Abstract
Microsatellite stable /microsatellite instable-low is the most common colorectal cancer genotype, counting for approximately 85% of common colorectal cancer patients. Treatment of advanced microsatellite stable/microsatellite instable-low colorectal cancer is difficult and successful pharmacological treatment options are currently lacking. Here, we report a case of a 37-year-old man with advanced colorectal cancer genotyping microsatellite stable/microsatellite instable-low with a Kirsten rat sarcoma viral oncogene (G12V) mutation. Following palliative surgery, the patient did not response to the common recommended chemotherapy FOLFIRI regimen and other chemotherapy options. Finally, the patient was successfully treated using a unique combinational immunotherapy, using nivolumab plus ipilimumab combined with regorafenib and irinotecan. Significant improvement in the Karnofsky Performance Status scores, liver function and well-being, reduction in serum tumor biomarkers, and reduction in the size of multiple liver metastatic tumors was evident. This report provides a rare case in which a unique and effective combinational immunotherapy for refractory advanced colon cancer patients is discussed. It encourages further research into combined immunotherapy for immuno-insensitive colon cancer patients.
Keywords: Microsatellite stable/microsatellite instable-low colorectal cancer, nivolumab, ipilimumab, regorafenib, irinotecan
Introduction
Colorectal cancer (CRC) is a common malignancy that ranks third in all cancers in terms of morbidity and mortality, accounting for more than 9% of all cancer incidence. 1 Microsatellite stable (MSS)/microsatellite instable-low (MSI-L) is the most common CRC genotype, approximately 85% of CRC patients are MSS/MSI-L. 2 Previous studies have shown that MSS/MSI-L CRC is resistant to existing immunological checkpoint inhibitors. 3 Therefore, understanding how to overcome drug resistance and improve tumor sensitivity, especially resistance to immunotherapies, has important clinical implications.
Immunological checkpoint inhibitor therapies have shown promising efficacy in the treatment of a variety of malignancies. Regorafenib is a multi-target inhibitor that not only inhibits vascular endothelial growth factor receptor (VEGFR) and platelet-derived growth factor receptor (PDGFR), but also has inhibitory effects on targets such as v-raf murine sarcoma viral oncogene homolog B (BRAF) protein.4–6 Irinotecan is an analogue of camptothecin, which has extensive anti-tumor activity in vitro and in vivo, and plays a pivotal role in the treatment of metastatic CRC. 7 Although a number of immunotherapy-based therapeutic methods for the treatment of CRC have recently become available, the therapeutic effect of these methods on MSS CRC is not optimistic and effective treatment is still lacking.3,8,9 Here, we report a successful case of combinational immunotherapy for MSS/MSI-L typed advanced colon cancer using two immune checkpoint inhibitors, nivolumab plus ipilimumab, combined with regorafenib and irinotecan.
Case presentation
At the end of May 2019, a male patient, aged 37, presented with right lower quadrant pain, decongested with thin stools. Positron emission tomography/computed tomography (PET/CT) results showed upper colon cancer with multiple lymph nodes, pelvic peritoneum, liver and bone multiple metastases, and incomplete intestinal obstruction (Figure 1). On 20 June 2019, right hepatic colon resection surgery plus liver tumor biopsies were performed. The postoperative pathology confirmed that the patient had colon adenocarcinoma with multiple lymph nodes, pelvic peritoneum, and liver and bone multiple metastasis T4N2M1 phase.
Figure 1.
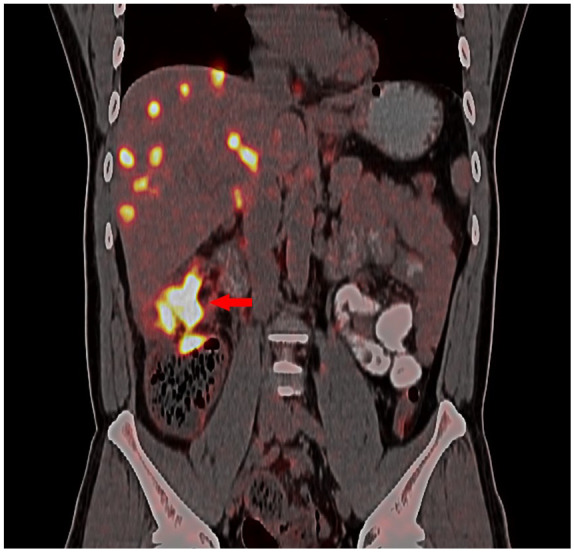
PET/CT scan of the abdomen. The scan was performed at admission, before treatment, showing ascending colon cancer with incomplete intestinal obstruction and multiple liver metastatic tumors.
Following the above palliative surgery, chemotherapy with FOLFIRI regimen (irinotecan 240 mg d1 + 5-fluorouracil 500 mg d1 and 3500 mg ci 46 h + calcium tetrahydrofolate 600 mg d1) was commenced on 9 July 2019. Approximately 2 weeks later (22 July 2019), the patient developed pain in the right upper quadrant. Serum tumor biomarkers were significantly increased (carcino-embryonic antigen (CEA), 44.56 → 219 ng/mL, cancer antigen (CA)-199, 3756 → 14,130 U/mL, and CA724, 35.73 → 119.1 U/mL) and liver function showed deterioration (alanine aminotransferase (ALT) 464 U/L, aspartate aminotransferase (AST) 206 U/L, total bilirubin (TBIL) 0.36 mg/dL, direct bilirubin (DB) 0.23 mg/dL, and indirect bilirubin (IB) 0.13 mg/dL). On 23 July 2019, abdominal magnetic resonance imaging (MRI) scans with enhancement results indicated multiple metastases in the liver, multiple lymph node metastases in the abdomen, pelvis and retroperitoneum, osseous thoracic, lumbar vertebrae, upper femoral and pelvic multiple metastases, and left upper abdomen small intestine local incomplete obstruction. Compared with the PET/CT results, the tumor was clearly progressive. The following day (24 July 2019), the patient exhibited mild yellow staining of the skin and sclera. The liver function was checked again, TBIL (0.36 → 1.99 mg/dL), DB (0.23 → 1.51 mg/dL), and IB (0.13 → 0.49 umol/L) were significantly elevated.
Gene sequencing data confirmed that the patient was MSS/MSI-L, with the presence of Kirsten rat sarcoma viral oncogene (KRAS) (G12V) mutation, BRAF gene (−). Tumor mutation burden (TMB) was 13/Mb. Gene sequencing was performed by next generation sequencing (NGS). Detailed methods can be found in Supplementary Material A. Since the immunohistochemistry results showed negative programmed cell death-ligand 1 (PD-L1) (<1%), (tested on 24 July 2019) treatment regimen changed to oxaliplatin 100 mg d1 + nivolumab 200 mg d1 + regofenib 120 mg, once a day. On completion of the regimen, symptoms of the right upper quadrant pain did not improve, and jaundice progressively worsened. On August 5, a review of tumor biomarkers (CEA, 219 → 266 ng/mL; CA-199, 14,130 → 20,181 U/mL; CA724, 119.1 → 146.0 U/mL) showed that the tumor was progressive. Therefore, the treatment regimen was changed to “Nivolumab 200 mg d1 + ipilimumab 50 mg d1 + irinotecan 280 mg d2 + regomafen 120 mg, once daily.”
Two days after treatment with the new regimen, the symptoms of right lower quadrant pain were relieved, and jaundice subsided gradually. Four weeks later (4 September 2019), the liver function was found to be significantly improved (ALT 110 U/L, AST 98 U/L, TBIL 0.48 mg/dL, DB 0.39 mg/dL, and IB 0.09 mg/dL) (Figure 2). The level of tumor biomarkers also significantly reduced (CEA 39.43 ng/mL, CA-199 8246 U/mL, and CA724 16.78 U/mL, as shown in Figure 3). Imaging results confirmed that the size of intrahepatic multiple metastases and abdominal pelvic and retroperitoneal lymph node metastases became smaller compared with the previous MRI scanning results. The overall improvement indicated that a partially response (PR) was achieved in this patient (Figure 3), the patient showing well-tolerance to the combinational treatment. After adding regofenib to the treatment regime, there was a skin reaction in the hands and feet, which was characterized by palm-foot erythema with pain. This was the only observed adverse reaction during the treatment, which is a grade 2 adverse event according to the guidelines of Common Terminology Criteria for Adverse Events (CTC AE) 5.0. After symptomatic management, the rash and pain subsided quickly, and the patient was discharged.
Figure 2.

Changes in tumor biomarkers and liver function following treatment with different regimens. (a) Serum biomarkers. Data from the time of admission were used as a baseline (0). All three markers (CEA: carcino-embryonic antigen, CA-199: cancer antigen 199, CA-724: cancer antigen 724) continued to rise despite surgery and FOLFIRI chemotherapy. As a result, the treatment regimen was changed to a combination of “Nivolumab + regomafenil + oxali” on July 24. The serum level of all three markers continued to increased further. However, all five markers’ level declined significantly after switching to a new combination therapy of “Nivolumab + ipilimumab + irinotecan + regorafenib” on 7 August 2019. The levels of CEA and CA-724 reduced to baseline within 3–4 weeks. (b) Levels of bilirubin and ALT and AST. FOLFIRI chemotherapy did not show any effect in controlling the rise in bilirubin. The treatment regimen changed on July 24 was ineffective. However, the bilirubin level declined sharply after switching to the “Nivolumab + ipilimumab + irinotecan + regorafenib” regimen on 7 August 2019.
Figure 3.
Comparison of abdominal MRI images before (a) and after (b) effective treatment. Images in panel (a) were taken on 23 July 2019 while panel (b) images were from scanning on 4 September 2019, 4 weeks after the reported combinational therapy. A significant reduction in all multiple liver metastatic tumor masses was observed.
Discussion
Currently, genotyping MSS/MSI-L with KRAS gene mutations in advanced CRCs are treated by surgery, radiotherapy, chemotherapy, and immunotherapy. For most of the patients with advanced metastatic CRC, radical surgery is no longer an option. A small number of patients can only be treated with palliative surgery. Therefore, the first-line therapies for MSS/MSI-L with KRAS mutations advanced CRC are still based on chemotherapy or chemotherapy plus targeted therapy. The available options include FOLFIRI, FOLFOX, or FOLFOXIRI ± bevacizumab. 10 The efficacy of FOLFIRI (irinotecan + calcium folic acid + fluorouracil) chemotherapy for MSS/MSI-L advanced CRC is between 39% and 56%. 11 Patients with KRAS mutation have a poorer clinical outcome. 11 FOLFOX and FOLFIRI shared similar safety and efficacy. 12 In addition, in a multicenter phase II clinical trial of advanced CRC using bevacizumab combined with FOLFIRI versus FORFIRI, the median progressive free survival (PFS) was found to be 8.5 months in the combination group and 5.1 months in the FOLFIRI group. The median overall survival (OS) between the two groups was also statistically significant (15.2 months vs 11.3 months, p < 0.01). 13 Bevacizumab combined with FOLFIRI was considered a more feasible treatment option, but the disadvantages include development of tumor resistance and adverse effect. Although immunotherapy shows promising clinical outcomes in the treatment of a variety of cancer types, immune checkpoint inhibitors when used as monotherapy is almost ineffective for patients with MSS/MSI-L CRC. The use of immune checkpoint inhibitors alone was shown to be effective for mismatch repair deficient dMMR-microsatellite instable-high (MSI-H) CRC, but not for mismatch repair pMMR-MSI-L CRC.14,15 In addition, efforts to combine immunotherapy with chemotherapy, antiangiogenic therapy, and MEK inhibitors failed.16,17
Use of dual immunological checkpoint inhibitors may be a feasible alternative approach in the management of MSS/MSI-L type CRC. In a large-scale phase II clinical study (CCTG CO.26) using a PD-L1 monoclonal antibody, durvalumab, combined with a CTLA-4 monoclonal antibody, tremelimumab, to treat 180 patients with refractory CRC (98 were MSS/MSI-L type), the findings showed that the OS was prolonged from 4.5 months in the control group to 6.9 months in the study group (p < 0.01). 18 The observation that the study endpoints reached a statistically different was encouraging; however, the main criticisms of the study included, the prolongation of OS (2.4 months) is a relatively brief reprieve, the objective response rate (ORR) in this study is relatively low, and there is no prolongation of PFS. The overall data suggest that the efficacy of durvalumab combined with tremelimumab in the treatment of patients with MSS/MSI-L is limited. Nevertheless, the study did suggest that combination therapy based on immunotherapy is a potentially feasible treatment option.
There is limited information on successful combinational therapy for MSS/MSI-L CRC. Here, we report that a patient with MSS/MSI-L advanced colon cancer responded well to the unique combinational therapy “Nivolumab + ipilimumab + regofenib + irinotecan” quadruple regimen. It is a successful case of turning the MSS/MSI-L CRC from a “cold tumor” to a “hot tumor” enabling the tumor sensitive to immunotherapy.
In this case report, the patient’s cancer genotyping was MSS/MSI-L with the presence of a KRAS (G12V) mutation, BRAF gene (−), which is considered as an immunologically cold (non-T-cell-inflamed) CRC. This type of cancer is usually non-responsive to immune checkpoint inhibitors (ICI) even with a relatively high TMB. 19 In addition, the presence of the KRAS (G12V) gene mutation is one factor contributing to poor prognosis in patients with CRC, making the treatment more challenging. When the KRAS gene is mutated, the abnormal RAS protein is not regulated by the upstream epidermal growth factor receptor (EGFR) signal and initiate downstream signal transduction, hence promoting tumor cell abnormally proliferation, tumor growth, and metastasis.8,20 Therefore, patients with CRC presenting with the MSS/MSI-L type/KRAS (G12V) mutation, similar to this patient case report, usually have a very poor prognosis. Successful treatment options for these patients are lacking.2,21
Despite huge advances in the treatment of MSS/MSI-L CRC, clinical outcomes have been disappointing. Advanced treatments including mono-immunotherapy, and combination immunotherapy (immunotherapy combined with chemotherapy and anti-angiogenesis therapy, or combined with MEK inhibitors), still result in poor prognosis.15–17 Therefore, there is huge potential to explore alternative effective treatment options for the MSS/MSI-L CRC patients. In exploring an effective treatment for the patient in this case, initially, “FOLFIRI regimen” chemotherapy was given empirically on the 19th day post-surgery. The treatment was not effective in controlling the tumor progression. Considering the reported efficacy of regorafenib in combination with nivolumab in the treatment of patients with advanced CRC of MSS, 22 we changed the treatment based on “Rigofini + nivolumab” combined therapy. In order to control the rapid progress of the tumor, oxaliplatin was added to form a triple therapy. This triple combination therapy was not effective in this patient and tumor progression continued. Inspired by previous preclinical studies, showing that dual immunological checkpoint inhibitors were effective in melanoma and non-small cell lung cancer,23–26 we developed a unique combinational therapy by adding a PD-L1 inhibitor, ipilimumab, into the original treatment protocol. In addition, taking into account the importance of irinotecan in the treatment of intestinal cancer, irinotecan replaced oxaliplatin in the final protocol.
Treatment of “Nivolumab + ipilimumab + irinotecan + regofenib” was started 2 weeks after the second course of chemotherapy. Following treatment using this new combinational treatment protocol, the symptoms of the patient were significantly relieved on the second day after treatment. The Karnofsky Performance Status (KPS) scores improved from 10 to 90 within 3 weeks after the treatment. The jaundice subsided on a daily basis, and all tumor-indicating biomarkers were significantly improved after 2 weeks. Four weeks after treatment, multiple metastases tumors shrunk significantly (Figures 2 and 3), confirming that a partial response (PR) treatment outcome was achieved.
In understanding why the final protocol was effective in the treatment of this case patient with an MSS/MSI-L/KRAS (G12V) mutation, it has been suggested that the addition of chemotherapeutic drugs into the immunotherapy regimen conferred an advantage by promoting a more effective immune response. Previous clinical studies have shown that chemotherapy not only interrupts the activity of the inhibitory immune cells, such as regulatory T cells (Treg), myeloid suppressor cells (MDSC), and tumor-associated macrophages (TAM), but also promotes the expansion and activation of immune effector cells, such as T cells, natural killer cells and dendritic cells, in order to promote the immune response.27–31
Conclusion
The positive outcome of this case study suggests that combinational therapy, combining checkpoint inhibitors, targeted therapy and chemotherapy, is effective in treating MSS/MSI-L advanced CRC, providing a more effective treatment option for refractory advanced colon cancer patients. This report also encourages further research into immunotherapy of immuno-insensitive colon cancer and supports clinical trials of dual immunological checkpoint inhibitors combining targeting drugs and chemotherapeutic agents for MSS/MSI-L advanced colon cancer.
Supplemental Material
Supplemental material, sj-pdf-1-sco-10.1177_2050313X211027737 for Effective management of advanced colon cancer genotyping microsatellite stable/microsatellite instable-low with Kirsten rat sarcoma viral oncogene mutation using nivolumab plus ipilimumab combined with regorafenib and irinotecan: A case report by Yingqiang Cui, Yimeng Ou, Yongping Luo, Jiongbiao Yu, Yiguang Lin and Size Chen in SAGE Open Medical Case Reports
Footnotes
Author contributions: Y.C. contributed to the data analysis/interpretation, and drafted the manuscript. Y.O., Yo.L., and J.Y. contributed to the acquisition of data, and analysis/interpretation of data, images/diagrams. Yi.L. and S.C. contributed to the conception, design, interpretation, and write-up of the final article.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical approval: Ethical approval to report this case series was obtained from the Ethics Committee of First Affiliated Hospital of Guangdong Pharmaceutical University (approval no. 2020-125)
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by research grants from the Yuexiu District Science and Technology Program of Guangzhou (grant no. 2017WS002), the Special Innovative Projects of Guangdong Province (grant no. 2017KTSCX104), and the Science and Technology Program of Guangzhou City (grant no. 2018059).
Informed consent: Written informed consent was obtained from the patient’s legally authorized representative for their anonymized information to be published in this article.
ORCID iD: Yiguang Lin  https://orcid.org/0000-0002-4637-9701
https://orcid.org/0000-0002-4637-9701
Supplemental material: Supplemental material for this article is available online.
References
- 1. Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018; 68(6): 394–424. [DOI] [PubMed] [Google Scholar]
- 2. Elizabeth A, Hendrik B, Viola W, et al. External validation of molecular subtype classifications of colorectal cancer based on microsatellite instability, CIMP, BRAF and KRAS. BMC Cancer 2019; 19(1): 681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ghiringhelli F, Fumet JD. Is there a place for immunotherapy for metastatic microsatellite stable colorectal cancer? Front Immunol 2019; 10: 1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dhillon S. Regorafenib: a review in metastatic colorectal cancer. Drugs 2018; 78(11): 1133–1144. [DOI] [PubMed] [Google Scholar]
- 5. Goel G. Evolution of regorafenib from bench to bedside in colorectal cancer: is it an attractive option or merely a “me too” drug. Cancer Manag Res 2018; 10: 425–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Roed Skarderud M, Polk A, Kjeldgaard Vistisen K, et al. Efficacy and safety of regorafenib in the treatment of metastatic colorectal cancer: a systematic review. Cancer Treat Rev 2018; 62: 61–73. [DOI] [PubMed] [Google Scholar]
- 7. Fujita K, Kubota Y, Ishida H, et al. Irinotecan, a key chemotherapeutic drug for metastatic colorectal cancer. World J Gastroenterol 2015; 21(43): 12234–12248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Allegra CJ, Rumble RB, Schilsky RL. Extended RAS gene mutation testing in metastatic colorectal carcinoma to predict response to anti-epidermal growth factor receptor monoclonal antibody therapy: American Society of Clinical Oncology Provisional Clinical Opinion Update 2015 summary. J Oncol Pract 2016; 12(2): 180–181. [DOI] [PubMed] [Google Scholar]
- 9. Hermel DJ, Sigal D. The emerging role of checkpoint inhibition in microsatellite stable colorectal cancer. J Pers Med 2019; 9(1): 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Benson AB, Venook AP, Al-Hawary MM, et al. NCCN guidelines insights: colon cancer, version 2.2018. J Natl Compr Canc Netw 2018; 16(4): 359–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Basso M, Strippoli A, Orlandi A, et al. KRAS mutational status affects oxaliplatin-based chemotherapy independently from basal mRNA ERCC-1 expression in metastatic colorectal cancer patients. Br J Cancer 2013; 108(1): 115–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tournigand C, Andre T, Achille E, et al. FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: a randomized GERCOR study. J Clin Oncol 2004; 22(2): 229–237. [DOI] [PubMed] [Google Scholar]
- 13. Cao R, Zhang S, Ma D, et al. A multi-center randomized phase II clinical study of bevacizumab plus irinotecan, 5-fluorouracil, and leucovorin (FOLFIRI) compared with FOLFIRI alone as second-line treatment for Chinese patients with metastatic colorectal cancer. Med Oncol 2015; 32(1): 325. [DOI] [PubMed] [Google Scholar]
- 14. Le DT, Durham JN, Smith KN, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science 2017; 357(6349): 409–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chung KY, Gore I, Fong L, et al. Phase II study of the anti-cytotoxic T-lymphocyte-associated antigen 4 monoclonal antibody, tremelimumab, in patients with refractory metastatic colorectal cancer. J Clin Oncol 2010; 28(21): 3485–3490. [DOI] [PubMed] [Google Scholar]
- 16. Eng C, Kim TW, Bendell J, et al. Atezolizumab with or without cobimetinib versus regorafenib in previously treated metastatic colorectal cancer (IMblaze370): a multicentre, open-label, phase 3, randomised, controlled trial. Lancet Oncol 2019; 20(6): 849–861. [DOI] [PubMed] [Google Scholar]
- 17. Grothey A, Tabernero J, Arnold D, et al. LBA19Fluoropyrimidine (FP) + bevacizumab (BEV) + atezolizumab vs FP/BEV in BRAFwt metastatic colorectal cancer (mCRC): findings from Cohort 2 of MODUL—a multicentre, randomized trial of biomarker-driven maintenance treatment following first-line induction therapy. Ann Oncol 2018; 29(Suppl. 8): viii714–viii715. [Google Scholar]
- 18. Chen EX, Jonker DJ, Kennecke HF, et al. CCTG CO.26 trial: a phase II randomized study of durvalumab (D) plus tremelimumab (T) and best supportive care (BSC) versus BSC alone in patients (pts) with advanced refractory colorectal carcinoma (rCRC). J Clin Oncol 2019; 37(4 Suppl.): 481–481.30620669 [Google Scholar]
- 19. Maleki Vareki S. High and low mutational burden tumors versus immunologically hot and cold tumors and response to immune checkpoint inhibitors. J Immunother Cancer 2018; 6(1): 157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Modest DP, Ricard I, Heinemann V, et al. Outcome according to KRAS-, NRAS- and BRAF-mutation as well as KRAS mutation variants: pooled analysis of five randomized trials in metastatic colorectal cancer by the AIO colorectal cancer study group. Ann Oncol 2016; 27(9): 1746–1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Smeby J, Sveen A, Merok MA, et al. CMS-dependent prognostic impact of KRAS and BRAFV600E mutations in primary colorectal cancer. Ann Oncol 2018; 29(5): 1227–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fukuoka S, Hara H, Takahashi N, et al. Regorafenib plus nivolumab in patients with advanced gastric (GC) or colorectal cancer (CRC): an open-label, dose-finding, and dose-expansion phase 1b trial (REGONIVO, EPOC1603). J Clin Oncol 2019; 37(15 Suppl.): 2522–2522. [DOI] [PubMed] [Google Scholar]
- 23. Antonia SJ, Lopez-Martin JA, Bendell J, et al. Nivolumab alone and nivolumab plus ipilimumab in recurrent small-cell lung cancer (CheckMate 032): a multicentre, open-label, phase 1/2 trial. Lancet Oncol 2016; 17(7): 883–895. [DOI] [PubMed] [Google Scholar]
- 24. Curran MA, Montalvo W, Yagita H, et al. PD-1 and CTLA-4 combination blockade expands infiltrating T cells and reduces regulatory T and myeloid cells within B16 melanoma tumors. Proc Natl Acad Sci U S A 2010; 107(9): 4275–4280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Valsecchi ME. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med 2015; 373(13): 1270. [DOI] [PubMed] [Google Scholar]
- 26. Hodi FS, Chiarion-Sileni V, Gonzalez R, et al. Nivolumab plus ipilimumab or nivolumab alone versus ipilimumab alone in advanced melanoma (CheckMate 067): 4-year outcomes of a multicentre, randomised, phase 3 trial. Lancet Oncol 2018; 19(11): 1480–1492. [DOI] [PubMed] [Google Scholar]
- 27. Galluzzi L, Buque A, Kepp O, et al. Immunological effects of conventional chemotherapy and targeted anticancer agents. Cancer Cell 2015; 28(6): 690–714. [DOI] [PubMed] [Google Scholar]
- 28. Kepp O, Galluzzi L, Martins I, et al. Molecular determinants of immunogenic cell death elicited by anticancer chemotherapy. Cancer Metastasis Rev 2011; 30(1): 61–69. [DOI] [PubMed] [Google Scholar]
- 29. Martins I, Wang Y, Michaud M, et al. Molecular mechanisms of ATP secretion during immunogenic cell death. Cell Death Differ 2014; 21(1): 79–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Obeid M, Tesniere A, Ghiringhelli F, et al. Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat Med 2007; 13(1): 54–61. [DOI] [PubMed] [Google Scholar]
- 31. Vincent J, Mignot G, Chalmin F, et al. 5-Fluorouracil selectively kills tumor-associated myeloid-derived suppressor cells resulting in enhanced T cell-dependent antitumor immunity. Cancer Res 2010; 70(8): 3052–3061. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-sco-10.1177_2050313X211027737 for Effective management of advanced colon cancer genotyping microsatellite stable/microsatellite instable-low with Kirsten rat sarcoma viral oncogene mutation using nivolumab plus ipilimumab combined with regorafenib and irinotecan: A case report by Yingqiang Cui, Yimeng Ou, Yongping Luo, Jiongbiao Yu, Yiguang Lin and Size Chen in SAGE Open Medical Case Reports



