Abstract
Background:
Inflammation is important in multiple myeloma (MM) pathogenesis, and regular aspirin use has been shown to confer a reduced risk of MM. The influence of aspirin on survival after MM diagnosis is unknown.
Methods:
We identified 436 men and women diagnosed with MM between 1980 and 2016 in the Health Professionals Follow-up Study (HPFS) and the Nurses’ Health Study (NHS) who reported aspirin intake biennially on follow-up questionnaires. Using multivariable Cox proportional hazards regression models, we estimated hazard ratios (HR) and 95% confidence intervals (CI) associated with the effect of aspirin use on MM-Specific and overall mortality.
Results:
Compared with nonusers, participants who used aspirin after diagnosis had a multivariable HR for MM−specific mortality of 0·61 (95% confidence interval [CI], 0·46, 0·79) and for overall mortality of 0·63 (95% CI, 0·49, 0·80), after adjustment for age at diagnosis, year of diagnosis, sex, body mass index, pre-diagnosis aspirin use, and number of comorbidities. For post-diagnosis aspirin quantity, we observed a modest trend of reduction in MM-specific and all-cause mortality with increasing number of 325 mg tablets of aspirin per week, although the confidence intervals for 1 to <6 and ≥6 tablets overlapped. Results were not materially different before or after the availability of novel therapies (before vs. after the year 2000). Pre-diagnosis frequency or duration of aspirin use was not significantly associated with MM-specific or overall mortality.
Conclusions:
Findings support the use of aspirin as a complementary strategy to enhance MM survival.
Impact:
Confirmation in samples that have comprehensive clinical information is encouraged.
INTRODUCTION
Multiple myeloma (MM) is an incurable, and indeed, lethal malignancy of permanently differentiated B-cells. The introduction of novel therapies after 2000 has improved the estimated life expectancy of MM patients, from a 5 year survival rate of 35% in 2000 to just over 50% today (1). However, uptake and utilization of these new therapies vary considerably across different sociographic subsets of the population (2–4), and the vast majority of patients eventually stop responding to treatment and relapse (5). Additional strategies available to clinicians and their patients for extending survival time are limited.
It is known that inflammation is important in MM pathogenesis, and both mechanistic and population-based data suggest that up-regulation of inflammatory pathways, including those mediated by nuclear factor (NF)-κB and interleukin (IL)-6, contributes to MM pathogenesis (6–8). Aspirin is a nonsteroidal anti-inflammatory drug (NSAID) that mediates inflammation, in part by down-regulating NF-κB and several of its downstream targets, such as cyclooxygenase (COX) and its production of prostaglandins. Aspirin can irreversibly inactivate COX-1 and COX-2 through covalent bond formation, although the effect of aspirin on COX-2 may be particularly relevant to MM prevention. Specifically, COX-2 is frequently expressed in MM cells (9, 10), and expression has been shown to predict poor outcome in MM patients (9). Therefore, investigations of the impact of pharmacologic agents such as aspirin, which can reduce COX-2 expression, is warranted in MM patients.
In the current study, we investigated aspirin use patterns in relation to MM-specific and all-cause mortality among men and women diagnosed with MM between 1980 and 2016 in two large cohort studies: the Nurses’ Health Study (NHS) and Health Professionals Follow-up Study (HPFS). We chose to focus on aspirin as opposed to a broader category of NSAIDs in the current manuscript because the interval of exposure assessment for aspirin began much earlier during cohort follow-up and was more complete than for other NSAIDS; available data also did not support a sufficiently detailed or statistically powered examination of the other analgesics as main effects. In addition, aspirin or other blood thinners are often recommended for MM patients who take modern immunomodulatory agents (which have been incorporated into MM first-line treatment regimens since 2006), to prevent venous thromboembolism. This may introduce confounding by indication to studies based solely in the modern era, and thus we sought to leverage the early inception and long follow-up of the NHS and HPFS to examine associations between aspirin use and survival of MM both before and after the widespread availability of such novel therapies. To our knowledge, this is the first epidemiological investigation of aspirin use patterns and mortality in MM patients.
MATERIALS AND METHODS
Study Participants and Identification of MM
The Nurses’ Health Study began in 1976 when 121,700 female US registered nurses ages 30 to 55 years returned an enrollment questionnaire (11). The Health Professionals Follow-up Study was established in 1986 as a parallel cohort of 51,529 US men who were dentists, optometrists, osteopathic physicians, podiatrists, pharmacists, and veterinarians ages 40 to 75 years at entry (12). In both cohorts, biennial follow-up questionnaires were used to update information on lifestyle and disease history, including whether participants received a diagnosis of MM. For any report of MM, participants gave written permission to obtain hospital medical records and pathology reports pertaining to their diagnosis, and trained study personnel, blinded to exposure data, reviewed the records to confirm the diagnosis. When the original medical records were unavailable, case confirmation was pursued via linkage to state tumor registries.
The current analysis includes individuals who were diagnosed with MM who reported their aspirin use patterns on one or more follow-up periods and had no history of cancer (except non-melanoma skin) prior to the first aspirin intake assessment (1980 in NHS; 1986 in HPFS). The study protocol was approved by the institutional review boards of the Brigham and Women’s Hospital and Harvard T.H. Chan School of Public Health, and those of participating registries as required. Informed consent was implied by return of the questionnaires.
Endpoint Ascertainment
Deaths were identified by next of kin, the postal system or routine searches of the National Death Index(13, 14). Mortality follow-up in these cohorts has been shown to be more than 98% complete.(13) Individuals blinded to exposure information ascertained cause of death from death certificates, which were supplemented with medical records or, for cancer deaths, tumor registry linkage when possible. Survival time was assessed as the interval of time from MM diagnosis to death or January 2016, whichever came first.
MM Clinical Characteristics
Clinical disease characteristics were not available on the full sample of MM cases in these cohorts; however, we manually abstracted select disease characteristics recorded at the time of diagnosis (prior to therapy) from available hospital medical records and pathology reports, as previously published (15).
Aspirin Use
Aspirin was first assessed in NHS in 1980 and every two years thereafter, except 1986. Men in HPFS were first asked about aspirin use at baseline (1986). Early in the cohort follow-up periods, the questions about aspirin use did not distinguish between low and standard-dose tablets, and frequency of intake was not asked until 1992. From 1992 through 1998, participants were asked to report their weekly use by converting baby aspirin intake to adult strength equivalents via the question, “On average, how many aspirin tablets do you take per week? (4 baby aspirin= 1 tablet).” From 2000 onward, the questionnaire was modified to allow participants to select their dose (e.g., 81 vs. 325 mg). The primary indications for aspirin use were determined using a survey among randomly-selected self-reported aspirin users (16). In NHS, top reasons for use included heart disease prevention (35%), muscle or joint pain (16%) and headache (13%); in HPFS, top reasons included cardiovascular disease prevention (58%), joint or musculoskeletal pain (33%) cardiovascular disease (25%), and headaches (25%)(17). Individual-level indication(s) for aspirin use were not available in either cohort.
Aspirin intake patterns were subdivided into pre- and post-diagnosis exposures. The pre-diagnosis aspirin exposures of interest included aspirin use status (use vs. non-use) and average weekly 325 mg aspirin intake (i.e., quantity) reported on the questionnaire returned before MM diagnosis, as well as years of continuous aspirin use (i.e., duration) before diagnosis. We used 325 mg as the unit of exposure for aspirin, because information on “low dose” aspirin could not be distinguished from adult strength use before the year 2000. Participants’ average pre-diagnosis quantity was computed by averaging the number of adult strength tablets taken weekly, as reported on all questionnaires up to the last returned questionnaire prior to the diagnosis of MM (18). Pre-diagnosis duration of use was calculated by summing the consecutive years in which a participant reported regular aspirin use up to the last questionnaire that was returned before the diagnosis of MM (18). If aspirin use information was missing on a given questionnaire, data from the previous follow-up interval were carried forward for one interval; the exposure variables were set to missing thereafter. This exposure captures the years of continuous duration of use most proximal to the MM diagnosis. The post-diagnosis aspirin exposures of interest included aspirin use status (use vs. non-use) as well as the weekly 325 mg aspirin intake (i.e., quantity) reported on the first questionnaire that was returned after diagnosis. We did not update aspirin use information after the first questionnaire cycle after diagnosis because fewer than half the participants with MM survived long enough to return two questionnaires (median survival was <4 years and the follow-up cycles biennial).
Covariates
Individuals were classified as regular users of acetaminophen and ibuprofen if they reported use at least twice per week. In NHS, this exposure was derived from a question on the regular use of other non-steroidal analgesics asked in 1980, as well as questions specifically pertaining to acetaminophen and ibuprofen use from 1990 onward. In HPFS, this exposure was derived from separate questions on the use of other analgesics beginning at baseline.
In both cohorts, height and weight were self-reported at baseline and current weight was updated on follow-up questionnaires. Height and weight measurements have been validated in these cohorts (r=0.94, recalled vs. previously measured height; 0.97 for recalled vs. technician measured weight) (19). We used self-reported height and weight to calculate the body mass index (BMI) of participants at each follow-up period. Medical comorbidities were also assessed in NHS and HPFS on follow-up questionnaires. A comorbidity index score was calculated by summing the number of cardiovascular-related comorbidities reported on each follow-up questionnaire(15, 20). Comorbidities of interest included high blood pressure, diabetes, elevated cholesterol, myocardial infraction, angina pectoris, coronary artery surgery or angioplasty, stroke, pulmonary embolism, paroxysmal atrial tachycardia, or other heart-rhythm disturbance.
Statistical Analysis
Cox proportional hazards regression models with time since diagnosis as the underlying time scale were used to estimate the hazard ratios (HR) and 95% confidence intervals (CI) for MM-specific and overall mortality for the exposures of pre- and post-diagnosis aspirin use. Models adjusted for the main covariates of age at diagnosis and year of diagnosis, BMI and comorbidity index; the post-diagnosis models also adjusted for aspirin use status self-reported prior to diagnosis (user/non-user). We could not adjust for clinically presenting characteristics of the MM diagnosis, or first line therapy, but adjustment for year of diagnosis may partially control for first line therapy in these cohorts. We considered additional adjustment for acetaminophen and/or other NSAID use (yes/no/unknown) by adding the corresponding term to the multivariable models. All analyses were conducted on a pooled data set with stratification for cohort (sex), after finding no consistent statistically significant interaction between aspirin use patterns and sex in relation to MM-specific or all-cause mortality. Models of pre-diagnosis aspirin use were run with and without a two-year exposure lag. To illustrate the post-diagnosis findings, we plotted survival curves by stratum of post-diagnosis aspirin use status (user vs. non-user) using Kaplan-Meier methods, testing their statistical significance with the log-rank test. We also performed competing risk analyses for causes of death: MM–specific mortality versus other causes of death using the Fine–Gray method(21).
Given that some MM treatments could influence a participant’s likelihood of taking aspirin, we explored whether the associations between post-diagnosis aspirin use patterns and mortality were substantially different across time periods of diagnosis. This was done by stratifying models of aspirin intake exposures by time period of diagnosis (before 2000 vs after 2000). In exploratory analyses, we examined joint associations of pre- and post-diagnosis aspirin use in models that adjusted for the same main covariates described above. Finally, we examined differences in MM clinical characteristics by aspirin use patterns in a subset of participants with available clinical data.
All statistical tests were two-sided, and the proportional hazards assumption was tested and satisfied in all models by including time dependent covariates in the Cox model.
RESULTS
In this cohort of 436 MM patients who were a mean age of 72 years at diagnosis, we identified 321 MM-specific and 383 total deaths over a median of 43 months of follow-up. Compared to individuals who did not take aspirin after a MM diagnosis, individuals who regularly took 6 or more aspirin tablets per week after MM diagnosis were slightly older and more racially/ethnically diverse, with more comorbidities (Table 1). The majority of individuals who reported taking aspirin after diagnosis also reported a history of aspirin use prior to the diagnosis of MM.
Table 1.
Age-Standardized Characteristics1 of Participants Enrolled in the Nurses’ Health Study (NHS) and Health Professionals Follow-up Study (HPFS) Stratified by Post-Diagnosis Aspirin Use Categories.
| Not current user N = 251 |
1 to <6 325 mg Tablets per Week N = 99 |
6+ 325 mg Tablets per Week N = 63 |
|
|---|---|---|---|
| Age at Diagnosis, years2 | 68.5 (9.2) | 68.5 (9.1) | 72.0 (7.5) |
| White, % | 92 | 95 | 88 |
| Body Mass Index at diagnosis3, kg/m2 | 26.0 (5.4) | 25.5 (4.4) | 26.5 (4.4) |
| Number of Comorbidities at diagnosis3 | 1.3 (1.3) | 2.0 (1.5) | 2.1 (1.3) |
| Time from Diagnosis to Return of Questionnaire Post-Diagnosis | 11.2 (6.9) | 11.6 (7.1) | 9.8 (7.1) |
| Pre-Diagnosis Continuous Duration (years) of Aspirin Use | 2.6 (4.8) | 8.5 (7.7) | 8.1 (7.2) |
| Pre-Diagnosis Aspirin Use, % | 30 | 86 | 79 |
note: Individuals with no analyzable information on post-diagnosis aspirin quantity are excluded from this table.
Values are means(SD) or percentages and are standardized to the age distribution of the study population.
Value is not age adjusted.
Body Mass Index and Comorbidities derived from first questionnaire returned after a diagnosis of multiple myeloma
No statistically significant associations were observed between pre-diagnosis aspirin use exposures with MM-specific or all-cause mortality (Table 2). These results were not materially different in models that included a 2-year exposure lag.
Table 2.
Associations Between Pre-Diagnosis Quantity and Duration of Aspirin and Multiple Myeloma-Specific and All-Cause Mortality.
| NHS | HPFS | Pooled | |||
|---|---|---|---|---|---|
|
| |||||
| N Events/ N at Risk | HR (95% CI)1 | N Events/ N at Risk | HR (95% CI)1 | HR (95% CI)1,2 | |
| Multiple Myeloma-Specific Mortality | |||||
|
| |||||
| Pre-Diagnosis Aspirin Use | |||||
| Non-users | 109/136 | ref | 72/103 | ref | ref |
| User | 116/153 | 0.80 (0.60, 1.05) | 57/83 | 0.89 (0.63, 1.27) | 0.84 (0.68, 1.04) |
| Pre-Diagnosis Number of 325 mg Tablets/Week | |||||
| Non-user | 109/136 | ref | 72/103 | ref | ref |
| 1 to <6 | 72/94 | 0.87 (0.63, 1.20) | 22/33 | 0.88 (0.55, 1.42) | 0.86 (0.67, 1.12) |
| ≥6 | 37/46 | 0.80 (0.54, 1.18) | 17/29 | 0.71 (0.40, 1.23) | 0.77 (0.57, 1.06) |
| P-trend3 | 0.26 | 0.21 | 0.10 | ||
| Pre-Diagnosis Continuous Duration (years) of Aspirin Use | |||||
| Never-user | 37/42 | ref | 40/55 | ref | ref |
| ≤5 | 121/158 | 1.04 (0.71, 1.53) | 64/89 | 1.07 (0.70, 1.61) | 1.06 (0.80, 1.41) |
| 6 to <11 | 29/40 | 0.82 (0.50, 1.35) | 16/24 | 0.93 (0.50, 1.73) | 0.87 (0.59, 1.28) |
| ≥11 | 55/72 | 1.02 (0.65, 1.60) | 12/22 | 0.84 (0.42, 1.70) | 0.99 (0.69, 1.42) |
| P-trend3 | 0.72 | 0.43 | 0.50 | ||
|
| |||||
| All-Cause Mortality | |||||
|
| |||||
| Pre-Diagnosis Aspirin Use | |||||
| Non-users | 115/136 | ref | 95/103 | ref | ref |
| User | 133/153 | 0.85 (0.65, 1.11) | 78/83 | 0.88 (0.65, 1.20) | 0.87 (0.71, 1.06) |
| Pre-Diagnosis Number of 325 mg Tablets/Week | |||||
| Non-user | 115/136 | ref | 72/103 | ref | ref |
| 1 to <6 | 82/94 | 0.92 (0.68, 1.25) | 23/33 | 0.89 (0.59, 1.35) | 0.88 (0.69, 1.12) |
| ≥6 | 41/46 | 0.86 (0.59, 1.25) | 17/29 | 0.68 (0.43, 1.08) | 0.81 (0.61, 1.08) |
| P-trend3 | 0.42 | 0.10 | 0.14 | ||
| Pre-Diagnosis Continuous Duration (years) of Aspirin Use | |||||
| Never-user | 40/42 | ref | 48/52 | ref | ref |
| ≤5 | 130/158 | 1.04 (0.72, 1.51) | 30/31 | 1.17 (0.80, 1.69) | 1.11 (0.86, 1.45) |
| 6 to <11 | 31/40 | 0.80 (0.49, 1.30) | 19/20 | 1.02 (0.60, 1.75) | 0.91 (0.64, 1.30) |
| ≥11 | 67/72 | 1.16 (0.76, 1.79) | 12/15 | 0.84 (0.46, 1.51) | 1.11 (0.79, 1.54) |
| P-trend3 | 0.74 | 0.33 | 0.89 | ||
Abbreviations: HR: Hazard Ratio; CI: Confidence Interval; NHS: Nurses’ Health Study; HPFS: Health Professionals Follow-Up Study; ref, reference category.
Cox proportional hazards models adjusted for age at multiple myeloma diagnosis (years), calendar year of diagnosis (<2000 vs. ≥2000), and pre-diagnosis body mass index and number of comorbidities.
Pooled models were stratified by cohort (sex).
P-values for trend tests modeled as an ordinal variable using the mid-point of each category of the respective variable in Cox proportional hazard models.
Aspirin use post diagnosis was inversely associated with MM-specific and all-cause mortality (Figures 1a-b). In multivariable models, compared with nonusers, participants who used aspirin after diagnosis had a HR for MM−specific mortality of 0·61 (95% confidence interval [CI], 0·46, 0·79) after adjustment for age at diagnosis, year of diagnosis, BMI, pre-diagnosis aspirin use, and number of comorbidities (Table 3). The effect estimates for overall mortality was of similar magnitude, suggesting a 37% reduction in risk among post-diagnosis aspirin users compared to non-users (HR: 0·63; 95% CI, 0·49, 0·80). For post-diagnosis aspirin quantity, we observed a modest trend of reduction in MM-specific and all-cause mortality with increasing number of 325 mg tablets of aspirin taken per week (or equivalent weekly use of low-dose tablets), although it is notable that the confidence intervals for 1 to <6 and ≥6 tablets per week overlapped. There were no marked differences in the effect estimates observed in models stratified by time-period of diagnosis (before vs. after 2000), although the effect estimates for the benefit of post-diagnosis aspirin use were slightly stronger in the models for the period after 2000 (Supplement Tables 1-2). We also confirmed that the time from diagnosis to the return of the first post-diagnosis questionnaire did not materially change the results reported herein. In addition, adjustment for concurrent use of other analgesics/NSAIDs did not materially change the effect estimates reported herein.
Figure 1.
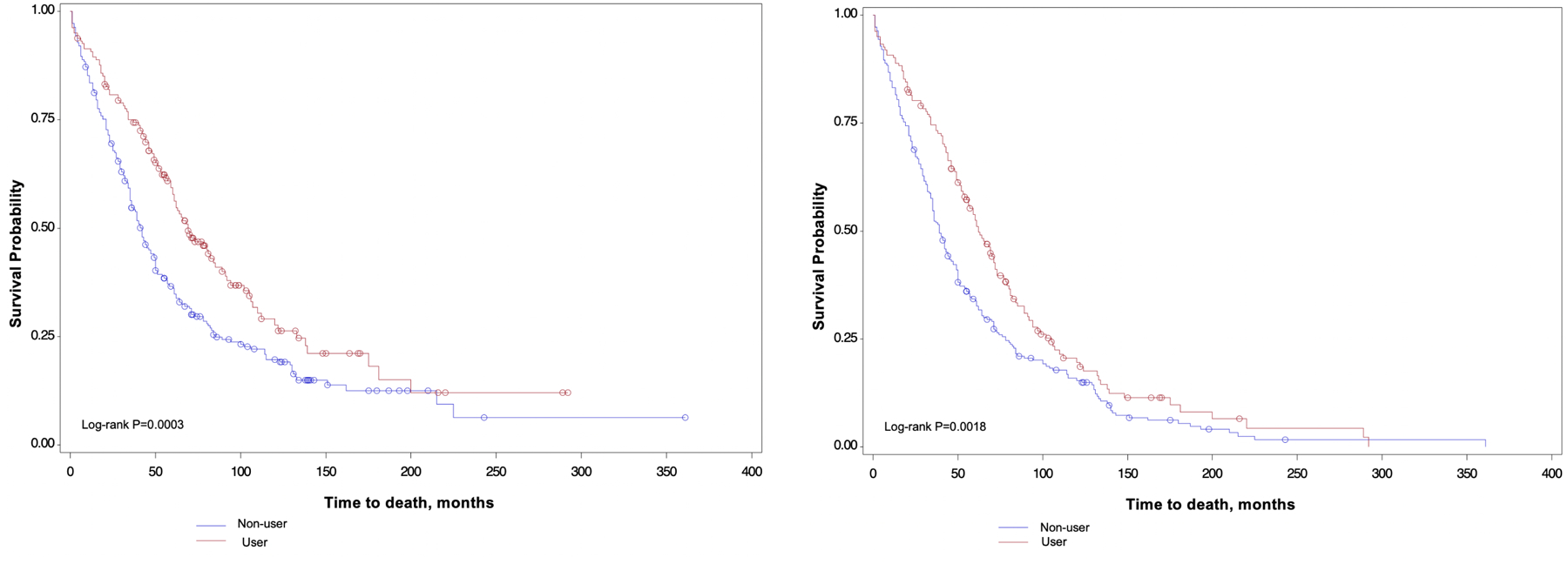
Kaplan-Meier (K-M) curves of multiple myeloma-specific and all-cause mortality by post-diagnosis aspirin use. A. K-M curves of multiple myeloma-specific mortality for the comparison of participants who reported using aspirin after diagnosis compared to participants who reported no aspirin use post-diagnosis. B. K-M curves of all-cause mortality for the comparison of individuals who reported using aspirin after diagnosis compared to participants who reported no aspirin use post-diagnosis.
Table 3.
Associations Between Post-Diagnosis Aspirin Use and Quantity and Multiple Myeloma-Specific and All-Cause Mortality.
| NHS | HPFS | Pooled | |||
|---|---|---|---|---|---|
| N Events/ N at Risk | HR (95% CI)1 | N Events/ N at Risk | HR (95% CI)1 | HR (95% CI)1,2 | |
| Multiple Myeloma-Specific Mortality | |||||
|
| |||||
| Post-Diagnosis Aspirin Use | |||||
| Non-users | 116/140 | ref | 83/111 | ref | ref |
| User | 88/125 | 0.72 (0.51, 1.02) | 34/60 | 0.45 (0.29, 0.71) | 0.61 (0.46, 0.79) |
| Post-Diagnosis Number of 325-mg Tablets/Week | |||||
| Non-user | 116/140 | ref | 83/111 | ref | ref |
| 1 to <6 | 58/80 | 0.75 (0.51, 1.11) | 15/19 | 0.62 (0.34, 1.14) | 0.70 (0.51, 0.96) |
| ≥6 | 18/29 | 0.58 (0.33, 0.99) | 16/34 | 0.39 (0.22, 0.73) | 0.49 (0.33, 0.74) |
| P-trend3 | 0.05 | 0.003 | 0.0005 | ||
|
| |||||
| All-Cause Mortality | |||||
|
| |||||
| Post-Diagnosis Aspirin Use | |||||
| Non-users | 125/140 | ref | 105/111 | ref | ref |
| User | 100/125 | 0.70 (0.50, 0.97) | 53/60 | 0.53 (0.36, 0.77) | 0.63 (0.49, 0.80) |
| Post-Diagnosis Number of 325-mg Tablets/Week | |||||
| Non-user | 125/140 | ref | 105/111 | ref | ref |
| 1 to <6 | 67/80 | 0.74, 0.51, 1.06) | 17/19 | 0.56 (0.32, 0.98) | 0.67 (0.49, 0.90) |
| ≥6 | 20/29 | 0.57 (0.34, 0.95) | 29/34 | 0.51 (0.31, 0.83) | 0.56 (0.40, 0.80) |
| P-trend3 | 0.03 | 0.008 | 0.002 | ||
Abbreviations: HR: Hazard Ratio; CI: Confidence Interval; NHS: Nurses’ Health Study; HPFS: Health Professionals Follow-Up Study.
Cox proportional hazards model adjusted for age at multiple myeloma diagnosis (years), calendar year of diagnosis (<2000 vs. ≥2000), post-diagnosis body mass index and number of comorbidities, and pre-diagnosis aspirin use (user vs. non-user).
Pooled models were stratified by cohort (sex).
P-values for trend tests modeled as an ordinal variable using the mid-point of each category of the respective variable in Cox proportional hazard models.
The analysis of joint associations of pre- and post-diagnosis aspirin supports the finding that post-diagnosis aspirin use was associated with enhanced survival irrespective of pre-diagnosis aspirin use. Compared to individuals who reported no aspirin use pre- or post-diagnosis, individuals who only reported aspirin use after diagnosis had a HR for MM-specific mortality of 0·50 (95% CI: 0·30, 0·83) and a HR for all-cause mortality of 0·56 (95% CI: 0·36, 0·87). As indicated by the sample sizes per category in Table 4, we observed that only 7% of participants began taking aspirin after MM diagnosis, whereas a much larger proportion of participants that took aspirin after diagnosis had also taken it before MM diagnosis. Joint models also suggest that risk of early mortality was not significantly different for individuals who discontinued aspirin after diagnosis compared to individuals who never took aspirin (Table 4).
Table 4.
Joint Models of Pre- and Post-Diagnosis Aspirin Intake Patterns and Multiple Myeloma-Specific and All-Cause Mortality.
| NHS |
HPFS |
Pooled |
|||
|---|---|---|---|---|---|
| N Events/ N at Risk | HR (95% CI)1 | N Events/ N at Risk | HR (95% CI)1 | HR (95% CI)1,2 | |
| Multiple Myeloma-Specific Mortality | |||||
|
| |||||
| Non-User | 88/104 | ref | 51/71 | ref | ref |
| Pre-Diagnosis Use Only | 28/36 | 0.81 (0.52, 1.25) | 32/40 | 1.28 (0.80, 2.06) | 1.02 (0.75, 1.39) |
| Post-Diagnosis Use Only | 9/19 | 0.43 (0.21, 0.87) | 9/17 | 0.55 (0.26, 1.17) | 0.50 (0.30, 0.83) |
| Use Pre- and Post-Diagnosis | 79/106 | 0.72 (0.52, 1.00) | 25/43 | 0.53 (0.32, 0.87) | 0.67 (0.51, 0.88) |
|
| |||||
| All-Cause Mortality | |||||
|
| |||||
| Non-User | 92/104 | ref | 66/71 | ref | ref |
| Pre-Diagnosis Use Only | 33/36 | 0.92 (0.61, 1.39) | 39/40 | 1.14 (0.74, 1.76) | 1.05 (0.78, 1.40) |
| Post-Diagnosis Use Only | 11/19 | 0.48 (0.25, 0.93) | 14/17 | 0.57 (0.30, 1.07) | 0.56 (0.36, 0.87) |
| Use Pre- and Post-Diagnosis | 89/106 | 0.75 (0.55, 1.03) | 39/43 | 0.58 (0.38 0.87) | 0.69 (0.54, 0.88) |
Abbreviations: HR: Hazard Ratio; CI: Confidence Interval; NHS: Nurses’ Health Study; HPFS: Health Professionals Follow-Up Study.
Cox proportional hazards model adjusted for age at multiple myeloma diagnosis (years), calendar year of diagnosis (<2000 vs. ≥2000), pre-diagnosis body mass index and number of comorbidities.
Pooled models were stratified by cohort (sex).
Exploratory analyses of differences in clinical characteristics of post-diagnosis aspirin use are limited by considerable missing data in the retrospective medical record review; however no marked differences in the clinically presenting features at diagnosis were apparent across post-diagnosis aspirin use pattern categories.
DISCUSSION
We previously reported an almost 40% reduction of MM risk in individuals with higher average quantity or longer duration of regular aspirin use compared to non-users in the combined populations of the Nurses’ Health Study (NHS) and Health Professionals Follow-up Study (HPFS)(18). In the current study, we observed that regular aspirin use after a diagnosis of MM was associated with an almost 40% reduction in both disease-specific and overall survival, independent of pre-diagnosis use. Notably, the association was similar during the time periods before and after the availability of novel MM therapies in the year 2000. While we are not able to infer causation from this observational study design, the strong association between post diagnosis aspirin use and MM-specific mortality, the directionally consistent (albeit non-significant) evidence of a survival benefit for individuals who reported aspirin use on the questionnaire cycle before diagnosis, and the observation that MM-specific deaths comprised the majority of all deaths suggest the benefit may be, in part, through antineoplastic properties in MM.
There are limited data on the association of aspirin and prognosis in MM patients. Nevertheless, our findings are consistent with trends observed in trials of aspirin for the prevention of vascular disease. In an intention-to-treat meta-analysis of eight randomized prevention trials, Rothwell and colleagues observed a reduced risk of death from hematologic cancer among those randomized to the aspirin group and who used it daily for at least 5 years (22). However, its notable that findings from that study were based on a small number of patients in the hematologic cancer subgroup, and the association did not reach statistical significance; it is also possible that the modest improvement in hematologic cancer survival in that analysis was driven by an effect of aspirin on reduction in hematologic cancer incidence. In addition, a post hoc analysis of data from a phase 2 trial of aspirin for the prevention of thrombotic events in MM patients receiving thalidomide (23) suggested better survival among aspirin users compared to non-users (24). Specifically, 89% of MM patients taking low dose aspirin (81-mg daily) were alive at year 1 compared to 68% of patients in the no-aspirin group (P=0·03). Despite the encouraging supportive evidence, we must also acknowledge that data from the Aspirin in Reducing Events in the Elderly (ASPREE) trial, which randomized 19,114 individuals to aspirin or a placebo, suggest an increased risk of cancer mortality among individuals in the aspirin group on the order of magnitude of 1.6 excess deaths per 1000 person-years for all cancers; however, the excess risk of cancer mortality in the aspirin group was largely driven by gastrointestinal cancers, and risk was not statistically significantly increased for hematologic cancers, although the hematologic cancer subgroup was small and MM-specific data were unavailable (25). Although other primary prevention trials of aspirin have not demonstrated similar adverse results (22, 26), results of the ASPREE trial do underscore the need to interpret the data we report with caution until more definitive studies have been conducted.
Recent population-based data consistently indicate that survival time after a diagnosis of MM has been steadily increasing over the past several decades due to the rapid expansion of standard of care therapies available. Indeed, the 5-year relative survival rate increased from 34·5% in 2000 to over 50% today (1). However, these improvements in survival have not been observed across all segments of the population equally, with older and minority patients receiving fewer gains for reasons that are thought to include treatment delays and unequal access to novel therapies (27–30). The finding that aspirin may enhance MM survival is particularly relevant in light of these disparities, given its affordability and accessibility.
Strengths of the current study include the large prospective design, with sufficient endpoints occurring before availability of novel MM therapies, which enabled us to examine heterogeneity in the effects we report by time period. In addition, participants provided biennially updated exposure information that enabled us to investigate pre- and post-diagnosis exposures separately.
The major limitation of the current study is the lack of comprehensive data on treatment or MM clinically presenting features with which to adjust for disease severity at diagnosis or first-line therapy. However, in the subsample of participants with clinical data available, we did not observe statistically significant differences in the clinically presenting features of MM by post diagnosis aspirin use, providing preliminary (albeit crude) indication that selection bias or residual confounding by clinical characteristics may not be strongly influencing the findings we report. We were also not able to specifically investigate the potential benefit of low-dose aspirin use (81mg) on MM outcomes, which is the dose commonly used for cardiovascular disease prevention. In addition, our study sample comprised a relatively homogeneous population of nurses and health professionals who we can infer may have had comparable access to medical care and MM therapies. Our sample is also comprised of predominantly white individuals and therefore these exposures warrant evaluation in more diverse cohorts. It is also notable that our results regarding an apparent benefit of aspirin in the post-diagnosis interval may only be generalizable to multiple myeloma patients who have lived long enough after diagnosis to report aspirin use on a post-diagnosis follow-up questionnaire; 79 individuals in our sample did not return a questionnaire after their MM diagnosis. Further, we did not see any evidence of confounding or effect measure modification by time interval between MM diagnosis and the return of the first post-diagnosis questionnaire, suggesting that questionnaire return was not related to the severity of illness. Finally, given the relatively short survival time for MM patients in these cohorts, we did not use repeated measures of post-diagnosis aspirin use and instead relied on a single self-report assessment. Therefore, this exposure may not reflect participants’ true long-run average post-diagnosis aspirin intake.
Our study provides preliminary support for the hypothesis that regular aspirin use may be associated with extended survival among MM patients. We urge others to seek confirmation in more diverse patient samples that have comprehensive clinical and treatment information and an ability to examine aspirin use by tablet dose so that stronger conclusions can be drawn. Until additional confirmation is achieved, the most conservative interpretation of our data is that aspirin use in the post-diagnosis interval appears to do no harm. If confirmed in other patient samples and randomized trials, regular aspirin use could be considered a safe, inexpensive, and complementary strategy to enhance survival in individuals living with MM.
Supplementary Material
ACKNOWLEDGEMENTS:
This study was funded in part by the National Cancer Institute of the National Institutes of Health under award numbers K07 CA115687 (B.M. Birmann), R01 CA127435 (G.A. Colditz), P01 CA87969 (B.M. Birmann, B. Rosner), UM1 CA186107 (B.M. Birmann, B. Rosner), U01 CA167552 (B.M. Birmann, B. Rosner), R21 CA198239 (B.M. Birmann), F32 CA220859 (C.R. Marinac), R03 CA204825 (D.H Lee), and K22 CA251648 (C.R. Marinac). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. This research was also supported by the American Cancer Society under award number PF-17–231-01-CCE (C.R. Marinac), and Clinical Research Professorship (G.A. Colditz), as well as institutional funds from the Dana Farber Cancer Institute, and Stand Up To Cancer under award number SU2C‐AACR‐DT‐28‐18 (I.M. Ghobrial). Stand Up To Cancer is a program of the Entertainment Industry Foundation. Research grants are administered by the American Association for Cancer Research, the scientific partner of Stand Up To Cancer. Opinions, interpretations, conclusions and recommendations are those of the author(s) and are not necessarily endorsed by Stand Up To Cancer, the Entertainment Industry Foundation, or the American Association for Cancer Research. Lastly, we would like to thank the participants and staff of the Health Professionals Follow-up Study and Nurses’ Health Study for their valuable contributions as well as the following state cancer registries for their help: AL, AZ, AR, CA, CO, CT, DE, FL, GA, ID, IL, IN, IA, KY, LA, ME, MD, MA, MI, NE, NH, NJ, NY, NC, ND, OH, OK, OR, PA, RI, SC, TN, TX, VA, WA, WY. The authors assume full responsibility for analyses and interpretation of these data.
ROLE OF THE FUNDING SOURCE: The funding sources had no role in the design, collection, analysis, interpretation or reporting of the study described herein, or in the decision to submit for publication.
Footnotes
CONFLICTS OF INTEREST: Irene M. Ghobrial has the following potential conflicts of interest to disclose: Honoraria: Celgene, Bristol-Myers Squibb, Takeda, Amgen, Janssen; Consulting or Advisory Role: Bristol-Myers Squibb, Novartis, Amgen, Takeda, Celgene, Cellectar, Sanofi, Janssen, Pfizer, Menarini Silicon Biosystems Oncopeptides, The Binding Site, GlazoSmithKlein, AbbVi Adaptive; Travel, Accommodations, Expenses: Bristol-Myers Squibb, Novartis, Celgene, Takeda, Janssen Oncology. Spouse: William Savage, MD, PhD is the Chief Medical Officer at Disc Medicine and holds equity in the company. All other authors declare no other potential conflicts of interest.
REFERENCES
- 1.Howlander N, Noon AM, Krapacho M, Miller D, Bishop K, Altekruse SF, et al. SEER Cancer Statistics Review, 1975–2013, National Cancer Institute. Bethesda, MD, http://seer.cancer.gov/csr/1975_2013/, based on November 2015 SEER data submission, posted to the SEER web site, April 2016. [Google Scholar]
- 2.Cowan AJ, Allen C, Barac A, Basaleem H, Bensenor I, Curado MP, et al. Global Burden of Multiple Myeloma: A Systematic Analysis for the Global Burden of Disease Study 2016. JAMA Oncol 2018;4(9):1221–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Al-Hamadani M, Hashmi SK, Go RS. Use of autologous hematopoietic cell transplantation as initial therapy in multiple myeloma and the impact of socio-geo-demographic factors in the era of novel agents. Am J Hematol 2014;89(8):825–30. [DOI] [PubMed] [Google Scholar]
- 4.Ailawadhi S, Frank RD, Advani P, Swaika A, Temkit M, Menghani R, et al. Racial disparity in utilization of therapeutic modalities among multiple myeloma patients: a SEER-medicare analysis. Cancer Med 2017;6(12):2876–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blade J, Rosinol L, Fernandez de Larrea C. How I treat relapsed myeloma. Blood 2015;125(10):1532–40. [DOI] [PubMed] [Google Scholar]
- 6.Demchenko YN, Kuehl WM. A critical role for the NFkB pathway in multiple myeloma. Oncotarget 2010;1(1):59–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kawano M, Hirano T, Matsuda T, Taga T, Horii Y, Iwato K, et al. Autocrine generation and requirement of BSF-2/IL-6 for human multiple myelomas. Nature 1988;332(6159):83–5. [DOI] [PubMed] [Google Scholar]
- 8.Hirano T. Interleukin 6 (IL-6) and its receptor: their role in plasma cell neoplasias. Int J Cell Cloning 1991;9(3):166–84. [DOI] [PubMed] [Google Scholar]
- 9.Ladetto M, Vallet S, Trojan A, Dell’Aquila M, Monitillo L, Rosato R, et al. Cyclooxygenase-2 (COX-2) is frequently expressed in multiple myeloma and is an independent predictor of poor outcome. Blood 2005;105(12):4784–91. [DOI] [PubMed] [Google Scholar]
- 10.Trojan A, Tinguely M, Vallet S, Seifert B, Jenni B, Zippelius A, et al. Clinical significance of cyclooxygenase-2 (COX-2) in multiple myeloma. Swiss Med Wkly 2006;136(25–26):400–3. [DOI] [PubMed] [Google Scholar]
- 11.Colditz GA, Manson JE, Hankinson SE. The Nurses’ Health Study: 20-year contribution to the understanding of health among women. J Womens Health 1997;6(1):49–62. [DOI] [PubMed] [Google Scholar]
- 12.Rimm EB, Giovannucci EL, Willett WC, Colditz GA, Ascherio A, Rosner B, et al. Prospective study of alcohol consumption and risk of coronary disease in men. Lancet 1991;338(8765):464–8. [DOI] [PubMed] [Google Scholar]
- 13.Rich-Edwards JW, Corsano KA, Stampfer MJ. Test of the National Death Index and Equifax Nationwide Death Search. Am J Epidemiol 1994;140(11):1016–9. [DOI] [PubMed] [Google Scholar]
- 14.Stampfer MJ, Willett WC, Speizer FE, Dysert DC, Lipnick R, Rosner B, et al. Test of the National Death Index. Am J Epidemiol 1984;119(5):837–9. [DOI] [PubMed] [Google Scholar]
- 15.Lee DH, Fung TT, Tabung FK, Marinac CR, Devore EE, Rosner BA, et al. Prediagnosis dietary pattern and survival in patients with multiple myeloma. Int J Cancer 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Manson JE, Stampfer MJ, Colditz GA, Willett WC, Rosner B, Speizer FE, et al. A prospective study of aspirin use and primary prevention of cardiovascular disease in women. JAMA 1991;266(4):521–7. [PubMed] [Google Scholar]
- 17.Giovannucci E, Rimm EB, Stampfer MJ, Colditz GA, Ascherio A, Willett WC. Aspirin use and the risk for colorectal cancer and adenoma in male health professionals. Ann Intern Med 1994;121(4):241–6. [DOI] [PubMed] [Google Scholar]
- 18.Birmann BM, Giovannucci EL, Rosner BA, Colditz GA. Regular aspirin use and risk of multiple myeloma: a prospective analysis in the health professionals follow-up study and nurses’ health study. Cancer Prev Res (Phila) 2014;7(1):33–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Troy LM, Hunter DJ, Manson JE, Colditz GA, Stampfer MJ, Willett WC. The validity of recalled weight among younger women. Int J Obes Relat Metab Disord 1995;19(8):570–2. [PubMed] [Google Scholar]
- 20.Downer MK, Kenfield SA, Stampfer MJ, Wilson KM, Dickerman BA, Giovannucci EL, et al. Alcohol Intake and Risk of Lethal Prostate Cancer in the Health Professionals Follow-Up Study. J Clin Oncol 2019;37(17):1499–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fine J, Gray R. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 1999;94:496–509. [Google Scholar]
- 22.Rothwell PM, Fowkes FG, Belch JF, Ogawa H, Warlow CP, Meade TW. Effect of daily aspirin on long-term risk of death due to cancer: analysis of individual patient data from randomised trials. Lancet 2011;377(9759):31–41. [DOI] [PubMed] [Google Scholar]
- 23.Baz R, Li L, Kottke-Marchant K, Srkalovic G, McGowan B, Yiannaki E, et al. The role of aspirin in the prevention of thrombotic complications of thalidomide and anthracycline-based chemotherapy for multiple myeloma. Mayo Clin Proc 2005;80(12):1568–74. [DOI] [PubMed] [Google Scholar]
- 24.Baz R, Hussein MA. Does low-dose aspirin have antineoplastic effects in multiple myeloma? Br J Haematol 2006;134(3):349–50. [DOI] [PubMed] [Google Scholar]
- 25.McNeil JJ, Nelson MR, Woods RL, Lockery JE, Wolfe R, Reid CM, et al. Effect of Aspirin on All-Cause Mortality in the Healthy Elderly. N Engl J Med 2018;379(16):1519–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rothwell PM, Wilson M, Price JF, Belch JF, Meade TW, Mehta Z. Effect of daily aspirin on risk of cancer metastasis: a study of incident cancers during randomised controlled trials. Lancet 2012;379(9826):1591–601. [DOI] [PubMed] [Google Scholar]
- 27.Waxman AJ, Mink PJ, Devesa SS, Anderson WF, Weiss BM, Kristinsson SY, et al. Racial disparities in incidence and outcome in multiple myeloma: a population-based study. Blood 2010;116(25):5501–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pulte D, Redaniel MT, Brenner H, Jansen L, Jeffreys M. Recent improvement in survival of patients with multiple myeloma: variation by ethnicity. Leukemia & Lymphoma 2014;55(5):1083–9. [DOI] [PubMed] [Google Scholar]
- 29.Costa LJ, Brill IK, Omel J, Godby K, Kumar SK, Brown EE. Recent trends in multiple myeloma incidence and survival by age, race, and ethnicity in the United States. Blood advances 2017;1(4):282–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marinac CR, Ghobrial IM, Birmann BM, Soiffer J, Rebbeck TR. Dissecting racial disparities in multiple myeloma. Blood Cancer J 2020;10(2):19. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


