Abstract
Objectives:
The rate and trend of transfusion transmissible infections (TTIs) in blood donations from 2012 to 2017 at the Bamenda Regional Hospital Blood Service (BRHBS), Cameroon was assessed.
Materials and Methods:
A six-year retrospective study was conducted by reviewing the records of donors. Blood was screened for HIV, hepatitis B, hepatitis C and syphilis. Data was analyzed using IBM SPSS Statistics version 21. Differences in seropositivity rates for the four TTIs were analyzed using Chi-squared test or Fisher’s exact test where appropriate. Associations between sociodemographic characteristics and the TTIs markers were assessed using multiple logistic regression analysis.
Results:
A total of 12,115 blood donations was included in the study and of these, the overall seropositivity rate of the four conventional TTIs markers was 10.5% (n=1,273). Of the seropositive cases, 23.8% (n=303) showed reactivity with at least two of the markers combined. When the markers were assessed individually, HBsAg recorded the highest seropositivity rate (4.7%), followed by anti-HIV and anti-syphilis (2.2%), then anti-HCV (1.7%). A significant decrease in the trend of the combined serological markers, HBsAg and anti-syphilis was observed over the years (p≤0.05).
Conclusion:
There is a decrease in seropositivity rates of TTIs markers in this blood service. Ongoing efforts toward the prevention of these infections is encouraged and should be intensified to improve blood safety.
Keywords: sero-positivity, transfusion transmissible infections, blood donation, Cameroon
Introduction
Blood donation remains vital in the saving of life and improvement of health for millions of people worldwide. However, this therapy is associated with various adverse effects including the transmission of infectious diseases. In 2010, Choudhury reported a 1–2 per 1000 risk for blood recipients of contaminated blood to get viral, bacterial or parasitic infections [1]. Approximately, 4 million people globally have been infected with HIV by transfusing unsafe blood [2]. Therefore, recipients of transfused blood stand a high risk of potential infection from transfusion transmissible infections (TTIs). The main infections associated to blood transfusions are human immunodeficiency virus (HIV), hepatitis B virus (HBV), hepatitis C virus (HCV), and syphilis and the spread of these infections progresses more in less developed countries [3,4]. Tagny et al. reported that approximately 25% of blood units donated in Francophone Africa are contaminated with blood borne pathogens [5]. Some studies on TTIs markers in some Cameroonian towns revealed a seroprevalence of 4.1% (HIV), 20.4% (HBV) 4.8% (HCV) and 8.1% for syphilis [6–8]. A steady decline in the prevalence of HCV and syphilis serological markers have been reported among blood donors in Yaoundé, Cameroon during a five-year period [9].
In an effort to improve the quality and safety of blood delivered at blood banks in Cameroon, various initiatives have been put in place by different structures. These bodies include the Ministry of Public Health (MoPH), the US Centers for Disease Control (CDC), the Safe Blood for Africa Foundation™ (SBFA), the World Health Organization (WHO), and the US CDC/PEPFAR (US President’s Emergency Plan for AIDS Relief). Strategies employed by these bodies include education, sensitization, immunization and trainings. Awareness is being created through regular campaigns, continuous education and free screening for TTIs. With regards to improving blood safety at the blood transfusion centers, these structures provide technical support which encompasses situational assessment, blood collection, blood testing, quality assurance and training of personnel. These actions are geared at establishing sustainable blood services according to the guidelines and recommendations of WHO for developing countries [10–12]. In line with WHO recommendations, a National Blood Transfusion Program (NBTP) and a national policy on blood transfusion were established in Cameroon in 2003 to oversee activities related to safe blood donation [13].
Although different studies related to blood donations and prevalence of TTIs have been reported in Cameroon [6,8,9,14,15], none so far, has been reported for the Bamenda Regional Hospital Blood Bank (BRHBB). Additionally, no systematic monitoring of TTIs markers has been conducted at this hospital which is a major transfusion center and one of SBFA initiative reference centers.
This study had as objective to provide novel data on the seropositivity rates and trend of serological markers of the four conventional TTIs in blood donations from 2012 to 2017 at the BRHBB.
Materials and Methods
Study design and site:
This was a retrospective cross-sectional study of all blood donations received at the Bamenda Regional Hospital Blood Bank (BRHBB), Cameroon. Data was collected spanning a six years duration from 2012 to 2017. Bamenda is the headquarters of the North West Region, one of the ten administrative Regions of the country. The Regional hospital is the largest government hospital in the region and serves as a referral hospital for approximately, two million people in the Region and beyond. This hospital is among the five that was chosen by SBFA with the expectation to serve as one of the reference blood transfusion centers in Cameroon. In line with guidelines, all potential donors go through an initial screening using a blood donor checklist. The hemoglobin concentration of those retained is measured before proceeding to test for TTIs. From the archives, we collected data on socio-demographic characteristics and screening test result. Files with incomplete records were excluded.
Serological testing and ethical issues:
Following the national algorithm for HIV testing for blood donations in Cameroon, all blood donations were screened for HIV infection using the rapid test, Determine® HIV-1/2 Ag/Ab Combo (Alere Medical Co., Ltd, Matsuhidai, Matsudo-Shi, China) for the first line. The ImmunoComb II HIV 1&2 BiSpot (Orgenics Ltd, Yavne, Israel) or the OraQuick (OraSure Technologies, Inc, Bethlehem) served for the second line, allowing for distinction between HIV 1 and 2. Sensitivity and specificity of these tests are respectively 100% and 98.93%. The screening of HBsAg was by Determine HBsAg Combo Rapid Test (Alere Medical Co., Ltd, Matsuhidai, Matsudo-Shi, Chiba) with reported sensitivity of 95.16% and specificity of 99.95%. Anti-HCV were screened using the Fastep Anti-HCV Test (Polymed Therapeutics, Inc, Houston, USA), while antibodies for syphilis was determined by the Treponema pallidum hemagglutination assay (Cypress Diagnostics, Langdorp, Belgium). Ethical approval for the study was got from the Regional Delegation of Public Health Review Board (N0 023/APP/RDPH/RHB/IRB). During blood donation, the donors sign a consent form accepting that their specimen and/or results can be used for research. All data were fully anonymized before having accessed to them.
Statistical analysis:
Data was analyzed using IBM SPSS Statistics version 21. The seropositivity rates for HBsAg, anti-HCV, anti-HIV and anti-syphilis was represented as percentage among the different demographic characteristics of the donors. Chi square (χ2) or Fisher’s Exact test was used where appropriate. The trend of seropositivity rates of the four TTI markers over time were examined using the Extended Mantel-Haenzel Chi-square test for linear trend. For univariate analysis, the association between the markers and factors was assessed using Odds ratios (ORs) with 95% confidence interval (CI). In order to adjust for confounding factors, multiple logistic regression was used, and the association was quantified using adjusted ORs with 95% CI. P-values less than or equal to 0.05 was considered statistically significant.
Results
General characteristics of blood donors and donations
Overall, there were 13,147 blood donations collected at the BRHBS during the six years’ study period. Of these, 1,032 were ineligible for our study because of incomplete data. Out of the 12,115 that we retained, 85.2% (n=10,322) of the donations were males and, 52.9% (n=6,403) from 20–29 years-old donors. Age ranged from 19 to 64 years with mean age of 29.7±8.37 years. When marital status was assessed, 63.6% (n=7,704) of the donations were from singles and 30.9% (n=3,744) from students. Most (97.8%) of the donations (11,848) were from Christians and 83.9% were from people residing in the city of Bamenda (10,164).
Positivity rates of the individual markers of TTIs
a. HIV:
The seropositivity rate of anti-HIV for the 12,115 donations was 2.2%. The highest rate was seen in 2012 (2.7%) and about a 2-fold drop observed in 2017 (1.7%). The odds of being HIV positive increased in 2013 [OR=1.6 (1.04–2.36)] and decreased in the latter years with the lowest odds recorded in 2017 [OR=1.0 (0.68–1.59)]. In the multivariate analyses, the ORs attenuated after adjusting for sociodemographic factors from [1.5 (1.01–2.32)] for 2012 to [1.1 (0.71–1.67)] for 2017. Table 1 shows the factors associated with HIV positivity rates. A statistically significant difference was seen in 2012.
Table 1:
Bivariate and multivariate analyses of factors associated with HIV positivity rates among the 12,115 donations between 2012–2017 at the Bamenda Regional Hospital Blood Bank
| Characteristics | Non Reactive (%) | Reactive (%) | Unadjusted odds ratio (95% CI) | P-value | Adjusted odds ratio (95% CI) | P-value |
|---|---|---|---|---|---|---|
|
| ||||||
| Gender (p = 0.08) | ||||||
| Male | 10101 (97.8) | 222 (2.2) | 1.4 (1.0–2.15) | 0.08 | 1.3(0.83–1.90) | 0.29 |
| Female | 1765 (98.5) | 27 (1.5) | 1 | 1 | ||
|
| ||||||
| Age range (in years; p = 0.51) | ||||||
| <20 | 1135 (98.4) | 19 (1.6) | 0.8(0.35–2.00) | 0.83 | 0.9(0.36–2.42) | 0.89 |
| 20 – 29 | 6279 (98.1) | 124 (2.0) | 1.0(0.45–2.11) | 0.98 | 1.0(0.45–2.21) | 0.97 |
| 30 – 39 | 3011 (97.7) | 72 (2.3) | 1.2(0.54–2.60) | 0.67 | 1.1(0.52–2.52) | 0.74 |
| 40 – 49 | 1094 (97.6) | 27 (2.4) | 1.2(0.53–2.83) | 0.64 | 1.2(0.51–2.73) | 0.71 |
| ≥50 | 347 (98.0) | 7 (2.0) | 1 | 1 | ||
|
| ||||||
| Marital status (p = 0.56) | ||||||
| Married | 4249(97.8) | 96(2.2) | 0.7(0.17–2.99) | 0.66 | 0.6(0.15–2.65) | 0.53 |
| Single | 7553(98.0) | 151(2.0) | 0.6(0.15–2.64) | 0.53 | 0.7(0.16–2.92) | 0.61 |
| Divorced/Widow | 64(97.0) | 2(3.0) | 1 | 1 | ||
|
| ||||||
| Level of education (p = 0.06) | ||||||
| Primary | 3566 (97.6) | 89 (2.4) | 1.5 (1.07–2.14) | 0.01 | 1.3(0.87–1.97) | 0.19 |
| Secondary | 5149 (97.9) | 108 (2.1) | 1.3 (0.91–1.78) | 0.16 | 1.2(0.86–1.76) | 0.26 |
| Tertiary | 3151 (98.4) | 52 (1.6) | 1 | 1 | ||
|
| ||||||
| Occupation (p = 0.09) | ||||||
| Civil servant | 1394 (98.0) | 30 (2.0) | 1.4(0.88–2.14) | 0.17 | 1.3(0.80–2.13) | 0.28 |
| Business | 1374 (98.0) | 29 (2.0) | 1.3 (0.86–2.10) | 0.20 | 1.1(0.65–1.79) | 0.77 |
| Farmer | 355 (98.1) | 7 (1.9) | 1.3 (0.57–2.77) | 0.58 | 1.0(0.42–2.31) | 0.97 |
| Driver | 5056 (97.6) | 125 (2.4) | 1.6 (1.15–2.15) | 0.005 | 1.3(0.88–1.93) | 0.18 |
| Student | 3687 (98.5) | 58 (1.5) | 1 | 1 | ||
|
| ||||||
| Religion (p = 0.000) | ||||||
| Christian | 11611 (98.0) | 243 (2.0) | 0.1 (0.03–0.28) | 0.000 | 0.1(0.03–0.26) | 0.000 |
| Muslim | 237 (99.2) | 2 (0.8) | 0.0 (0.01–0.22) | 0.000 | 0.0(0.00–0.19) | 0.00 |
| Pagan | 18 (81.8) | 4 (18.2) | 1 | 1 | ||
|
| ||||||
| Residence (p = 0.49) | ||||||
| Bamenda | 9964 (98.0) | 205 (2.0) | 0.9(0.64–1.24) | 0.485 | 0.9(0.66–1.30) | 0.63 |
| Out of Bamenda | 1902 (97.7) | 44 (2.3) | 1 | 1 | ||
|
| ||||||
| Year of donation (p = 0.21) | ||||||
| 2012 | 1479(97.3) | 41(2.7) | 1.6(1.04–2.36) | 0.03 | 1.5(1.01–2.32) | 0.04 |
| 2013 | 2372(98.1) | 45(1.9) | 1.1.(0.72–1.59) | 0.73 | 1.0(0.65–1.47) | 0.92 |
| 2014 | 1874(97.7) | 45(2.3) | 1.3(0.91–2.02) | 0.13 | 1.3(0.87–1.93) | 0.21 |
| 2015 | 1085(97.6) | 27(2.4) | 1.4(0.88–2.24) | 0.15 | 1.4(0.86–2.19 | 0.19 |
| 2016 | 1950(98.2) | 36(1.8) | 1.0(0.68–1.59) | 0.85 | 1.1(0.71–1.67) | 0.70 |
| 2017 | 3106(98.3) | 55(1.7) | 1 | 1 | ||
OR: odd ratio; aOR: adjusted odds ratio (odds ratios adjusted for socio-demographic characteristics); CI: confidence interval
b. HBV:
This infection recorded the highest seropositivity rate of 4.7% (n=571/12,115) among the four markers. Of these 571 cases, the highest rate of 6.0% (n=146) was recorded in 2013 while the lowest rate of 3.4% (n=67) in 2016. The respective odds ratios and adjusted odds are detailed in Table 2. Figure 1 shows the trend of the four markers over the years. A significant decrease was observed with HBsAg (p=0.00003).
Table 2:
Bivariate and multivariate analyses of factors associated with HBV positivity rates among the 12,115 donations between 2012–2017 at the Bamenda Regional Hospital Blood Bank
| Characteristics | Non Reactive (%) | Reactive (%) | Unadjusted odds ratio (95% CI) | P-value | Adjusted odds ratio (95% CI) | P-value |
|---|---|---|---|---|---|---|
|
| ||||||
| Gender (p = 0.01) | ||||||
| Male | 9814 (95.1) | 509 (4.9) | 1.4 (1.10–1.86) | 0.009 | 1.3 (0.97–1.69) | 0.07 |
| Female | 1729 (96.5) | 63 (3.5) | 1 | 1 | ||
|
| ||||||
| Age range (in years; (p = 0.03) | ||||||
| <20 | 1108 (96.0) | 46 (4.0) | 0.7 (0.42–1.27) | 0.27 | 0.9(0.48–1.60) | 0.66 |
| 20 – 29 | 6070 (94.8) | 333 (5.2) | 0.9 (0.60–1.56) | 0.89 | 1.1 (0.64–1.78) | 0.83 |
| 30 – 39 | 2946 (96.1) | 137 (4.9) | 0.8 (0.50–1.34) | 0.43 | 0.8 (0.50–1.36) | 0.46 |
| 40 – 49 | 1084 (96.7) | 37 (3.3) | 0.6 (0.34–1.06) | 0.08 | 0.6 (0.34–1.06) | 0.07 |
| ≥50 | 335 (94.6) | 19 (5.4) | 1 | 1 | ||
|
| ||||||
| Marital status (p = 0.80) | ||||||
| Married | 4138(95.2) | 207(4.8) | 1.6(0.39–6.59) | 0.51 | 1.3(0.32–5.48) | 0.70 |
| Single | 7341(95.3) | 363(4.7) | 1.6(0.39–6.49 | 0.52 | 1.2(0.29–5.06) | 0.78 |
| Divorced/Widow | 64(97.0) | 2(3.0) | 1 | 1 | ||
|
| ||||||
| Level of education (p = 0.04) | ||||||
| Primary | 3458 (94.6) | 197 (5.4) | 1.3 (1.06–1.66) | 0.14 | 1.2 (1.00–1.65) | |
| Secondary | 5014 (95.4) | 243 (4.6) | 1.1 (0.91–1.40) | 0.28 | 1.1 (0.85–1.37) | 0.09 |
| Tertiary | 3071 (95.9) | 132 (4.1) | 1 | 1 | 0.52 | |
|
| ||||||
| Occupation (p = 0.68) | ||||||
| Civil servant | 1361 (95.6) | 63 (4.4) | 1.0 (0.75–1.36) | 0.94 | 1.1(0.78–1.50) | 0.63 |
| Business | 1335 (95.2) | 68 (4.8) | 1.1 (0.83–1.49) | 0.47 | 1.0(0.73–1.40) | 0.94 |
| Farmer | 345 (95.3) | 17 (4.7) | 1.1 (0.65–1.79) | 0.78 | 1.1(0.61–1.83) | 0.85 |
| Driver | 4921 (95.0) | 260 (5.0) | 1.2 (0.94–1.41) | 0.16 | 1.0(0.79–1.30) | 0.93 |
| Student | 3581 (95.6) | 164 (4.4) | 1 | 1 | ||
|
| ||||||
| Religion (p = 0.27) | ||||||
| Christian | 11289 (95.2) | 565 (4.8) | 1.1 (0.14–7.83) | 0.96 | 1.3(0.18–10.1) | 0.77 |
| Muslim | 233 (97.5) | 6 (2.5) | 0.5 (0.62–4.71) | 0.54 | 0.7(0.07–5.87) | 0.72 |
| Pagan | 21 (95.5) | 1 (4.5) | 1 | 1 | ||
|
| ||||||
| Residence (p = 0.83) | ||||||
| Bamenda | 9687 (95.3) | 482 (4.7) | 1.0 (0.82–1.29) | 0.83 | 1.0(0.80–1.29) | 0.88 |
| Out of Bamenda | 1856 (95.4) | 90 (4.6) | 1 | 1 | ||
|
| ||||||
| Year of donation (p = 0.000) | ||||||
| 2012 | 1439(94.7) | 81(5.3) | 1.4(1.02–1.81) | 0.04 | 1.3(1.00–1.77) | |
| 2013 | 2271(94.0) | 146(6.0) | 1.5(1.21–2.00) | 0.00 | 1.5(1.17–1.92) | 0.05 |
| 2014 | 1819(94.8) | 100(5.2) | 1.3(1.01–1.73) | 0.04 | 1.3(0.98–1.68) | 0.00 |
| 2015 | 1060(95.3) | 52(4.7) | 1.2(0.85–1.65) | 0.32 | 1.2(0.83–1.61) | 0.07 |
| 2016 | 1919(96.6) | 67(3.4) | 0.8(0.62–1.14) | 0.26 | 0.9(0.63–1.16) | 0.40 |
| 2017 | 3035(96.0) | 126(4.0) | 1 | 1 | 0.32 | |
OR: odd ratio; aOR: adjusted odds ratio (odds ratios adjusted for socio-demographic characteristics); CI: confidence interval
Figure 1:
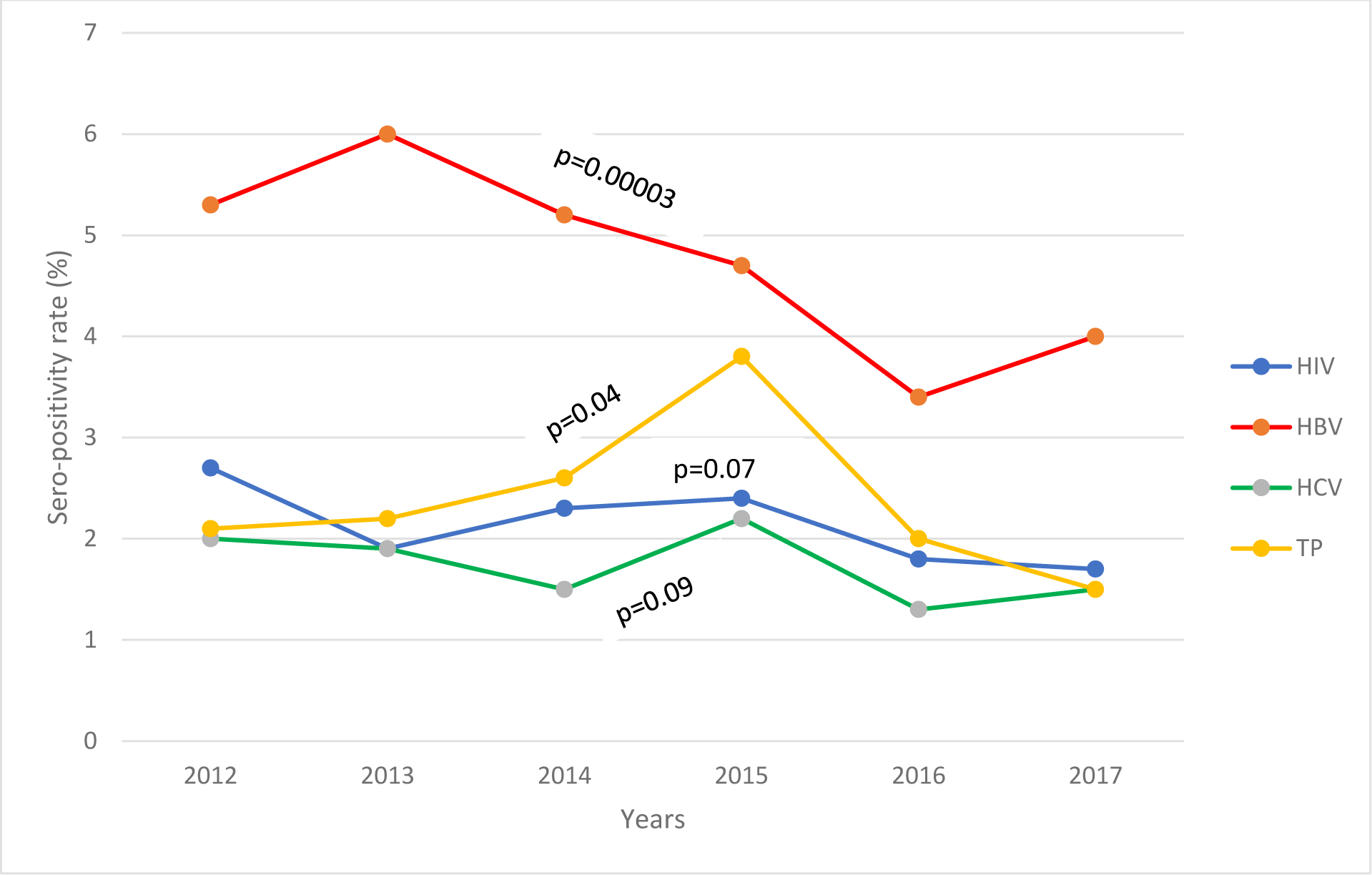
Trend in seropositivity rate of individual TTID markers among 12,115 blood donations between 2012 – 2017 at the Bamenda Regional Hospital Blood Service
c. HCV:
The overall seropositivity rate was 1.7% (n=202/12,115). The highest positivity rate of 2.2% for HCV was recorded in 2015 followed by a decrease to 1.3% in 2016. The odds of having an anti-HCV reactive test decreased in 2016 [OR=0.9(0.55–1.46)] Females, drivers and those with primary level of education had the highest reactivity to anti-HCV (Table 3).
Table 3:
Bivariate and multivariate analyses of factors associated with HCV positivity rates among the 12,115 donations between 2012–2017 at the Bamenda Regional Hospital Blood Bank
| Characteristics | Non-Reactive (%) | Reactive (%) | Unadjusted odds ratio (95% CI) | P-Value | Adjusted odds ratio (95% CI) | P-Value |
|---|---|---|---|---|---|---|
|
| ||||||
| Gender (p = 0.41) | ||||||
| Male | 10155 (98.4) | 168 (1.6) | 0.9 (0.59–1.24) | 0.41 | 0.9(0.57–1.24) | 0.37 |
| Female | 1758 (98.1) | 34 (1.9) | 1 | 1 | ||
|
| ||||||
| Age range (in years; (p = 0.31) | ||||||
| <20 | 1133 (98.2) | 21 (1.8) | 2.2 (0.64–7.31) | 0.21 | 2.1(0.59–7.67) | 0.25 |
| 20 – 29 | 6307 (98.5) | 96 (1.55) | 1.8 (0.56–5.65) | 0.33 | 1.8(0.56–6.01) | 0.32 |
| 30 – 39 | 3022 (98) | 61 (2) | 2.4 (0.74–7.57) | 0.15 | 2.5(0.78–8.11) | 0.12 |
| 40 – 49 | 1100 (98.1) | 21 (1.9) | 2.2 (0.66–7.53) | 0.20 | 2.2(0.66–7.58) | 0.19 |
| ≥50 | 351 (99.2) | 3 (0.8) | 1 | 1 | ||
|
| ||||||
| Marital status (p = 0.80) | ||||||
| Married | 4249(97.8) | 96(2.2) | 0.9(0.68–1.21) | 0.50 | 1.0(0.68–1.40) | 0.88 |
| Single | 7553(98) | 151(2) | 0.9(0.12–6.22) | 0.88 | 0.9(0.12–6.50) | 0.90 |
| Divorced/Widow | 64(97) | 2(3) | 1 | 1 | ||
|
| ||||||
| Level of education (p=0.80) | ||||||
| Primary | 3590 (98.2) | 65 (1.8) | 1.1 (0.79–1.66) | 0.49 | 1.0(0.65–1.59) | 0.93 |
| Secondary | 5170 (98.3) | 87 (1.7) | 1.1 (0.75–1.51) | 0.74 | 1.0(0.69–1.43) | 0.99 |
| Tertiary | 3153 (98.4) | 50 (1.6) | 1 | 1 | ||
|
| ||||||
| Occupation (p = 0.13) | ||||||
| Civil servant | 1405 (98.7) | 19 (1.3) | 0.8 (0.46–1.30) | 0.34 | 0.6(0.34–1.19) | 0.17 |
| Business | 1386 (98.8) | 17 (1.2) | 0.7 (0.41–1.21) | 0.20 | 0.6(0.33–1.11) | 0.11 |
| Farmer | 351 (97) | 11 (3) | 1.8 (0.94–3.45) | 0.08 | 1.3(0.63–2.75) | 0.47 |
| Driver | 5090 (98.2) | 91 (1.8) | 1.0 (0.75–1.42) | 0.87 | 0.9(0.61–1.40) | 0.71 |
| Student | 3681 (98.3) | 64 (1.7) | 1 | 1 | ||
|
| ||||||
| Religion (p = 0.50) | ||||||
| Christian | 11658 (98.4) | 196 (1.6) | 0.4 (0.05–2.64) | 0.31 | 0.4(0.05–2.85) | 0.34 |
| Muslim | 234 (97.9) | 5 (2.1) | 0.5 (0.05–4.02) | 0.48 | 0.5(0.05–4.53) | 0.53 |
| Pagan | 21 (95.5) | 1 (4.5) | 1 | 1 | ||
|
| ||||||
| Residence (p = 0.001) | ||||||
| Bamenda | 10017 (98.5) | 152 (1.5) | 0.6 (0.42–0.80) | 0.001 | 0.6(0.43–0.84) | 0.003 |
| Out of Bamenda | 1896 (97.4) | 50 (2.6) | 1 | 1 | ||
|
| ||||||
| Year of donation (p = 0.25) | ||||||
| 2012 | 1490(98.0) | 30(2.0) | 1.4(0.86–2.17) | 0.19 | 1.4(0.85–2.16) | 0.21 |
| 2013 | 2371(98.1) | 46(1.9) | 1.3(0.87–1.98) | 0.19 | 1.3(0.88–2.03) | 0.17 |
| 2014 | 1890(98.5) | 29(1.5) | 1.0(0.65–1.66) | 0.87 | 1.1(0.67–1.71) | 0.79 |
| 2015 | 1087(97.8) | 25(2.2) | 1.6(0.95–2.55) | 0.08 | 1.6(0.98–2.62) | 0.06 |
| 2016 | 1960(98.7) | 26(1.3) | 0.9(0.55–1.46) | 0.66 | 0.9(0.54–1.44) | 0.63 |
| 2017 | 3115(98.5) | 46(1.5) | 1 | 1 | ||
OR: odd ratio; aOR: adjusted odds ratio (odds ratios adjusted for socio-demographic characteristics); CI: confidence interval
d. Syphilis:
A positivity rate of 2.2% (266/12,115) was noticed for anti-syphilis with the highest (3.8%) seen in 2015 and the least (1.5%) in 2017. Table 4 shows a significant difference in reactivity among the various age groups, marital status, level of education and occupation.
Table 4:
Bivariate and multivariate analyses of factors associated with Syphilis positivity rates among the 12,115 donations between 2012–2017 at the Bamenda Regional Hospital Blood Bank
| Characteristics | Non Reactive (%) | Reactive (%) | Unadjusted odd ratio (95% CI) | P-Value | Adjusted odds ratio (95% CI) | P-Value |
|---|---|---|---|---|---|---|
|
| ||||||
| Gender (p = 0.45) | ||||||
| Male | 10092 (97.8) | 231 (2.2) | 1.1 (0.80–1.65) | 0.45 | 0.9(0.61–1.29) | 0.53 |
| Female | 1757 (98) | 35 (2) | 1 | 1 | ||
|
| ||||||
| Age range (in years; (p = 0.000) | ||||||
| <20 | 1146 (99.3) | 8 (0.7) | 0.1 (0.04)–0.17 | 0.000 | 0.1 (0.04–0.24) | 0.000 |
| 20 – 29 | 6298 (98.4) | 105 (1.6) | 0.2 (0.12–0.29) | 0.000 | 0.2 (1.13–0.36) | 0.000 |
| 30 – 39 | 3012 (97.7) | 71 (2.3) | 0.3 (0.17–0.41) | 0.000 | 0.3 (0.17–0.43) | 0.000 |
| 40 – 49 | 1068 (95.3) | 53 (4.7) | 0.6 (0.35–0.89) | 0.014 | 0.6 (0.35–0.89) | 0.015 |
| ≥50 | 325 (91.8) | 29 (8.2) | 1 | 1 | ||
|
| ||||||
| Marital status (p = 0.000) | ||||||
| Married | 4138(95.2) | 207(4.8) | 1.6 (0.39–6.59) | 0.92 | 1.1(0.25–4.48) | 0.93 |
| Single | 7341(95.3) | 363(4.7) | 1.6 (0.39–6.49) | 0.37 | 0.9(0.22–4.01) | 0.93 |
| Divorced/Widow | 64(97.0) | 2(3.0) | 1 | 1 | ||
|
| ||||||
| Level of education (p = 0.000) | ||||||
| Primary | 3539 (96.9) | 116 (3.1) | 1.9 (1.36–2.60) | 0.000 | 1.6 (1.11–2.42) | 0.01 |
| Secondary | 5162 (98.2) | 95 (1.8) | 1.1 (0.75–1.47) | 0.76 | 1.2 (0.80–1.66) | 0.44 |
| Tertiary | 3148 (98.3) | 55 (1.7) | 1 | 1 | ||
|
| ||||||
| Occupation (p = 0.000) | ||||||
| Civil servant | 1387 (97.5) | 37 (2.5) | 2.1 (1.38–3.32) | 0.001 | 1.2 (0.75–1.97) | 0.44 |
| Business | 1368 (97.5) | 35 (2.5) | 2.1 (1.32–3.21) | 0.001 | 1.0 (0.62–1.71) | 0.90 |
| Farmer | 350 (96.7) | 12 (3.3) | 2.8 (1.45–5.23) | 0.002 | 1.0 (0.50–2.13) | 0.94 |
| Driver | 5045 (97.4) | 136 (2.6) | 2.1 (1.55–3.04) | 0.000 | 1.1 (0.74–1.69) | 0.59 |
| Student | 3699 (98.8) | 46 (1.2) | 1 | 1 | ||
|
| ||||||
| Religion (p = 0.73) | ||||||
| Christian | 11594 (97.8) | 260 (2.2) | N/A | N/A | ||
| Muslim | 233 (97.5) | 6 (2.5) | ||||
| Pagan | 22 (100) | 0 (0) | ||||
|
| ||||||
| Residence (p = 0.16) | ||||||
| Bamenda | 9954 (97.9) | 215 (2.1) | 0.8 (0.59–1.09) | 0.16 | 1.0(0.70–1.33) | 0.84 |
| Out of Bamenda | 1895 (97.4) | 51 (2.6) | 1 | 1 | ||
|
| ||||||
| Year of donation (p = 0.000) | ||||||
| 2012 | 1488(97.9) | 32(2.1) | 1.5(0.92–2.30) | 0.11 | 1.6(1.00–2.53) | 0.05 |
| 2013 | 2362(97.7) | 55(2.3) | 1.6(1.06–2.34) | 0.02 | 1.7(1.14–2.54) | 0.00 |
| 2014 | 1868(97.3) | 51(2.7) | 1.8(1.24–2.77) | 0.00 | 1.8(1.20–2.70) | 0.01 |
| 2015 | 1070(96.2) | 42(3.8) | 2.7(1.74–4.06) | 0.00 | 2.6(1.71–4.02) | 0.00 |
| 2016 | 1946(98.0) | 40(2.0) | 1.4(0.91–2.13) | 0.13 | 1.7(1.08–2.57) | 0.02 |
| 2017 | 3115(98.5) | 46(1.5) | 1 | 1 | ||
OR: odd ratio; aOR: adjusted odds ratio (odds ratios adjusted for socio-demographic characteristics); CI: confidence interval
Positivity rates of the combined TTIs markers
Overall, the positivity rates of all the four markers among the 12,115 donations was 10.5% (n=1,273). A steady decline was observed from 2012 (12.1%) to 2014 (11.7%) followed by slight increase to 13.1% in 2015. There was a significant decrease (p=0.000) in the overall combined positivity rate for these markers over time. Of the 1,273 reactive cases, HIV/HBV co-infection recorded the highest (23.8%) while co-infection with HIV/HCV was the least (6%). Those co-infected with HIV, HBV and HCV were 436 (3.6%) followed by 290 (2.4%) for HBV, HCV and syphilis. Out of the 1,273 positive cases, 145 (1.2%) were reactive for all the four markers.
Discussion
We observed an overall decrease in the seropositivity rate of all the four markers with the decline being significant for HBsAg and anti-syphilis. A noticeable trend of decline in the positivity rate was also observed when these markers were combined. Remarkably, the highest positivity rate for all the combined markers was in 2015 after a gradual decrease from 2012 to 2014 while the lowest rate for the individual markers was observed in either 2016 (for HBV and HCV) or 2017 (for HIV and syphilis).
There was a 2-fold decrease in the seropositivity rate for HIV in 2017 when compared to 2012. Our observation in this study is slightly lower than those seen in Ethiopia [16,17] but higher than those in Yaoundé [15], Nigeria [18], Iran, Thailand, Australia and China [19–22]. Possible explanation for the observed decline could be the progressive increase in the number of campaigns on the prevention of HIV transmission by structures such as SBFA, the National AIDS Control Committee [14].
Our findings for HBsAg showed contradictory results to those of other studies. For example, we got a higher seropositivity rate compared to other studies in Africa and Asia [16–20,22], but lower when compared to studies conducted in other cities of Cameroon [7,9,23]. One of the reasons for the above observation could be the initiation of children immunization program against HBV which went operational in 2000. We noticed that drivers, those aged >50 years-old, and primary school attendants recorded the highest positivity rate of this infection. This may be due to several reasons: Firstly, the elderlies are prone to infection because of possible exposure to multiple sexual activities. Secondly, this observation could be linked to the consequence of a birth cohort effect reflecting the past expansion and contemporary restraint of this infection. Even though we did not classify the donors as either first time or repeated, we think that most of the elderly who were infected could be first time thus explaining our findings.
The positivity rate of HCV was the lowest among all the four markers of TTIs in this study though the decline trend was not significant over the years. Interestingly, this is also the lowest observed when compared to other studies in Cameroon [6,8,15], Kenya and Burkina Faso [25,26] although higher than the observation in other Africa countries [3,16–18,24]. HCV infection is linked to geographical distribution and cultural practices. This include tattooing, multiple sexual partners and scarification which are more common in villages [27]. This could justify our observations which showed that those residing in the city recorded a lower positivity rate when compared to their counterparts from the rural areas.
Our observation on the trend of syphilis is consistent with other studies in Ethiopia and Nigeria [3,17,18]. The risk of sexually transmitted syphilis is higher in those who practice unprotected sex. This is associated with the level of knowledge and could partly justify why those living in villages, farmers and having attained only primary level of education recorded the highest positivity rate.
Almost a quarter of those who showed positive reaction to any of the serologic markers were co-infected with at least two. Our observation of HIV and HBV co-infections being the highest could be explained because these two viruses have the same route of transmission. Other causes of multiple infections in this study could be linked to combination of practices including behavioral, cultural inclinations such as shared use of invasive objects and level of awareness.
One limitation recognized in this study was the absence of confirmatory test of all the seropositive subjects. Therefore, seropositivity rate might be different if, in addition to these Enzyme Immuno Assays (EIAs) diagnostic methods used, a more sensitive method is employed. Nonetheless, being a retrospective study, we could only report on what was used which also reflects the method previously used by others in such related studies. In addition, the consistent use of the same method over the years in the study site provide dependable information suitable to assess trend without any bias.
Conclusion:
We have provided first-line data on the seropositivity rate of TTIs in blood donation units at the BRHBB. Overall, there is a significant decrease over time. Those who have attained only primary level of education recorded the highest seropositivity rate with all the serological markers. Efforts geared at the prevention of serological markers of TTIs in the community, is highly encouraged.
Acknowledgements
The authors are grateful to the Management of the Bamenda Regional Hospital and the staff of the Hospital’s Blood Bank for their cooperation in making data available. We wish to also acknowledge the support from the University of California, San Francisco’s International Traineeships in AIDS Prevention Studies (ITAPS), U.S. NIMH, R25MH064712, the CV Starr Foundation and the NIH Fogarty International Center (Grant # D43-TW010345). The authors declare that they have no competing interest.
References
- 1.Choudhury N Transfusion transmitted infections: How many more? Asian J Transfus Sci 2010:4:71–2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO Factsheet. Blood safety and availability. Available from: http://www.who.int/news-room/fact-sheets/detail/blood-safety-and-availability. Access on July 15 2018. [Google Scholar]
- 3.Kebede W, Zeleke M, Asfaw G, et al. : Transfusion-transmissible infection surveillance among blood donors in Southwest Ethiopia: A six years retrospective study. 2017 Asian Pac J Trop Dis 2017; 7(3): 156–161 [Google Scholar]
- 4.Yang S, Danmei J, Changjun L, et al. : Seroprevalence of human immunodeficiency virus, hepatitis B and C viruses, and Treponema pallidum infections among blood donors at Shiyan, Central China. BMC Infectious Diseases. 2016; 16:531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tagny CT, Kouao MD, Touré H, et al. : Transfusion safety in francophone African countries: an analysis of strategies for the medical selection of blood donors. Transfus. 2012;52(1):134–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Noubiap JJ, Joko WY, Nansseu JR, et al. : Sero-epidemiology of human immunodeficiency virus, hepatitis B and C viruses, and syphilis infections among first-time blood donors in Edéa, Cameroon. Int J Infect Dis 2013, s1201–s9712. [DOI] [PubMed] [Google Scholar]
- 7.Ducancelle A, Abgueguen P, Birguel J, et al. : High endemicity and low molecular diversity of hepatitis B virus infections in pregnant women in a rural district of North Cameroon. PloS One. 2013;8(11):e80346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moukoko CEE, Françoise NS, Estelle GES, et al. : HIV, HBV, HCV and T. pallidum infections among blood donors and Transfusion-related complications among recipients at the Laquintinie hospital in Douala, Cameroon. BMC Hematol 2014; 14:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tagny CT, Ndoumba A, Laperche S, et al. : Reducing risks of Transfusion-transmitted infections in a resource-limited hospital-based blood bank: the case of the Yaoundé University Teaching Hospital, Cameroon. ISBT Sci Ser. 2016;11(2):82–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.US President’s Emergency Plan for AIDS Relief (PEPFAR). Cameroon Operational Plan Report FY 2013, Available from: https://www.pepfar.gov/documents/organization/222156.pdf
- 11.Safe Blood for Africa, 2016; http://www.safebloodforafrica.org/index.php/cameroon Accessed: May 13 2018. [Google Scholar]
- 12.Centers for Disease Control and Prevention, Global Health, Cameroon Available from: https://www.cdc.gov/globalhealth/countries/cameroon/default.htm, Accessed on July 10 2018 [Google Scholar]
- 13.WHO, 2017. National Blood Transfusion Program, Cameroon. Available from: https://www.afro.who.int/news/national-blood-transfusion-program, Accessed on June 1 2018 [Google Scholar]
- 14.Mbanya D, Sama M, Tchounwou P: Current status of HIV/AIDS in Cameroon: how effective are control strategies? Int J Environ Res Public Health. 2008;5(5):378–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tagny CT, Nguefack-Tsague G, Fopa D, et al. : Risk factors for human immunodeficiency virus among blood donors in Cameroon: evidence for the design of an Africa-specific donor history questionnaire. Transfus. 2017;57(8):1912–21. [DOI] [PubMed] [Google Scholar]
- 16.Biadgo B, Shiferaw E, Woldu B, et al. : Transfusion-transmissible viral infections among blood donors at the North Gondar district blood bank, northwest Ethiopia: A three year retrospective study. PloS One. 2017;12(7):e0180416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Deressa T, Birhan W, Enawgaw B, et al. : Proportion and predictors of transfusion-transmissible infections among blood donors in North Shewa Zone, Central North Ethiopia. PloS One. 2018;13(3):e0194083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Okoh DA, Omunakwe HE, Iyalla C: Trends in transfusion-transmissible infections in potential donors in a specialist hospital in Rivers State. ISBT Sci Ser. 2014;9(2):334–8. [Google Scholar]
- 19.Nantachit N, Robison V, Wongthanee A, et al. : Temporal trends in the prevalence of HIV and other transfusion-transmissible infections among blood donors in northern Thailand, 1990 through 2001. Transfus. 2003;43(6):730–5. [DOI] [PubMed] [Google Scholar]
- 20.Lucky TTA, Seed CR, Keller A, et al. : Trends in transfusion-transmissible infections among Australian blood donors from 2005 to 2010. Transfus. 2013;53(11):2751–62. [DOI] [PubMed] [Google Scholar]
- 21.Farshadpour F, Taherkhani R, Tajbakhsh S, et al. : Prevalence and Trends of Transfusion-Transmissible Viral Infections among Blood Donors in South of Iran: An Eleven-Year Retrospective Study. PloS One. 2016;11(6):e0157615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Song Y, Bian Y, Petzold M, et al. : Prevalence and Trend of Major Transfusion-Transmissible Infections among Blood Donors in Western China, 2005 through 2010. Plos One. 2014. 8;9(4):e94528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mbanya DN, Takam D, Ndumbe PM: Serological findings amongst first-time blood donors in Yaoundé, Cameroon: is safe donation a reality or a myth? Transfus Med Oxf Engl. 2003;13(5):267–73. [DOI] [PubMed] [Google Scholar]
- 24.Shiferaw E, Tadilo W, Melkie I, et al. : Sero-prevalence and trends of transfusion-transmissible infections among blood donors at Bahir Dar district blood bank, northwest Ethiopia: A four year retrospective study. PLoS One. 2019; 14(4): e0214755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Onyango CG, Ogonda L, Guyah B, et al. : Correction to: Seroprevalence and determinants of transfusion transmissible infections among voluntary blood donors in Homabay, Kisumu and Siaya counties in western Kenya. BMC Res Notes. 2018; 26;11(1):410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nagalo BM, Bisseye C, Sanou M, et al. : Seroprevalence and incidence of transfusion-transmitted infectious diseases among blood donors from regional blood transfusion centres in Burkina Faso, West Africa. Trop Med Int Health TM IH. 2012;17(2):247–53. [DOI] [PubMed] [Google Scholar]
- 27.Agbor VN, Tagny CT, Kenmegne J-B, et al. : Prevalence of anti-hepatitis C antibodies and its co-infection with HIV in rural Cameroon. BMC Res Notes. 2018;11(1):459. [DOI] [PMC free article] [PubMed] [Google Scholar]


