Abstract
First reported in Wuhan, China, Novel Coronavirus Disease-19 rapidly spread causing an outbreak of viral pneumonia and became a pandemic in early 2020. It was later discovered to be caused by Severe Acute Respiratory Syndrome Coronavirus 2, a novel coronavirus. Although the vast majority of cases have primarily involved the respiratory system, some serious cases have started to emerge with central and peripheral nervous system complications. We present the case of a 30-year-old morbidly obese male who initially presented to the emergency department with seizures, altered mental status, and COVID-19 pneumonia. After a 21-day hospital course including 14 days of intensive care unit management, he was stabilized and discharged to a rehabilitation facility. He returned 1 day later with worsening respiratory distress and was found to have acute pulmonary embolism requiring placement of an inferior vena cava filter. After an additional 6 days in the hospital, he was discharged back to the outpatient facility. He returned for a third time with altered mental status, visual and auditory hallucinations, and confabulation. This report provides critical information in revealing a peculiar neurological sequela of COVID-19 induced leukoencephalopathy and its disease course. We hope to shed light on this sequence of events by providing possible mechanisms to aid clinicians in the identification and management of this complication.
Keywords: Cardiovascular, neurology, infectious diseases
Introduction
As of 14 June 2020, the World Health Organization (WHO) has reported 7,690,708 cases and 427,630 deaths globally attributed to Novel Coronavirus Disease-19 (COVID-19). 1 Recent studies show the mean age to be 51.97 years old, 55.9% being male with 36.8% having hypertension, cardiovascular disease, and diabetes as the most common co-morbidities. 2 Clinical features commonly reported include fever, cough, and dyspnea. 2
Laboratory findings may include decreased albumin, elevated lactate dehydrogenase, leukopenia, lymphopenia, thrombocytopenia, as well as, elevations in C-reactive peptide, erythrocyte sedimentation, aminotransferases, creatine kinase, and D-dimer.2,3
Testing for Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) involves sample collection with nasopharyngeal or oropharyngeal swabs with subsequent amplification through a reverse transcription polymerase chain reaction (RT-PCR). On chest X-ray or computed tomography (CT), bilateral lung involvement with multi-lobar ground-glass opacities (GGOs), is the predominant finding with the onset of symptoms. Peripheral GGO accurately distinguishing COVID-19 from other viral etiologies in up to 80% of cases.4,5
The data regarding neurological presentations of COVID-19 are sparse but current evidence suggests that the central nervous system (CNS), peripheral nervous system (PNS), and skeletal system may all be affected.6,7
In this report, we provide further clinical evidence of CNS involvement in COVID-19. Our patient presented with seizure activity and alteration in mental status with no prior history of organic or psychiatric pathologies. We will further examine possible mechanisms for our patient’s peculiar clinical presentation.
Case section
A 30-year-old morbidly obese male with a body mass index (BMI) of 50.7 kg/m2 and a history of gastroesophageal reflux disease was brought in by ambulance to the emergency department (ED) after his wife witnessed him make grunting sounds, fall from the bed, and subsequently have convulsive movements. According to his wife, he had never had any similar episodes and she denied he had any complaints of fever, chills, cough, diarrhea, or vomiting nor had he traveled recently or any sick contacts.
On admission of the patient to the ED, the temperature was 38.5°C, the pulse 128 beats per minute, the blood pressure of 104/75 mm Hg, the respiratory rate 30 breaths per minute, and the oxygen saturation 99% on a non-rebreather mask at 70% fraction of inspired oxygen. On examination, he was unresponsive, and his eyes remained open throughout with bilateral upper extremity myoclonus.
Initial labs were concerning for elevated lactic acid (2.3 mmol/L) and elevated D-dimer (2.09 mg/L). RT-PCR testing for SARS-CoV-2 was positive. Electrocardiogram (EKG) showed sinus tachycardia, cardiac enzymes were negative. Initial chest X-ray on presentation (see Figure 1) showed a right lower lobe patchy density with blunting of the left costophrenic angle suggesting early COVID-19 pneumonia.
Figure 1.

Anterior–posterior portable chest radiograph shows a right lower lobe patchy density (red arrow) and blunting of the left costophrenic angle (red arrow) suggestive of early pneumonia.
In the ED, he was given levetiracetam, lorazepam, and phenytoin for seizure prevention. Given his neurological status and imaging suggestive of pneumonia, he was intubated and mechanically ventilated immediately in the ED and transferred to the intensive care unit (ICU).
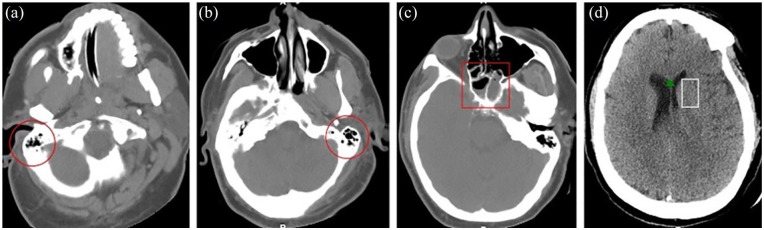
Head CT without contrast (see Figure 2) during day 7 revealed near-complete bilateral opacification of the sphenoid sinuses and several ethmoid air cells consistent with a sinus infection and a mild hypodensity of the white matter surrounding left basal ganglia and thalamus with apparent mass effect on the left lateral ventricle. Cerebrospinal fluid (CSF) testing was contraindicated due to the mass appearing lesion on initial head CT. Serial head CT scans were all subsequently negative for intracranial abnormality; however, they continuously demonstrated sinusitis. Electroencephalogram, complete metabolic panel, and serology for herpes simplex virus (HSV) were all non-diagnostic. Magnetic resonance imaging (MRI) could not be completed due to body habitus. He was managed with antiepileptics, antibiotics, anticoagulation, and acyclovir for seizures, pneumonia, and encephalopathy, respectively. After 14 days in the ICU, he had significant clinical improvement, and he was downgraded to the general medical floor. Acyclovir was discontinued after completion of a 10-day course.
Figure 2.
Axial non-enhanced CT. (a) Image through the right mastoid shows mild opacification of the mastoid air cells (red circle). (b) Image through the left mastoid shows mild opacification of the mastoid air cells (red circle). (c) Image through the sinuses shows bilateral nearly complete opacification of the sphenoidal sinuses (red rectangle). (d) Image through the brain shows a mild hypodensity of the white matter (white rectangle) surrounding the left basal ganglia and thalamus with apparent mass effect on the left lateral ventricle (green arrow) which is lower than the right. These findings could be secondary to early ischemia or encephalitis.
On day 17 of hospital stay, despite therapeutic anticoagulation with enoxaparin, he began complaining of leg pain and duplex ultrasound confirmed a bilateral deep vein thrombosis (DVT). He was subsequently started on heparin sodium intravenously, which was discontinued on the same day as he had developed hematuria. He was subsequently switched to apixaban and discharged after a 21-day course for management at an outpatient treatment facility.
One day after discharge, he was brought back to the hospital due to respiratory distress with oxygen saturation at 84% on room air. A chest CT angiography (see Figure 3) demonstrated suboptimal pulmonary arterial opacification for probable bilateral pulmonary embolism (PE). Ultimately on day 5 of this stay, the decision was made to place an Option™ ELITE inferior vena cava (IVC) filter that was placed in the infrarenal portion of IVC to prevent future pulmonary emboli. Following this procedure, he was again discharged on day 6 to an outpatient service.
Figure 3.
Axial IV contrast–enhanced CT image through the chest. (a–c) Images show bilateral lower lung filling defects; opacification of the arterial system was limited due to poor timing bolus.
He returned to the hospital again 4 days later for altered mental status and hallucinations. During the examination, he endorsed visual and audio hallucinations of voices telling him to kill himself, he denied being suicidal. The patient also confidently believed he was in another state, and he would continuously change his story when interviewed in the subsequent days. A head CT was done but showed no acute pathology. MRI of the head without contrast (see Figure 4) on day 3 of current stay revealed an ill-defined confluent high T2 FLAIR signal lesions in the deep and subcortical white matter of both cerebral hemispheres which are nonspecific, although no restricted diffusion or acute infarct was seen. At the time of writing, he remained hemodynamically stable, and SARS-CoV-2 testing was repeatedly negative; however, his mental status continued to wax and wane with periods of clear cognition interspersed with wild autobiographical stories.
Figure 4.
Axial head MRI T2-FLAIR weighted. (a–c) Images through the brain show nonspecific bilateral ill-defined confluent high T2-FLAIR signal lesion in the deep and subcortical white matter of both cerebral hemispheres (blue arrows).
Discussion
Concerning neurological complications of COVID-19 including seizure activity, Guillain–Barré syndrome, and encephalopathy have been previously reported.7–11 However, the constellation of neurological findings of COVID-19 seen in our young patient has not been previously reported.
In addition to acute onset neurological findings, which persisted nearly a month after presentation and continued at the time of writing, MRI imaging demonstrated lesions in the deep and subcortical white matter of both hemispheres, seen as hyperintensities. Similar findings have been recently reported in a 59-year-old male with confirmed COVID-19, which demonstrated diffuse hyperintensities in the posterior predominant white matter along with microhemorrhages in the corpus callosum (not seen in our patient). 12 This suggests that there may be SARS-CoV-2 tropism for the white matter in patients with CNS involvement. We suspect that the neurological sequelae seen in our case are a result of COVID-19-induced leukoencephalopathy, which refers to any pathology of the brain that results in interference with the white matter of the CNS. Broadly, encephalopathy manifests as an altered mental state that may present as confusion, disorientation, behavioral changes, with or without inflammatory changes in the brain.
SARS-CoV-2 entry into the host cell is mediated by its spike glycoprotein S1 subunit-receptor fusion with the angiotensin-converting enzyme (ACE)-2 receptor. Surface glycoprotein (S) has also been implicated in inducing neuronal injury. 13 ACE-2 receptor expression has been found to be positively correlated with CoV-2 infection and has abundantly expressed in the CNS. 14 In addition, SARS-CoV autopsy findings have demonstrated viral particles in the neurons of the hypothalamus and cortex. 15 Given the close link between SARS-CoV and SARS-COV-2, we suspected direct neural involvement may play a role in our patient’s findings.
In a study of mice transgenic for human ACE-2 receptor, intranasal viral inoculation with SARS rapidly led to viral particles affecting other parts of the brain. 16 Given the proclivity of SARS-CoV-2 for ACE-2 receptor, abundant on the nasal epithelium, a potential route for CNS invasion may be through the nasal epithelium, olfactory nerves, and olfactory bulb. 17
The proposed mechanism involves the movement of SARS-CoV-2 across the cribriform plate which is adjacent to the olfactory bulb.14,18 An early manifestation of COVID-19 has been anosmia, loss of sensation of smell, which supports entrance of SARS-CoV-2 across the cribriform plate into the olfactory bulb.14,18 The inflammatory response from SARS-CoV-2 may contribute to this by impeding odorants from reaching the receptor neurons. 16 In most cases, the loss of smell resolves in weeks. 16
Netland et al. 19 have demonstrated in murine models that SARS-CoV invades the CNS primarily through the olfactory nerve, allowing further spread through structures within the CNS contiguous the olfactory bulb.
HCoV-OC43, HCoV-229E, and mouse hepatitis virus (MHV) are other coronaviruses which have been implicated in CNS infections. HCoV-OC43 uses axonal transport mechanism to invade the CNS and directly damaging neurons leading to cellular injury and death. 18 Furthermore, HCoV-OC43 has been detected in tissues of Alzheimer’s, Parkinson’s, and multiple sclerosis patients. 18 HCoV-229E has been implicated in febrile seizures and death. 18 Studies of MHV infection in both mice and primates have also shown viral entry into the CNS through intranasal and intravenous pathways leading to acute encephalomyelitis and focal demyelination from a local inflammatory response leading to microglial activation and expression of inflammatory mediators. Although some have suggested that the blood–brain barrier’s role in containing SARS-CoV-2 need to be investigated, other studies hypothesize that the large particle size of SARS-CoV-2 supports an alternative mechanism of CNS entry.14,18
The white matter in the CNS is constructed by oligodendrocytes which create a myelin sheath around the nerves to aid in conduction among other functions. Demyelination can result from pathogens that invade the CNS and afflict injury onto the oligodendrocytes resulting on the loss of the protection sheath around the nerves. Studies of MHV were found to be involved in acute and chronic demyelination in mice. 20 The virus was found to disrupt the hosts’ genetic and immune response in specific mice strains. This suggests that certain patients infected with SARS-CoV-2 may have a genetic predisposition that allows for development of leukoencephalopathy. Interaction of SARS-CoV-2 with ACE-2 receptors in neuronal cells can generate a cyclical pattern of viral budding followed by damage to neuronal cells. 14
Another proposed mechanism for the entrance of SARS-CoV-2 into brain parenchyma is viremia of the microcirculation of the cerebrum, where blood flow velocity is decreased may allow for increased interaction between ACE-2 receptors expressed on the capillary endothelium and the SARS-CoV-2 virus spike protein. 14 Secondarily, release of SARS-CoV-2 via budding from the capillary endothelium results in additional damage to the endothelial lining, facilitating entrance into the brain. 14 However, endothelial rupture in the form of hemorrhage within the cerebrum would occur before the manifestations of neuronal injury.14,21
The true mechanism of SARS-CoV-2 entrance into the CNS is likely a combination of viremia in the microcirculation of the cerebrum inciting endothelial injury via binding of the spike protein to ACE-2 receptors and budding of packaged virus from the endothelium as well as early entrance via the cribriform plate.14,18 An alternative explanation for the findings in our patient is a nervous system injury secondary to systemic inflammation.
SARS-CoV 2 has shown to induce a severe and life-threatening inflammatory response, as evidenced by descriptions of cytokine storm in severely ill patients. Increased inflammatory mediators in COVID-19 infected patients including interleukin (IL)-6 and IL-1, as well as procoagulant inflammatory cytokines such as tumor necrosis factor-alpha (TNF-alpha) and IL-2R, are elevated in COVID-19 patients. 22 Neurotropic viruses have been shown to induce the release of TNF-alpha and IL-6 from glial cells.23,24 The resultant pro-inflammatory response may wreak havoc on the CNS.
COVID-19 has also shown a prothrombotic response related to inflammation resulting in thromboembolism of veins and arteries. In a study of 184 ICU patients with confirmed COVID-19 pneumonia, thrombotic events were seen in 31% of patients, despite all patients receiving thromboprophylaxis. 25 81% of these patients developed a PE. 18 In another study examining 388 COVID-19 patients with diagnosed thromboembolic events, 50% occurred within 24 h of hospital admission. 26 During the latter part of the disease course, our patient developed both a PE and bilateral DVT, raising suspicion that systemic inflammation may also play a role in our patient’s neurological findings.
Conclusion
Our case was limited by the inability to obtain an initial MRI due to the patients’ body habitus. The initial CT was also delayed, 7 days after patient presentation. These factors limited the visualization of any acute pathology if it was present.
The relationship between SARS-CoV-2 and the CNS has been widely speculated. In this report, we provide clinical evidence of COVID-19 affecting the CNS with bizarre and unexplained sequelae. Further studies are needed to clarify the mechanism of injury.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethical approval: Our institution does not require ethical approval for reporting individual cases or case series.
Informed consent: Written informed consent was obtained from a legally authorized representative(s) for anonymized patient information to be published in this article. Given the patients altered mental status, his legally authorized representative provided written consent on his behalf.
ORCID iD: Zohaib Khan  https://orcid.org/0000-0002-1702-0388
https://orcid.org/0000-0002-1702-0388
Sukhdev Singh  https://orcid.org/0000-0002-7922-9590
https://orcid.org/0000-0002-7922-9590
Allison Foster  https://orcid.org/0000-0001-5900-7969
https://orcid.org/0000-0001-5900-7969
References
- 1. COVID-19 situation reports, https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports (accessed 14 June 2020).
- 2. Rodriguez-Morales AJ, Cardona-Ospina JA, Gutiérrez-Ocampo E, et al. Clinical, laboratory and imaging features of COVID-19: a systematic review and meta-analysis. Travel Med Infect Dis 2020; 34: 101623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Guan W, Ni Z, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020; 382: 1708–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wang Y, Dong C, Hu Y, et al. Temporal changes of CT findings in 90 patients with COVID-19 pneumonia: a longitudinal study. Radiology 2020; 296(2): E55–E64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Simpson S, Kay FU, Abbara S, et al. Radiological society of North America expert consensus statement on reporting chest CT findings related to COVID-19: endorsed by the society of thoracic radiology, the American college of radiology, and RSNA. Radiology Cardiothorac Imag 2020; 2: e200152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mao L, Jin H, Wang M, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol 2020; 77: 683–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Whittaker A, Anson M, Harky A. Neurological manifestations of COVID-19: a systematic review and current update. Acta Neurol Scand 2020; 142: 14–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhao H, Shen D, Zhou H, et al. Guillain-Barré syndrome associated with SARS-CoV-2 infection: causality or coincidence? Lancet Neurol 2020; 19: 383–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Vollono C, Rollo E, Romozzi M, et al. Focal status epilepticus as unique clinical feature of COVID-19: a case report. Seizure 2020; 78: 109–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Filatov A, Sharma P, Hindi F, et al. Neurological complications of coronavirus disease (COVID-19): encephalopathy. Cureus 2020; 12: e7352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Poyiadji N, Shahin G, Noujaim D, et al. COVID-19–associated acute hemorrhagic necrotizing encephalopathy: CT and MRI Features. Radiology 2020; 31: 201187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sachs JR, Gibbs KW, Swor DE, et al. COVID-19-associated Leukoencephalopathy. Radiology 2020; 14: 201753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Desforges M, Le Coupanec A, Brison E, et al. Neuroinvasive and neurotropic human respiratory coronaviruses: potential neurovirulent agents in humans. In: R Adhikari, S Thapa. (eds) Infectious diseases and nanomedicine I. New Delhi: Springer, 2014, pp. 75–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Baig AM, Khaleeq A, Ali U, et al. Evidence of the COVID-19 virus targeting the CNS: tissue distribution, host–virus interaction, and proposed neurotropic mechanisms. ACS Chem Neurosci 2020; 11(7): 995–998. [DOI] [PubMed] [Google Scholar]
- 15. Gu J, Gong E, Zhang B, et al. Multiple organ infection and the pathogenesis of SARS. J Experim Med 2005; 202: 415–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Soler ZM, Patel ZM, Turner JH, et al. A primer on viral-associated olfactory loss in the era of COVID-19. Int Forum Allergy Rhinol 2020; 10(7): 814–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hoffmann M, Kleine-Weber H, Schroeder S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 2020; 181: 271–280.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Matías-Guiu J, Gomez-Pinedo U, Montero-Escribano P, et al. Should we expect neurological symptoms in the SARS-CoV-2 epidemic. Neurology 2020; 35(3): 170–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Netland J, Meyerholz DK, Moore S, et al. Severe acute respiratory syndrome coronavirus infection causes neuronal death in the absence of encephalitis in mice transgenic for human ACE2. J Virol 2008; 82(15): 7264–7275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Stohlman SA, Hinton DR. Viral induced demyelination. Brain Pathol 2001; 11: 92–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Radmanesh A, Raz E, Zan E, et al. Brain imaging utilization and findings in COVID-19: a single academic center experience in the epicenter of disease in the United States. Am J Neurol 2020; 41: A6610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Morelli N, Rota E, Terracciano C, et al. The baffling case of ischemic stroke disappearance from the casualty department in the COVID-19 era. Eur Neurol 2020; 83(2): 213–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Li Y, Fu L, Gonzales DM, et al. Coronavirus neurovirulence correlates with the ability of the virus to induce proinflammatory cytokine signals from astrocytes and microglia. J Virol 2004; 78(7): 3398–3406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bohmwald K, Gálvez NMS, Ríos M, et al. Neurologic alterations due to respiratory virus infections. Front Cell Neurosci 2018; 12: 386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Klok FA, Kruip MJHA, van der Meer NJM, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thrombos Res 2020; 191: 145–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lodigiani C, Iapichino G, Carenzo L, et al. Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thromb Res 2020; 191: 9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]