Abstract
Post-transplant cyclophosphamide (PTCy) combined with tacrolimus (TAC) as graft-versus-host disease (GvHD) prophylaxis post-hematopoietic cell transplantation (HCT) is safe and effective. Optimal serum levels of TAC in this combination remain undetermined. We hypothesized that TAC at initial steady state (TISS) of <10 ng/mL could promote optimal transplant outcomes and prevent TAC-associated toxicities. We retrospectively analyzed a consecutive case series of 210 patients who received PTCy/TAC-based prophylaxis post-HCT from 1/2013–6/2018. Patients received HCT from haploidentical (n=172) or mismatched donors (n=38), and flat dose (FD) or weight-based dose (WBD) TAC. Twenty-four-month overall survival (OS), disease free survival (DFS), and relapse rate (RR) were 61%, 56%, and 22%, respectively, in TISS <10 ng/mL cohort (n=176), and 50%, 43%, and 35%, respectively, in TISS ≥10 ng/mL cohort (n=34) (OS, P=0.71; DFS, P=0.097; RR, P=0.031). OS, DFS, RR, non-relapse mortality, acute GvHD grade II-IV, grade III-IV or chronic GvHD by TISS were similar in multivariable analysis. TISS ≥10 ng/mL conferred increased risk of viral infection (P=0.003). More patients receiving FD vs. WBD had TISS <10 ng/mL (P=0.001). Overall, TISS <10 ng/mL early post HCT conferred similar survival outcomes and lowered risk of viral infection and toxicities compared to TISS ≥10 ng/mL.
INTRODUCTION
Allogeneic hematopoietic cell transplantation (HCT) remains the only potential curative treatment for many hematologic and immunologic diseases. However, prolonged time to identify appropriate matched donors and limited availability of HLA-matched donors, especially for minority and multiracial patient populations, are significant limitations for HCT(1, 2). The recent introduction of post-transplant cyclophosphamide (PTCy) as graft-versus-host disease (GvHD) prophylaxis has overcome the barrier of HLA matching, leading to increased use of mismatched related (haploidentical; Haplo) donors or unrelated donors(3, 4). PTCy-based GvHD prophylaxis is safe and promising in transplants using bone marrow (BM)(2) or peripheral blood stem cells (PBSC)(5) grafts. Multiple clinical studies (e.g. NCT03959241) are currently ongoing to assess PTCy in different HCT donor types(3, 4, 6–8).
PTCy targets alloreactive T cells while sparing stem cells and immunity to infection. PTCy may be combined with other immunosuppressants, including tacrolimus (TAC) and mycophenolate mofetil (MMF), that work through distinct mechanisms of action. TAC is a calcineurin inhibitor that inhibits T-cell activation by binding to FK506 binding intracellular proteins, and MMF is a prodrug of mycophenolic acid that prevents T- and B-cell proliferation by depleting guanosine nucleotides. The PTCy, TAC and MMF combination can effectively prevent GvHD; however, the optimal serum level of TAC in this combination has not been determined. Since TAC has a narrow therapeutic window and supratherapeutic levels are associated with numerous toxicities including renal impairment, neurotoxicity and posterior reversible encephalopathy syndrome (PRES)(9–14), it is crucial to balance TAC serum concentration to provide sufficient immunosuppression while avoiding toxicities. Target serum levels of TAC combined with methotrexate (10–20 ng/mL TAC)(15, 16) and sirolimus (5–10 ng/mL TAC)(13, 17) for HCT with matched-related or matched-unrelated donors have been established(9, 12–14), and provide guidance for serum levels in other combinations. Because PTCy inhibits alloreactive T cells and TAC broadly inhibits T-cell activation, we hypothesized that lower TAC serum levels (<10 ng/mL) could promote optimal transplant outcomes without increasing toxicity. While we did not aim to achieve a specific TAC value, we identified 10 ng/mL as a clinically relevant cut off for serum TAC levels for our analyses based on TAC use in other combinations(9, 12–14).
Weight-based dosing (WBD) has been utilized with TAC when combined with sirolimus or methotrexate(12, 17), while an initial flat dose (FD) of 1 mg TAC has been used in PTCy-based regimens(2, 18). However, TAC dosing is not standardized in PTCy-based regimens, and it is unknown whether different dosing strategies correlate with specific therapeutic TAC levels at initial steady state (TISS).
We assessed the use of TAC in a PTCy-based GvHD prophylaxis regimen in patients who received HCT. We described transplant outcomes and toxicity focusing on the effect of TISS (<10 ng/mL vs. ≥10 ng/mL) and the dosing method used to achieve TISS.
SUBJECTS AND METHODS
Patients and study design
This retrospective study was approved by the City of Hope (COH) Institutional Review Board. We retrospectively identified a consecutive case series of 210 patients who received their first allogeneic HCT with PTCy, TAC and MMF as GvHD prophylaxis at COH from January 1, 2013–June 30, 2018. Patients who received PTCy but did not initiate TAC by day +7 post-HCT or discontinued TAC before achieving TISS, as defined below, were excluded. Serum levels of TAC were assessed twice weekly from the day following initiation of TAC until discontinuation per our institution standard of practice. Peak and trough levels were not routinely measured. TISS was defined as the first serum level >48 hours post-initiation of TAC, which is ~4–5 times the TAC half-life (12 hours).
Conditioning regimen, GVHD regimen and supportive care
Conditioning regimens were selected based on patient age, comorbidities, disease type and disease status at HCT. Myeloablative (MAC) regimens included total body irradiation (TBI)-based when TBI dose was >800 cGy (e.g. fludarabine/fractionated TBI and fludarabine/cyclophosphamide/total marrow and lymph node irradiation [TMLI]) and non-TBI-based regimens (e.g. busulfan/fludarabine and busulfan/fludarabine/cyclophosphamide). Reduced intensity/non-myeloablative conditioning (RIC/NMA) included fludarabine/cyclophosphamide/TBI, fludarabine/melphalan and fludarabine/melphalan/TBI.
GvHD prophylaxis was PTCy (50 mg/kg) on day +3 and +4, TAC at WBD (0.02–0.03 mg/kg) or FD (1 mg), selected randomly per discretion of the treating physician, and MMF (15 mg/kg or 1000 mg maximum TID) both starting on day +5 post-HCT through day +90, or through day +35 in absence of severe GvHD. Patients received continuous intravenous infusion of TAC until engraftment, and then switched to equivalent oral dose in patients capable of tolerating oral administration. Granulocyte-colony stimulating factor (5 mcg/kg/day) was started on day +5 until absolute neutrophil count (ANC) reached 1500 cells/mm3 for 3 consecutive days. All patients completed a pre-transplant workup and met creatinine clearance >60 ml/min.
All patients received supportive care and antimicrobial prophylaxis for bacterial, fungal, viral and Pneumocystitis jiroveci (PJP) infection per institutional practice. Patients were tested weekly for cytomegalovirus (CMV) infection and treated preemptively if CMV was detectable by polymerase chain reaction; 18 patients were started on CMV prophylaxis (letermovir) for CMV seropositivity per a change in our institutional practice in March 2018.
Endpoints
The primary endpoint was disease-free survival (DFS) after HCT. Secondary endpoints were acute GvHD (aGvHD), chronic GVHD (cGvHD), relapse rate (RR), non-relapse mortality (NRM), GvHD-free relapse-free survival (GRFS) and overall survival (OS).
DFS was defined as time from HCT to first observation of disease relapse or death from any cause without evidence of disease. DFS was censored at last follow-up if patients remained alive and disease-free. aGvHD and cGvHD were graded according to established criteria(19, 20). RR and NRM were defined as incidence of relapse and death from any cause without evidence of relapse, respectively; relapse and NRM were competing risk events and were censored at last follow-up if patients were alive and free of relapse. GRFS was defined as time from transplant to first observation of the following: grade III-IV aGvHD, moderate/severe cGvHD, relapse or death, and it was censored at last follow-up if patients were alive and free of any aforementioned events. OS was defined as time from HCT to death from any cause and was censored at last follow-up if the patient was alive.
Statistical methods
Wilcoxon tests and chi-square tests were used to compare differences in baseline demographic, disease, and transplant by TISS (<10 ng/mL vs. ≥10 ng/mL). OS, DFS, and GRFS were analyzed using Kaplan-Meier curves and log-rank tests in univariate analyses. Cumulative incidence curves and Gray’s tests were used for RR, NRM, aGvHD, cGvHD, and infections. Association between TAC dosing method and TISS was examined by chi-square test. Multivariable Cox proportional hazards regression models were used for OS, DFS, and GRFS when adjusting for baseline characteristics. Multivariable Fine and Gray proportional hazards regression models were used to assess RR, NRM, aGvHD, cGvHD, and infections when controlling for baseline characteristics. Stepwise selection was used to choose covariates that were significantly associated with outcomes at 0.1 level in the multivariable models. For multivariable analyses, TISS was categorized as <10 ng/mL as the reference group and ≥10 ng/mL as comparison group. P-values were 2 sided at a significance level of 0.05. Analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC). The sample size was chosen to have 80% power to detect a clinically meaningful difference (HR=2.0) in DFS by TISS using a 0.05 level two-sided log-rank test.
RESULTS
Patient characteristics
Patient characteristics are shown in Table 1. Patients received HCT from haploidentical (n=172) or mismatched donors (n=38). Median age was 49 years (range, 4–73), and 122 of 210 (58.1%) patients were male. Eighty-nine of 210 (42.4%) patients received MAC, with 71 of 89 (79.8%) patients receiving a TBI-based regimen. The primary diagnosis of 125 of 210 (59.5%) patients was leukemia. The graft source for 166 of 210 (79%) patients was PBSC, while 44 of 210 (21%) patients received BM.
Table 1.
Patient Characteristics
| Tacrolimus level at initial steady state | ||||
|---|---|---|---|---|
| <10 ng/mL (N=176) | ≥10 ng/mL (N=34) | Total (N=210) | p value* | |
| Age at HSCT, years | 0.008 | |||
| Median | 52 | 39 | 49 | |
| Range | (4–73) | (8–65) | (4–73) | |
| Recipient sex, n (%) | 0.071 | |||
| Male | 107 (60.8%) | 15 (44.1%) | 122 (58.1%) | |
| Female | 69 (39.2%) | 19 (55.9%) | 88 (41.9%) | |
| Female donor to male recipient, n (%) | 0.31 | |||
| Yes | 31 (17.6%) | 3 (8.8%) | 34 (16.2%) | |
| No | 145 (82.4%) | 31 (91.2%) | 176 (83.8%) | |
| Donor age | 0.68 | |||
| Median | 33 | 32 | 33 | |
| Range | (10–68) | (14–49) | (10–68) | |
| Primary diagnosis HCT, n (%) | 0.023 | |||
| AML | 68 (38.6%) | 10 (29.4%) | 78 (37.1%) | |
| ALL | 35 (19.9%) | 12 (35.3%) | 47 (22.4%) | |
| MDS/CML/MPN | 42 (23.9%) | 2 (5.9%) | 44 (21%) | |
| Lymphoma | 16 (9.1%) | 5 (14.7%) | 21 (10%) | |
| Non-Malignant | 15 (8.5%) | 5 (14.7%) | 20 (9.5%) | |
| DRI, n (%) | 0.32 | |||
| Low | 29 (16.5%) | 2 (5.9%) | 31 (14.8%) | |
| Intermediate | 68 (38.6%) | 13 (38.2%) | 81 (38.6%) | |
| High/Very high | 64 (36.4%) | 14 (41.2%) | 78 (37.1%) | |
| Non-Malignant | 15 (8.5%) | 5 (14.7%) | 20 (9.5%) | |
| Karnofsky performance status %, n (%) | 0.44 | |||
| 80–100 | 146 (83%) | 30 (88.2%) | 176 (83.8%) | |
| <80 | 30 (17%) | 4 (11.8%) | 34 (16.2%) | |
| HCT comorbidity index, n (%) | 0.78 | |||
| 0 | 54 (30.7%) | 12 (35.3%) | 66 (31.4%) | |
| 1–2 | 49 (27.8%) | 10 (29.4%) | 59 (28.1%) | |
| ≥3 | 73 (41.5%) | 12 (35.3%) | 85 (40.5%) | |
| Donor Type | 0.051 | |||
| Haploidentical | 140 (79.5%) | 32 (94.1%) | 172 (81.9%) | |
| Mismatch unrelated | 36 (20.5%) | 2 (5.9%) | 38 (18.1%) | |
| Graft source | <0.001 | |||
| Peripheral Stem Cells | 150 (85.2%) | 16 (47.1%) | 166 (79%) | |
| Bone Marrow | 26 (14.8%) | 18 (52.9%) | 44 (21%) | |
| ABO blood group compatibility, n (%) | 0.76 | |||
| ABO compatible | 116 (65.9%) | 23 (67.6%) | 139 (66.2%) | |
| Minor mismatch (donor is O) | 26 (14.8%) | 6 (17.6%) | 32 (15.2%) | |
| Major mismatch (Recipient is O) | 20 (11.4%) | 4 (11.8%) | 24 (11.4%) | |
| Bidirectional (None are O) | 14 (8%) | 1 (2.9%) | 15 (7.1%) | |
| Donor/Recipient CMV serostatus, n (%) | 0.064 | |||
| D−/R− | 18 (10.2%) | 2 (5.9%) | 20 (9.5%) | |
| D−/R+ | 43 (24.4%) | 4 (11.8%) | 47 (22.4%) | |
| D+/R− | 8 (4.5%) | 5 (14.7%) | 13 (6.2%) | |
| D+/R+ | 107 (60.8%) | 23 (67.6%) | 130 (61.9%) | |
| Conditioning regimen, n (%) | 0.55 | |||
| MAC | 73 (41.5%) | 16 (47.1%) | 89 (42.4%) | |
| TBI-based | 60 (82.2%) | 11 (68.8%) | 71 (79.8%) | |
| Not TBI-based | 13 (17.8%) | 5 (31.3%) | 18 (20.2%) | |
| RIC/NMA | 103 (58.5%) | 18 (52.9%) | 121 (57.6%) | |
| GvHD prophylaxis, n (%) | 1.00 | |||
| PTCy / TAC / MMF | 174 (98.9%) | 34 (100%) | 208 (99%) | |
| PTCy / TAC | 2 (1.1%) | 0 (0%) | 2 (1%) | |
| Year of HCT, n (%) | 0.10 | |||
| ≤2016 | 87 (49.4%) | 22 (64.7%) | 109 (51.9%) | |
| >2016 | 89 (50.6%) | 12 (35.3%) | 101 (48.1%) | |
| Letermovir | 0.081 | |||
| No | 159 (90.3%) | 34 (100%) | 193 (91.9%) | |
| Yes | 17 (9.7%) | 0 (0%) | 17 (8.1%) | |
| Tacrolimus initial dosing method, n (%) | 0.001 | |||
| Flat dose | 118 (67%) | 13 (38.2%) | 131 (62.4%) | |
| Weight base dose | 58 (33%) | 21 (61.8%) | 79 (37.6%) | |
P value was based on two-sample Wilcoxon test for age and donor age, Fisher’s exact test or χ2 test for other characteristics.
Overall, 176 of 210 (83.8%) had TISS <10 ng/mL and 34 of 210 (16.2%) had TISS ≥10 ng/mL. The TISS <10 ng/mL cohort included patients with subtherapeutic TISS (i.e. <5 ng/mL). Patients with TISS ≥10 ng/mL were younger than those with <10 ng/mL (median age 39 vs. 52 years, respectively; P=0.008). The majority of patients across both cohorts had leukemia (103 of 176 [58.5%] for TISS <10 ng/mL and 22 of 34 [64.7%] for TISS ≥10 ng/mL); however, there were more patients with myelodysplastic syndrome/chronic myeloid leukemia/myeloproliferative neoplasm (23.9 vs. 5.9%), but fewer patients with non-malignant diseases (8.5% vs. 14.7%) in the TISS <10 ng/mL vs. TISS ≥10 ng/mL group, respectively. The majority with TISS <10 ng/mL received PBSC grafts (150 of 176 [85.2%] patients), and 18 of 34 (52.9%) of patients with TISS ≥10 ng/mL received BM grafts (P<0.001). The majority (131 of 210 [62.4%] patients) received FD TAC, while 79 of 210 (37.6%) patients received WBD TAC. More patients with TISS <10 ng/mL received FD than WBD TAC (67% vs. 33%), whereas more patients with TISS ≥10 ng/mL received WBD than FD TAC (61.8% vs. 38.2%) (P=0.001).
Engraftment
Median time to neutrophil engraftment was 17 days (range, 12–35) and was not statistically different by TISS (at day 28: 94% in <10 ng/mL vs. 88% in ≥10 ng/mL; Gray’s test P=0.24) (Supplemental Table 1). Median time to platelet engraftment was 28 days (range, 8–101) and was not statistically different by TISS (at day 42: 80% in <10 ng/mL vs 74% in ≥10 ng/mL; Gray’s test p=0.27).
Relapse & NRM
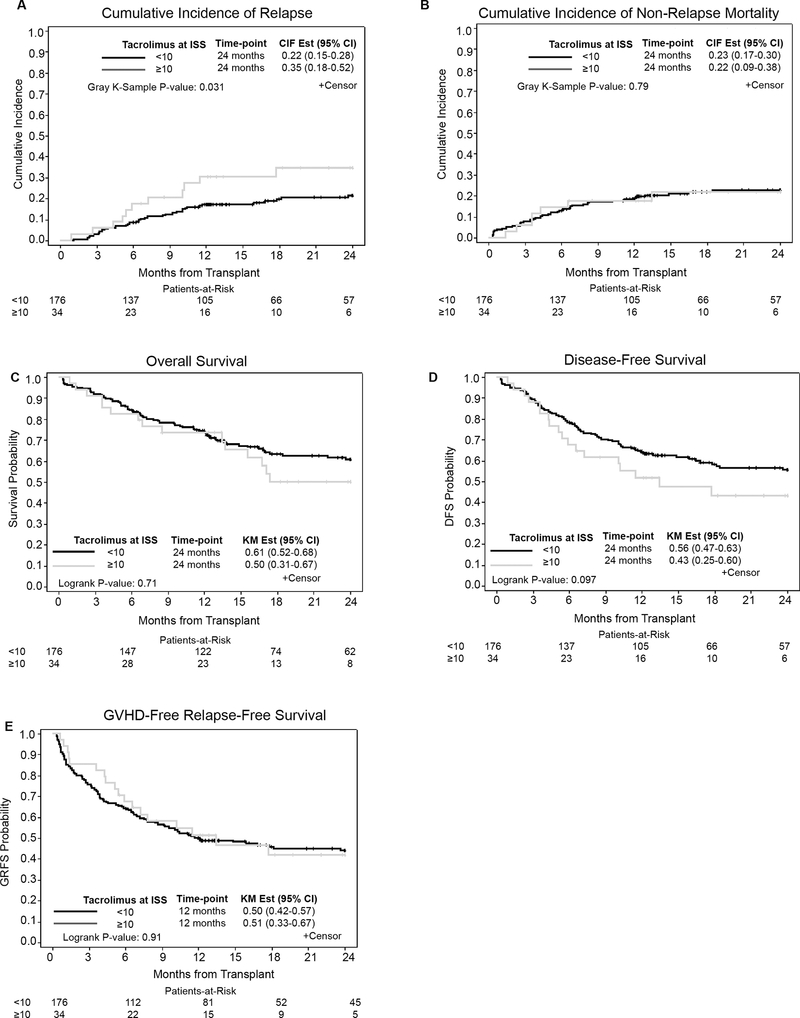
Two-year RR was 24% overall (95% CI: 18%−30%). In univariate analysis (Supplemental Table 1), patients with TISS <10 ng/mL had significantly lower RR than patients with TISS ≥10 ng/mL (22% vs. 35%, P=0.031) (Figure 1A). However, the association between RR and TISS was not significant in multivariable regression models when adjusting for disease risk, graft source and conditioning regimen (Table 2). In multivariable analysis, high/very high disease risk index (28.6% vs. 16.1% or 16.4%, HR=5.47, 95% CI:1.59–18.77, P=0.014) and BM graft source were associated with increased risk of relapse (30.1% vs. 16.5%, HR=3.63, 95% CI:1.81–7.29, P<0.001). Overall cumulative incidence of NRM at 24 months (Figure 1B) was 23% (95% CI: 17%−29%) and was similar in both cohorts (23% in <10 ng/mL and 22% in ≥10 ng/mL; P=0.79).
Figure 1.
Outcomes comparing <10 ng/mL vs. ≥10 ng/mL TAC at ISS cohorts. Cumulative incidence of A) relapse and B) Non-relapse mortality. Kaplan Meier curves of C) Overall survival, D) Disease-free survival and E) GVHD-Free Relapse-Free survival.
Table 2.
Multivariable Analysis of Engraftment, Relapse and Non-Relapse Mortality
| Neutrophil Engraftment* | Relapse† | Non-Relapse Mortality‡ | ||||||
|---|---|---|---|---|---|---|---|---|
| N | HR (95%CI) | P | HR (95%CI) | P | HR (95%CI) | P | ||
| Age | <60 | 144 | Reference | 0.058 | Reference | 0.75 | Reference | 0.005 |
| ≥60 | 66 | 0.72(0.52,1.01) | 0.89(0.46,1.75) | 3.42(1.45,8.07) | ||||
| Sex | M | 122 | Reference | 0.57 | Reference | 0.054 | Reference | 0.096 |
| F | 88 | 1.08(0.83,1.42) | 0.56(0.31,1.01) | 1.65(0.92,2.97) | ||||
| Female donor to male recipient | No | 176 | Reference | 0.83 | Reference | 0.16 | Reference | 0.42 |
| Yes | 34 | 0.97(0.71,1.32) | 1.59(0.83,3.04) | 0.71(0.31,1.63) | ||||
| Disease Risk Index | Low | 31 | Reference | 0.26 | Reference | 0.014 | Reference | 0.14 |
| Intermediate | 81 | 0.74(0.51,1.06) | 4.01(0.99,16.29) | 3.23(0.97,10.73) | ||||
| High/Very high | 78 | 0.84(0.58,1.22) | 5.47(1.59,18.77) | 3.18(0.97,10.49) | ||||
| Conditioning regimen | MAC-TBI | 71 | Reference | 0.51 | Reference | 0.21 | Reference | 0.77 |
| MAC-NonTBI | 18 | 1.01(0.61,1.68) | 0.23(0.05,1.19) | 0.61(0.14,2.68) | ||||
| RIC/NMA | 121 | 0.83(0.57,1.20) | 0.75(0.39,1.44) | 0.80(0.30,2.12) | ||||
| Donor type | Haploidentical | 172 | Reference | 0.058 | Reference | 0.076 | Reference | 0.57 |
| Mismatched unrelated | 38 | 1.40(0.99,1.99) | 0.34(0.10,1.12) | 1.23(0.60,2.49) | ||||
| ABO blood group compatibility | ABO Compatible | 139 | Reference | 0.18 | Reference | 0.63 | Reference | 0.29 |
| Minor | 32 | 1.50(1.04,2.17) | 0.98(0.52,1.86) | 0.31(0.09,1.05) | ||||
| Major | 24 | 1.05(0.75,1.49) | 0.50(0.17,1.43) | 0.75(0.31,1.79) | ||||
| Bidirectional | 15 | 1.18(0.70,1.99) | 0.97(0.32,2.96) | 0.94(0.31,2.87) | ||||
| Donor CMV serostatus | D+ | 143 | Reference | 0.74 | Reference | 0.48 | Reference | 0.23 |
| D− | 67 | 1.05(0.79,1.40) | 1.23(0.69,2.17) | 0.66(0.34,1.30) | ||||
| Recipient CMV serostatus | R+ | 177 | Reference | 0.70 | Reference | 0.81 | Reference | 0.30 |
| R− | 33 | 1.09(0.70,1.70) | 0.90(0.38,2.13) | 0.59(0.21,1.61) | ||||
| Graft source | Peripheral blood stem cells | 166 | Reference | 0.047 | Reference | <0.001 | Reference | 0.47 |
| Bone marrow | 44 | 0.70(0.50,1.00) | 3.63(1.81,7.29) | 0.73(0.32,1.68) | ||||
| Karnofsky Performance Status, % | ≥80 | 176 | Reference | 0.76 | Reference | 0.37 | Reference | 0.13 |
| <80 | 34 | 0.94(0.63,1.40) | 1.41(0.66,2.99) | 1.69(0.86,3.32) | ||||
| HCT-Comorbidity Index | 0–2 | 125 | Reference | 0.037 | Reference | 0.85 | Reference | 0.16 |
| ≥3 | 85 | 0.74(0.56,0.98) | 1.05(0.62,1.81) | 1.51(0.85,2.68) | ||||
| Tacrolimus initial dosing method | Flat | 131 | Reference | 0.59 | Reference | 0.34 | Reference | 0.41 |
| Weight-based | 79 | 1.08(0.82,1.43) | 1.31(0.75,2.27) | 1.27(0.72,2.25) | ||||
| Tacrolimus at initial steady state, ng/mL | <10 | 176 | Reference | 0.36 | Reference | 0.53 | Reference | 0.80 |
| ≥10 | 34 | 0.83(0.56,1.23) | 1.25(0.62,2.52) | 1.11(0.49,2.54) | ||||
Adjusted for Age, Graft source, HCT-Comorbidity Index and Conditioning regimen
Adjusted for Disease Risk Index, Graft source and Conditioning regimen
Adjusted for Age and Conditioning regimen
Survival Outcomes
With median follow-up of 24 months (range: 6.0–61.7), the 2-year OS and DFS were 59% (95% CI: 51%−66%) and 54% (95% CI: 46%−61%), respectively. There was no difference in OS by TISS (61% for <10 ng/mL vs. 50% for ≥10 ng/mL, P=0.71) (Figure 1C). Patients with TISS <10 ng/mL vs. ≥10 ng/mL had a trend of higher DFS (56% vs. 43%, respectively P=0.097) (Figure 1D). However, no significant associations were found between TISS and OS or DFS in multivariable analysis (Table 3). Overall 1-year GRFS was 50% (95% CI: 43%−56%). There was no difference in GRFS by TISS (Table 3). At 12 months, GRFS was 50% (95% CI: 42%−57%) for <10 ng/mL and 51% (95% CI: 33%−67%) for ≥10 ng/mL (P=0.91) (Figure 1E). Full univariate and multivariable analyses of survival outcomes are in Supplemental Table 2 and Table 3, respectively.
Table 3.
Multivariable Analysis of Survival Outcomes
| Overall Survival* | GvHD-Free, Relapse-Free Survival† | Disease-Free Survival‡ | ||||||
|---|---|---|---|---|---|---|---|---|
| N | HR (95%CI) | Wald test P | HR (95%CI) | Wald test P | HR (95%CI) | Wald test P | ||
| Age | <60 | 144 | Reference | 0.009 | Reference | 0.34 | Reference | 0.009 |
| ≥60 | 66 | 2.15(1.21,3.82) | 1.26(0.79,2.02) | 2.10(1.20,3.67) | ||||
| Sex | M | 122 | Reference | 0.38 | Reference | 0.57 | Reference | 0.83 |
| F | 88 | 1.22(0.78,1.91) | 1.11(0.77,1.61) | 1.05(0.69,1.59) | ||||
| Female donor to male recipient | No | 176 | Reference | 0.35 | Reference | 0.88 | Reference | 0.88 |
| Yes | 34 | 0.74(0.40,1.38) | 0.97(0.60,1.55) | 0.96(0.56,1.64) | ||||
| Disease Risk Index | Low | 31 | Reference | 0.018 | Reference | 0.002 | Reference | <0.001 |
| Intermediate | 81 | 4.42(1.41,13.87) | 4.45(1.71,11.59) | 6.36(2.20,18.38) | ||||
| High/Very high | 78 | 4.48(1.59,12.64) | 4.85(2.03,11.57) | 7.83(2.98,20.58) | ||||
| Conditioning regimen | MAC-TBI | 71 | Reference | 0.58 | Reference | 0.14 | Reference | 0.37 |
| MAC-NonTBI | 18 | 0.52(0.15,1.78) | 0.50(0.20,1.30) | 0.44(0.14,1.38) | ||||
| RIC/NMA | 121 | 0.95(0.52,1.74) | 1.21(0.82,1.78) | 0.88(0.48,1.63) | ||||
| Donor type | Haploidentical | 172 | Reference | 0.95 | Reference | 0.50 | Reference | 0.88 |
| Mismatched unrelated | 38 | 0.98(0.54,1.77) | 0.84(0.50,1.39) | 0.95(0.52,1.76) | ||||
| ABO blood group compatibility | ABO Compatible | 139 | Reference | 0.12 | Reference | 0.21 | Reference | 0.034 |
| Minor | 32 | 0.46(0.22,0.95) | 0.57(0.33,1.00) | 0.47(0.25,0.89) | ||||
| Major | 24 | 0.56(0.26,1.21) | 0.72(0.41,1.28) | 0.47(0.24,0.93) | ||||
| Bidirectional | 15 | 0.90(0.39,2.07) | 0.87(0.42,1.83) | 0.81(0.36,1.83) | ||||
| Donor CMV serostatus | D+ | 143 | Reference | 0.39 | Reference | 0.75 | Reference | 0.65 |
| D− | 67 | 0.80(0.49,1.33) | 0.94(0.64,1.38) | 0.90(0.57,1.42) | ||||
| Recipient CMV serostatus | R+ | 177 | Reference | 0.98 | Reference | 0.74 | Reference | 0.73 |
| R− | 33 | 1.01(0.49,2.06) | 0.91(0.51,1.60) | 0.89(0.45,1.74) | ||||
| Graft source | Peripheral blood stem cells | 166 | Reference | 0.35 | Reference | 0.83 | Reference | 0.008 |
| Bone marrow | 44 | 1.32(0.73,2.37) | 1.05(0.65,1.72) | 2.12(1.22,3.70) | ||||
| Karnofsky Performance Status, % | ≥80 | 176 | Reference | 0.053 | Reference | 0.085 | Reference | 0.024 |
| <80 | 34 | 1.70(0.99,2.91) | 1.50(0.95,2.38) | 1.81(1.08,3.02) | ||||
| HCT-Comorbidity Index | 0–2 | 125 | Reference | 0.035 | Reference | 0.32 | Reference | 0.23 |
| ≥3 | 85 | 1.61(1.03,2.51) | 1.20(0.84,1.73) | 1.29(0.85,1.95) | ||||
| Tacrolimus initial dosing method | Flat | 131 | Reference | 0.30 | Reference | 0.82 | Reference | 0.046 |
| Weight-based | 79 | 1.27(0.81,2.00) | 1.05(0.72,1.53) | 1.53(1.01,2.32) | ||||
| Tacrolimus at initial steady state, ng/mL | <10 | 176 | Reference | 0.32 | Reference | 1.00 | Reference | 0.29 |
| ≥10 | 34 | 1.35(0.75,2.42) | 1.00(0.62,1.63) | 1.38(0.77,2.47) | ||||
Adjusted for Age, Disease Risk Index, Karnofsky Performance Status, HCT-Comorbidity Index and Conditioning Regimen
Adjusted for Disease Risk Index and Conditioning Regimen
Adjusted for Age, Disease Risk Index, ABO blood group compatibility, Graft source, Karnofsky Performance Status and Conditioning Regimen
GvHD
The 100-day cumulative incidence of grade II-IV aGvHD was 43% (95% CI: 37%−50%) and grade III-IV aGvHD was 15% (95% CI: 10%−20%), and were not significantly different by TISS <10 ng/mL vs. ≥10 ng/mL (45.5% vs. 32.4%; P=0.16 for grade II-IV; and 15.9% vs. 8.8%; P=0.29 for grade III-IV; Supplemental Table 3). The overall cumulative incidence of cGvHD at 24 months was 42% (95% CI: 35%−49%) and was similar by TISS (40.2% and 31.1% for <10 ng/mL and ≥10 ng/mL, respectively; P=0.21; Supplemental Table 3). In multivariate analysis (Table 4), BM graft source was associated with lower risk of aGvHD grade II-IV than PBSC graft source (29.5% vs. 47%; HR=0.49, 95% CI: 0.25–0.97, P=0.041).
Table 4.
Multivariable Analysis of GvHD
| Grade II-IV aGvHD* | Grade III-IV aGvHD† | Any cGvHD‡ | Extensive cGvHD† | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | HR (95%CI) | P | HR (95%CI) | P | HR (95%CI) | P | HR (95%CI) | P | ||
| Age | <60 | 144 | Reference | 0.11 | Reference | 0.071 | Reference | 0.24 | Reference | 0.51 |
| ≥60 | 66 | 0.63(0.36,1.11) | 0.47(0.20,1.07) | 0.71(0.40,1.26) | 0.79(0.39,1.59) | |||||
| Sex | M | 122 | Reference | 0.33 | Reference | 0.52 | Reference | 0.69 | Reference | 0.12 |
| F | 88 | 0.81(0.52,1.25) | 0.79(0.37,1.65) | 1.10(0.68,1.77) | 1.57(0.89,2.76) | |||||
| Female donor to male recipient | No | 176 | Reference | 0.32 | Reference | 0.73 | Reference | 0.043 | Reference | 0.041 |
| Yes | 34 | 0.74(0.41,1.34) | 0.84(0.30,2.32) | 1.68(1.02,2.77) | 1.81(1.02,3.21) | |||||
| Disease Risk Index | Low | 31 | Reference | 0.50 | Reference | 0.50 | Reference | 0.81 | Reference | 0.38 |
| Intermediate | 81 | 0.88(0.47,1.66) | 1.35(0.38,4.82) | 0.87(0.47,1.62) | 1.64(0.72,3.72) | |||||
| High/Very high | 78 | 1.17(0.62,2.21) | 1.93(0.56,6.64) | 0.81(0.43,1.53) | 1.21(0.52,2.84) | |||||
| Conditioning regimen | MAC-TBI | 71 | Reference | 0.48 | Reference | 0.20 | Reference | 0.22 | Reference | 0.22 |
| MAC-Non- TBI | 18 | 1.51(0.64,3.59) | 0.85(0.09,8.14) | 0.64(0.27,1.55) | 0.72(0.28,1.85) | |||||
| RIC/NMA | 121 | 0.92(0.58,1.44) | 1.97(0.88,4.44) | 0.69(0.45,1.07) | 0.64(0.38,1.06) | |||||
| Donor type | Haploidentical | 172 | Reference | 0.22 | Reference | 0.43 | Reference | 0.41 | Reference | 0.64 |
| Mismatched unrelated | 38 | 1.41(0.82,2.45) | 1.39(0.61,3.17) | 1.26(0.73,2.20) | 1.17(0.61,2.23) | |||||
| ABO blood group compatibility | ABO Compatible | 139 | Reference | 0.70 | Reference | 0.76 | Reference | 0.64 | Reference | 0.95 |
| Minor | 32 | 1.19(0.66,2.14) | 0.55(0.17,1.78) | 1.38(0.76,2.49) | 0.96(0.47,1.95) | |||||
| Major | 24 | 1.17(0.60,2.29) | 1.02(0.34,3.05) | 1.13(0.60,2.12) | 1.08(0.49,2.36) | |||||
| Bidirectional | 15 | 1.50(0.75,3.00) | 1.17(0.34,4.01) | 1.45(0.65,3.21) | 1.31(0.50,3.44) | |||||
| Donor CMV serostatus | D+ | 143 | Reference | 0.31 | Reference | 0.40 | Reference | 0.069 | Reference | 0.100 |
| D− | 67 | 1.50(0.69,3.25) | 1.38(0.65,2.92) | 1.51(0.97,2.36) | 1.54(0.92,2.59) | |||||
| Recipient CMV serostatus | R+ | 177 | Reference | 0.31 | Reference | 0.67 | Reference | 0.36 | Reference | 0.56 |
| R− | 33 | 1.50(0.69,3.25) | 0.76(0.21,2.72) | 1.30(0.74,2.28) | 1.22(0.63,2.35) | |||||
| Graft source | Peripheral blood stem cells | 166 | Reference | 0.041 | Reference | 0.11 | Reference | 0.17 | Reference | 0.30 |
| Bone marrow | 44 | 0.49(0.25,0.97) | 0.36(0.10,1.27) | 0.58(0.26,1.27) | 0.58(0.21,1.60) | |||||
| Karnofsky Performance Status, % | ≥80 | 176 | Reference | 0.68 | Reference | 0.37 | Reference | 0.61 | Reference | 0.47 |
| <80 | 34 | 1.14(0.62,2.08) | 1.48(0.63,3.47) | 0.85(0.46,1.57) | 0.75(0.35,1.63) | |||||
| HCT-Comorbidity Index | 0–2 | 125 | Reference | 0.81 | Reference | 0.36 | Reference | 0.21 | Reference | 0.76 |
| ≥3 | 85 | 0.95(0.62,1.45) | 1.39(0.69,2.82) | 0.75(0.48,1.17) | 0.92(0.56,1.54) | |||||
| Tacrolimus initial dosing method | Flat | 131 | Reference | 0.23 | Reference | 0.58 | Reference | 0.074 | Reference | 0.36 |
| Weight-based | 79 | 0.76(0.48,1.19) | 0.81(0.38,1.71) | 0.65(0.41,1.04) | 0.78(0.46,1.32) | |||||
| Tacrolimus at initial steady state, ng/mL | <10 | 176 | Reference | 0.55 | Reference | 0.78 | Reference | 0.28 | Reference | 0.81 |
| ≥10 | 34 | 0.81(0.41,1.62) | 0.83(0.22,3.07) | 0.69(0.36,1.34) | 1.08(0.56,2.12) | |||||
Adjusted for Donor/Recipient CMV serostatus, Graft source, and Conditioning regimen
Adjusted for Graft source and Conditioning regimen
Adjusted for Female donor to male recipient and Conditioning regimen
Infection
There were no significant differences in incidence of bloodstream infection (BSI) or fungal infection by TISS in univariable (Supplemental Table 4) and multivariable (Table 5) analyses. In multivariate analysis, factors associated with BSI included KPS (<80% vs. ≥80%: 50% vs. 27.3%; HR=2.44, 95% CI: 1.38–4.32, P=0.002) and HCT-CI (≥3 vs. 0–2: 40% vs. 24.8%; HR=1.86, 95% CI: 1.02–3.37, P=0.041). Variables associated with fungal infection in multivariate analysis included age ≥60 years (P=0.016), recipient being female (P=0.033), donor CMV positivity (P=0.037) and KPS <80% (P=0.01) (Table 5). Patients with TISS ≥10 ng/mL had significantly higher risk of viral infection than patients with <10 ng/mL (91.2% vs. 77.3%) in univariable (P=0.003) and multivariable analyses (P=0.014). Recipient CMV positivity and absence of letermovir prophylaxis were associated with increased risk for CMV infection (P<0.001 and P=0.019, respectively), while no variables were associated with BK infection (Supplemental Table 5).
Table 5.
Multivariable Analysis of Infection
| Bloodstream Infection | Fungal Infection | Viral Infection* | ||||||
|---|---|---|---|---|---|---|---|---|
| N | HR (95% CI)† | P | HR (95% CI)‡ | P | HR (95% CI)§ | P | ||
| Age | <60 | 144 | Reference | 0.31 | Reference | 0.016 | Reference | 0.20 |
| ≥60 | 66 | 1.31(0.78,2.18) | 3.35(1.25,9.01) | 0.76(0.50,1.15) | ||||
| Sex | M | 122 | Reference | 0.84 | Reference | 0.033 | Reference | 0.10 |
| F | 88 | 0.95(0.58,1.55) | 3.12(1.10,8.88) | 0.77(0.57,1.05) | ||||
| Female donor to male recipient | No | 176 | Reference | 0.59 | Reference | 0.72 | Reference | 0.61 |
| Yes | 34 | 1.18(0.64,2.18) | 1.25(0.36,4.30) | 0.90(0.61,1.34) | ||||
| Disease Risk Index | Low | 31 | Reference | 0.80 | Reference | 0.71 | Reference | 0.15 |
| Intermediate | 81 | 1.26(0.53,3.00) | 2.24(0.26,19.15) | 0.78(0.49,1.26) | ||||
| High/Very high | 78 | 1.33(0.57,3.09) | 2.41(0.29,19.90) | 1.12(0.71,1.76) | ||||
| Conditioning regimen | MAC-TBI | 71 | Reference | 0.95 | Reference | 0.79 | Reference | <0.001 |
| MAC-Non- TBI | 18 | 1.01(0.35,2.92) | 1.05(0.11,9.65) | 2.22(1.22,4.05) | ||||
| RIC/NMA | 121 | 1.09(0.64,1.84) | 1.45(0.47,4.51) | 0.68(0.49,0.93) | ||||
| Donor type | Haploidentical | 172 | Reference | 0.88 | Reference | 0.48 | Reference | 0.082 |
| Mismatched unrelated | 38 | 1.05(0.57,1.90) | 0.49(0.06,3.66) | 0.69(0.46,1.05) | ||||
| ABO blood group compatibility | ABO Compatible | 139 | Reference | 0.68 | Reference | 0.85 | Reference | 0.53 |
| Minor | 32 | 0.92(0.45,1.88) | 1.52(0.42,5.48) | 0.85(0.57,1.28) | ||||
| Major | 24 | 1.57(0.72,3.41) | 0.71(0.09,5.64) | 1.34(0.78,2.29) | ||||
| Bidirectional | 15 | 1.03(0.46,2.33) | 1.54(0.35,6.77) | 1.15(0.60,2.18) | ||||
| Donor CMV serostatus | D+ | 143 | Reference | 0.88 | Reference | 0.037 | Reference | 0.70 |
| D− | 67 | 0.96(0.57,1.62) | 0.15 (0.03, 0.89) | 1.22(0.45,3.31) | ||||
| Recipient CMV serostatus | R+ | 177 | Reference | 0.61 | Reference | 0.51 | Reference | 0.012 |
| R− | 33 | 1.20(0.59,2.41) | 0.54 (0.09, 3.36) | 0.45(0.24,0.84) | ||||
| Graft source | Peripheral blood stem cells | 166 | Reference | 0.49 | Reference | 0.88 | Reference | 0.28 |
| Bone marrow | 44 | 1.24(0.67,2.28) | 0.90(0.25,3.21) | 1.26(0.83,1.90) | ||||
| Karnofsky Performance Status, % | ≥80 | 176 | Reference | 0.002 | Reference | 0.010 | Reference | 0.058 |
| <80 | 34 | 2.44(1.38,4.32) | 3.74(1.37,10.17) | 1.42(0.99,2.05) | ||||
| HCT-Comorbidity Index | 0–2 | 125 | Reference | 0.041 | Reference | 0.89 | Reference | 0.63 |
| ≥3 | 85 | 1.86(1.02,3.37) | 0.93(0.35,2.46) | 0.93(0.68,1.26) | ||||
| Tacrolimus initial dosing method | Flat | 131 | Reference | 0.78 | Reference | 0.28 | Reference | 0.062 |
| Weight-based | 79 | 1.07(0.66,1.74) | 1.75(0.64,4.82) | 1.36(0.98,1.88) | ||||
| Tacrolimus at initial steady state, ng/mL | <10 | 176 | Reference | 0.74 | Reference | 0.098 | Reference | 0.014 |
| ≥10 | 34 | 1.12(0.59,2.10) | 2.40(0.85,6.78) | 1.58(1.10,2.27) | ||||
Earliest viral infection of CMV, ADV, HHV6, BKV, or EBV
Adjusted for Karnofsky Performance Score and HCT-Comorbidity Index
Adjusted for Donor/Recipient CMV serostatus
Adjusted for Conditioning regimen and Donor/Recipient CMV serostatus
Day 30 discontinuation
Overall, 92% of patients continued TAC on day +30 post-HCT. Patients with TISS ≥10 ng/mL had higher early discontinuation rate on day +30 than patients with <10 ng/mL (11.8% vs 7.4%, respectively) (Table 6). Reasons for TAC discontinuation included renal insufficiency (6 of 17), neurotoxicity/PRES (7 of 17), death (2 of 17) or engraftment failure (2 of 17).
Table 6.
Transplant-related Morbidity
| Tacrolimus at Initial Steady State | |||
|---|---|---|---|
| <10 ng/mL (N=176) | ≥10 ng/mL (N=34) | Total (N=210) | |
| Continue Tacrolimus on day +30, n (%) | |||
| No | 13 (7.4%) | 4 (11.8%) | 17 (8.1%) |
| Yes | 163 (92.6%) | 30 (88.2%) | 193 (91.9%) |
| Reason for Tacrolimus discontinuation, n/total n who discontinued TAC | |||
| Renal insufficiency | 4/13 | 2/4 | 6/17 |
| Neurotoxicity/PRES | 7/13 | 0 | 7/17 |
| Engraftment failure | 0 | 2/4 | 2/17 |
| Death | 1/13 | 1/4 | 2/17 |
| Hemodialysis within day +100, n (%) | |||
| No | 171 (97.2%) | 31 (91.2%) | 202 (96.2%) |
| Yes | 5 (2.8%) | 3 (8.8%) | 8 (3.8%) |
| ICU Stay, n (%) | |||
| No | 161 (91.5%) | 29 (85.3%) | 190 (90.5%) |
| Yes | 15 (8.5%) | 5 (14.7%) | 20 (9.5%) |
| Sinusoidal obstruction syndrome, n (%) | |||
| No | 173 (98.3%) | 33 (97.1%) | 206 (98.1%) |
| Yes | 3 (1.7%) | 1 (2.9%) | 4 (1.9%) |
| Hemorrhagic Cystitis Grade, n (%) | |||
| No | 123 (69.9%) | 28 (82.4%) | 151 (71.9%) |
| Yes | 53 (30.1%) | 6 (17.6%) | 59 (28.1%) |
Transplant-Related Morbidities
Transplant-related morbidities are summarized in Table 6. Eight of 210 (3.8%) patients required hemodialysis within 100 days of HCT, 5 patients (2.8%) with TISS <10 ng/mL and 3 patients (8.8%) with TISS ≥10 ng/mL. Twenty of 210 (9.5%) patients were admitted to the ICU within 100 days of HCT, 8.5% with TISS <10 ng/mL and 14.7% with TISS ≥10 ng/mL. Four of 210 patients (1.9%) experienced sinusoidal obstruction syndrome, 3 patients (1.7%) with TISS <10 ng/mL and 1 patient (2.9%) with TISS ≥10 ng/mL. Fifty-nine patients had hemorrhagic cystitis within 100 days of HCT (grade 1=50 patients, grade 2=9 patients). There was no significant difference in hemorrhagic cystitis comparing TISS <10 and ≥10 ng/mL groups (30.1% vs 17.6%; P=0.14).
DISCUSSION
PTCy-based platforms effectively prevent GvHD regardless of recipient-donor HLA disparity(2, 6, 21). Despite the increased use of PTCy combined with TAC, there are currently no standard dosing guidelines or therapeutic target serum levels for TAC. It is assumed that immunosuppressive levels pre-engraftment could impact T cell repertoire and recovery, which may dictate the incidence and severity of GvHD(22–29), the graft-versus-leukemia (GvL) effect and transplant outcomes. TISS represents an early adjustable timepoint where intervention could lead to better outcomes. Thus, it is beneficial to determine optimal TISS.
When combined with methotrexate, higher early (first week) TAC level was associated with lower risk of aGvHD grade II-IV in RIC setting(22, 29), but less GvL with higher RR(29). We observed a trend of better DFS with lower RR in patients with TISS <10 ng/mL vs. ≥10 ng/mL without affecting GvHD and NRM. This trend was not upheld in multivariable analysis when adjusted for graft source, which was previously shown to be predictive of relapse risk in patients with leukemia(5), possibly due to small sample size. One reasonable explanation is that early lower levels of suppression (TISS <10 ug/mL) permitted GvL that likely contributed to lower RR. TISS was not correlated with aGvHD or cGvHD in our analysis, as noted in TAC/methotrexate-based regimen(22, 29), which is possibly due to the upfront effect of PTCy on T cells before TAC is introduced. In our analysis, PBSCs as the graft source was the only predictor of risk of aGvHD grade II-IV, which is comparable to published data(5, 7).
Butts et al examined their institutional practice using initial 1 mg FD TAC to achieve the target serum level of 10–15 ng/mL(18), which they concluded required at least two dose adjustments and a median of 10 days to achieve. We found that lower TISS (<10 ng/mL) was as effective with less toxicity than higher (≥10 ng/mL), and that TISS <10ng/mL did not affect engraftment. Moreover, we observed a greater likelihood of achieving TISS <10 ng/mL with FD TAC. Our observation that TISS <10 ng/mL was sufficient to promote engraftment might be due to our study population, the majority of which received PBSC grafts; higher levels of TAC might be required to achieve engraftment with BM grafts. There were some differences in graft source and dosing strategy in our cohort due to subgroups of patients being treated on clinical protocols that required specific dosing strategies and/or graft sources. Additionally, certain pediatric transplant protocols required BM grafts and WBD. However, these subgroups represent a minority in our overall study population, and the number of patients in these subgroups were not sufficient to draw meaningful conclusions. Although we focused this study on TISS, levels of TAC beyond TISS likely influence transplant outcomes, which should be examined in future studies.
Viral infection, particularly by CMV or BK, is a main cause of treatment-related mortality following HCT. In our study, patients with TISS <10 ng/mL had lower risk of viral infection overall, which was partially due to decreased infection by BK or CMV. Lower TISS may result in lower levels of T-cell suppression and decreased incidence of viral infection. Patients with lower TISS were less likely to have TAC-associated toxicities and early discontinuation.
To our knowledge, this is the largest analysis correlating TISS with outcomes for patients who received PTCy-based GvHD prophylaxis. Our study is limited by heterogeneity of patient disease and conditioning intensity, both of which contribute to infection and transplant-related toxicities and morbidities. Additionally, few patients had early TAC dosing adjustments prior to achieving TISS, which could have affected assignment of patients to TISS cohorts. Concurrent use of azoles and other medications metabolized by CYP enzymes could affect TISS and interfere with levels of TAC beyond ISS, which could have affected our results. However, per our institution practice, patients were on micafungin during conditioning and switched to azole prophylaxis after TISS was achieved; only 2 of 210 patients were on azoles (due to prior fungal infection) concurrently with TAC at TISS assessment. Thus, it is unlikely that azole use affected our results. It is common practice for our clinicians to avoid using medications that interfere with TAC during TAC treatment and, therefore, it is unlikely that drug-drug interactions impacted this study.
We demonstrated that lower TISS (<10 ng/mL) in the PTCy platform was acceptable, led to similar outcomes when compared to TISS >10 ng/mL, and lowered risk of viral infection and TAC-related toxicities. Our results indicate that FD TAC was sufficient to achieve <10 ng/mL in our patient population.
Supplementary Material
Competing Interests:
Conflict of Interest Disclosure
Research reported in this publication included work performed in the Biostatistics and Mathematical Modeling Core supported by the National Cancer Institutes of Health under grant number P30CA033572. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors have no relevant conflicts of interest to declare.
REFERENCES
- 1.Ciurea SO, Zhang MJ, Bacigalupo AA, Bashey A, Appelbaum FR, Aljitawi OS, et al. Haploidentical transplant with posttransplant cyclophosphamide vs matched unrelated donor transplant for acute myeloid leukemia. Blood. 2015;126(8):1033–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Luznik L, O’Donnell PV, Symons HJ, Chen AR, Leffell MS, Zahurak M, et al. HLA-haploidentical bone marrow transplantation for hematologic malignancies using nonmyeloablative conditioning and high-dose, posttransplantation cyclophosphamide. Biol Blood Marrow Transplant. 2008;14(6):641–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Al Malki MM, Tsai N-C, Palmer J, Mokhtari S, Cao T, Ali H, et al. A Phase II Trial of Post-Transplant Cyclophosphamide As Graft-Versus-Host Disease Prophylaxis in HLA-Mismatched Unrelated Donor Hematopoietic Cell Transplantation. Biology of Blood and Marrow Transplantation. 2020;26(3, Supplement):S188. [Google Scholar]
- 4.Shaw BE, Burns LJ, Logan B, Jimenez-Jimenez AM, Khimani F, Shaffer BC, et al. Transplantation Using Bone Marrow from a (very) HLA Mismatched Unrelated Donor in the Setting of Post-Transplant Cyclophosphamide Is Feasible and Expands Access to Underserved Minorities. Biology of Blood and Marrow Transplantation. 2020;26(3, Supplement):S283–S4. [Google Scholar]
- 5.Bashey A, Zhang MJ, McCurdy SR, St Martin A, Argall T, Anasetti C, et al. Mobilized Peripheral Blood Stem Cells Versus Unstimulated Bone Marrow As a Graft Source for T-Cell-Replete Haploidentical Donor Transplantation Using Post-Transplant Cyclophosphamide. J Clin Oncol. 2017;35(26):3002–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carnevale-Schianca F, Caravelli D, Gallo S, Coha V, D’Ambrosio L, Vassallo E, et al. Post-Transplant Cyclophosphamide and Tacrolimus-Mycophenolate Mofetil Combination Prevents Graft-versus-Host Disease in Allogeneic Peripheral Blood Hematopoietic Cell Transplantation from HLA-Matched Donors. Biol Blood Marrow Transplant. 2017;23(3):459–66. [DOI] [PubMed] [Google Scholar]
- 7.Ruggeri A, Labopin M, Bacigalupo A, Afanasyev B, Cornelissen JJ, Elmaagacli A, et al. Post-transplant cyclophosphamide for graft-versus-host disease prophylaxis in HLA matched sibling or matched unrelated donor transplant for patients with acute leukemia, on behalf of ALWP-EBMT. J Hematol Oncol. 2018;11(1):40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Battipaglia G, Labopin M, Kröger N, Vitek A, Afanasyev B, Hilgendorf I, et al. Posttransplant cyclophosphamide vs antithymocyte globulin in HLA-mismatched unrelated donor transplantation. Blood. 2019;134(11):892–9. [DOI] [PubMed] [Google Scholar]
- 9.Shayani S, Palmer J, Stiller T, Liu X, Thomas SH, Khuu T, et al. Thrombotic microangiopathy associated with sirolimus level after allogeneic hematopoietic cell transplantation with tacrolimus/sirolimus-based graft-versus-host disease prophylaxis. Biol Blood Marrow Transplant. 2013;19(2):298–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hodnett P, Coyle J, O’Regan K, Maher MM, Fanning N. PRES (posterior reversible encephalopathy syndrome), a rare complication of tacrolimus therapy. Emerg Radiol. 2009;16(6):493–6. [DOI] [PubMed] [Google Scholar]
- 11.Przepiorka D, Nash RA, Wingard JR, Zhu J, Maher RM, Fitzsimmons WE, et al. Relationship of tacrolimus whole blood levels to efficacy and safety outcomes after unrelated donor marrow transplantation. Biol Blood Marrow Transplant. 1999;5(2):94–7. [DOI] [PubMed] [Google Scholar]
- 12.Cutler C, Kim HT, Hochberg E, Ho V, Alyea E, Lee SJ, et al. Sirolimus and tacrolimus without methotrexate as graft-versus-host disease prophylaxis after matched related donor peripheral blood stem cell transplantation. Biol Blood Marrow Transplant. 2004;10(5):328–36. [DOI] [PubMed] [Google Scholar]
- 13.Nakamura R, Rodriguez R, Nademanee A, Palmer J, Senitzer D, Snyder D, et al. The Use of Sirolimus Combined with Tacrolimus and Low-Dose Methotrexate Is Effective in Preventing Graft-Versus-Host Disease after Unrelated Donor Hematopoietic Stem Cell Transplantation. Blood. 2006;108(11):2866–. [Google Scholar]
- 14.Khaled SK, Palmer JM, Herzog J, Stiller T, Tsai NC, Senitzer D, et al. Influence of Absorption, Distribution, Metabolism, and Excretion Genomic Variants on Tacrolimus/Sirolimus Blood Levels and Graft-versus-Host Disease after Allogeneic Hematopoietic Cell Transplantation. Biol Blood Marrow Transplant. 2016;22(2):268–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wingard JR, Nash RA, Przepiorka D, Klein JL, Weisdorf DJ, Fay JW, et al. Relationship of tacrolimus (FK506) whole blood concentrations and efficacy and safety after HLA-identical sibling bone marrow transplantation. Biol Blood Marrow Transplant. 1998;4(3):157–63. [DOI] [PubMed] [Google Scholar]
- 16.Przepiorka D, Devine S, Fay J, Uberti J, Wingard J. Practical considerations in the use of tacrolimus for allogeneic marrow transplantation. Bone Marrow Transplant. 1999;24(10):1053–6. [DOI] [PubMed] [Google Scholar]
- 17.Cutler C, Logan B, Nakamura R, Johnston L, Choi S, Porter D, et al. Tacrolimus/sirolimus vs tacrolimus/methotrexate as GVHD prophylaxis after matched, related donor allogeneic HCT. Blood. 2014;124(8):1372–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Butts AR, Brown VT, McBride LD, Bolaños-Meade J, Bryk AW. Factors associated with optimized tacrolimus dosing in hematopoietic stem cell transplantation. J Oncol Pharm Pract. 2016;22(2):275–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shulman HM, Sullivan KM, Weiden PL, McDonald GB, Striker GE, Sale GE, et al. Chronic graft-versus-host syndrome in man. A long-term clinicopathologic study of 20 Seattle patients. Am J Med. 1980;69(2):204–17. [DOI] [PubMed] [Google Scholar]
- 20.Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995;15(6):825–8. [PubMed] [Google Scholar]
- 21.Kasamon YL, Luznik L, Leffell MS, Kowalski J, Tsai HL, Bolaños-Meade J, et al. Nonmyeloablative HLA-haploidentical bone marrow transplantation with high-dose posttransplantation cyclophosphamide: effect of HLA disparity on outcome. Biol Blood Marrow Transplant. 2010;16(4):482–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ganetsky A, Shah A, Miano TA, Hwang WT, He J, Loren AW, et al. Higher tacrolimus concentrations early after transplant reduce the risk of acute GvHD in reduced-intensity allogeneic stem cell transplantation. Bone Marrow Transplant. 2016;51(4):568–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cristiano S, Giorgia F, Sec Julie H, Marco R, Estefania Nova L, Mitalee S, et al. Impact of immunosuppressive drugs on the therapeutic efficacy of ex vivo expanded human regulatory T cells. Haematologica. 2016;101(1):91–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.García Cadenas I, Valcarcel D, Martino R, Piñana JL, Barba P, Novelli S, et al. Impact of Cyclosporine Levels on the Development of Acute Graft versus Host Disease after Reduced Intensity Conditioning Allogeneic Stem Cell Transplantation. Mediators of Inflammation. 2014;2014:620682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.de Kort EA, de Lil HS, Bremmers MEJ, van Groningen LFJ, Blijlevens NMA, Huls G, et al. Cyclosporine A trough concentrations are associated with acute GvHD after non-myeloablative allogeneic hematopoietic cell transplantation. PLoS One. 2019;14(3):e0213913–e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Malard F, Szydlo RM, Brissot E, Chevallier P, Guillaume T, Delaunay J, et al. Impact of cyclosporine-A concentration on the incidence of severe acute graft-versus-host disease after allogeneic stem cell transplantation. Biol Blood Marrow Transplant. 2010;16(1):28–34. [DOI] [PubMed] [Google Scholar]
- 27.Yee GC, Self SG, McGuire TR, Carlin J, Sanders JE, Deeg HJ. Serum Cyclosporine Concentration and Risk of Acute Graft-versus-Host Disease after Allogeneic Marrow Transplantation. New England Journal of Medicine. 1988;319(2):65–70. [DOI] [PubMed] [Google Scholar]
- 28.Martin P, Bleyzac N, Souillet G, Galambrun C, Bertrand Y, Maire PH, et al. Relationship between CsA trough blood concentration and severity of acute graft-versus-host disease after paediatric stem cell transplantation from matched-sibling or unrelated donors. Bone Marrow Transplantation. 2003;32(8):777–84. [DOI] [PubMed] [Google Scholar]
- 29.Sharma N, Zhao Q, Ni B, Elder P, Puto M, Benson DM, et al. Effect of Early Post-Transplantation Tacrolimus Concentration on the Risk of Acute Graft-Versus-Host Disease in Allogenic Stem Cell Transplantation. Cancers. 2021;13(4):613. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.