Abstract
Transgenerational immune priming is the process of increased resistance to infection in offspring due to parental pathogen exposure. Honey bees (Apis mellifera L. (Hymenoptera: Apidae)) are hosts to multiple pathogens, and this complex immune function could help protect against overwhelming infection. Honey bees have demonstrated transgenerational immune priming for the bacterial pathogen Paenibacillus larvae; however, evidence for viral transgenerational immune priming is lacking across insects in general. Here we test for the presence of transgenerational immune priming in honey bees with Deformed wing virus (DWV) by injecting pupae from DWV-exposed queens and measuring virus titer and immune gene expression. Our data suggest that there is evidence for viral transgenerational immune priming in honey bees, but it is highly context-dependent based on route of maternal exposure and potentially host genetics or epigenetic factors.
Keywords: Deformed wing virus, queen, vertical transmission, Apis mellifera, social immunity
Social behavior and large colony sizes of honey bees (Apis mellifera L. (Hymenoptera: Apidae)) provide an ideal environment to study pathogen survival and defense. Social living encourages disease spread with dense populations of closely related individuals constantly interacting (Cremer et al. 2018). However, social insects have various social, behavioral, and physiological tactics for fighting off disease (Evans and Spivak 2010). Given that Deformed wing virus (DWV) is the most prevalent honey bee virus and has been associated with overwintering colony loss (Tentcheva et al. 2004, Genersch et al. 2010, Dainat et al. 2012, Francis et al. 2013, Traynor et al. 2016), it provides an excellent opportunity to understand how immune responses, such as transgenerational immune priming, may mitigate viral effects. DWV is a quasispecies consisting of multiple master variants that exhibit different levels of prevalence and effects on colony health, with DWV-A being the most prevalent (Di Prisco et al. 2011, Martin et al. 2012, Kevill et al. 2019). However, DWV-B has been increasing in prevalence (Martin et al. 2012, McMahon et al. 2016, Ryabov et al. 2017, Norton et al. 2020). DWV is not only capable of infecting all castes of honey bees at all life stages, but it can also be transmitted horizontally (worker–worker, worker–drone, worker–queen, and drone–queen) and vertically (queen–offspring) (Chen et al. 2006, De Miranda and Fries 2008). The virus has a nearly worldwide distribution largely mirroring the distribution of the virus’s major vector, Varroa destructor Anderson & Trueman (Mesostigmata: Varroidae) (Wilfert et al. 2016). Symptoms of DWV include deformed wings, decreased body size and weight, discoloration of adults, premature pupal death, and a severely reduced adult lifespan (De Miranda and Genersch 2010). DWV is a major threat to honey bees due to its high prevalence and ability to develop lethal symptoms in overt infections (Dainat et al. 2012, Traynor et al. 2016). In addition, sub-lethal effects from covert or asymptomatic, while less understood, are known to exist (e.g., reduced lifespan and impaired foraging) (Benaets et al. 2017).
Given the high prevalence of viral infections in honey bees, a greater understanding of honey bee antiviral defense mechanisms is much needed, particularly those that might be operating at the colony level. Transgenerational immune priming is the process where offspring have increased resistance to infection due to parental immune experience being passed down to their progeny (Little et al. 2003). Despite previous thoughts that insects are not equipped with the immune machinery to have pathogen specificity, and that they are incapable of passing on any type of immune memory, transgenerational immune priming has been shown in some insects (Sadd et al. 2005, Moret 2006, Freitak et al. 2009, Roth et al. 2010, Tidbury et al. 2011, Zanchi et al. 2011, Freitak et al. 2014, Salmela et al. 2015, Mondotte et al. 2020). Although in many cases there has been mixed evidence for and against the existence of this trait due to route of transmission, type of pathogen, life stages tested, host, and pathogen genetics among many other potentially interacting factors (Tetreau et al. 2019). Specifically for honey bees, transgenerational immune priming has been shown for Paenibacillus larvae White (Bacillales: Paenibacillaceae), the causal agent of the brood disease American foulbrood, and for Escherichia coli Migula (Enterobacterales: Enterobacteriaceae) (López et al. 2014, Salmela et al. 2015). López and colleagues (2014), however, noted a context-dependency in the effect of transgenerational immune priming as larvae from some colonies exhibited effects, whereas others did not based on their six experimental colonies.
While there is an increasing body of literature documenting immune priming for bacteria in insects (Sadd et al. 2005, Zanchi et al. 2011, López et al. 2014, Gegner et al. 2019, Cole et al. 2020), the relationship of insect-virus immune priming is not well understood. Previous research has shown conflicting evidence for and against viral immune priming in studies on a DNA virus infecting Plodia interpunctella Hübner (Lepidoptera: Pyralidae) (Tidbury et al. 2011) and RNA viruses infecting Drosophila melanogaster Meigen (Diptera: Drosophilidae) and Aedes aegypti L. (Diptera: Culcidae) (Longdon et al. 2013, Mondotte et al. 2020). One recent study found inconclusive evidence for transgenerational immune priming for Israeli acute paralysis virus in honey bees due to high variability among queens (Amiri et al. 2020). Insect immune systems vary widely between orders; therefore, the ability of honey bees to elicit an antiviral response through transgenerational immune priming should be further explored. The goal of this study was to explore the potential for transgenerational immune priming for DWV-A in honey bees by inoculating queens and then later exposing their pupal offspring. We included different viral transmission routes and the influence of queen source as a preliminary measure of any influence of genetic factors on expression of transgenerational immune priming.
Methods
Insects
Queens were grafted from two Italian source colonies (referred to as QS1 and QS2) and reared following standard protocols (Büchler et al. 2013). Larvae were transferred to a single ‘cell-builder’ as first-instar larvae to generate queens. Therefore, experimental queens were reared in the same environment shortly after egg hatching, reducing the influence of environmental factors on downstream effects. In this way, QS1 and QS2 represent two genotypes in the broadest sense; however, they were not further characterized and epigenetic influences cannot be entirely ruled out. Following emergence, queens were placed in a queenless colony for 1 wk before receiving their treatments via feeding and artificial insemination in May 2018. Artificial inseminations followed modifications of standard protocols (Cobey et al. 2013).
A total of 18 queens were used for this experiment, 9 per queen source. Queens were exposed to the virus through oral and venereal transmission, mimicking natural transmission pathways. All queens were individually pipette fed 5 µl of 50% sugar water solution and artificially inseminated with 5 µl of semen (a homogenized mixture from 1,200 drones) and 1 µl of insemination buffer, a solution made with 400 ml of sterilized water, 5.0 g of NaCl, and 10 g of dihydrostreptomycin sesquisulfate (Cobey et al. 2013). All virus treatments given corresponded to 107 viral copies of DWV, a dose found in Varroa mites infesting asymptomatic pupae (Gisder et al. 2009). Viral inoculum was extracted and quantified with qPCR from DWV symptomatic adult bees that were injected with DWV as pupae following established methods (Simone-Finstrom et al. 2018, Penn et al. 2021). Orally exposed queens received DWV in their feeding solution. Venereally exposed queens received DWV in the insemination buffer. Control queens were fed and inseminated without virus. Approximately 150 white-eyed pupae (~12 d post-egg laying) used for transgenerational immune priming experiments were collected in the first week of October 2018, 5 mo following queen treatment, from each of 18 colonies (3 colonies per treatment for both queen sources) and verified as being uninfested by Varroa. Colonies were maintained in the same location to reduce impacts of potential variation due to environmental factors and were all of similar strength with low natural Varroa infestation.
Pupae treatments consisted of uninjected, injected with 3 µl of sterile 1X PBS, and injected with 3 µl of DWV-A suspended in 1X PBS at a titer level of 107. Pupae were incubated at 34°C and 80% relative humidity until emergence, and mortality was recorded daily (de Miranda et al. 2013). At 3 d post-treatment, a subset of pupae were stored at −80°C for molecular analysis.
Viral and Gene Expression
RNA was extracted from three individual pupae per colony per treatment using Promega Maxwell simplyRNA Tissue kits, and cDNA synthesis was performed with Qiagen QuantiTect Reverse Transcription kits. Pupae were analyzed using qPCR to quantify DWV-A, DWV-B, dicer-like (XM_006571316.1), relish, and the reference gene Ndufa38 using established protocols (Supp Table S1 [online only]; Cameron et al. 2013). Dicer-like is highly involved in antiviral responses (Kingsolver et al. 2013, Brutscher et al. 2017) and relish has been identified as a candidate marker for transgenerational immune priming in termites (Cole et al. 2020). DWV-A and DWV-B quantification using plasmid standards was performed on the Applied Biosystems QuantStudio 6 Flex Real-Time PCR System with the PowerUpTM SYBR Green PCR Master Mix. Relative quantification of dicer-like and relish in relation to Ndufa38 as performed on the BioRad CFX Connect using BioRad SsoAdvancedTM Universal SYBR Green Supermix. Ndufa38 expression was stable across queen and pupal treatment groups (F4,155 = 0.626, P = 0.64).
Statistical Analysis
Analyses were carried out to determine if queen source, virus treatments for queens, and/or virus treatments for pupae had significant impact on the level of wing deformity, days to emergence, virus presence and titer for DWV-A and DWV-B, and relative quantification of dicer-like and relish. Relative quantification of dicer-like and relish was determined by calculating the fold change in their expression using the 2−ΔΔCt method (Livak and Schmittgen 2001).
Percent mortality, days to emergence, log transformed viral titers, and log transformed 2−ΔΔCt were analyzed by three-way ANOVA using least squares means model with queen source, queen viral treatment, and pupal viral treatment as fixed effects and colony as a random effect. Post hoc analyses consisted of least squares means differences with Tukey HSD. Wing deformity, detection of DWV-A, and detection of DWV-B were analyzed by logistic regression using general linear models with binomial distribution. Post hoc analyses of wing deformity, DWV-A, and DWV-B consisted of categorical response analysis and response homogeneity tests with Pearson’s chi-square and Fisher’s exact. All statistical analyses were performed in JMP Pro 14.
Results and Discussion
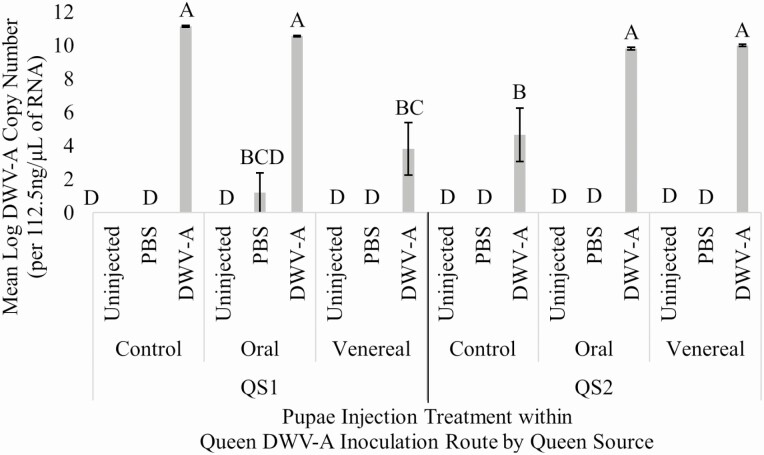
Queen sources differed in their expression of transgenerational immune priming based on the route of viral exposure, suggesting that there is a strong context-dependent component to viral transgenerational immune priming in honey bees. Queen source refers to the maternal origin of queens that were differentially exposed to virus and subsequently used to rear pupae for this experiment, and so represents two distinct queen genotypes, broadly speaking, in all tests. Given that all queens were inseminated with the same homogenate of semen from 1,200 drones and that they were reared in the same environment (post-egg hatching), it is suggestive that the maternally derived genes are more likely driving the differences seen between the two queen sourcesthan epistatic or epigenetic effects. Our data also show that for the QS1 queen source, the evidence for transgenerational immune priming was based on route of viral exposure, where venereal inoculation of queens led to more DWV-A resistance in offspring compared to queens that were orally inoculated (Fig. 1). Virus titer of DWV-A was significantly affected by an interaction between queen source, queen DWV-A exposure route, and pupal injection treatment (F4,144 = 18.855, P < 0.0001; Fig. 1). DWV-A titer in QS1 was lower in DWV-A injected pupae from venereally exposed queens compared to DWV-A injected pupae from non-exposed and orally exposed queens. However, this was not observed in QS2 with offspring of primed queens actually having increased DWV-A with respect to controls. Previous research has shown evidence for variation in transgenerational immune priming between different colonies, further supporting a possible genetic basis for variation of expression for this trait (López et al. 2014). Although interesting, this is not wholly unexpected as genetic variation and epistasis can influence the development of virus symptoms, virus titer, and antiviral response (Kulinčević and Rothenbuhler 1975, Rinderer et al. 1975, Boncristiani et al. 2013, Khongphinitbunjong et al. 2015, Penn et al. 2021, Weaver et al. 2021).
Fig. 1.
Mean log DWV-A copy number (per 112.5 ng/μl RNA) for pupae injections within queen DWV-A exposure routes by queen source n = 162 (9 individuals per bar). Bars accompanied by the same letter represent non-significance (P > 0.05; Tukey HSD). Error bars represent one standard error from the mean.
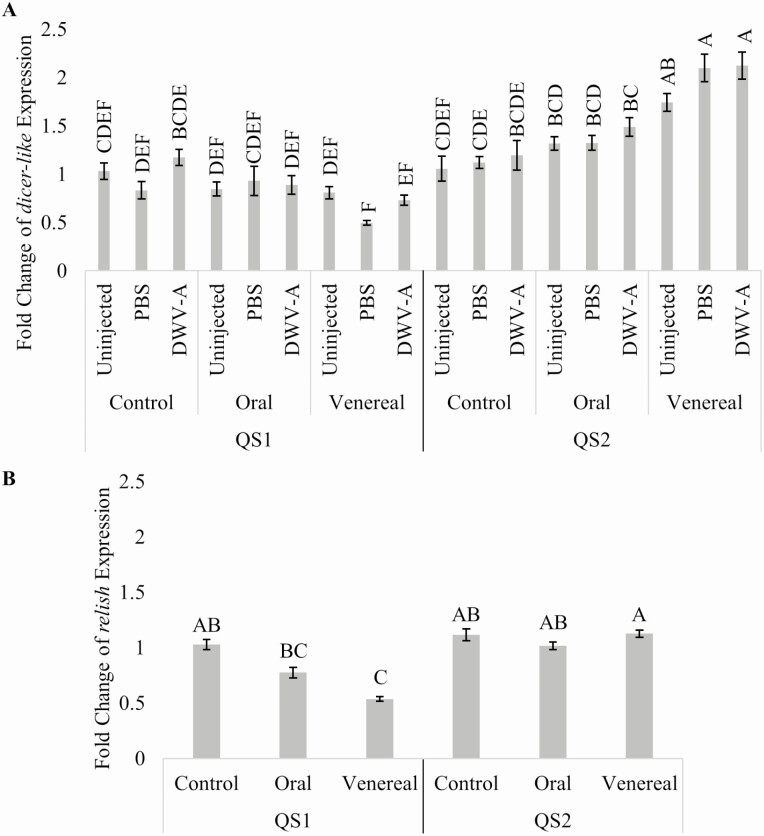
Within the same pupae from QS1 venereally inoculated queens, we also found significantly lower fold change expression of dicer-like and relish, two genes involved in the honey bee immune pathways, RNAi and Imd, respectively. The relative quantity of dicer-like was significantly affected by interactions between queen source, queen DWV-A exposure route, and pupal injection treatment (F4,144 = 2.479, P = 0.0471; Fig. 2A). QS2 pupae from venereally exposed queens had higher fold change of dicer-like expression compared with QS1 pupae and QS2 pupae from non-exposed and orally exposed queens. QS1 injected pupae from venereally inoculated queens had lower fold change of dicer-like expression compared with all groups within QS2, except for uninjected pupae from non-exposed queens. The relative quantity of relish was significantly affected by an interaction between queen source and queen DWV-A exposure route (F2,156 = 6.105, P = 0.0148; Fig. 2B), where pupae from QS1 venereally exposed queens had significantly lower fold change of relish than uninjected QS1 pupae and pupae from QS2 queens. Two previous studies have assessed regulation of dicer-like in response to viral infections, the first study by Ryabov et al. (2014) found no change in dicer-like expression in pupae exposed to Varroa mites and high DWV levels, and the second by Galbraith et al. (2015) found upregulation of dicer-like in adult workers fed virus extracts from bees infected with IAPV, DWV, BQCV, KBV, and SBV.
Fig. 2.
Antiviral response to pupal injection with DWV-A from two genotypes, QS1 and QS2, where queens were exposed to DWV-A orally and venereally. (A) Fold change relative quantity of dicer-like where pupal injection treatments were significant, n = 162 (9 individuals per bar). Overall, venereally exposed queens from QS2 had upregulated dicer-like expression, whereas those from QS1 had reduced expression, tracking their DWV-A levels in Fig. 1. (B) Fold change relative quantity of relish where queen exposure was significant n = 162 (27 individuals per bar). Bars accompanied by the same letter represent non-significance (P > 0.05; Tukey HSD). Error bars represent one standard error from the mean.
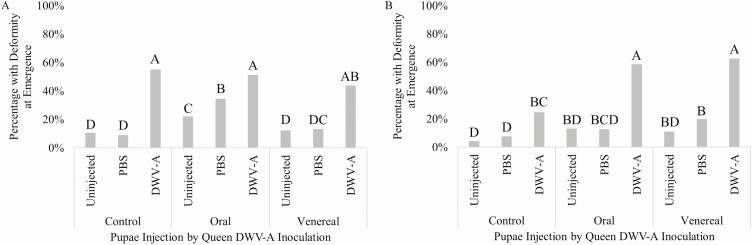
The influence of queen source was also observed in the development of DWV symptoms where wing deformity was significantly affected by an interaction between queen source, queen DWV-A exposure route, and pupal injection treatment (χ 2 = 13.968, df = 2, P = 0.0074; Fig. 3). Wing deformity symptoms in pupae injected with DWV-A exhibited different patterns between the two queen sources in relation to the DWV-A exposure route to queens. For QS1, pupal injection with DWV-A led to increased percentage of pupae developing wing deformities regardless of queen exposure (Fig. 3A). However, for QS2, pupae reared from queens exposed to DWV-A, orally or venereally, had significantly more symptom development compared to pupae reared from non-exposed queens (Fig. 3B). We also found an interaction between queen source, queen DWV-A exposure route, and pupal injection with DWV-A reducing emergence time (F4,2030 = 14.666, P < 0.0001; Supp Fig. S1 [online only]) but not mortality (F4,2072 = 0.729, P = 0.5785; Supp Fig. S2 [online only]).
Fig. 3.
Percentage of pupae that emerged with wing deformities for each pupae injection treatment within each queen DWV-A exposure route for (A) QS1 n = 9 colonies (an average of 122 individuals ± 16 per colony; an average of 122 individuals per bar), and (B) QS2 n = 9 colonies (an average of 106 individuals ± 34 per colony; an average of 106 individuals per bar). Bars accompanied by the same letter represent nonsignificance (P > 0.05; Fisher’s exact).
Conclusions
In summary, there is evidence of context-dependent viral transgenerational immune priming in honey bees. Based on our results, it appears that viral transgenerational immune priming may be dependent on the route of parental exposure and that host genetics, epistatic effects, or epigenetics may also influence expression of this phenomenon. Given that the queen treatment and pupal experiment were done 5 mo apart, it is possible that some effects or the strength of noted effects waned over time. Queens in this experiment were exposed to DWV-A on a single occasion; multiple exposures to the virus may have different effects, especially in regard to oral transmission since the queen is fed by workers which allows for oral transmission of pathogens multiple times over the lifespan of the queen. Given the high prevalence of DWV (Martin et al. 2012, Traynor et al. 2016, Kevill et al. 2019), queens may have been previously exposed to DWV during their own development. However, that exposure would have been similar at least within queens from the same queen source as eggs and first-instar larvae and for all queens during queen rearing. The more acute exposures presented at the time of insemination appear to be the most influential in the current study. Clearly, given the idiosyncratic nature of the development of this trait, timing, number, and route of exposure, along with duration of any effects, needs to be the subject of subsequent research. This context-dependency of transgenerational immune priming was also recently documented in Tenebrio molitor L. (Coleoptera: Tenebrionidae), where the mother’s size along with differential investment in her own immunity versus that transferred to her eggs influenced expression of priming (Moreau et al. 2012).
Further research must also be conducted to determine heritability of this potential trait and if it could in turn be selectively bred to produce DWV resistant genotypes. The potential mechanism for viral transgenerational immune priming is unknown. The phospholipoglycoprotein vitellogenin is associated with bacterial transgenerational immune priming in honey bees; vitellogenin is believed to have rapidly evolved in honey bees which may allow for different vitellogenin variants that are able to bind to a wide array of pathogens (Kent et al. 2011, Salmela et al. 2015). Although the vitellogenin pathogen binding pattern is predominantly a gram-positive bacteria signature (Salmela et al. 2015), its role in transgenerational immune priming outside of bacterial challenges should be investigated. Additionally it is possible that transgenerational immune priming may be more effective for orally transmitted pathogens (e.g. bacteria) and less so for those that are transmitted primarily via injection (e.g., Varroa vectored viruses). Future studies should further test for viral transgenerational immune priming with more and fully sequenced queen genotypes to determine how regularly it is observed, if it is seen only for specific viruses, and how beneficial it could be. Although DWV-A is capable of multiple strategies for survival and transmission, viral transgenerational immune priming could be a defense against DWV and reduce colony loss.
Supplementary Material
Acknowledgments
This work was supported by funding from United States Department of Agriculture-National Institute of Food & Agriculture grant 2017-69004-26515 awarded to KH and MSF and the United States Department of Agriculture, Agricultural Research Service research plan 6050-21000-014-00D. We thank Garrett Dodds, Phil Tokarz, Hannah Penn, and Christopher Fellows, for key contributions to the completion of this study. Mention of trade names or commercial products in this publication is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the U.S. Department of Agriculture. U.S. Department of Agriculture is an equal opportunity provider and employer. Samples were collected and treated according to standard protocols, minimizing distress of individual bees. Ethics committee approval was not required.
References Cited
- Amiri, E., Strand M. K., Tarpy D. R., and Rueppell O.. . 2020. Honey bee queens and virus infections. Viruses 12: 322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benaets, K., Van Geystelen A., Cardoen D., De Smet L., de Graaf D. C., Schoofs L., Larmuseau M. H., Brettell L. E., Martin S. J., and Wenseleers T.. . 2017. Covert deformed wing virus infections have long-term deleterious effects on honeybee foraging and survival. Proc. R. Soc. B. 284: 20162149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boncristiani, H. F., Evans J. D., Chen Y., Pettis J., Murphy C., Lopez D. L., Simone-Finstrom M., Strand M., Tarpy D. R., and Rueppell O.. . 2013. In vitro infection of pupae with Israeli acute paralysis virus suggests disturbance of transcriptional homeostasis in honey bees (Apis mellifera). PLoS One 8: e73429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brutscher, L. M., Daughenbaugh K. F., and Flenniken M. L.. . 2017. Virus and dsRNA-triggered transcriptional responses reveal key components of honey bee antiviral defense. Sci. Rep. 7: 6448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Büchler, R., Andonov S., Bienefeld K., Costa C., Hatjina F., Kezic N., Kryger P., Spivak M., Uzunov A., and Wilde J.. . 2013. Standard methods for rearing and selection of Apis mellifera queens. J. Apic. Res. 52: 1-30. [Google Scholar]
- Cameron, R. C., Duncan E. J., and Dearden P. K.. . 2013. Stable reference genes for the measurement of transcript abundance during larval caste development in the honeybee. Apidologie 44: 357-366. [Google Scholar]
- Chen, Y., Evans J., and Feldlaufer M.. . 2006. Horizontal and vertical transmission of viruses in the honey bee, Apis mellifera. J. Invertebr. Pathol. 92: 152-159. [DOI] [PubMed] [Google Scholar]
- Cobey, S. W., Tarpy D. R., and Woyke J.. . 2013. Standard methods for instrumental insemination of Apis mellifera queens. J. Apic. Res. 52: 1-18. [Google Scholar]
- Cole, E. L., Empringham J. S., Biro C., Thompson G. J., and Rosengaus R. B.. . 2020. Relish as a candidate marker for transgenerational immune priming in a dampwood termite (Blattodae: Archeotermopsidae). Insects 11: 149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cremer, S., Pull C. D., and Fürst M. A.. . 2018. Social immunity: emergence and evolution of colony-level disease protection. Annu. Rev. Entomol. 63: 105–123. [DOI] [PubMed] [Google Scholar]
- Dainat, B., Evans J. D., Chen Y. P., Gauthier L., and Neumann P.. . 2012. Dead or alive: deformed wing virus and Varroa destructor reduce the life span of winter honeybees. Appl. Environ. Microbiol. 78: 981-987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Miranda, J., and Fries I.. . 2008. Venereal and vertical transmission of deformed wing virus in honeybees (Apis mellifera L.). J. Invertebr. Pathol. 98: 184-189. [DOI] [PubMed] [Google Scholar]
- De Miranda, J. R., and Genersch E.. . 2010. Deformed wing virus. J. Invertebr. Pathol. 103: S48-S61. [DOI] [PubMed] [Google Scholar]
- de Miranda, J. R., Bailey L., Ball B. V., Blanchard P., Budge G. E., Chejanovsky N., Chen Y.-P., Gauthier L., Genersch E., and De Graaf D. C.. . 2013. Standard methods for virus research in Apis mellifera. J. Apic. Res. 52: 1-56. [Google Scholar]
- Di Prisco, G., Zhang X., Pennacchio F., Caprio E., Li J., Evans J. D., DeGrandi-Hoffman G., Hamilton M., and Chen Y. P.. . 2011. Dynamics of persistent and acute deformed wing virus infections in honey bees, Apis mellifera. Viruses 3: 2425-2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans, J. D., and Spivak M.. . 2010. Socialized medicine: individual and communal disease barriers in honey bees. J. Invertebr. Pathol. 103: S62-S72. [DOI] [PubMed] [Google Scholar]
- Francis, R. M., Nielsen S. L., and Kryger P.. . 2013. Varroa-virus interaction in collapsing honey bee colonies. PLoS One 8: e57540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freitak, D., Heckel D. G., and Vogel H.. . 2009. Dietary-dependent trans-generational immune priming in an insect herbivore. Proc. R. Soc. Lond. B: Biol. Sci. 276: 2617-2624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freitak, D., Schmidtberg H., Dickel F., Lochnit G., Vogel H., and Vilcinskas A.. . 2014. The maternal transfer of bacteria can mediate trans-generational immune priming in insects. Virulence 5: 547-554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galbraith, D. A., Yang X., Nino E. L., Yi S., and Grozinger C.. . 2015. Parallel epigenomic and transcriptomic responses to viral infection in honey bees (Apis mellifera). PLoS Pathog. 11: e1004713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gegner, J., Baudach A., Mukherjee K., Halitschke R., Vogel H., and Vilcinskas A.. . 2019. Epigenetic mechanisms are involved in sex-specific trans-generational immune priming in the lepidopteran model host Manduca sexta. Front. Physiol. 10: 137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genersch, E., Von Der Ohe W., Kaatz H., Schroeder A., Otten C., Büchler R., Berg S., Ritter W., Mühlen W., and Gisder S.. . 2010. The German bee monitoring project: a long term study to understand periodically high winter losses of honey bee colonies. Apidologie 41: 332-352. [Google Scholar]
- Gisder, S., Aumeier P., and Genersch E.. . 2009. Deformed wing virus: replication and viral load in mites (Varroa destructor). J. Gen. Virol. 90: 463-467. [DOI] [PubMed] [Google Scholar]
- Kent, C. F., Issa A., Bunting A. C., and Zayed A.. . 2011. Adaptive evolution of a key gene affecting queen and worker traits in the honey bee, Apis mellifera. Mol. Ecol. 20: 5226-5235. [DOI] [PubMed] [Google Scholar]
- Kevill, J. L., de Souza F. S., Sharples C., Oliver R., Schroeder D. C., and Martin S. J.. . 2019. DWV-A lethal to honey bees (Apis mellifera): a colony level survey of DWV variants (A, B, and C) in England, Wales, and 32 states across the US. Viruses 11: 426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khongphinitbunjong, K., de Guzman L. I., Tarver M. R., Rinderer T. E., Chen Y., and Chantawannakul P.. . 2015. Differential viral levels and immune gene expression in three stocks of Apis mellifera induced by different numbers of Varroa destructor. J. Insect Physiol. 72: 28-34. [DOI] [PubMed] [Google Scholar]
- Kingsolver, M. B., Huang Z., and Hardy R. W.. . 2013. Insect antiviral innate immunity: pathways, effectors, and connections. J. Mol. Biol. 425: 4921-4936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulinčević, J. M., and Rothenbuhler W. C.. . 1975. Selection for resistance and susceptibility to hairless-black syndrome in the honeybee. J. Invertebr. Pathol. 25: 289-295. [DOI] [PubMed] [Google Scholar]
- Little, T. J., O’Connor B., Colegrave N., Watt K., and Read A. F.. . 2003. Maternal transfer of strain-specific immunity in an invertebrate. Curr. Biol. 13: 489-492. [DOI] [PubMed] [Google Scholar]
- Livak, K. J., and Schmittgen T. D.. . 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. Methods 25: 402-408. [DOI] [PubMed] [Google Scholar]
- Longdon, B., Cao C., Martinez J., and Jiggins F. M.. . 2013. Previous exposure to an RNA virus does not protect against subsequent infection in Drosophila melanogaster. PLoS One 8: e73833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López, J. H., Schuehly W., Crailsheim K., and Riessberger-Gallé U.. . 2014. Trans-generational immune priming in honeybees. Proce. R. Soc. B. 281: 20140454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin, S. J., Highfield A. C., Brettell L., Villalobos E. M., Budge G. E., Powell M., Nikaido S., and Schroeder D. C.. . 2012. Global honey bee viral landscape altered by a parasitic mite. Science 336: 1304-1306. [DOI] [PubMed] [Google Scholar]
- McMahon, D. P., Natsopoulou M. E., Doublet V., Fürst M., Weging S., Brown M. J., Gogol-Döring A., and Paxton R. J.. . 2016. Elevated virulence of an emerging viral genotype as a driver of honeybee loss. Proc. R. Soc. B: Biol. Sci. 283: 20160811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondotte, J. A., Gausson V., Frangeul L., Yasutsugu S., Vazeille M., Mongelli V., Blanc H., Faillous A., and Saleh M.. . 2020. Evidence for long-lasting transgenerational antiviral immunity in insects. Cell Reports 33(11): 108506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreau, J., Martinaud G., Troussard J. P., Zanchi C., and Moret Y.. . 2012. Trans-generational immune priming is constrained by the maternal immune response in an insect. Oikos 121(11): 1828-1832. [Google Scholar]
- Moret, Y. 2006. ‘Trans-generational immune priming’: specific enhancement of the antimicrobial immune response in the mealworm beetle, Tenebrio molitor. Proc. R. Soc. Lond. B: Biol. Sci. 273: 1399-1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton, A. M., Remnant E. J., Buchmann G., and Beekman M.. . 2020. Accumulation and competition amongst deformed wing virus genotypes in naïve Australian honeybees provides insight into the increasing global prevalence of genotype B. Front. Microbiol. 11: 620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penn H. J., Simone-Finstrom M., Lang S., Chen J., and Healy K.. . 2021. Host genotype and tissue type determine DWV infection intensity. Front. Insect Sci. 1: 756690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinderer, T. E., Rothenbuhler W. C., and Kulinčević J. M.. . 1975. Responses of three genetically different stocks of the honeybee to a virus from bees with hairless-black syndrome. J. Invertebr. Pathol. 25: 297-300. [DOI] [PubMed] [Google Scholar]
- Roth, O., Joop G., Eggert H., Hilbert J., Daniel J., Schmid‐Hempel P., and Kurtz J.. . 2010. Paternally derived immune priming for offspring in the red flour beetle, Tribolium castaneum. J. Anim. Ecol. 79: 403-413. [DOI] [PubMed] [Google Scholar]
- Ryabov, E. V., Childers A. K., Chen Y., Madella S., Nessa A., and Evans J. D.. . 2017. Recent spread of Varroa destructor virus-1, a honey bee pathogen, in the United States. Sci. Rep. 7: 17447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryabov, E. V., Wood G. R., Fannon J. M., Moore J. D., Bull J. C., Chandler D., Mead A., Burroughs N., and Evans D. J.. . 2014. A virulent strain of deformed wing virus (DWV) of honeybees (Apis mellifera) prevails after Varroa destructor-mediated, or in vitro, transmission. PLoS Pathogens 10: e1004230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadd, B. M., Kleinlogel Y., Schmid-Hempel R., and Schmid-Hempel P.. . 2005. Trans-generational immune priming in a social insect. Biol. Lett. 1: 386-388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmela, H., Amdam G. V., and Freitak D.. . 2015. Transfer of immunity from mother to offspring is mediated via egg-yolk protein vitellogenin. PLoS Pathogens 11: e1005015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simone-Finstrom, M., Aronstein K., Goblirsch M., Rinkevich F., and De Guzman L.. . 2018. Gamma irradiation inactivates honey bee fungal, microsporidian, and viral pathogens and parasites. J. Invertebr. Pathol. 153: 57-64. [DOI] [PubMed] [Google Scholar]
- Tentcheva, D., Gauthier L., Zappulla N., Dainat B., Cousserans F., Colin M. E., and Bergoin M.. . 2004. Prevalence and seasonal variations of six bee viruses in Apis mellifera L. and Varroa destructor mite populations in France. Appl. Environ. Microbiol. 70: 7185-7191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tetreau, G., Dhinaut J., Gourbal B., and Moret Y.. . 2019. Trans-generational immune priming in invertebrates: current knowledge and future prospects. Front. Immunol. 10: 1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tidbury, H. J., Pedersen A. B., and Boots M.. . 2011. Within and transgenerational immune priming in an insect to a DNA virus. Proc. R. Soc. B: Biol. Sci. 278: 871-876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traynor, K. S., Rennich K., Forsgren E., Rose R., Pettis J., Kunkel G., Madella S., Evans J., and Lopez D.. . 2016. Multiyear survey targeting disease incidence in US honey bees. Apidologie 47: 325-347. [Google Scholar]
- Weaver, D. B., Cantarel B. L., Elsik C., Lopez D. L., and Evans J.. . 2021. Multi-tiered analyses of honey bees that resist or succumb to parasitic mites and viruses. BMC Genomics 22: 720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilfert, L., Long G., Leggett H., Schmid-Hempel P., Butlin R., Martin S., and Boots M.. . 2016. Deformed wing virus is a recent global epidemic in honeybees driven by Varroa mites. Science 351: 594-597. [DOI] [PubMed] [Google Scholar]
- Zanchi, C., Troussard J. P., Martinaud G., Moreau J., and Moret Y.. . 2011. Differential expression and costs between maternally and paternally derived immune priming for offspring in an insect. J. Anim. Ecol. 80: 1174-1183. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.