Abstract
Varroa destructor (Mesostigmata: Varroidae) is arguably the most damaging parasitic mite that attacks honey bees worldwide. Since its initial host switch from the Asian honey bee (Apis cerana) (Hymenoptera: Apidae) to the Western honey bee (Apis mellifera) (Hymenoptera: Apidae), Varroa has become a widely successful invasive species, attacking honey bees on almost every continent where apiculture is practiced. Two haplotypes of V. destructor (Japanese and Korean) parasitize A. mellifera, both of which vector various honey bee-associated viruses. As the population of Varroa grows within a colony in the spring and summer, so do the levels of viral infections. Not surprisingly, high Varroa parasitization impacts bees at the individual level, causing bees to exhibit lower weight, decreased learning capacity, and shorter lifespan. High levels of Varroa infestation can lead to colony-wide varroosis and eventually colony death, especially when no control measures are taken against the mites. Varroa has become a successful parasite of A. mellifera because of its ability to reproduce within both drone cells and worker cells, which allows populations to expand rapidly. Varroa uses several chemical cues to complete its life cycle, many of which remain understudied and should be further explored. Given the growing reports of pesticide resistance by Varroa in several countries, a better understanding of the mite’s basic biology is needed to find alternative pest management strategies. This review focuses on the genetics, behavior, and chemical ecology of V. destructor within A. mellifera colonies, and points to areas of research that should be exploited to better control this pervasive honey bee enemy.
Keywords: Western honey bee, parasitic mite, host–parasite interaction, chemical ecology, behavioral ecology
Varroa destructor (Mesostigmata: Varroidae) is a cosmopolitan ectoparasitic mite known for its successful infestation of Western honey bee (Apis mellifera L. (Hymenoptera: Apidae)) colonies worldwide. The Varroa mite is the leading driver of colony mortality in the United States, causing colony collapse and/or death if highly infested colonies are left untreated (Guzmán-Novoa et al. 2010, Kulhanek et al. 2017, Brodschneider et al. 2018, Steinhauer et al. 2018). Since its introduction in the 1980’s, Varroa has caused significant damage to the U.S. beekeeping industry in terms of colony and economic losses (Brodschneider et al. 2018). The average loss of honey bee colonies in the U.S. was 40.5 and 45.5% in 2015–2016 and 2020–2021, respectively, with both surveys showing Varroa mites as the top culprit for colony mortality reported by surveyed beekeepers (Kulhanek et al. 2017, Steinhauer et al. 2021). Similar patterns of high colony losses due to Varroa parasitization have been seen around the world. For example, regional surveys in Europe reported an average winter loss of 20.9% in 2016–2017 (Brodschneider et al. 2018) and 16.7% in 2018–2019 (Gray et al. 2020). While colony losses have not been tracked as closely in Latin America as they have in the U.S. or Europe (Requier et al. 2018), In Uruguay, the average winter colony loss was 18.3% in 2013–2014, with 61.5% of the reported losses being caused by parasites and disease (Antunez et al. 2017). Given the negative impact that Varroa has caused on Western honey bee populations, multiple lines of integrated pest management have been deployed for Varroa control around the world. However, to date, none of the existing management options have been able to fully eliminate Varroa from infested colonies, and instead, have only allowed us to maintain infestations below damaging levels (Lee et al. 2015, Kulhanek et al. 2017, Brodschneider et al. 2018, Jack and Ellis 2021).
The overall reduction of colony health and longevity caused by high mite infestation is known as Varroa disease or varroosis (Boecking and Benersch 2008). The severity of varroosis-caused symptoms depends on the level of mite infestation and is often associated with a steady and linear increase in a colony’s Varroa population through the spring and mid-summer (Wegener et al. 2016). Around July, colonies with varroosis show a high brood-to-adult bee ratio and bees exhibit higher expression of phenol oxidase (POX) and glucose oxidase (GOX) enzymes (Wegener et al. 2016). The high expression of bee immune responses, at least partially, could be explained by increased levels in the viruses vectored by Varroa (Wegener et al. 2016). By late August, GOX and POX are expressed at low levels, possibly allowing viral loads to increase (Wegener et al. 2016). In the fall, the weight of overwintering workers is lower in infested colonies compared to uninfested ones, regardless of infestation levels (Aronstein et al. 2012, Wegener et al. 2016). Throughout fall and winter, as the infestation threshold is reached, varroosis can lead to colony collapse. However, the infestation thresholds vary depending on environmental factors (e.g., average precipitation, geographic region, and food availability), genetics (e.g., differences between Africanized and European lineages or the presence of hygienic behavior), as well as their interaction (Dainet et al. 2012, Wegener et al. 2016, Dechatre et al. 2021). For example, the growth of Varroa mite populations fluctuates with the weather, with years in which the amount of rainfall is below the annual average showing lower mite growth rates than wetter years (Harris et al. 2003). Furthermore, colonies with higher amounts of honey and brood show higher levels of infestation levels than colonies with lower amounts of honey and brood, likely caused by the increased amount of available brood cells to invade (Lodesani et al. 2002). Interestingly, even though hygienic behavior has been a phenotype selected by beekeepers to control mite loads, this behavior does not always show a significant correlation with lower Varroa infestation levels (Arechavaleta-Velasco et al. 2001, Lodesani et al. 2002).
V. destructor feeds on the fat bodies of developing and adult honey bees (Ramsey et al. 2019), all while transmitting several honey bee-associated viruses (Francis et al. 2013, Mondet et al. 2014, Emsen et al. 2015). These include Deformed wing virus (DWV), Acute bee paralysis virus (ABPV), Israeli acute paralysis virus (IAPV), Kashmir bee virus (KBV), and Sacbrood virus (SBV) (Ball 1983, Ball and Allen 1988, Chen et al. 2004, Yue and Genersch 2005, Boecking and Genersch 2008). Because DWV has evolved alongside A. mellifera, the virus was found in most hives at covert levels before the introduction of Varroa as an invasive parasite of Western honey bees (Wilfert et al. 2016). However, with the spread of Varroa the symptoms caused by DWV have become much more prevalent because the mite spreads the virus from bee to bee as it feeds on different hosts. Furthermore, increased time spent by mites on adult bees can increase the chance of DWV spread to bee brood (Prisco et al. 2011, Piou et al. 2016). It is unclear if all honey bee-associated viruses harm and/or replicate within the Varroa mite, however. For instance, Posada-Florez et al. (2019a) found that V. destructor is a nonpropagative vector of DWV-type A, which means that the virus does not replicate within the mite. More studies are needed to determine if other honey bee-associated viruses vectored by Varroa mites can replicate within the mite, as well as the health consequences to the mite from carrying the viruses, which would give insight on its ability to spread pathogens within and among honey bee colonies.
Varroa parasitization causes several physiological problems for bees at the individual and colony levels (for reviews on these topics see Noel et al. 2020 and Traynor et al. 2020). At the individual level, the constant feeding of mites using their sucking-piercing mouthparts causes an open wound on parasitized pupae, resulting in the development of scar tissue and bacterial infections (Kanbar and Engels 2003). Varroa feeding also leads to decreased hemocyte concentrations and reduced POX expression, both of which are important for immune function (Koleoglu et al. 2018). Parasitized worker and drone brood also exhibit lower weight at emergence compared with unparasitized brood and are unable to regain the lost weight upon emergence (De Jong et al. 1982, Duay et al. 2003, Van Dooremalen et al. 2013). Workers parasitized by Varroa also tend to have smaller hypopharyngeal glands and, when parasitized by three or more mites, typically have smaller mandibular glands (Ayoub et al. 2015). Infested bees also exhibit increased metabolic rates, likely because of their immune response and the mites’ consumption of their fat bodies (Ramsey et al. 2019, Aldea and Bozinovic 2020). Large infestations also show decreased learning capacity in workers, particularly in their homing ability, which causes highly parasitized foragers to get disoriented when returning to the hive (Kraji and Fuchs 2006). Moreover, drones within Varroa infested colonies weigh less and have significantly lighter testes than drones in noninfested colonies (Omar 2017). At the colony level, high Varroa infestation levels cause a large proportion of the worker population to be inactive (Annoscia et al. 2015). Colonies with high mite infestations also have lower success in the retention of newly introduced queens (Rateb et al. 2010).
While many studies have focused on the health, management, and economic impacts of Varroa parasitization on A. mellifera (see Jack and Ellis 2021), there is still a need for greater understanding of the life history of V. destructor within A. mellifera colonies. This review is a compilation of influential studies regarding V. destructor’s genetics, behavior, and chemical ecology, and points to those aspects of the mite’s biology that should be studied further. A growing body of knowledge about the mechanisms of Varroa parasitization of A. mellifera colonies will help us better understand how this mite has become so successful in attacking colonies and will help inform the future development of improved management strategies against this devastating honey bee enemy.
Varroa Destructor’s Initial Host Switch and Genetics
The Asian honey bee, Apis cerana (Hymenoptera: Apidae), is the original obligate host of V. destructor. It is also the host of another mite species in the same genus, V. jacobsoni (Anderson and Trueman 2000). The two Varroa species are very similar genetically, sharing 99.7% of their genome in common (Techer et al. 2019). Their morphological similarities require genetic testing using amplified fragment length polymorphism (AFLP) to accurately identify each species (Anderson and Trueman 2000, Roberts et al. 2015, Techer et al. 2019). While V. jacobsoni is found mostly within A. cerana colonies throughout Asia, in 2008 the mite was found parasitizing worker and drone brood of A. mellifera in Papua New Guinea (Roberts et al. 2015). This shows that V. jacobsoni can spread to A. mellifera colonies, although more studies are needed to fully understand the extent of this novel parasitism. Originally it was believed that V. jacobsoni was the mite species that switched hosts and began infesting A. mellifera colonies. However, in the year 2000, V. destructor was identified as the infesting mite and was separated phylogenetically from V. jacobsoni soon after (Anderson and Trueman 2000). Because of this confusing misidentification of mites, the studies referencing V. jacobsoni parasitization of A. mellifera published before 2000 actually refer to V. destructor.
In 1952, a host switching event by V. destructor from A. cerana to A. mellifera colonies in eastern Russia produced the Korean haplotype of this mite (Oldroyd 1999). A second host switching event occurred around 1957, which led to the description of the Japanese haplotype (Oldroyd 1999). While both V. destructor haplotypes have spread west from their countries of origin, the Korean haplotype has become dominant (Anderson and Trueman 2000, Guerra Junior et al. 2010, Gajic et al. 2013, Ayan et al. 2017, Kelomey et al. 2017, Octaviano-Salvadé et al. 2017, Hajializadeh et al. 2018, Muntaabski et al. 2020, Ogihara et al. 2020). Several variants of the Korean and Japanese haplotypes have been identified in parts of Asia (Navajas et al. 2010). Even though only the Japanese and Korean haplotypes are known to invade A. mellifera colonies, a recent study looking at the mite’s genome suggests a higher genomic diversity among mite populations across the world than previously thought (Techer et al. 2021). In that study, the authors analyzed the genome-wide variation and divergence among V. destructor and V. jacobsoni females from their original and novel hosts, confirming Varroa species identity by aligning single nucleotide polymorphisms (SNPs) within the mitogenomes, along with known reference sequences of the mitochondrial DNA (mtDNA) COX1 458-bp standard marker. They found multiple previously undiscovered mitochondrial lineages on the novel hosts of each mite, as well the genetic equivalent of tens of individuals that were involved in the initial host switch. Therefore, contrary to previous beliefs, modest gene flow remains between mites adapted to different hosts. The low genetic diversity of V. destructor within A. mellifera colonies is thought to be caused by three factors: i) a genetic bottleneck that occurred during the host switching events, ii) the founder effect that occurred after the spread of the two haplotypes into new regions, and iii) the mite’s sibling–sibling mating system (Solignac et al. 2005, Navajas et al. 2010, Dynes et al. 2017).
Varroa Destructor’s Life Cycle
Reproductive Phase
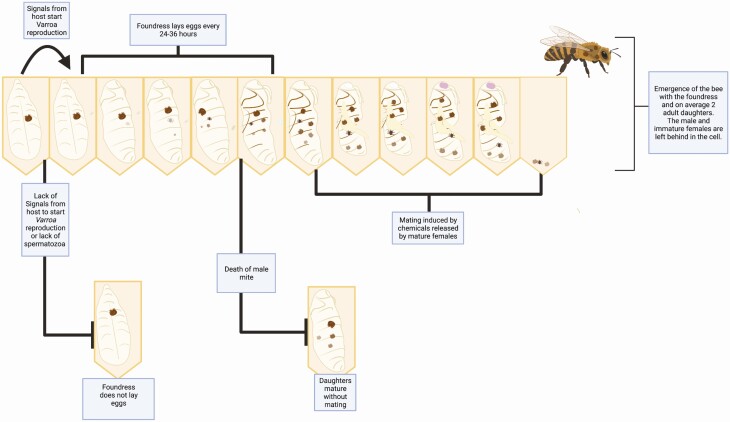
There are two phases in the life cycle of V. destructor: the reproductive phase and the dispersal phase (for a review of Varroa’s life cycle see Rosenkranz et al. 2010). To begin the reproductive phase, a gravid adult female mite, referred to as a foundress, invades the cell of a 5th instar bee larva (Figs. 1 and 2). Upon invasion, the foundress is temporarily trapped in the brood food found at the bottom of the cell and remains trapped for up to six hours in workers cells and 20 hr in drone cells before the cell is capped for the bee to initiate pupation (Ifantidis 1988, Aumeier et al. 2002). The capped bee pupa then releases an unknown chemical signal that initiates egg production by the mite (Garrido and Rosenkranz 2003).
Fig. 1.
A simplified diagram showing the life cycle of Varroa destructor. During the reproductive phase, a gravid female mite enters the cell of a worker or drone larva before it being capped (A). Once the cell is capped, the foundress mite produces a son and several daughters who undergo sibling–sibling mating, all while feeding on the bee pupa (B). Upon the emergence of the adult bee, the mites leave the cell and begin the dispersal phase (C), during which newly gravid females get transported by bees to reach a bee brood cell to invade, starting the cycle again.
Fig. 2.
Reproductive cycle of Varroa destructor after a gravid female foundress invades a developing honey bee cell. Once the adult bee completes pupation and emerges from its cell, the mites that developed therein also exit to begin the dispersal phase.
Varroa destructor undergoes arrhenotokous parthenogenesis (Rehm and Ritter 1989, Häußermann et al. 2018, 2020). Because of its haploid–diploid sex determining system, the first egg laid by the foundress mite (approximately 60 hr postcapping) is unfertilized and develops into a male (Ifantidis 1983). The rest of the eggs, which are laid every 24–36 hr, are fertilized and develop into females (Rehm and Ritter 1989, Martin 1994). A foundress typically produces four to five offspring in a worker cell and five to six offspring in a drone cell (Ifantidis 1983, Martin 1995). However, the average production of mature daughters in single-infested cells is 1.8 and 3.0 for worker and drone cells, respectively (Donze et al. 1996, Martin 1998). A feeding site is established by the foundress along with a fecal accumulation site on the cell wall (Donze and Guerin 1994). Feeding by the mites occurs every 1.6 hr and competition for food is common at the feeding site (Donze and Guerin 1994). Based on the amount of excretion and fecal matter produced, it is estimated that mites can consume up to 1 µl of host fluids per day (Posada-Florez et al. 2019b). The development of mite offspring takes approximately 5.5 d within worker cells and 7.5 d within drone cells (Ifantidis 1983, Martin 1994).
After maturation, mating among mites occurs at the fecal accumulation site and is bimodal, being either three or six minutes in duration (Donze et al. 1996). Mite mating is activated by volatile compounds released by the female (Ziegelmann et al. 2013) and females who mature first usually mate more times. Because mating events are often interrupted, it is thought that several mating events are required for proper fertilization. This may explain why females that mate only once typically do not become fertilized (Donze et al. 1996). Successful mating is also not possible sometimes due to the lack of a mature male within the cell. In fact, an estimated average of 17% of worker cells and 23% of drone cells is thought to contain no mature male mites at all (Donze et al. 1996).
Varroa mites typically invade a cell individually, leading to the offspring of one foundress to mate with one another. However, multiple foundresses can invade the same cell, which leads to mating between offspring from different mothers (Beaurepaire et al. 2019). When more than one male is present in the cell, female mites exhibit polyandry (Donze et al. 1996). After fertilization, a female’s full spermatheca generally contains up to 35 spermatozoa (Donze et al. 1996). Since Varroa foundresses generally lay between 4 and 7 eggs per invasion, and one egg is a haploid male, it could be estimated that one Varroa foundress can invade a bee cell up to five times in her lifespan if her spermatheca is full of spermatozoa (Ifantidis 1983, Donze et al. 1996). However, the actual number of times that a foundress can invade cells varies and is likely to be between 1.5 and 3 (Fries and Rosenkranz 1996, Martin and Kemp 1997). Once the female mites are fully mated, they can begin the dispersal phase upon the emergence of the adult bee host.
Dispersal Phase
The second component of the mite’s life cycle was recently renamed the ‘dispersal’ phase instead of the ‘phoretic’ phase. This is because we now know that feeding by the mite does occur on adult bees as they get transported to potential sites for invasion, whereas in a truly phoretic species, the host is only used as a mode of transport and no feeding occurs (Ramsey et al. 2019). The dispersal phase starts when the adult bee emerges from a mite-invaded cell (Fig. 1). The foundress and her adult daughters attach themselves to the bee before it exits, while the male and all immature females are left behind and die soon after (Donze et al. 1996). After leaving the cell, the female mites switch from riding on the original newly emerged bee to riding on a nurse bee (Kuenen and Calderone 1997). Mites are not often found walking directly on the comb (Kuenen and Calderone 1997). The number of times that a mite switches from one bee host to the next, as well as the duration of each transport event is still unknown.
The most likely spot to find mites on adult bees is between sternites on the ventral side of the abdomen. Interestingly, mites show a preference for attaching on the left side of the bee’s abdomen for reasons still undetermined (Delfinado-Baker et al. 1992, Fernández et al. 1993). Varroa mites exhibit a preference for dispersing atop nurse bees, likely because nurses give mites the access to brood cells (Xie et al. 2016). However, mite preference for adult hosts can change during the year. For instance, when there are high Varroa loads within a colony, mites can be more frequently found atop foragers (DeGrandi-Hoffman et al. 2016).
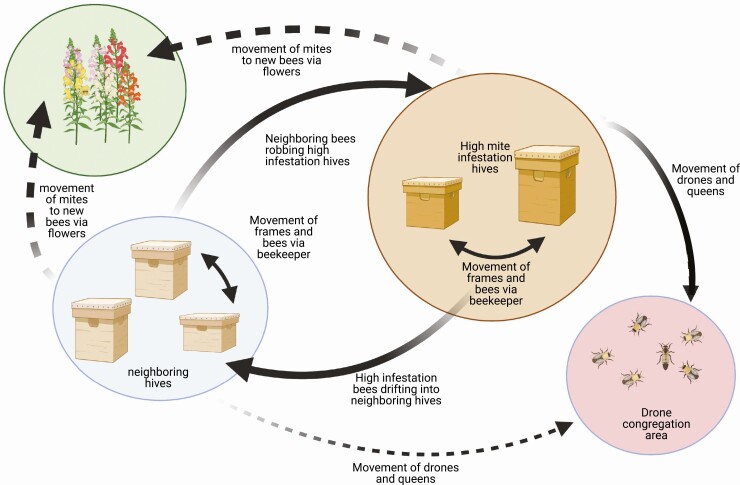
Mites can also gain access to new colonies during the dispersal phase (Fig. 3). This can happen through robbing and drifting behaviors performed by the bees, as well as through beekeeping practices (Peck et al. 2016, Peck and Seeley 2019). For example, as a colony’s mite population increases to the point that it begins to collapse, robbing by foragers from other colonies is common. Mites can also jump hosts from robbing bees to other workers and infest a new colony (Fig. 3). They can also attach to foragers of their original colony and, in the event those foragers accidentally drift, mites gain access to other colonies (Peck and Seeley 2019). Additionally, mites can be introduced into foreign colonies by beekeepers when they transfer supplies and bees between hives (Fig. 3). Studies have also found mites on drones collected at drone congregation areas, or ‘DCAs’ (Mortensen et al. 2018, Galindo-Cardona et al. 2020). Moreover, Galindo-Cardona et al. (2020) showed that drones returning from mating flights could drift into neighboring colonies, spreading mites throughout the apiary. However, it has not been confirmed that mites can access new colonies after getting infested at DCAs, and thus, more information on how adult drones and Varroa mites interact is needed to further understand how drones play a role in the spread of Varroa within and among colonies.
Fig. 3.
Diagram depicting the potential ways in which Varroa mites can move between honey bee colonies. Solid arrows represent those paths that have been confirmed by previous studies and dashed arrows represent suspected (yet mostly understudied) methods of how Varroa mites spread between hives.
Additionally, the bees’ own behavior may contribute to the mite’s success during the dispersal phase. For example, Rivera-Marchand et al. (2012) found that commercial honey bees of Italian maternal descent habituated faster to mite attempts at ‘catching a ride’ on adult bees than mites in colonies of Africanized maternal descent. Finally, there is some evidence that mites can spread to new colonies via flowers (Peck et al. 2016), but more studies are needed to understand how likely this is to occur.
Behavior and Chemical Ecology
Chemical-Producing Organs in Varroa
Varroa destructor lacks visual organs and relies on chemical cues for orientation within the confined darkness of a honey bee hive. The mite uses the front pair of legs as sensory organs (Diller et al. 2006, Nganso et al. 2020). Each front leg has a sensory pit organ with nine sensillae within and nine sensillae around the organ, respectively (Liu 1988). This pit organ has been compared to the Haller’s organ in ticks (Diller et al. 2006) and has been shown to react to compounds of honey bee brood pheromone (Eliash et al. 2014). The mite also has chemoreceptive sensillae on the palptarsi of the front two legs, which are responsive to brood chemicals such as methyl oleate and methyl palmitate, as well as alarm pheromones such as 2-heptanone (Liu and Peng 1990, Light et al. 2020).
Host Preference
There is a clear host preference exhibited by Varroa during both the reproductive and the dispersal phases. It is thought that mites detect cuticle compounds in bee brood to choose their host and to make their way around the hive depending on what phase of the life cycle they are in. During the dispersal phase, mites show a preference for attaching onto nurse bees over foragers or adult drones (Kraus 1994, Kuenen and Calderone 1997, Pernal et al. 2005, Del Piccolo et al. 2010, Xie et al. 2016). Varroa’s preference for attaching to nurse bees during the dispersal phase is thought to be mediated by chemical cues. Foragers exhibit shorter straight-chain cuticular hydrocarbons (CHC) profiles compared to nurses, which have higher amounts of longer straight-chain CHCs (Del Piccolo et al. 2010). The cuticle of foragers also has higher amounts of (Z)-8-heptadecene, which acts as a repellent to mites (Del Piccolo et al. 2010). As the population of V. destructor increases in a colony, the distinct preference of mites for attaching onto nurses over foragers decreases (Cervo et al. 2014, Xie et al. 2016). This could be explained by the fact that the CHC profiles of bee foragers and nurses are more similar in colonies that have high mite loads compared to those with low mite loads (Cervo et al. 2014, Xie et al. 2016). The increased presence of mites on foragers could be a way for Varroa to spread to new colonies. This change in host preference during the dispersal phase does not come without a cost, however, as mites that attach to foragers have increased infertility rates and lower fitness after invading a cell (Xie et al. 2016). It is still unclear if the increased infertility is caused by a decreased nutritional state in the forager, the host age of the mite, or other factors (Xie et al. 2016). Interestingly, Lin et al. (2018) showed that V. destructor did not exhibit different larval host preferences within A. mellifera and A. cerana colonies. From that study, we could infer that Varroa’s host preferences during the dispersal phase within A. cerana also does not differ from mites within A. mellifera colonies. However, there is an overall lack of studies focusing on the host preferences of V. destructor during the dispersal phase within A. cerana colonies, and thus more studies are needed to understand this important life phase of the mite.
The decision-making mechanisms through which Varroa mites invade bee cells to begin the reproductive phase are not fully understood. Within Asian honey bee colonies, V. destructor can only successfully reproduce in drone cells because bees remove parasitized worker larvae, which interrupts Varroa reproduction in worker cells (Lin et al. 2018). However, the development of parasitized worker larvae is not normally interrupted in Western honey bee colonies, allowing the mite to successfully reproduce in both worker and drone cells. Nevertheless, Varroa displays a clear preference for invading cells of drone larvae over worker larvae in A. mellifera colonies (Fuchs 1990, Boot et al. 1995). This preference was originally attributed to differences in CHC profiles between drone and worker larvae (Le Conte et al. 1989, 1990; Trouiller et al. 1991; Rickli et al. 1992, 1994; Del Piccolo et al. 2010; Cervo et al. 2014). Several compounds within the honey bee brood pheromone have been identified as kairomones for V. destructor. Of these compounds, the most active is methyl palmitate, which is found in higher amounts in drone brood compared to worker brood (Le Conte et al. 1989, Trouiller et al. 1992).
In the past, methyl palmitate and other brood pheromones were used to explain the mite’s preference for invading drone brood over worker brood. In fact, for some time it was believed that methyl palmitate could be exploited as a potential method for mite management. Continued studies showed contradicting results, however, as methyl palmitate and other cuticular compounds did not always elicit clear host choices by mites between the drone and worker brood (Boot 1994, Calderone and Lin 2001, Nazzi et al. 2001, Pernal et al. 2005). A study also revealed that topical application of methyl palmitate onto brood cell caps resulted in bee larval death at high doses (Boot 1994). Furthermore, even though drone and worker larvae produce different amounts of brood pheromone (Trouiller et al. 1992), choice tests showed that Varroa is equally attracted to drone and worker cuticle extracts (Calderone and Lin 2001), showing that these compounds likely do not help the mite in choosing between bee larval types. Instead, these compounds may simply help the mites determine if a cell is empty or contains a larva (Boot 1994, Calderone and Lin 2001). Other compounds within larval cells have also been studied as possible mite attractants. For example, a particular compound in the brood food, 2-hydroxyhexanioc acid, elicits a behavioral response from Varroa (Nazzi et al. 2001, 2004). However, this attraction does not differ between brood types (Calderone and Lin 2001). Therefore, while mites have the ability to detect bee larvae through chemical cues, olfactory stimuli alone may not be enough to elicit the invasion of a specific type of bee cell (Kraus 1994).
The distance between a bee larva and the top of the cell’s opening may also be a key factor in cell invasion by Varroa. Once this distance is 7.0–7.5 mm, the cell becomes attractive to mites and invasion begins (Goetz and Koeniger 1993, Beetsma et al. 1999). If this distance is achieved earlier, the attractive period is longer (Boot et al. 1995). Comb that is older is also more attractive to mites, possibly because the cells are smaller from use and thus the distance from a larva to the top of a cell is shorter sooner (Piccirillo and Jong 2004). This suggests that beekeepers should remove older comb or only use it for honey supers to reduce mite invasion. Interestingly, small cell comb, which was once suggested as a possible mechanical management system to reduce Varroa populations (Martin and Kryger 2002, McMullan and Brown 2006), has now been deemed ineffective in reducing Varroa loads compared to normal comb (Seeley and Griffin 2011) and can actually elicit a higher chance of cell invasion. Thus, small cell comb should be discontinued as a mite treatment option (Berry et al. 2010, Coffey et al. 2010).
The visitation rates of larvae by nurse bees could also be a factor in Varroa’s cell invasion process. Drone larvae are attractive to mites for around 40 hr before capping, while worker larvae are attractive for only 20 hr before capping (Boot et al. 1992). This extended period of mite attraction toward drone larvae could contribute to the preference of Varroa for invading drone cells. Drone larvae also have roughly 2.5 times higher visitation rates by nurse bees than worker larvae (Calderone and Kuenen 2003, Reams et al. unpublished data). Brood that has a higher visitation rate is likely exposed to Varroa mites more often and thus has a higher chance of being invaded. More studies are needed to understand how nurse visitation rates influence Varroa cell invasion, however.
Cell Invasion
The cell invasion rate of Varroa within a colony throughout the year varies and is heavily influenced by the presence of adult and larval bees, as well as the choices made by other mites. As the number of available brood cells to be invaded in the spring increases, so does the Varroa invasion rate (Boot et al. 1994a, Martin 1998). This increase is caused by the higher number of larvae that are at the right developmental stage for invasion. As the population of adult bees increases, Varroa invasion rate decreases (Boot et al. 1994b). This brood-to-adult bee ratio is important for studying Varroa invasion: as the population of adult bees increases, the chance that a mite is exposed to brood cells goes down, thus decreasing invasion and vice versa. As the number of larvae in the 5th instar increases, the invasion of Varroa should increase, since this is the appropriate time period for mite invasion.
The invasion of Varroa into brood cells can be looked at in multiple ways: the invasion rate of both larval types, the drone cell preference, and the worker cell acceptance. The invasion rates of drone and worker larvae are calculated by the number of mites per cell (Fuchs 1990). Drone cell invasion rates are usually higher than worker cell invasion rates (Fuchs 1990, Boot et al. 1995). Drone cell preference is the drone cell infestation rate divided by the worker cell infestation rate (Fuchs 1990). As the ratio of drone cells to worker cells increases, the drone cell preference decreases (Fuchs 1990, 1992). This means that with less drone brood, drone cells are preferred, and thus, drone cell preference is not constant but fluctuates throughout the year (Fuchs 1990, 1992). Drone cell acceptance also depends on the population of mites within the entire colony. As the mite population increases (typically over the spring and summer) the preference for drone cells decreases (Fuchs 1990).
Worker cell acceptance is the threshold at which Varroa will begin invading worker cells (Fuchs 1992). This threshold is measured as the total number of mites within the hive, which is around 300. After this threshold is reached, worker cell acceptance begins to increase (Fuchs 1992). This is because after the threshold is reached, there are so many mites within the colony that it becomes more optimal for a mite to invade a worker cell as the sole foundress than to invade a drone cell that already contains mites. This shows that both drone and worker cell invasion can fluctuate throughout the year and as the mite population changes. Interestingly, while Varroa prefers to invade drone and worker cells, they can also be found invading queen cells. In fact, queen cell invasion can range from 1 to 9.1%, depending on the presence or absence of brood (Harizanis 1991). Mites that invade queen cells cannot complete their reproductive cycle, but do not typically affect the grafting success of queen cells (Harizanis 1991).
Multiple mites can invade the same cell and this occurs more often with higher mite infestation rates (Martin 1995, Floris et al. 2020). This may be advantageous to the overall mite population within a hive because it increases the chance of outbreeding. However, mites that invade the same cell are likely to be related, so the offspring may not achieve an increased genetic diversity from outbreeding (Beaurepaire et al. 2019). Multiple invasions also have a negative impact on the mite. As the number of invasions per cell increases, fewer eggs are laid per mite and offspring mortality increases (Martin 1995). Interestingly, even after a successful invasion of a brood cell, female mite infertility is relatively high. Low fertility is caused by several factors including male mortality, such as by crushing or dislodging by the pupa, which leads to unfertilized mites and an ‘unsuccessful’ mite invasion (Martin 1997, 2001; Nganso et al. 2020). Male mortality tends to be higher during the winter (Martin 2001) and can lead to mature females leaving the cell without mating (Martin et al. 1997, Häußermann et al. 2020). The failure of a foundress to lay eggs within the brood cell can also be caused by delayed oviposition or by a low number of spermatozoa stored in her spermatheca (Harris and Harbo 1999).
Egg Laying and Mating
Semiochemicals are also involved in V. destructor’s egg laying behavior within a brood cell. The capping of the cell triggers oocyte activation in the foundress, which is caused by a signal lasting about 14 hr postcapping (Garrido et al. 2000, Garrido and Rosenkranz 2003, Rosenkranz and Garrido 2004). Varroa mating occurs within the cell and is also started by chemical cues. The male mite detects six chemicals emitted from the newly mature females, which lead to mating: oleic acid, palmitic acid, stearic acid, ethyl palmitate, ethyl oleate, and ethyl stearate (Ziegelmann et al. 2013). Once a younger female mite matures, the male stops mating with the older female and mates with the newly mature female (Ziegelmann et al. 2013).
Camouflage
Varroa mites rely on chemical ecology to remain hidden inside a honey bee hive and uses passive chemical camouflage to stay undetected by the bees. The CHCs of bee pupae have a high alkene-to-methyl alkane ratio, while the CHCs in adult bees have a low alkene-to-methyl alkane ratio (Kather et al. 2015). This means that the mite has to change its chemical profile with every pupa-to-adult or adult-to-pupa host switch. The mite needs direct contact with the bee’s cuticle to make this change, which takes between three and nine hours. This has been shown to occur with dead mites as well as live mites, meaning that the change in CHC profiles is passive (Kather et al. 2015). There are also some chemicals in the hive that have possible negative or repulsive reactions on V. destructor. For example, (Z)-8-heptadecene has been shown to reduce mite reproduction when applied to larval cells (Milani et al. 2004), resulting in a significant reduction in the number of possibly mated daughters. This reduction could disrupt the Varroa population within a colony, but more studies on this aspect of Varroa’s chemical ecology are still needed.
Concluding Remarks
Varroa destructor is the biggest enemy currently faced by the beekeeping industry worldwide. The swift spread of the Varroa mite has caused detrimental impacts on A. mellifera populations, from substantial viral spread to massive colony losses around the world. The genetics of the mite is important for understanding and developing novel methods of chemical control against the mites. Recent studies suggest that the genetic diversity of V. destructor has been historically underestimated and thus, more studies are needed to measure the mite’s genetic structure in all the countries where it is present. While the life cycle of the mite is generally well understood, there is still a lack of behavioral studies on the dispersal phase. A better understanding of the dispersal phase would shed light on the decision-making process of cell invasion by Varroa. Moreover, the mite’s reliance on chemical communication for mating and host selection is an important component of the mite’s behavior that begs to be studied further. Determining how chemical ecology is involved in mite host choice during the reproductive phase would be a substantial step for future mite control methods. In conclusion, the behavioral ecology of the Varroa mite needs to be fully understood before we are able to truly understand and control this devastating honey bee parasite on a global scale.
Acknowledgments
We thank the guest editors and two anonymous reviewers for their insightful comments, which helped us strengthen this paper. We are also indebted to the members of the Rangel Honey Bee Research Program that helped during the development of this manuscript. All figures were created with BioRender.com.
Author Contributions
T.R.: Conceptualization, investigation, methodology, visualization, writing - original draft, writing – review and editing. J.R.: Conceptualization, funding acquisition, methodology, resources, supervision, writing - original draft, writing – review and editing.
References Cited
- Aldea, P., and Bozinovic F.. . 2020. The energetic and survival costs of Varroa parasitism in honeybees. Apidologie. 51: 997–1005. [Google Scholar]
- Anderson, D. L., and Trueman J. W.. . 2000. Varroa jacobsoni (Acari: Varroidae) is more than one species. Exp. Appl. Acarol. 24: 165–189. [DOI] [PubMed] [Google Scholar]
- Annoscia, D., Del Piccolo F., Covre F., and Nazzi F.. . 2015. Mite infestation during development alters the in-hive behaviour of adult honeybees. Apidologie. 46: 306–314. [Google Scholar]
- Antúnez, K., Invernizzi C., Mendoza Y., vanEngelsdorp D., and Zunino P.. . 2017. Honeybee colony losses in Uruguay during 2013–2014. Apidologie. 48: 364–370. [Google Scholar]
- Arechavaleta-Velasco, M. E., and Guzmán-Novoa E.. . 2001. Relative effect of four characteristics that restrain the population growth of the mite Varroa destructor in honey bee (Apis mellifera) colonies. Apidologie. 32: 157–174. [Google Scholar]
- Aronstein, K. A., Saldivar E., Vega R., Westmiller S., and Douglas A. E.. . 2012. How Varroa parasitism affects the immunological and nutritional status of the honey bee, Apis mellifera. Insects. 3: 601–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aumeier, P., Rosenkranz P., and Francke W.. . 2002. Cuticular volatiles, attractivity of worker larvae and invasion of brood cells by Varroa mites. A comparison of Africanized and European honey bees. Chemoecology. 12: 65–75. [Google Scholar]
- Ayan, A., Aldemır O. S., and Selamoglu Z.. . 2017. Analysis of COI gene region of Varroa destructor in honey bees (Apis mellifera) in province of Siirt. Turkish J. Vet. Res. 1: 20–23. [Google Scholar]
- Ayoub, Z. N., Mosa M. H., Abdulla M., and Ahmed D. S.. . 2015. Impact of Varroa mite infestation on the mandibular and hypopharyngeal glands of honey bee workers. Acarina. 23: 92–97. [Google Scholar]
- Ball, B. V. 1983. association of Varroa jacobsoni with virus diseases of honey bees. In R. Cavalloro (ed.), Varroa jacobsoni Oud. affecting honey bees: present status and needs: pro. Meet. EC Exp. Gr., Wageningen, 7–9 February 1983, Rotterdam: Published for the Commission of the European Communities by AA Balkema. [Google Scholar]
- Ball, B. V., and Allen M. F.. . 1988. The prevalence of pathogens in honey bee (Apis mellifera) colonies infested with the parasitic mite Varroa jacobsoni. Ann. Appl. Biol. 113: 237–244. [Google Scholar]
- Beaurepaire, A. L., Ellis J. D., Krieger K. J., and Moritz R. F. A.. . 2019. Association of Varroa destructor females in multiply infested cells of the honeybee Apis mellifera. Insect Sci. 26: 128–134. [DOI] [PubMed] [Google Scholar]
- Beetsma, J., Boot W. J., and Calis J.. . 1999. Invasion behaviour of Varroa jacobsoni Oud.: from bees into brood cells. Apidologie. 30: 125–140. [Google Scholar]
- Berry, J. A., Owens W. B., and Delaplane K. S.. . 2010. Small-cell comb foundation does not impede Varroa mite population growth in honey bee colonies. Apidologie. 41: 40–44. [Google Scholar]
- Boecking, O., and Genersch E.. . 2008. Varroosis–the ongoing crisis in bee keeping. J. Ver. Leb. 3: 221–228. [Google Scholar]
- Boot, W. J. 1994. Methyl palmitate does not elicit invasion of honeybee brood cells by Varroa mites. Exp. Appl. Acarol. 18: 587–592. [Google Scholar]
- Boot, W. J., Calis J. N., and Beetsma J.. . 1992. Differential periods of Varroa mite invasion into worker and drone cells of honey bees. Exp. Appl. Acarol. 16: 295–301. [Google Scholar]
- Boot, W. J., Beetsma J., and Calis J. N. M.. 1994a. Behavior of Varroa mites invading honey bee brood cells. Exp. Appl. Acarol. 18: 371–379. [Google Scholar]
- Boot, W. J., Sisselaar D. J., Calis J. N., and Beetsma J.. . 1994b. Factors affecting invasion of Varroa jacobsoni (Acari: Varroidae) into honeybee, Apis mellifera (Hymenoptera: Apidae), brood cells. Bull. Entomol. Res. 84: 3–10. [Google Scholar]
- Boot, W. J., Schoenmaker J., Calis J. N. M., and Beetsma J.. . 1995. Invasion of Varroa jacobsoni into drone brood cells of the honey bee, Apis mellifera. Apidologie. 26: 109–118. [Google Scholar]
- Brodschneider, R., Gray A., Adjlane N., Ballis A., Brusbardis V., Charrière J. D., Chlebo R., Coffey M. F., Dahle B., de Graaf D. C., . et al. 2018. Multi-country loss rates of honey bee colonies during winter 2016/2017 from the COLOSS survey. J. Apicult. Res. 57: 452–457. [Google Scholar]
- Calderone, N. W., and Kuenen L. P. S.. . 2003. Differential tending of worker and drone larvae of the honey bee, Apis mellifera, during the 60 hours prior to cell capping. Apidologie. 34: 543–552. [Google Scholar]
- Calderone, N. W., and Lin S.. . 2001. Behavioural responses of Varroa destructor (Acari: Varroidae) to extracts of larvae, cocoons and brood food of worker and drone honey bees, Apis mellifera (Hymenoptera: Apidae). Physiol. Entomol. 26: 341–350. [Google Scholar]
- Cervo, R., Bruschini C., Cappa F., Meconcelli S., Pieraccini G., Pradella D., and Turillazzi S.. . 2014. High Varroa mite abundance influences chemical profiles of worker bees and mite-host preferences. J. Exp. Biol. 217: 2998–3001. [DOI] [PubMed] [Google Scholar]
- Chen, Y., Pettis J. S., Evans J. D., Kramer M., and Feldlaufer M. F.. . 2004. Transmission of Kashmir bee virus by the ectoparasitic mite Varroa destructor. Apidologie. 35: 441–448. [Google Scholar]
- Coffey, M. F., Breen J., Brown M. J., and McMullan J. B.. . 2010. Brood-cell size has no influence on the population dynamics of Varroa destructor mites in the native western honey bee, Apis mellifera mellifera. Apidologie. 41: 522–530. [Google Scholar]
- Dainat, B., Evans J. D., Chen Y. P., Gauthier L., and Neumann P.. . 2012. Predictive markers of honey bee colony collapse. PLoS One. 7: e32151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Jong, D., P. HDe Jong., and Goncalves L. S.. . 1982. Weight loss and other damage to developing worker honeybees from infestation with Varroa jacobsoni. J. Apicult. Res. 21: 165–167. [Google Scholar]
- Del Piccolo, F., Nazzi F., Della Vedova G., and Milani N.. . 2010. Selection of Apis mellifera workers by the parasitic mite Varroa destructor using host cuticular hydrocarbons. Parasitology. 137: 967–973. [DOI] [PubMed] [Google Scholar]
- DeGrandi-Hoffman, G., Ahumada F., Zazueta V., Chambers M., Hidalgo G., and deJong E. W.. . 2016. Population growth of Varroa destructor (Acari: Varroidae) in honey bee colonies is affected by the number of foragers with mites. Exp. Appl. Acarol. 69: 21–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delfinado-Baker, M., Rath W., and Boecking O.. . 1992. Phoretic bee mites and honeybee grooming behavior. Int. J. Acarol. 18: 315–322. [Google Scholar]
- Dechatre, H., Michel L., Soubeyrand S., Maisonnasse A., Moreau P., Poquet Y., Pioz M., Vidau C., Basso B., Mondet F., and Kretzschmar A.. . 2021. To treat or not to treat bees? handy varload: a predictive model for Varroa destructor load. Pathogens. 10: 678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillier, F. X., Fluri P., and Imdorf A.. . 2006. Review of the orientation behaviour in the bee parasitic mite Varroa destructor: sensory equipment and cell invasion behaviour. Rev. Suisse Zool. 113: 857–877. [Google Scholar]
- Donzé, G., and Guerin P. M.. . 1994. Behavioral attributes and parental care of Varroa mites parasitizing honeybee brood. Behav. Ecol. Sociobiol. 34: 305–319. [Google Scholar]
- Donzé, G., Herrmann M., Bachofen B., and Guerin P. M.. . 1996. Effect of mating frequency and brood cell infestation rate on the reproductive success of the honeybee parasite Varroa jacobsoni. Physiol. Entomol. 21: 17–26. [Google Scholar]
- van Dooremalen, C., Stam E., Gerritsen L., Cornelissen B., van der Steen J., van Langevelde F., and Blacquière T.. . 2013. Interactive effect of reduced pollen availability and Varroa destructor infestation limits growth and protein content of young honey bees. J. Insect Physiol. 59: 487–493. [DOI] [PubMed] [Google Scholar]
- Duay, P., De Jong D., and Engels W.. . 2003. Weight loss in drone pupae (Apis mellifera) multiply infested by Varroa destructor mites. Apidologie. 34: 61–65. [Google Scholar]
- Dynes, T. L., De Roode J. C., Lyons J. I., Berry J. A., Delaplane K. S., and Brosi B. J.. . 2017. Fine scale population genetic structure of Varroa destructor, an ectoparasitic mite of the honey bee (Apis mellifera). Apidologie. 48: 93–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliash, N., Singh N. K., Kamer Y., Pinnelli G. R., Plettner E., and Soroker V.. . 2014. Can we disrupt the sensing of honey bees by the bee parasite Varroa destructor? PLoS One. 9: e106889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emsen, B., Hamiduzzaman M. M., Goodwin P. H., and Guzman-Novoa E.. . 2015. Lower virus infections in Varroa destructor-infested and uninfested brood and adult honey bees (Apis mellifera) of a low mite population growth colony compared to a high mite population growth colony. PLoS One. 10: e0118885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández, N., Eguaras M., and Hernández D.. . 1993. Distribution patterns of Varroa jacobsoni Oud on Apis mellifera L during winter in Argentina. Apidologie. 24: 397–401. [Google Scholar]
- Floris, I., Pusceddu M., and Satta A.. . 2020. How the infestation level of Varroa destructor affects the distribution pattern of multi-infested cells in worker brood of Apis mellifera. Vet. Sci. 7: 136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis, R. M., Nielsen S. L., and Kryger P.. . 2013. Varroa-virus interaction in collapsing honey bee colonies. PLoS One. 8: e57540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fries, I., and Rosenkranz P.. . 1996. Number of reproductive cycles of Varroa jacobsoni in honey-bee (Apis mellifera) colonies. Exp. Appl. Acarol. 20: 103–112. [Google Scholar]
- Fuchs, S. 1990. Preference for drone brood cells by Varroa jacobsoni Oud in colonies of Apis mellifera carnica. Apidologie. 21: 193–199. [Google Scholar]
- Fuchs, S. 1992. Choice in Varroa jacobsoni Oud. between honey bee drone or worker brood cells for reproduction. Behav. Ecol. Sociobiol. 31: 429–435. [Google Scholar]
- Gajic, B., Radulovic Z., Stevanovic J., Kulisic Z., Vucicevic M., Simeunovic P., and Stanimirovic Z.. . 2013. Variability of the honey bee mite Varroa destructor in Serbia, based on mtDNA analysis. Exp. Appl. Acarol. 61: 97–105. [DOI] [PubMed] [Google Scholar]
- Galindo-Cardona, A., Scannapieco A. C., Russo R., Escalante K., Geria M., Lepori N., Ayup M. M., Muntaabski I., Liendo M. C., Landi L., Giray T., and Monmany-Garzia A.. . 2020. Varroa destructor parasitism and genetic variability at honey bee (Apis mellifera) drone congregation areas and their associations with environmental variables in Argentina. Front. Ecol. Evol. 8: 394. [Google Scholar]
- Garrido, C., and Rosenkranz P.. . 2003. The reproductive program of female Varroa destructor mites is triggered by its host, Apis mellifera. Exp. Appl. Acarol. 31: 269–273. [DOI] [PubMed] [Google Scholar]
- Garrido, C., Rosenkranz P., Stürmer M., Rübsam R., and Büning J.. . 2000. Toluidine blue staining as a rapid measure for initiation of oocyte growth and fertility in Varroa jacobsoni Oud. Apidologie. 31: 559–566. [Google Scholar]
- Goetz, B., and Koeniger N.. . 1993. The distance between larva and cell opening triggers broodcell invasion by Varroa jacobsoni. Apidologie. 24: 67–72. [Google Scholar]
- Gray, A., Adjlane N., Arab A., Ballis A., Brusbardis V., Charrière J. D., Chlebo R., Coffey M. F., Cornelissen B., de Costa C. A., . et al. 2020. Honey bee colony winter loss rates for 35 countries participating in the COLOSS survey for winter 2018–2019, and the effects of a new queen on the risk of colony winter loss. J. Apicult. Res. 59: 744–751. [Google Scholar]
- Guerra, J. C., Jr, Issa M. R., Carneiro F. E., Strapazzon R., and Moretto G.. . 2010. RAPD identification of Varroa destructor genotypes in Brazil and other regions of the Americas. Genet. Mol. Res. 9: 303–308. [DOI] [PubMed] [Google Scholar]
- Guzmán-Novoa, E., Eccles L., Calvete Y., Mcgowan J., Kelly P. G., and Correa-Benítez A.. . 2010. Varroa destructor is the main culprit for the death and reduced populations of overwintered honey bee (Apis mellifera) colonies in Ontario, Canada. Apidologie. 41: 443–450. [Google Scholar]
- Hajializadeh, Z., Asadi M., and Kavousi H.. . 2018. First report of Varroa genotype in western Asia based on genotype identification of Iranian Varroa destructor populations (Mesostigmata: Varroidae) using RAPD marker. Syst. Appl. Acarol. 23: 199–205. [Google Scholar]
- Harizanis, P. C. 1991. Infestation of queen cells by the mite Varroa jacobsoni. Apidologie. 22: 533–538. [Google Scholar]
- Harris, J. W., and Harbo J. R.. . 1999. Low sperm counts and reduced fecundity of mites in colonies of honey bees (Hymenoptera: Apidae) resistant to Varroa jacobsoni (Mesostigmata: Varroidae). J. Econ. Entomol. 92: 83–90. [Google Scholar]
- Harris, J. W., Harbo J. R., Villa J. D., and Danka R. G.. . 2003. Variable population growth of Varroa destructor (Mesostigmata: Varroidae) in colonies of honey bees (Hymenoptera: Apidae) during a 10-year period. Environ. Entomol. 32: 1305–1312. [Google Scholar]
- Häußermann, C. K., Ziegelmann B., and Rosenkranz P.. . 2018. Spermatozoa production in male Varroa destructor and its impact on reproduction in worker brood of Apis mellifera. Exp. Appl. Acarol. 74: 43–54. [DOI] [PubMed] [Google Scholar]
- Häußermann, C. K., Giacobino A., Munz R., Ziegelmann B., Palacio M. A., and Rosenkranz P.. . 2020. Reproductive parameters of female Varroa destructor and the impact of mating in worker brood of Apis mellifera. Apidologie. 51: 1–14. [Google Scholar]
- Ifantidis, M. D. 1983. Ontogenesis of the mite Varroa jacobsoni in worker and drone honeybee brood cells. J. Apicult. Res. 22: 200–206. [Google Scholar]
- Ifantidis, M. D. 1988. Some aspects of the process of Varroa jacobsoni mite entrance into honey bee (Apis mellifera) brood cells. Apidologie. 19: 387–396. [Google Scholar]
- Jack, C. J., and Ellis J. D.. . 2021. Integrated pest management control of Varroa destructor (Acari: Varroidae), the most damaging pest of (Apis mellifera L. (Hymenoptera: Apidae)) colonies. J. Insect Sci. 21: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanbar, G., and Engels W.. . 2003. Ultrastructure and bacterial infection of wounds in honey bee (Apis mellifera) pupae punctured by Varroa mites. Parasitol. Res. 90: 349–354. [DOI] [PubMed] [Google Scholar]
- Kather, R., Drijfhout F. P., Shemilt S., and Martin S. J.. . 2015. Evidence for passive chemical camouflage in the parasitic mite Varroa destructor. J. Chem. Ecol. 41: 178–186. [DOI] [PubMed] [Google Scholar]
- Kelomey, A. E., Paraiso A., Sina H., Legout H., Garnery L., and Baba-Moussa L.. . 2017. Genetic characterization of the honeybee ectoparasitic mite Varroa destructor from Benin (West Africa) using mitochondrial and microsatellite markers. Exp. Appl. Acarol. 72: 61–67. [DOI] [PubMed] [Google Scholar]
- Koleoglu, G., Goodwin P. H., Reyes-Quintana M., Hamiduzzaman M. M., and Guzman-Novoa E.. . 2018. Varroa destructor parasitism reduces hemocyte concentrations and prophenol oxidase gene expression in bees from two populations. Parasitol. Res. 117: 1175–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kralj, J., and Fuchs S.. . 2006. Parasitic Varroa destructor mites influence flight duration and homing ability of infested Apis mellifera foragers. Apidologie. 37: 577–587. [Google Scholar]
- Kraus, B. 1994. Factors influencing host choice of the honey bee parasite Varroa jacobsoni Oud. Exp. Appl. Acarol. 18: 435–443. [Google Scholar]
- Kuenen, L. P. S., and Calderone N. W.. . 1997. Transfers of Varroa mites from newly emerged bees: preferences for age- and function-specific adult bees (Hymenoptera: Apidae). J. Insect Behav. 10: 213–228. [Google Scholar]
- Kulhanek, K., Steinhauer N., Rennich K., Caron D. M., Sagili R. R., Pettis J. S., Ellis J. D., Wilson M. E., Wilkes J. T., Tarpy D. R., . et al. 2017. A national survey of managed honey bee 2015–2016 annual colony losses in the USA. J. Apicult. Res. 56: 328–340. [Google Scholar]
- Le Conte, Y., Arnold G., Trouiller J., Masson C., Chappe B., and Ourisson G.. . 1989. Attraction of the parasitic mite varroa to the drone larvae of honey bees by simple aliphatic esters. Science. 245: 638–639. [DOI] [PubMed] [Google Scholar]
- Le Conte, Y., Arnold G., Trouiller J., Masson C., and Chappe B.. . 1990. Identification of a brood pheromone in honeybees. Naturwissenschaften. 77: 334–336. [Google Scholar]
- Lee, K. V., Steinhauer N., Rennich K., Wilson M. E., Tarpy D. R., Caron D. M., Rose R., Delaplane K. S., Baylis K., Lengerich E. J., . et al. 2015. A national survey of managed honey bee 2013–2014 annual colony losses in the USA. Apidologie. 46: 292–305. [Google Scholar]
- Light, M., Shutler D., Cutler G. C., and Hillier N. K.. . 2020. Electrotarsogram responses to synthetic odorants by Varroa destructor, a primary parasite of western honey bees (Apis mellifera). Exp. Appl. Acarol. 81: 515–530. [DOI] [PubMed] [Google Scholar]
- Lin, Z., Qin Y., Page P., Wang S., Li L., Wen Z., Hu F., Neumann P., Zheng H., and Dietemann V.. . 2018. Reproduction of parasitic mites Varroa destructor in original and new honeybee hosts. Ecol. Evol. 8: 2135–2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, T. P. 1988. The morphology of the tarsal sensilla in the female mite Varroa jacobsoni. Pan-Pac. Entomol. 64: 261–265. [Google Scholar]
- Liu, T. P., and Peng Y. S.. . 1990. Palpal tarsal sensilla of the female mite, Varroa jacobsoni Oudemans (Acari: Varroidae). Can. Entomol. 122: 295–300. [Google Scholar]
- Lodesani, M., Crailsheim K., and Moritz R. F. A.. . 2002. Effect of some characters on the population growth of mite Varroa jacobsoni in Apis mellifera L colonies and results of a bi‐directional selection. J. Appl. Entomol. 126: 130–137. [Google Scholar]
- Martin, S. J. 1994. Ontogenesis of the mite Varroa jacobsoni Oud. in worker brood of the honeybee Apis mellifera L. under natural conditions. Exp. Appl. Acarol. 18: 87–100. [Google Scholar]
- Martin, S. J. 1995. Reproduction of Varroa jacobsoni in cells of Apis mellifera containing one or more mother mites and the distribution of these cells. J. Apicult. Res. 34: 187–196. [Google Scholar]
- Martin, S. 1998. A population model for the ectoparasitic mite Varroa jacobsoni in honey bee (Apis mellifera) colonies. Ecol. Model. 109: 267–281. [Google Scholar]
- Martin, S. J. 2001. Varroa destructor reproduction during the winter in Apis mellifera colonies in UK. Exp. Appl. Acarol. 25: 321–325. [DOI] [PubMed] [Google Scholar]
- Martin, S. J., and Kemp D.. . 1997. Average number of reproductive cycles performed by Varroa jacobsoni in honey bee (Apis mellifera) colonies. J. Apicult. Res. 36: 113–123. [Google Scholar]
- Martin, S. J., and Kryger P.. . 2002. Reproduction of Varroa destructor in South African honey bees: does cell space influence Varroa male survivorship? Apidologie. 33: 51–61. [Google Scholar]
- Martin, S., Holland K., and Murray M.. . 1997. Non-reproduction in the honeybee mite Varroa jacobsoni. Exp. Appl. Acarol. 21: 539–549. [Google Scholar]
- McMullan, J. B., and Brown M. J. F.. 2006. The influence of small-cell brood combs on the morphometry of honeybees (Apis mellifera). Apidologie. 37: 665–672. [Google Scholar]
- Milani, N., Della Vedova G., and Nazzi F.. . 2004. (Z)-8-Heptadecene reduces the reproduction of Varroa destructor in brood cells. Apidologie. 35: 265–273. [DOI] [PubMed] [Google Scholar]
- Mondet, F., de Miranda J. R., Kretzschmar A., Le Conte Y., and Mercer A. R.. . 2014. On the front line: quantitative virus dynamics in honeybee (Apis mellifera L.) colonies along a new expansion front of the parasite Varroa destructor. PLoS Pathog. 10: e1004323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortensen, A. N., Jack C. J., and Ellis J. D.. . 2018. The discovery of Varroa destructor on drone honey bees, Apis mellifera, at drone congregation areas. Parasitol. Res. 117: 3337–3339. [DOI] [PubMed] [Google Scholar]
- Muntaabski, I., Russo R. M., Liendo M. C., Palacio M. A., Cladera J. L., Lanzavecchia S. B., and Scannapieco A. C.. . 2020. Genetic variation and heteroplasmy of Varroa destructor inferred from ND4 mtDNA sequences. Parasitol. Res. 119: 411–421. [DOI] [PubMed] [Google Scholar]
- Navajas, M., Anderson D. L., De Guzman L. I., Huang Z. Y., Clement J., Zhou T., and Le Conte Y.. . 2010. New Asian types of Varroa destructor: a potential new threat for world apiculture. Apidologie. 41: 181–193. [Google Scholar]
- Nazzi, F., Milani N., Della Vedova G., and Nimis M.. . 2001. Semiochemicals from larval food affect the locomotory behaviour of Varroa destructor. Apidologie. 32: 149–155. [Google Scholar]
- Nazzi, F., Milani N., and Della Vedova G.. . 2004. A semiochemical from larval food influences the entrance of Varroa destructor into brood cells. Apidologie. 35: 403-410. [Google Scholar]
- Nganso, B. T., Mani K., Altman Y., Rafaeli A., and Soroker V.. . 2020. How crucial is the functional pit organ for the Varroa mite? Insects. 11: 395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noël, A., Le Conte Y., and Mondet F.. . 2020. Varroa destructor: how does it harm Apis mellifera honey bees and what can be done about it? Emerg. Top. Life Sci. 4: 45–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Octaviano-Salvade, C. E., Leher C. E., David D. E., Pinto P. M., Delgado-Canedo A., and Boldo J. T.. . 2017. A scientific note on genetic profile of the mite Varroa destructor infesting apiaries in Rio Grande do Sul state, Brazil. Apidologie. 48: 621–622. [Google Scholar]
- Ogihara, M. H., Yoshiyama M., Morimoto N., and Kimura K.. . 2020. Dominant honeybee colony infestation by Varroa destructor (Acari: Varroidae) K haplotype in Japan. Appl. Entomol. Zool. 55: 189–197. [Google Scholar]
- Oldroyd, B. P. 1999. Coevolution while you wait: Varroa jacobsoni, a new parasite of western honeybees. Trends Ecol. Evol. 14: 312–315. [DOI] [PubMed] [Google Scholar]
- Omar, R. E. M. 2017. Effect of Varroa infestation on the development of body weight and some reproductive organs of honeybee drones, Apis mellifera L. Sciences. 7: 272–279. [Google Scholar]
- Peck, D. T., Smith M. L., and Seeley T. D.. . 2016. Varroa destructor mites can nimbly climb from flowers onto foraging honey bees. PLoS One. 11: e0167798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peck, D. T., and Seeley T. D.. . 2019. Mite bombs or robber lures? The roles of drifting and robbing in Varroa destructor transmission from collapsing honey bee colonies to their neighbors. PLoS One. 14: e0218392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pernal, S. F., Baird D. S., Birmingham A. L., Higo H. A., Slessor K. N., and Winston M. L.. . 2005. Semiochemicals influencing the host-finding behaviour of Varroa destructor. Exp. Appl. Acarol. 37: 1–26. [DOI] [PubMed] [Google Scholar]
- Piccirillo, G. A., and De Jong D.. . 2004. Old honey bee brood combs are more infested by the mite Varroa destructor than are new brood combs. Apidologie. 35: 359–364. [Google Scholar]
- Piou, V., Tabart J., Urrutia V., Hemptinne J. L., and Vétillard A.. . 2016. Impact of the phoretic phase on reproduction and damage caused by Varroa destructor (Anderson and Trueman) to its host, the European honey bee (Apis mellifera L.). PLoS One. 11: e0153482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posada-Florez, F., Childers A. K., Heerman M. C., Egekwu N. I., Cook S. C., Chen Y., Evans J. D., and Ryabov E. V.. . 2019a. Deformed wing virus type A, a major honey bee pathogen, is vectored by the mite Varroa destructor in a non-propagative manner. Sci. Rep. 9: 12445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posada-Florez, F., Sonenshine D. E., Egekwu N. I., Rice C., Lupitskyy R., and Cook S. C.. . 2019b. Insights into the metabolism and behaviour of Varroa destructor mites from analysis of their waste excretions. Parasitology. 146: 527–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prisco, G. D., Zhang X., Pennacchio F., Caprio E., Li J., Evans J. D., Degrandi-Hoffman G., Hamilton M., and Chen Y. P.. . 2011. Dynamics of persistent and acute deformed wing virus infections in honey bees, Apis mellifera. Viruses. 3: 2425–2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsey, S. D., Ochoa R., Bauchan G., Gulbronson C., Mowery J. D., Cohen A., Lim D., Joklik J., Cicero J. M., Ellis J. D., . et al. 2019. Varroa destructor feeds primarily on honey bee fat body tissue and not hemolymph. Proc. Natl. Acad. Sci. U. S. A. 116: 1792–1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rateb, S. H., Abdel-Rahman M. F., and Sanad R. E.. . 2010. The relationship between Varroa destructor infestation and virgin queen’s acceptance, mating success and onset of oviposition in honeybee colonies. Egypt. Acad. J. Biol. Sci. Entomol. 3: 207–212. [Google Scholar]
- Rehm, S., and Ritter W.. . 1989. Sequence of the sexes in the offspring of Varroa jacobsoni and the resulting consequences for the calculation of the developmental period. Apidologie. 20: 339–343. [Google Scholar]
- Requier, F., Antúnez K., Morales C. L., Aldea Sánchez P., Castilhos D., Garrido P. M., Giacobino A., Reynaldi F., Rosso Londono M. R., Santos E., and Garibaldi L. A.. . 2018. Trends in beekeeping and honey bee colony losses in Latin America. J. Apicult. Res. 57: 657–662. [Google Scholar]
- Rickli, M., Guerin P. M., and Diehl P. A.. 1992. Palmitic acid released from honeybee worker larvae attracts the parasitic mite Varroa jacobsoni on a servosphere. Naturwissenschaften. 79: 320–322. [Google Scholar]
- Rickli, M., Diehl P. A., and Guerin P. M.. . 1994. Cuticle alkanes of honeybee larvae mediate arrestment of bee parasite Varroa jacobsoni. J. Chem. Ecol. 20: 2437–2453. [DOI] [PubMed] [Google Scholar]
- Rivera-Marchand, B., Oskay D., and Giray T.. . 2012. Gentle Africanized bees on an oceanic island. Evol. Appl. 5: 746–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts, J. M., Anderson D. L., and Tay W. T.. . 2015. Multiple host shifts by the emerging honeybee parasite, Varroa jacobsoni. Mol. Ecol. 24: 2379–2391. [DOI] [PubMed] [Google Scholar]
- Rosenkranz, P., and Garrido C.. . 2004. Volatiles of the honey bee larva initiate oogenesis in the parasitic mite Varroa destructor. Chemoecology. 14: 193–197. [Google Scholar]
- Rosenkranz, P., Aumeier P., and Ziegelmann B.. . 2010. Biology and control of Varroa destructor. J. Invertebr. Pathol. 103: S96–119. [DOI] [PubMed] [Google Scholar]
- Seeley, T. D., and Griffin S. R.. . 2011. Small-cell comb does not control Varroa mites in colonies of honeybees of European origin. Apidologie. 42: 526–532. [Google Scholar]
- Solignac, M., Cornuet J. M., Vautrin D., Le Conte Y., Anderson D., Evans J., Cros-Arteil S., and Navajas M.. . 2005. The invasive Korea and Japan types of Varroa destructor, ectoparasitic mites of the Western honeybee (Apis mellifera), are two partly isolated clones. Proc. Biol. Sci. 272: 411–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinhauer, N., Kulhanek K., Antúnez K., Human H., Chantawannakul P., Chauzat M. P., and vanEngelsdorp D.. . 2018. Drivers of colony losses. Curr. Opin. Insect Sci. 26: 142–148. [DOI] [PubMed] [Google Scholar]
- Steinhauer, N., Aurell D., Bruckner S., Wilson M., Rennich K., vanEngelsdorp D., and Williams G.. . 2021. United States honey bee colony losses 2020–2021: preliminary results.https://beeinformed.org/wp-content/uploads/2021/06/BIP_2020_21_Losses_Abstract_2021.06.14_FINAL_R1.pdf
- Techer, M. A., Rane R. V., Grau M. L., Roberts J. M. K., Sullivan S. T., Liachko I., Childers A. K., Evans J. D., and Mikheyev A. S.. . 2019. Divergent evolutionary trajectories following speciation in two ectoparasitic honey bee mites. Commun. Biol. 2: 357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Techer, M. A., Roberts J. M. K., Cartwright R. A., and Mikheyev A. S.. . 2021. The first steps toward a global pandemic: Reconstructing the demographic history of parasite host switches in its native range. Mol Ecol. doi: 10.1111/mec.16322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traynor, K. S., Mondet F., de Miranda J. R., Techer M., Kowallik V., Oddie M. A. Y., Chantawannakul P., and McAfee A.. . 2020. Varroa destructor: a complex parasite, crippling honey bees worldwide. Trends Parasitol. 36: 592–606. [DOI] [PubMed] [Google Scholar]
- Trouiller, J., Arnold G., Le Conte Y., Masson C., and Chappe B.. . 1991. Temporal pheromonal and kairomonal secretion in the brood of honeybees. Naturwissenschaften. 78: 368–370. [Google Scholar]
- Trouiller, J., Arnold G., Chappe B., Le Conte Y., and Masson C.. . 1992. Semiochemical basis of infestation of honey bee brood by Varroa jacobsoni. J. Chem. Ecol. 18: 2041–2053. [DOI] [PubMed] [Google Scholar]
- Xie, X., Huang Z. Y., and Zeng Z.. . 2016. Why do Varroa mites prefer nurse bees? Sci. Rep. 6: 28228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue, C., and Genersch E.. . 2005. RT-PCR analysis of deformed wing virus in honeybees (Apis mellifera) and mites (Varroa destructor). J. Gen. Virol. 86: 3419–3424. [DOI] [PubMed] [Google Scholar]
- Wegener, J., Ruhnke H., Scheller K., Mispagel S., Knollmann U., Kamp G., and Bienefeld K.. . 2016. Pathogenesis of varroosis at the level of the honey bee (Apis mellifera) colony. J. Insect Physiol. 91–92: 1–9. [DOI] [PubMed] [Google Scholar]
- Wilfert, L., Long G., Leggett H. C., Schmid-Hempel P., Butlin R., Martin S. J., and Boots M.. . 2016. Deformed wing virus is a recent global epidemic in honeybees driven by Varroa mites. Science. 351: 594–597. [DOI] [PubMed] [Google Scholar]
- Ziegelmann, B., Tolasch T., Steidle J. L. M., and Rosenkranz P.. . 2013. The mating behavior of Varroa destructor is triggered by a female sex pheromone. Part 2: identification and dose-dependent effects of components of the Varroa sex pheromone. Apidologie. 44: 481–490. [Google Scholar]