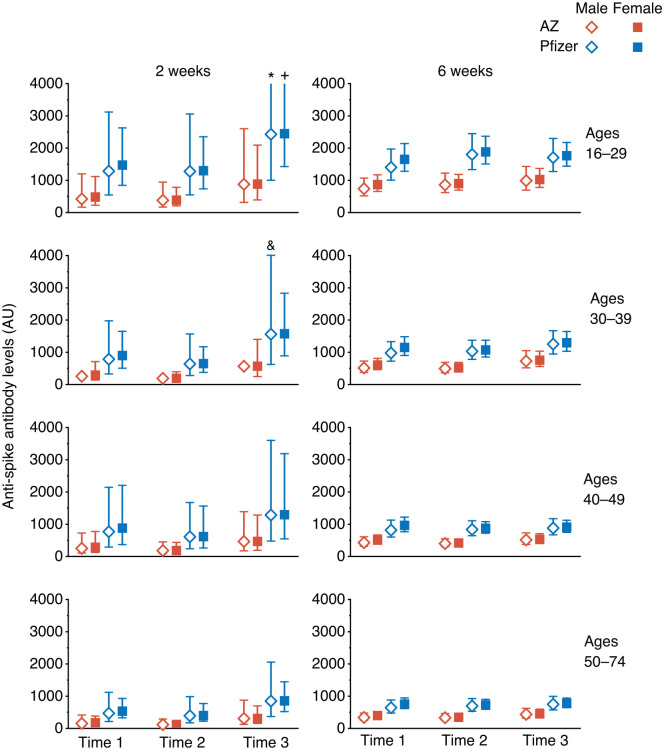
Figure 1.
Estimated Anti-Spike antibody levels at 2 and 6 weeks after first SARS-CoV-2 vaccination, partitioned by age, sex, and time of day of vaccination (Time 1, 0700-1059 h; Time 2, 1100-1459 h; Time 3, 1500-2059 h). Mean value (symbol) with 95% confidence values (vertical line). Three confidence intervals extend beyond the Y-axis limits (* = 4275, + = 5996 and & = 4028). Abbreviation: SARS-CoV-2 = severe acute respiratory syndrome coronavirus 2.
Using a linear mixed-model approach, we found that anti-Spike responses were higher in those who were vaccinated later in the day (p = 0.013), in those who received the Pfizer mRNA vaccine (p < 0.0001), in women (p = 0.013), and in younger participants (p < 0.0001) (Table 2). We observed significant interactions between days post-vaccination and vaccine type (p < 0.0001) and age (p = 0.032), but not with vaccine time (p = 0.238). Analysing the data using two time intervals (before or after 1 pm) gave similar results. We did not observe a significant effect of time of day of sample collection (using the same time intervals as for vaccination times) (p = 0.097), and this parameter was not included in the final model; results from the model including sample times are shown in Supplementary Table S1. Sixty-seven samples gave values beneath the cutoff (<50) in the anti-Spike assay and were classified as ‘non-responders’; we found no significant association with the time of day of vaccination for these samples (linear mixed-effects logistic regression, p = .23).
Table 2.
Type III tests of fixed effects from mixed-effects model.
| Effect | Num DF | F Value† | Probability |
|---|---|---|---|
| Main effects | |||
| Vaccination_Time (Time 2, Time 3 vs. Time 1)‡ |
2 | 4.33 | 0.0133 |
| Vaccine type (AstraZeneca vs. Pfizer) |
1 | 148.31 | <0.0001 |
| Age (30-39, 40-49, 50-74 vs.16-29) |
3 | 51.15 | <0.0001 |
| Sex (Female vs. Male) |
1 | 6.16 | 0.0131 |
| Days post-vaccination | 6 | 18.78 | <0.0001 |
| Interaction terms | |||
| Days × Vaccination_Time | 12 | 1.26 | 0.2380 |
| Days × Vaccine type | 6 | 7.24 | <0.0001 |
| Days × Age | 18 | 1.70 | 0.0319 |
| Days × sex | 6 | 1.03 | 0.4010 |
| Vaccination_Time × Vaccine type | 2 | 1.22 | 0.2945 |
| Vaccination_Time × Age | 6 | 0.71 | 0.6446 |
| Vaccination_Time × Sex | 2 | 0.44 | 0.6412 |
Details of the linear mixed modeling are: Time of vaccination (Time 1, 07:00-10:59; Time 2, 11:00-14:59; Time 3, 15:00-21:59), vaccine type (Pfizer mRNA or AstraZeneca Adenovirus), age groups (from Table 1A), sex, and days post-vaccination were treated as fixed factors. A B-spline transformation of days post-vaccination was used to model the non-linear pattern of anti-Spike antibody responses (log10 transformed) post vaccination.
Abbreviation: DF = Degrees of Freedom.
For all F tests the denominator DF was 3359.
For each F test, the fixed effect referent is the last term shown, the F and P values are the Type III tests of overall fixed effects.
Our analysis of 2784 health care workers reveals a significant effect of the time of vaccination on anti-Spike antibody levels following the administration of two alternative SARS-CoV-2 vaccines (mRNA or Adenovirus based). A recent report studying a small cohort of health care workers immunised with an inactivated SARS-CoV-2 vaccine in the morning (0900-1100 h, n = 33) or afternoon (1500 1700 h, n = 30) showed increased B-cell responses and anti-Spike antibodies in participants vaccinated in the morning (Zhang et al., 2021). This contrasts with our observations and may reflect the use of an inactivated whole virus immunogen that will likely induce polytypic responses to a range of SARS-CoV-2 encoded proteins. Our observation contrasts with earlier studies in elderly men that reported higher anti-influenza titers in the morning (Phillips et al., 2008; Long et al., 2016). This may reflect differences between the cohorts studied, particularly with regard to immune status; we studied seronegative participants whereas responses to influenza vaccination will involve the stimulation of memory responses. Sample collection time in this study showed no significant association with anti-Spike levels, in contrast to previous reports (Kurupati et al., 2017; McNaughton et al., 2021). These data highlight the importance of recording the time of vaccination in clinical and research studies, and highlight the importance of considering time-of-day factors in future study designs that may reduce inter-individual variance and the number of participants needed to obtain statistical significance.
Additional studies are warranted to evaluate the circadian regulation of natural and vaccine-induced SARS-CoV-2 immunity. McNaughton and colleagues reported a diurnal variation in SARS-CoV-2 PCR test results, showing a 2-fold variation in Ct values implying higher viral RNA levels in the afternoon (McNaughton et al., 2021). These data are consistent with our recent study showing a role for the circadian component BMAL1 in regulating SARS-CoV-2 replication (Zhuang et al., 2021) that could influence the induction of host innate and adaptive responses.
It is worth noting that despite the significant differences in anti-Spike levels detected in participants receiving Pfizer mRNA or AstraZeneca Adenoviral vaccines, both show comparable efficacies highlighting the robust nature of the host antibody response. Limitations of this retrospective observational study include: (a) relatively few participants had more than one anti-Spike antibody measurement, limiting our ability to study both longitudinal immune responses and the effect of time of day of sample collection; (b) the health profiles of our health care workers may differ from the general population and no information was available on their medical or medication history, except that they had no prior infection with SARS-CoV-2 and were seronegative; (c) there was limited serological sampling following second vaccination, precluding the analysis of time-of-day effects following a 2-dose schedule; (d) the extent to which anti-Spike levels are a correlate of clinical efficacy is not known; (e) the sleep and shift-work patterns of the participants, that are known to influence vaccine responses (Spiegel et al., 2002; Lange et al., 2003; Prather et al., 2021), were not available; and (e) our cohort does not include children or high-risk groups, such as the elderly or immunocompromised. We recommend future studies address these limitations when documenting natural and vaccine-induced SARS-CoV-2 immune responses.
Supplemental Material
Supplemental material, sj-docx-1-jbr-10.1177_07487304211059315 for Time of Day of Vaccination Affects SARS-CoV-2 Antibody Responses in an Observational Study of Health Care Workers by Wei Wang, Peter Balfe, David W. Eyre, Sheila F. Lumley, Denise O’Donnell, Fiona Warren, Derrick W. Crook, Katie Jeffery, Philippa C. Matthews, Elizabeth B. Klerman and Jane A. McKeating in Journal of Biological Rhythms
Supplemental material, sj-docx-2-jbr-10.1177_07487304211059315 for Time of Day of Vaccination Affects SARS-CoV-2 Antibody Responses in an Observational Study of Health Care Workers by Wei Wang, Peter Balfe, David W. Eyre, Sheila F. Lumley, Denise O’Donnell, Fiona Warren, Derrick W. Crook, Katie Jeffery, Philippa C. Matthews, Elizabeth B. Klerman and Jane A. McKeating in Journal of Biological Rhythms
Supplemental material, sj-pdf-3-jbr-10.1177_07487304211059315 for Time of Day of Vaccination Affects SARS-CoV-2 Antibody Responses in an Observational Study of Health Care Workers by Wei Wang, Peter Balfe, David W. Eyre, Sheila F. Lumley, Denise O’Donnell, Fiona Warren, Derrick W. Crook, Katie Jeffery, Philippa C. Matthews, Elizabeth B. Klerman and Jane A. McKeating in Journal of Biological Rhythms
Acknowledgments
We thank Helene Borrmann, Xiaodong Zhuang, and Tanya Wilson for early discussions on this project, and Tess Lambe and Merryn Voysey for their constructive comments on the article. We also thank all OUH staff who participated in the staff testing programme and the staff and medical students who ran the programme. This work uses data provided by health care workers and collected by the UK’s National Health Service as part of their care and support. We thank all the people of Oxfordshire who contributed to the Infections in Oxfordshire Research Database. Research Database Team: L Butcher, H Boseley, C Crichton, O Freeman, J Gearing (community), R Harrington, M Landray, A Pal, TEA Peto, TP Quan, J Robinson (community), J Sellors, B Shine, AS Walker, D Waller. Patient and Public Panel: G Blower, C Mancey, P McLoughlin, and B Nichols.
EBK is funded by NIH K24-HL105664, P01-AG009975, and R01-HL128538. JAM is funded by a Wellcome Investigator Award 200838/Z/16/Z, UK Medical Research Council project grant MR/R022011/1, and Chinese Academy of Medical Sciences Innovation Fund for Medical Science, China (grant number: 2018-I2M-2-002). WW is funded by NCATS Harvard Clinical and Translational Science Center grant 5UL1TR002541-02. DWE is a Robertson Foundation Fellow. DCA is funded by the NIHR Oxford Biomedical Research Centre. The report presents independent research. The views expressed in this publication are those of the authors and not necessarily those of the NHS, NIHR, or the UK Department of Health. PCM is funded by a Wellcome intermediate fellowship grant Ref 110110/Z/15/Z.
Supplementary material is available for this article online.
Footnotes
Conflict of Interest Statement: WW has a consultancy for the National Sleep Foundation. PB has no relevant disclosures. DWE declares lecture fees from Gilead, outside the submitted work. EBK declares travel support from Gordon Research Conference, Sleep Research Society, Santa Fe institute, DGSM (German Sleep Society); consultancy for Circadian Therapeutics, National Sleep Foundation, Puerto Rico Science Technology Trust, Sanofi-Genzyme; partner owns Chronsulting. JAM has no relevant disclosures.
ORCID iD: Jane A. McKeating  https://orcid.org/0000-0002-7229-5886
https://orcid.org/0000-0002-7229-5886
References
- Ainsworth M, Andersson M, Auckland K, Baillie JK, Barnes E, Beer S, Beveridge A, Bibi S, Blackwell L, Borak M, et al. (2020) Performance characteristics of five immunoassays for SARS-CoV-2: a head-to-head benchmark comparison. Lancet Infect Dis 20:1390-1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allada R, Bass J. (2021) Circadian mechanisms in medicine. N Engl J Med 384:550-561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar RS, Stangherlin A, Nagy AD, Nicoll MP, Efstathiou S, O’Neill JS, Reddy AB. (2016) Cell autonomous regulation of herpes and influenza virus infection by the circadian clock. PNAS 113:10085-10090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers A, Xie W, Agapov E, Brown S, Steinberg D, Tidwell R, Sajol G, Schutz R, Weaver R, Yu H, et al. (2018) BMAL1 links the circadian clock to viral airway pathology and asthma phenotypes. Mucosal Immunol 11:97-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyre DW, Lumley SF, Wei J, Cox S, James T, Justice A, Jesuthasan GO’ Donnell D, Howarth A, Hatch SB, et al. (2021) Quantitative SARS-CoV-2 anti-spike responses to Pfizer-BioNTech and Oxford-AstraZeneca vaccines by previous infection status. Clin Microbiol Infect 27:1516.e7-1516.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ince LM, Zhang Z, Beesley S, Vonslow RM, Saer BR, Matthews LC, Begley N, Gibbs JE, Ray DW, Loudon ASI. (2019) Circadian variation in pulmonary inflammatory responses is independent of rhythmic glucocorticoid signaling in airway epithelial cells. FASEB J 33:126-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurupati RK, Kossenkoff A, Kannan S, Haut LH, Doyle S, Yin X, Schmader KE, Liu Q, Showe L, Ertl HCJ. (2017) The effect of timing of influenza vaccination and sample collection on antibody titers and responses in the aged. Vaccine 35:3700-3708. [DOI] [PubMed] [Google Scholar]
- Lange T, Perras B, Fehm HL, Born J. (2003) Sleep enhances the human antibody response to hepatitis A vaccination. Psychosom Med 65:831-835. [DOI] [PubMed] [Google Scholar]
- Long JE, Drayson MT, Taylor AE, Toellner KM, Lord JM, Phillips AC. (2016) Morning vaccination enhances antibody response over afternoon vaccination: a cluster-randomised trial. Vaccine 34:2679-2685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumley SF, Wei J, O’Donnell D, Stoesser NE, Matthews PC, Howarth A, Hatch SB, Marsden BD, Cox S, James T, et al. (2021) The duration, dynamics, and determinants of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) antibody responses in individual healthcare workers. Clin Infect Dis 73:e699-e709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNaughton CD, Adams NM, Johnson CH, Ward MJ, Lasko TA. (2021) Diurnal variation in SARS-CoV-2 PCR test results: test accuracy may vary by time of day. J Biol Rhythms 36:595-601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maidstone R, Anderson SG, Ray DW, Rutter MK, Durrington HJ, Blaikley JF. (2021) Shift work is associated with positive COVID-19 status in hospitalised patients. Thorax 76:601-606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips AC, Gallagher S, Carroll D, Drayson M. (2008) Preliminary evidence that morning vaccination is associated with an enhanced antibody response in men. Psychophysiology 45:663-666. [DOI] [PubMed] [Google Scholar]
- Prather AA, Pressman SD, Miller GE, Cohen S. (2021) Temporal links between self-reported sleep and antibody responses to the influenza vaccine. Int J Behav Med 28:151-158. [DOI] [PubMed] [Google Scholar]
- Ray S, Reddy AB. (2020) COVID-19 management in light of the circadian clock. Nat Rev Mol Cell Biol 21:494-495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengupta S, Ince L, Sartor F, Borrmann H, Zhuang X, Naik A, Curtis A, McKeating JA. (2021) Clocks, viruses, and immunity: lessons for the COVID-19 pandemic. J Biol Rhythms 36:23-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengupta S, Tang SY, Devine JC, Anderson ST, Nayak S, Zhang SL, Valenzuela A, Fisher DG, Grant GR, Lopez CB, et al. (2019) Circadian control of lung inflammation in influenza infection. Nature Commun 10:4107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegel K, Sheridan JF, Van Cauter E. (2002) Effect of sleep deprivation on response to immunization. JAMA 288:1471-1472. [DOI] [PubMed] [Google Scholar]
- Wyse C, O’Malley G, Coogan AN, McConkey S, Smith DJ. (2021) Seasonal and daytime variation in multiple immune parameters in humans: evidence from 329,261 participants of the UK Biobank cohort. iScience 24:102255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Liu Y, Liu D, Zeng Q, Li L, Zhou Q, Li M, Mei J, Yang N, Mo S, et al. (2021) Time of day influences immune response to an inactivated vaccine against SARS-CoV-2. Cell Res 31:1215-1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang X, Tsukuda S, Wrensch F, Wing PA, Schilling M, Harris JM, Borrmann H, Morgan SB, Cane JL, Mailly L, et al. (2021) The circadian clock component BMAL1 regulates SARS-CoV-2 entry and replication in lung epithelial cells. iScience 24:103144. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-jbr-10.1177_07487304211059315 for Time of Day of Vaccination Affects SARS-CoV-2 Antibody Responses in an Observational Study of Health Care Workers by Wei Wang, Peter Balfe, David W. Eyre, Sheila F. Lumley, Denise O’Donnell, Fiona Warren, Derrick W. Crook, Katie Jeffery, Philippa C. Matthews, Elizabeth B. Klerman and Jane A. McKeating in Journal of Biological Rhythms
Supplemental material, sj-docx-2-jbr-10.1177_07487304211059315 for Time of Day of Vaccination Affects SARS-CoV-2 Antibody Responses in an Observational Study of Health Care Workers by Wei Wang, Peter Balfe, David W. Eyre, Sheila F. Lumley, Denise O’Donnell, Fiona Warren, Derrick W. Crook, Katie Jeffery, Philippa C. Matthews, Elizabeth B. Klerman and Jane A. McKeating in Journal of Biological Rhythms
Supplemental material, sj-pdf-3-jbr-10.1177_07487304211059315 for Time of Day of Vaccination Affects SARS-CoV-2 Antibody Responses in an Observational Study of Health Care Workers by Wei Wang, Peter Balfe, David W. Eyre, Sheila F. Lumley, Denise O’Donnell, Fiona Warren, Derrick W. Crook, Katie Jeffery, Philippa C. Matthews, Elizabeth B. Klerman and Jane A. McKeating in Journal of Biological Rhythms