Abstract
The last several years have seen an emergence of literature documenting the utility of combination antimicrobial therapy, particularly in the salvage of refractory methicillin-resistant Staphylococcus aureus (MRSA) bacteremia. Recent clinical data are shaping conundrums of which regimens may be more beneficial, which can be potentially harmful, and which subset of patients stand to benefit from more aggressive treatment regimens than called for by current standards. In addition, the incorporation of combination therapy for MRSA bacteremia should be accompanied by the reminder that antimicrobial therapy does not need to be uniform for the entire duration, with an early intensive phase in high inoculum infections (eg, with combination therapy), followed by a consolidation phase (ie, monotherapy). This review and perspective consolidates the recent data on this subject and directs future goals in filling the knowledge gaps to methodically move forward towards improving patient outcomes.
Keywords: bacteremia, MRSA, combination therapy
This review consolidates the recent data on combination therapy for methicillin-resistant Staphylococcus aureus (MRSA) bacteremia, provides perspective on potential use and applicable patients, and directs future goals in filling the knowledge gaps to methodically move forward toward improving patient outcomes.
STANDARD-OF-CARE (SOC) FOR MRSA BACTEREMIA: ROOM FOR IMPROVEMENT?
The first consideration when assessing combination versus SOC monotherapy regimens (vancomycin or daptomycin) for MRSA bacteremia is the SOC performance in clinical practice and clinical trials. After all, medical need for alternatives would be low if SOC reflected steady progress with successive improvement in patient outcomes over time. Unfortunately, this is not the case for serious MRSA infections such as bacteremia and endocarditis, as vancomycin has served as the cornerstone of therapy from the time MRSA was a sporadic pathogen in the 1970s, to a common healthcare pathogen in the 1980s, to a community-based pathogen around 2000 [1]. Although daptomycin costs have fallen dramatically after generic introduction, vancomycin remains the standard initial treatment of MRSA bacteremia at nearly every institution. Previous reviews note the use and limitations of these primary therapies for MRSA bacteremia [2, 3]. The discordance between antibiotic susceptibility testing and clinical success with vancomycin remains; S. aureus vancomycin resistance is exceedingly rare in traditional bacterial medium; however, vancomycin treatment failure, including persistent bacteremia is quite common [4, 5]. Concurrently, despite progress and improvements in many other common medical conditions—for example, 30-day mortality for acute myocardial infarction decreased by 50% from 1984 to 2008—patient mortality rates in S. aureus bacteremia in general and MRSA in particular have remained fairly constant over this time period [2].
COMBINATION THERAPIES FOR MRSA BACTEREMIA
Vancomycin Plus β-Lactams
Interest in the specific combination of vancomycin with β-lactams against MRSA bacteremia first emerged due to the so-called seesaw effect, an inverse relationship between vancomycin and β-lactam susceptibility observed in some vancomycin intermediate-resistant S. aureus (VISA) strains [6]. Indeed, early studies revealed better activity of ampicillin-sulbactam over vancomycin against these strains [7].
MRSA susceptibility to β-lactams is enhanced coincident with the emergence of decreased susceptibility to vancomycin, despite the presence of the mecA product, PBP2a. In rare instances, deletion of mecA and conversion back to MSSA occurs under vancomycin selection pressure [6]. The mechanism for the seesaw effect may lie in chaperon protein PrsA altering PBP2A maturation [8], and clinical validation of this notion holds merit and may solidify the combination against S. aureus. As such, the potential clinical utility of the combination is extensive and the inspiration for numerous investigations. Experimental models consistently validate vancomycin synergy with different β-lactams in vancomycin susceptible MRSA potentially through dual effects on peptidoglycan synthesis [7, 8].
In study by Truong et al, vancomycin combined with various β-lactams resulted in a significant reduction in clinical failure in vancomycin susceptible strains (minimum inhibitory concentration (MIC) ≤ 2 mg/L) [9]. A similar investigation by Casapao et al found no significant difference in clinical failure but revealed shorter median duration of bacteremia in patients receiving combination [10]. In the Combination Antibiotics for Methicillin-Resistant Staphylococcus aureus-1 (CAMERA-1) randomized controlled trial, a decrease in average duration of bacteremia by 1 day (P = .06) was observed in patients receiving flucloxacillin plus vancomycin relative to vancomycin alone [11]. The follow-up CAMERA-2 study, the most rigorous clinical trial on the subject, will be discussed in detail in a separate section below.
Daptomycin Plus Antistaphylococcal β-Lactams
The mechanisms underpinning daptomycin synergy with β-lactams are well established [12]. As a functional cationic peptide, daptomycin is attracted to the negatively charged cellular bacteria surface. In MRSA, β-lactams enhance daptomycin binding to cell membranes by inducing more negatively charged cell surfaces resulting in potent bactericidal synergy [13]. However, select β-lactams such as meropenem may not increase overall daptomycin binding, but rather focally enhances divisome-specific binding, where daptomycin exerts its most potent effect [14, 15]. In addition, β-lactam inhibition of select penicillin-binding proteins (PBPs), such as PBP1, in MRSA may provide added synergistic mechanism and prevent resistance development [14, 16]. Importantly, corresponding in vivo animal models of daptomycin plus β-lactam treatment demonstrate enhanced clearance of MRSA in target tissues [17].
In a small case series of patients with persistent MRSA bacteremia, Dhand and colleagues described 7 cases of daptomycin combined with an antistaphylococcal β-lactam (primarily nafcillin) resulting in rapid clearance and infection resolution, including examples in daptomycin-resistant MRSA. This synergistic effect was linked to enhanced daptomycin binding and cidality [13]. Subsequent case series and cohort studies were limited by small sample sizes and use of β-lactam plus daptomycin as salvage therapy [18–21]. A large (n = 229) retrospective, comparative cohort study by Jorgensen et al addressed these limitations by evaluating MRSA bacteremia patients treated with daptomycin alone versus daptomycin combined with a β-lactam for at least 72 hours and initiated within 5 days of culture [19]. The β-lactams were diverse and included cefepime, cefazolin, ceftaroline, ceftriaxone, meropenem, piperacillin/tazobactam, ertapenem, and ampicillin-sulbactam; significantly reduced odds of clinical failure occurred in the dual therapy cohort (adjusted odds ratio [OR] = 0.386) [19]. This study identifies potential benefits of using β-lactams in combination with daptomycin when added early in the bacteremia course.
Daptomycin Plus Ceftaroline
An important limitation of the above β-lactam studies for MRSA is that, although synergistic with SOC agents and known to enhance innate immunity [22], the β-lactams under study do not possess direct bactericidal MRSA activity. In contrast, ceftaroline is active against MRSA by binding to PBP2A and inhibiting peptidoglycan transpeptidation, but it is not approved by the Food and Drug Administration (FDA) for bacteremia [23]. Like other β-lactams, ceftaroline enhances daptomycin cell membrane binding [24]. In one of the first case reports, ceftaroline was added to daptomycin to treat a patient who developed persistent, daptomycin-resistant MRSA. Interestingly, this not only cleared persistent bacteremia but also reverted the MRSA back to a daptomycin-susceptible phenotype [25]. Subsequent case series of this combination therapy demonstrated similar success [26, 27].
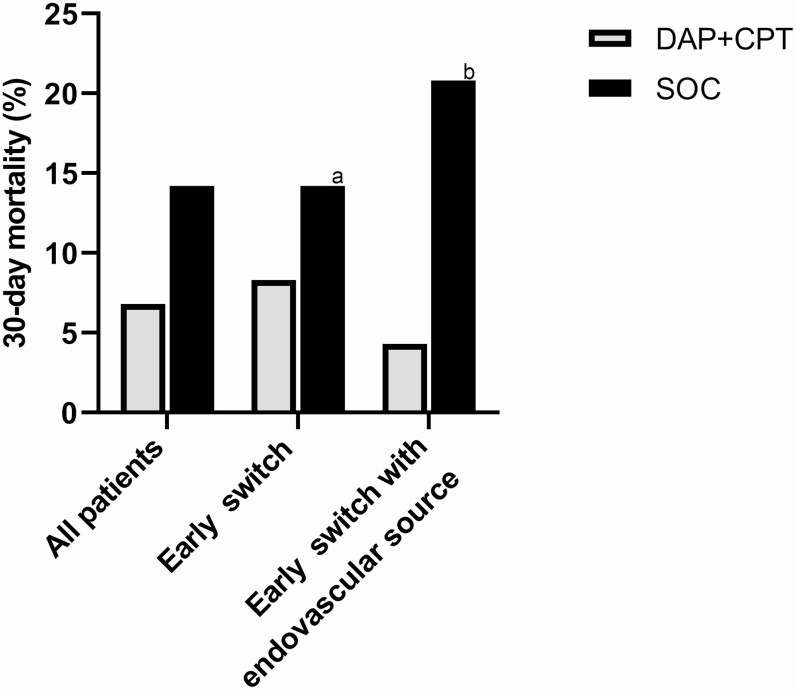
A large retrospective matched cohort study among 3 institutions compared patients with MRSA bacteremia treated with SOC versus daptomycin plus ceftaroline [28]. Many of the combination patients were inherently high-risk because the combination was used in a salvage role, but despite this consideration, daptomycin-ceftaroline resulted in clearance of persistent MRSA bacteremia and a numerically lower 30-day mortality rate than SOC (8.3% vs 14.2% at 30 days). In patients switched to combination early in the course of bacteremia with an endovascular source, there was a further trend for improved survival (Figure 1).
Figure 1.
Data from McCreary et al [28] demonstrating mortality in patients treated with SOC vancomycin or daptomycin versus DAP + CPT in all patients, those switched to DAP + CPT early in the course of bacteremia (<72 hrs), and those switched early with an endovascular source of bacteremia. Abbreviations: DAP+CPT, daptomycin plus ceftaroline; SOC, standard of care. aSOC represents all patients since no switch occurred. bSOC represents patients with an endovascular source.
A randomized-controlled open-label pilot study attempted to provide clarity on the value of this combination [29]. Patients with MRSA bacteremia at 3 institutions were randomized to receive daptomycin 6–8 mg/kg daily plus ceftaroline 600 mg every 8 hours or SOC monotherapy. The study was designed to measure both bacteremia duration and mortality; however, interim analysis showed an in-hospital mortality rate of 26% for the SOC arm and 0% for daptomycin plus ceftaroline group (P = .029), resulting in early study termination due to the ethics of continuing such a study in an open-label format. The median duration of bacteremia was similar between the 2 groups. Importantly, there were minimal treatment-related adverse events [29]. Some critiques of this study have been proposed including questions on premature termination without predefined designated data safety monitoring board and stopping rules, and external validity [30]. However, it sheds light on the potential utility of this combination in high-risk MRSA bacteremia patients and provides a baseline for future studies.
Daptomycin Plus Fosfomycin
Fosfomycin inhibits the formation of the peptidoglycan precursor UDP N-acetylmuramic acid, an early step in peptidoglycan synthesis, there is potential for dual mechanistic synergy with daptomycin [31]. In vitro results confirm synergy in various assays and model systems [32–34], and the combination has been shown to slow daptomycin resistance development [33]. Animal models of experimental infective endocarditis and osteomyelitis also show effective bacterial killing [34–36].
Clinical success of daptomycin plus fosfomycin was described in 3 patients with left-sided staphylococcal endocarditis [37], leading to the design of a randomized controlled clinical trial in MRSA bacteremia. The trial compared daptomycin plus fosfomycin (10 mg/kg intravenously daily and 2 g intravenously every 6 hours, respectively) to daptomycin 10 mg/kg monotherapy. Initial results with the combination appear promising––54% cure with combination therapy versus 42% with monotherapy (relative risk, 1.29 [95% confidence interval, .93–1.8]; P = .135) and greater bacteremia clearance by day 7 [38]. But again, there were few patients with complicated bacteremia; only 12% of patients had confirmed endocarditis, although 45% of patients had catheter sources of bacteremia. Furthermore, more patients in the combination therapy group had treatment-limiting adverse events (P = .018), so the safety profile appears to favor monotherapy. Although intravenous fosfomycin is not yet available in several countries including the United States, this combination therapy holds promise for future evaluation and consideration.
Putting CAMERA-2 Under the Microscope
In one of the most ambitious study designs of MRSA combination therapy, CAMERA-2 compared SOC monotherapy to combination therapy of a SOC agent with flucloxacillin/cloxacillin or cefazolin in those with a history of mild penicillin allergy. Although the protocol allowed daptomycin or vancomycin, 348/352 (99%) patients in the trial received vancomycin, rendering this in essence a vancomycin plus β-lactam versus vancomycin study. Similar to CAMERA-1, the authors found shorter bacteremia duration with addition of a β-lactam but detected acute kidney injury (AKI) of sufficient magnitude in the combination therapy group to terminate the study at the recommendation of the data safety monitoring board. Overall, the study showed no benefit of adding a β-lactam to vancomycin, with the shortening of duration of bacteremia being negated by the AKI [39]. Therefore, although numerically large, the negative results of CAMERA-2 cannot be extended to all MRSA combination therapy [40]. Some salient features of the trial bear discussion for conclusions to be drawn in context.
First, the CAMERA-2 patients showed an 11% all-cause mortality at 42 days for the standard vancomycin monotherapy arm, which is approximately half of the 20–25% generally accepted in the literature. This may have been due to the fact that only 4.3% of patients in the study had confirmed endocarditis, although 38% had skin or intravenous line sources of bacteremia, a low-risk group where there is no medical need for combination therapy. Regardless, such low mortality does not reflect real world practice settings and hardly left room for improvement with any alternative comparison.
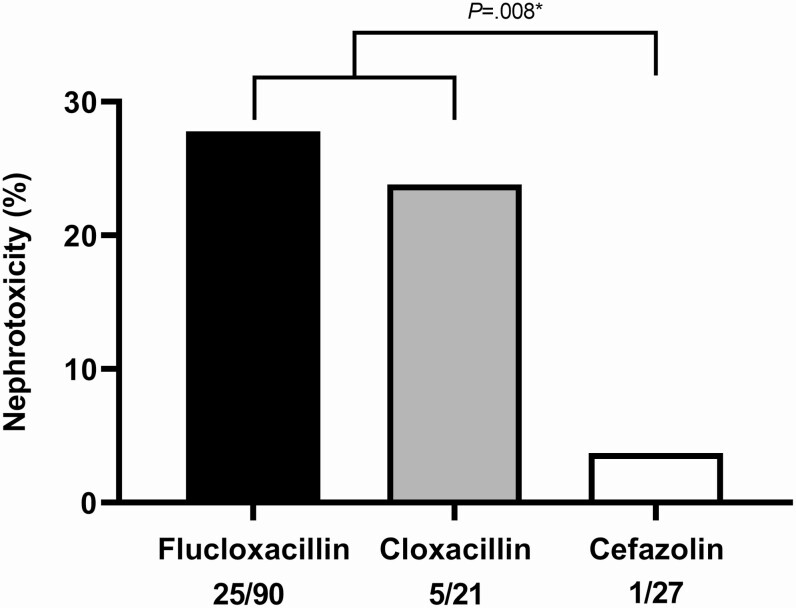
Second, with the data that emerged since development of the CAMERA-2 study protocol pointing to AKI risk with vancomycin plus piperacillin/tazobactam, these results confirm nephrotoxicity in vancomycin plus some penicillins [41]. Importantly, the addition of cefazolin, a β-lactam with a better safety profile compared to antistaphylococcal penicillins [42], to vancomycin in the small subset of 27 patients found only 1 patient developed AKI (Figure 2). Other clinical studies with vancomycin combined with cephalosporins, including cefazolin, demonstrate improved clinical response, and low nephrotoxicity potential, but these studies are limited by retrospective design, small sample size, and suboptimal evaluation of nephrotoxicity [43–45].
Figure 2.
CAMERA-2 results demonstrating significantly less nephrotoxicity when combining vancomycin with cefazolin as compared to an antistaphylococcal penicillin (*Fisher exact test) [36]. Abbreviation: CAMERA, combination sntibiotics for methicillin-resistant Staphylococcus aureus.
Some helpful insights regarding nephrotoxicity potential of different β-lactams when combined with vancomycin can be made when reviewing the study by Wolman et al showing that lipophilic beta-lactams are recognized by the organic anion transporter 3 (OAT-3), allowing for drug accumulation within proximal tubular cells [46]. Coupled with vancomycin, which may already be placing mitochondrial stress on the proximal tubular cells [47], OAT-3 mediated intracellular accumulation of lipophilic β-lactams may cause mitochondrial injury in these highly metabolically active cells. Given that the endosymbiosis theory places primordial origins of mitochondria as intracellular bacteria [48], the potency of combination therapy may have a price on these organelles if β-lactam plus vancomycin concentrations are high enough in the cell. Indeed, when one reviews the partition coefficient (relative solubility in organic versus aqueous solvents) of the various β-lactams, striking parallels are seen between nephrotoxic hydrophobic β-lactams with vancomycin (eg, piperacillin, anti-staphylococcal penicillins) versus hydrophilic ones with less nephrotoxic potential (eg, ampicillin, most cephalosporins). Therefore, until more definitive data exist, we recommend utilizing β-lactams with vancomycin with these points in mind.
ROLE FOR PATIENT RISK STRATIFICATION FOR THERAPY SELECTION
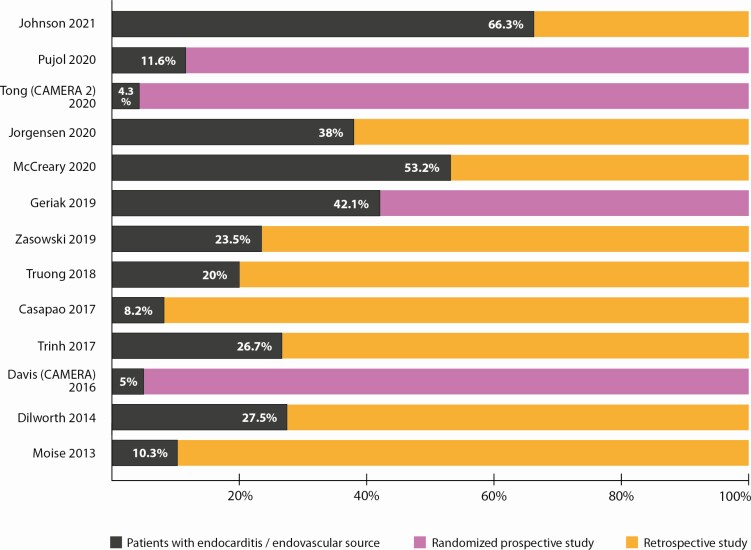
Consistent limitations of clinical trials in S. aureus bacteremia is the “one size fits all” approach where all patients are treated in the same manner regardless of outcome risk. S. aureus bacteremia trials enroll patients with bacteremia from skin and soft tissue infection and catheter sources as well as endovascular sources. Although these might be equally distributed among the randomization groups, a superior or inferior therapy is hard to discern because it is not powered for the high-risk endovascular source patients who may benefit from combination or other novel therapy where animal modeling supports their role. Figure 3 highlights the limitation of the low percentage of patients with endovascular/endocarditis sources in the prospective and retrospective combination studies discussed. Retrospective analyses of combination therapy are significantly biased because clinicians frequently deploy combination antibiotic therapy for MRSA bacteremia in the highest risk patients outside of clinical trial settings, usually in a salvage approach. Therefore, retrospectively comparing these outcomes is unlikely to provide a fair assessment, no matter how rigorous the post hoc matching. There is significant need to stratify patients based on clinical features and potentially apply new approaches in those high-risk patients, but questions remain on how.
Figure 3.
Overview of endovascular/endocarditis source of bacteremia among patients included in retrospective and prospective studies of MRSA combination therapy. The percentage of endovascular/endocarditis source represents patients from each study with endovascular or endocarditis source explicitely stated in the study results. Abbreviations: CAMERA, combination sntibiotics for methicillin-resistant Staphylococcus aureus; MRSA, methicillin-resistant Staphylococcus aureus.
Along with clinical judgement of infection severity, source of infection (eg, endovascular vs nonendovascular), and comorbidities, some novel applications of biomarkers show promise. In the randomized pilot study by Geriak et al, no patients randomized to daptomycin plus ceftaroline died, but 6 patients receiving SOC died. Among these 6 patients, 5 had interleukin (IL)-10 concentrations upon admission of >5 pg/mL (threshold for elevated IL-10), whereas no patients died in the combination group with elevated IL-10. The anti-inflammatory cytokine IL-10, driven by endovascular bacterial burden, is a recently identified but consistent host biomarker for increased S. aureus bacteremia mortality risk [29, 49–53]. A recent study that applied high resolution proteomic and metabolomic techniques, coupled with advanced computational strategies, on blood samples drawn at the time of admission revealed a more extensive cadre of biomarkers that may be useful in early stratification of mortality risk in patients with S. aureus bacteremia [54].
MRSA THAT ARE Β-LACTAM SUSCEPTIBLE IN VIVO
Recent reports have reinforced the potential clinical utility of standard β-lactams for MRSA infections by demonstrating that some clinical MRSA strains have a “bicarbonate responsive” β -lactam susceptible phenotype: in the presence of bicarbonate buffer, they are phenotypically methicillin-susceptible and potentially treatable with β-lactams alone [55–57]. This is highly relevant given that bicarbonate is the main in vivo physiological buffer but absent in the bacteriological media used in standard clinical antimicrobial susceptibility testing. In this line of investigation, MRSA strains were effectively treated by β-lactam monotherapy in rabbit models of endocarditis. Because clinical strains are not routinely tested for this bicarbonate responsiveness, the addition of β-lactams to standard MRSA backbone therapy (daptomycin, vancomycin) may be providing some direct antimicrobial activity along with enhancing activity of the backbone drug or innate host defense peptides.
THE DEBATE ON DURATION OF COMBINATION THERAPY
MRSA bacteremia treatment guidelines recommending “4–6 weeks” of antibiotic therapy fail to account for individual patient and infection factors, including inoculum size and host immunocompromising comorbidities. Interestingly, oncologists frequently deploy early intensive “induction chemotherapy” and subsequently back off to less intense “maintenance chemotherapy” regimens. It is understood in oncology that killing tumor cells more efficiently comes at the price of patient toxicity, but that early in the treatment course, this is a price willing to be paid. After some critical initial period of tumor reduction, there is the need to continue to provide antineoplastic therapy but the side effects risks to the patient becomes an equally important consideration.
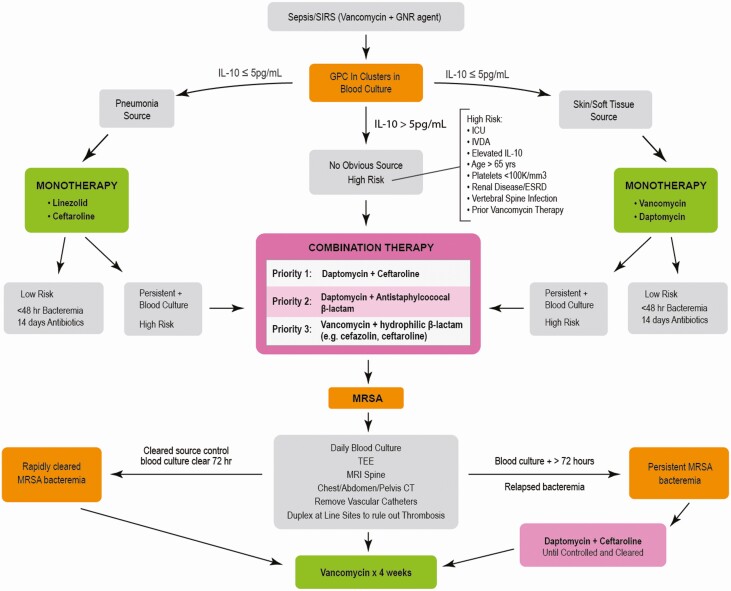
With these points in mind, combination antimicrobial therapy is important to initiate early, within the first 72 hours of onset, and ideally within the first 24 hours to prevent complications of persistent bacteremia [58–60]. However, combination therapy may not be needed for the entire 4–6 weeks in S. aureus bacteremia. In fact, starting with a failing monotherapy up front that results in combination salvage therapy may result in longer durations of salvage combination and greater total antibiotic exposure on the tail end. Instead, we advocate intensive therapy up front and narrow down for the remainder of treatment based on patient response. Figure 4 provides a conceptual approach for implementing early combination therapy based on risk stratification and appropriate de-escalation and duration. In the study by McCreary et al, only 55% of patients completed treatment on daptomycin plus ceftaroline, while the remainder were de-escalated to daptomycin, ceftaroline, or vancomycin monotherapy [28]. Understanding which patients are candidates and when to de-escalate to monotherapy are important antimicrobial stewardship interventions. This can be applicable to outpatient parenteral antimicrobial therapy (OPAT), where MRSA bacteremia patients can be streamlined to a single antibiotic after the inpatient induction phase. In this regard, novel predictive biomarkers may have a role. A recent study by Volk et al found that β-lactams, including those inactive against MRSA, stimulate IL-1β and reduce IL-10 during the first 7 days of treatment, improving the patient innate immune response to infection [49]. Future studies should consider using these and other host biomarkers to identify optimal duration of combination therapy and best approaches for de-escalation on an individual patient basis.
Figure 4.
Conceptual decision pathway for risk stratification and antibiotic therapies for MRSA bacteremia. Abbreviations: CT, computerized tomography; ESRD, end state renal disease; GNR, gram-negative rod; GPC, gram-positive cocci; ICU, intensive care unit; IL, interleukin; IVDA, intravenous drug abuse, MRI, magnetic resonance imaging; MRSA, methicillin-resistant Staphylococcus aureus; SIRS, systemic inflammatory response syndrome; TEE, transesophogeal echocardiogram.
CONCLUSION
MRSA bacteremia is the final common pathway of a heterogeneous group of deep-seated infections that possess tissue-specific differences in inocula and host-pathogen interactions. As such, it will be necessary for clinical investigators to simultaneously evolve diagnostics of risk stratification with matching optimal pharmacotherapies. We began this discussion asking whether combination therapy works, which combinations are best, and for which patients. Early combination therapy offers advantages over monotherapy in high-risk endovascular infection. However, vancomycin plus hydrophobic β-lactam combinations (eg, antistaphylococcal penicillins) best be avoided due to nephrotoxicity, and if such agents are to be used in combination, they may be better suited with daptomycin. Using daptomycin plus ceftaroline appeared very promising even in a trial with a very small number of patients. More studies will be needed to examine relative differences on efficacy, safety, and pharmacoeconomic advantages of different combinations. With dropping costs of non-vancomycin MRSA antibiotics via expiration of drug patents and generic availability, financial factors will play less of a role in directed MRSA therapy in the evolution away from vancomycin.
Notes
Acknowledgments. The authors thank Sally Griffith-Oh for creating the graphic and figure designs.
Potential conflicts of interest. W. E. R. has received grant funding from Merck, Theravance, and Paratek; honoraria from Melinta, Paratek, and Merck; and is on the Board of Directors: Society of Infectious Diseases Pharmacists (unpaid). G. S. has consulted for Allergan, Paratek, and Octapharma. V. N. has consulted for Cellics Therapeutics, Vaxcyte, Clarametyx Biosciences, SNIPR Biome, Boehringer Ingelheim, and Iogen. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Contributor Information
Warren Rose, School of Pharmacy, University of Wisconsin-Madison, Madison, Wisconsin, USA.
Michael Fantl, School of Pharmacy, University of Wisconsin-Madison, Madison, Wisconsin, USA.
Matthew Geriak, Pharmacy Department, Sharp Memorial Hospital, San Diego, California, USA.
Victor Nizet, Division of Host-Microbe Systems and Therapeutics, Center for Immunity, Infection and Inflammation, University of California-San Diego School of Medicine, La Jolla, California, USA.
George Sakoulas, Division of Host-Microbe Systems and Therapeutics, Center for Immunity, Infection and Inflammation, University of California-San Diego School of Medicine, La Jolla, California, USA.
References
- 1. Turner NA, Sharma-Kuinkel BK, Maskarinec SA, et al. Methicillin-resistant Staphylococcus aureus: an overview of basic and clinical research. Nat Rev Microbiol 2019; 17:203–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. van Hal SJ, Jensen SO, Vaska VL, Espedido BA, Paterson DL, Gosbell IB. Predictors of mortality in Staphylococcus aureus bacteremia. Clin Microbiol Rev 2012; 25:362–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kullar R, Sakoulas G, Deresinski S, van Hal SJ. When sepsis persists: a review of MRSA bacteraemia salvage therapy. J Antimicrob Chemother 2016; 71:576–86. [DOI] [PubMed] [Google Scholar]
- 4. Machado H, Seif Y, Sakoulas G, et al. Environmental conditions dictate differential evolution of vancomycin resistance in Staphylococcus aureus. bioRxiv 2020: 2020.06.07.138933. Available at: https://www.biorxiv.org/content/10.1101/2020.06.07.138933v1.full [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Levine DP, Fromm BS, Reddy BR. Slow response to vancomycin or vancomycin plus rifampin in methicillin-resistant Staphylococcus aureus endocarditis. Ann Intern Med 1991; 115:674–80. [DOI] [PubMed] [Google Scholar]
- 6. Adhikari RP, Scales GC, Kobayashi K, Smith JM, Berger-Bächi B, Cook GM. Vancomycin-induced deletion of the methicillin resistance gene mecA in Staphylococcus aureus. J Antimicrob Chemother 2004; 54:360–3. [DOI] [PubMed] [Google Scholar]
- 7. Backo M, Gaenger E, Burkart A, Chai YL, Bayer AS. Treatment of experimental staphylococcal endocarditis due to a strain with reduced susceptibility in vitro to vancomycin: efficacy of ampicillin-sulbactam. Antimicrob Agents Chemother 1999; 43:2565–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Renzoni A, Kelley WL, Rosato RR, et al. Molecular bases determining daptomycin resistance-mediated resensitization to beta-lactams (seesaw effect) in methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother 2017; 61:e01634-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Truong J, Veillette JJ, Forland SC. Outcomes of vancomycin plus a beta-lactam versus vancomycin only for treatment of methicillin-resistant Staphylococcus aureus Bacteremia. Antimicrob Agents Chemother 2018; 62:e01554-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Casapao AM, Jacobs DM, Bowers DR, Beyda ND, Dilworth TJ; REACH-ID Study Group . Early administration of adjuvant β-lactam therapy in combination with vancomycin among patients with methicillin-resistant Staphylococcus aureus bloodstream infection: a retrospective, multicenter analysis. Pharmacotherapy 2017; 37:1347–56. [DOI] [PubMed] [Google Scholar]
- 11. Davis JS, Sud A, O’Sullivan MVN, et al. Combination of vancomycin and beta-lactam therapy for methicillin-resistant Staphylococcus aureus bacteremia: a pilot multicenter randomized controlled trial. Clin Infect Dis 2016; 62:173–80. [DOI] [PubMed] [Google Scholar]
- 12. Rand KH, Houck HJ. Synergy of daptomycin with oxacillin and other beta-lactams against methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother 2004; 48:2871–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dhand A, Bayer AS, Pogliano J, et al. Use of antistaphylococcal beta-lactams to increase daptomycin activity in eradicating persistent bacteremia due to methicillin-resistant Staphylococcus aureus: role of enhanced daptomycin binding. Clin Infect Dis 2011; 53:158–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Berti AD, Theisen E, Sauer JD, et al. Penicillin binding protein 1 is important in the compensatory response of Staphylococcus aureus to daptomycin-induced membrane damage and is a potential target for β-lactam-daptomycin synergy. Antimicrob Agents Chemother 2016; 60:451–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pogliano J, Pogliano N, Silverman JA. Daptomycin-mediated reorganization of membrane architecture causes mislocalization of essential cell division proteins. J Bacteriol 2012; 194:4494–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Berti AD, Sakoulas G, Nizet V, Tewhey R, Rose WE. β-Lactam antibiotics targeting PBP1 selectively enhance daptomycin activity against methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother 2013; 57:5005–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yang SJ, Xiong YQ, Boyle-Vavra S, Daum R, Jones T, Bayer AS. Daptomycin-oxacillin combinations in treatment of experimental endocarditis caused by daptomycin-nonsusceptible strains of methicillin-resistant Staphylococcus aureus with evolving oxacillin susceptibility (the “seesaw effect”). Antimicrob Agents Chemother 2010; 54:3161–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Moise PA, Amodio-Groton M, Rashid M, et al. Multicenter evaluation of the clinical outcomes of daptomycin with and without concomitant β-lactams in patients with Staphylococcus aureus bacteremia and mild to moderate renal impairment. Antimicrob Agents Chemother 2013; 57:1192–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jorgensen SCJ, Zasowski EJ, Trinh TD, et al. Daptomycin plus β-lactam combination therapy for methicillin-resistant Staphylococcus aureus bloodstream infections: a retrospective, comparative cohort study. Clin Infect Dis 2020; 71:1–10. [DOI] [PubMed] [Google Scholar]
- 20. Kale-Pradhan PB, Giuliano C, Jongekrijg A, Rybak MJ. Combination of vancomycin or daptomycin and beta-lactam antibiotics: a meta-analysis. Pharmacotherapy 2020; 40:648–58. [DOI] [PubMed] [Google Scholar]
- 21. Alosaimy S, Sabagha NL, Lagnf AM, et al. Monotherapy with vancomycin or daptomycin versus combination therapy with β-lactams in the treatment of methicillin-resistant Staphylococcus aureus bloodstream infections: a retrospective cohort analysis. Infect Dis Ther 2020; 9:325–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sakoulas G, Okumura CY, Thienphrapa W, et al. Nafcillin enhances innate immune-mediated killing of methicillin-resistant Staphylococcus aureus. J Mol Med (Berl) 2014; 92:139–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Steed ME, Rybak MJ. Ceftaroline: a new cephalosporin with activity against resistant gram-positive pathogens. Pharmacotherapy 2010; 30:375–89. [DOI] [PubMed] [Google Scholar]
- 24. Werth BJ, Sakoulas G, Rose WE, Pogliano J, Tewhey R, Rybak MJ. Ceftaroline increases membrane binding and enhances the activity of daptomycin against daptomycin-nonsusceptible vancomycin-intermediate Staphylococcus aureus in a pharmacokinetic/pharmacodynamic model. Antimicrob Agents Chemother 2013; 57:66–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rose WE, Schulz LT, Andes D, et al. Addition of ceftaroline to daptomycin after emergence of daptomycin-nonsusceptible Staphylococcus aureus during therapy improves antibacterial activity. Antimicrob Agents Chemother 2012; 56:5296–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sakoulas G, Moise PA, Casapao AM, et al. Antimicrobial salvage therapy for persistent staphylococcal bacteremia using daptomycin plus ceftaroline. Clin Ther 2014; 36:1317–33. [DOI] [PubMed] [Google Scholar]
- 27. Duss FR, Garcia de la Mària C, Croxatto A, et al. Successful treatment with daptomycin and ceftaroline of MDR Staphylococcus aureus native valve endocarditis: a case report. J Antimicrob Chemother 2019; 74:2626–30. [DOI] [PubMed] [Google Scholar]
- 28. McCreary EK, Kullar R, Geriak M, et al. Multicenter cohort of patients with methicillin-resistant Staphylococcus aureus bacteremia receiving daptomycin plus ceftaroline compared with other MRSA treatments. Open Forum Infect Dis 2020; 7:ofz538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Geriak M, Haddad F, Rizvi K, et al. Clinical data on daptomycin plus ceftaroline versus standard of care monotherapy in the treatment of methicillin-resistant Staphylococcus aureus bacteremia. Antimicrob Agents Chemother 2019; 63:e02483-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kalil AC, Holubar M, Deresinski S, Chambers HF. Is daptomycin plus ceftaroline associated with better clinical outcomes than standard of care monotherapy for Staphylococcus aureus bacteremia? Antimicrob Agents Chemother 2019; 63:e00900-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Falagas ME, Vouloumanou EK, Samonis G, Vardakas KZ. Fosfomycin. Clin Microbiol Rev 2016; 29:321–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Aktas G, Derbentli S. In vitro activity of daptomycin combinations with rifampicin, gentamicin, fosfomycin and fusidic acid against MRSA strains. J Glob Antimicrob Resist 2017; 10:223–7. [DOI] [PubMed] [Google Scholar]
- 33. Berti AD, Wergin JE, Girdaukas GG, Hetzel SJ, Sakoulas G, Rose WE. Altering the proclivity towards daptomycin resistance in methicillin-resistant Staphylococcus aureus using combinations with other antibiotics. Antimicrob Agents Chemother 2012; 56:5046–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Reed JM, Gardner SG, Mishra NN, Bayer AS, Somerville GA. Metabolic interventions for the prevention and treatment of daptomycin non-susceptibility in Staphylococcus aureus. J Antimicrob Chemother 2019; 74:2274–83. [DOI] [PubMed] [Google Scholar]
- 35. Poeppl W, Tobudic S, Lingscheid T, et al. Daptomycin, fosfomycin, or both for treatment of methicillin-resistant Staphylococcus aureus osteomyelitis in an experimental rat model. Antimicrob Agents Chemother 2011; 55:4999–5003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Garcia-de-la-Maria C, Gasch O, Garcia-Gonzalez J, et al. The combination of daptomycin and fosfomycin has synergistic, potent, and rapid bactericidal activity against methicillin-resistant Staphylococcus aureus in a rabbit model of experimental endocarditis. Antimicrob Agents Chemother 2018; 62:e02633-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Miró JM, Entenza JM, Del Río A, et al. ; Hospital Clinic Experimental Endocarditis Study Group . High-dose daptomycin plus fosfomycin is safe and effective in treating methicillin-susceptible and methicillin-resistant Staphylococcus aureus endocarditis. Antimicrob Agents Chemother 2012; 56:4511–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Pujol M, Miro JM, Shaw E, et al. Daptomycin plus fosfomycin versus daptomycin alone for methicillin-resistant Staphylococcus aureus bacteremia and endocarditis. a randomized clinical trial. Clin Infect Dis 2021; 72:1517–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tong SYC, Lye DC, Yahav D, et al. ; Australasian Society for Infectious Diseases Clinical Research Network . Effect of vancomycin or daptomycin with vs without an antistaphylococcal β-lactam on mortality, bacteremia, relapse, or treatment failure in patients with MRSA bacteremia: a randomized clinical trial. JAMA 2020; 323:527–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Gandhi TN, Malani PN. Combination therapy for methicillin-resistant Staphylococcus aureus bacteremia: not ready for prime time. JAMA 2020; 323:515–6. [DOI] [PubMed] [Google Scholar]
- 41. Watkins RR, Deresinski S. Increasing Evidence of the nephrotoxicity of piperacillin/tazobactam and vancomycin combination therapy-what is the clinician to do? Clin Infect Dis 2017; 65:2137–43. [DOI] [PubMed] [Google Scholar]
- 42. Eljaaly K, Alshehri S, Erstad BL. Systematic review and meta-analysis of the safety of antistaphylococcal penicillins compared to cefazolin. Antimicrob Agents Chemother 2018; 62:e01816-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Trinh TD, Zasowski EJ, Lagnf AM, et al. Combination vancomycin/cefazolin (VAN/CFZ) for methicillin-resistant Staphylococcus aureus (MRSA) bloodstream infections (BSI). Open Forum Infect Dis 2017; 4:S281-S. [Google Scholar]
- 44. Zasowski EJ, Trinh TD, Atwan SM, et al. The impact of concomitant empiric cefepime on patient outcomes of methicillin-resistant Staphylococcus aureus bloodstream infections treated with vancomycin. Open Forum Infect Dis 2019; 6:ofz079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Dilworth TJ, Ibrahim O, Hall P, Sliwinski J, Walraven C, Mercier RC. β-lactams enhance vancomycin activity against methicillin-resistant Staphylococcus aureus bacteremia compared to vancomycin alone. Antimicrob Agents Chemother 2014; 58:102–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wolman AT, Gionfriddo MR, Heindel GA, et al. Organic anion transporter 3 interacts selectively with lipophilic β-lactam antibiotics. Drug Metab Dispos 2013; 41:791–800. [DOI] [PubMed] [Google Scholar]
- 47. Sakamoto Y, Yano T, Hanada Y, et al. Vancomycin induces reactive oxygen species-dependent apoptosis via mitochondrial cardiolipin peroxidation in renal tubular epithelial cells. Eur J Pharmacol 2017; 800:48–56. [DOI] [PubMed] [Google Scholar]
- 48. Roger AJ, Muñoz-Gómez SA, Kamikawa R. The origin and diversification of mitochondria. Curr Biol 2017; 27:R1177–92. [DOI] [PubMed] [Google Scholar]
- 49. Volk CF, Burgdorf S, Edwardson G, Nizet V, Sakoulas G, Rose WE. Interleukin (IL)-1β and IL-10 host responses in patients with Staphylococcus aureus bacteremia determined by antimicrobial therapy. Clin Infect Dis 2020; 70:2634–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Rose WE, Eickhoff JC, Shukla SK, et al. Elevated serum interleukin-10 at time of hospital admission is predictive of mortality in patients with Staphylococcus aureus bacteremia. J Infect Dis 2012; 206:1604–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Rose WE, Shukla SK, Berti AD, et al. Increased endovascular Staphylococcus aureus inoculum is the link between elevated serum interleukin 10 concentrations and mortality in patients with bacteremia. Clin Infect Dis 2017; 64:1406–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Guimaraes AO, Cao Y, Hong K, et al. A prognostic model of persistent bacteremia and mortality in complicated Staphylococcus aureus bloodstream infection. Clin Infect Dis 2019; 68:1502–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Minejima E, Bensman J, She RC, et al. A dysregulated balance of proinflammatory and anti-inflammatory host cytokine response early during therapy predicts persistence and mortality in Staphylococcus aureus bacteremia. Crit Care Med 2016; 44:671–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Wozniak JM, Mills RH, Olson J, et al. Mortality risk profiling of Staphylococcus aureus bacteremia by multi-omic serum analysis reveals early predictive and pathogenic signatures. Cell 2020; 182:1311–27 e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ersoy SC, Abdelhady W, Li L, Chambers HF, Xiong YQ, Bayer AS. Bicarbonate resensitization of methicillin-resistant Staphylococcus aureus to beta-lactam antibiotics. Antimicrob Agents Chemother 2019; 63:e00496-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Rose WE, Bienvenida AM, Xiong YQ, Chambers HF, Bayer AS, Ersoy SC. Ability of bicarbonate supplementation to sensitize selected methicillin-resistant Staphylococcus aureus strains to beta-lactam antibiotics in an ex vivo simulated endocardial vegetation model. Antimicrob Agents Chemother 2020; 64:e02072-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Ersoy SC, Otmishi M, Milan VT, et al. Scope and predictive genetic/phenotypic signatures of bicarbonate (NaHCO3) responsiveness and beta-lactam sensitization in methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother 2020; 64:e02445-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Kuehl R, Morata L, Boeing C, et al. ; International Staphylococcus aureus collaboration study group and the ESCMID Study Group for Bloodstream Infections, Endocarditis and Sepsis . Defining persistent Staphylococcus aureus bacteraemia: secondary analysis of a prospective cohort study. Lancet Infect Dis 2020; 20:1409–17. [DOI] [PubMed] [Google Scholar]
- 59. Minejima E, Mai N, Bui N, et al. Defining the breakpoint duration of Staphylococcus aureus bacteremia predictive of poor outcomes. Clin Infect Dis 2020; 70:566–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Souli M, Ruffin F, Choi SH, et al. Changing characteristics of Staphylococcus aureus bacteremia: results from a 21-year, prospective, longitudinal study. Clin Infect Dis 2019; 69:1868–77. [DOI] [PMC free article] [PubMed] [Google Scholar]