Abstract
Animals that ingest toxins can become unpalatable and even toxic to predators and parasites through toxin sequestration. Because most animals rapidly eliminate toxins to survive their ingestion, it is unclear how populations transition from susceptibility and toxin elimination to tolerance and accumulation as chemical defence emerges. Studies of chemical defence have generally focused on species with active toxin sequestration and target-site insensitivity mutations or toxin-binding proteins that permit survival without necessitating toxin elimination. Here, we investigate whether animals that presumably rely on toxin elimination for survival can use ingested toxins for defence. We use the A4 and A3 Drosophila melanogaster fly strains from the Drosophila Synthetic Population Resource (DSPR), which respectively possess high and low metabolic nicotine resistance among DSPR fly lines. We find that ingesting nicotine increased A4 but not A3 fly survival against Leptopilina heterotoma wasp parasitism. Further, we find that despite possessing genetic variants that enhance toxin elimination, A4 flies accrued more nicotine than A3 individuals, likely by consuming more medium. Our results suggest that enhanced toxin metabolism can allow greater toxin intake by offsetting the cost of toxin ingestion. Passive toxin accumulation that accompanies increased toxin intake may underlie the early origins of chemical defence.
Keywords: xenobiotic metabolism, chemical defence, multi-trophic selection, bioaccumulation, enemy-free space
1. Introduction
Most animals survive toxin ingestion by eliminating toxins through metabolic detoxification [1–3]. Some chemically defended animals subvert this paradigm by sequestering dietary toxins to deter predators or parasites [4]. Because metabolic detoxification serves to prevent toxin accumulation, toxin-sequestering taxa often employ resistance mechanisms that do not degrade toxins [5]. For example, target-site insensitivity (TSI), which results from mutations in a protein that prevent toxins from binding, is common in toxin-sequestering insects [6,7]. TSI sometimes co-occurs with toxin-binding proteins that scavenge toxins and prevent them from binding to targets [8–11]. Such non-metabolic resistance mechanisms may facilitate the transition from toxin elimination to sequestration by decreasing reliance on toxin breakdown for survival [12].
Although metabolic detoxification degrades toxins, it is unclear whether reliance on this mechanism constrains chemical defence evolution. Metabolic detoxification permits toxin consumption and may ultimately lead to toxin sequestration so long as consumption outpaces degradation. To test this idea, we obtained two isofemale, homozygous strains of Drosophila melanogaster from the Drosophila Synthetic Population Resource (DSPR [13]) that possess high and low nicotine resistance (A3 and A4, Bloomington stocks 3852 and 3844), and exposed them to nicotine, a plant allelochemical that targets acetylcholine receptors [14]. Although some drosophilids do feed on toxic food sources [15,16] and the A4 fly strain may have experienced incidental nicotine exposure on tobacco farms that were prevalent at its collection site [17], drosophilids are not known to select nicotine-producing plants as hosts. Nevertheless, the genetic basis of nicotine resistance in D. melanogaster is extensively characterized, making this toxin well-suited to modelling the evolutionary origins of chemical defence [18]. In contrast to A3, A4 flies possess duplicate copies of cytochrome p450 genes Cyp28d1 and Cyp28d2 that are constitutively expressed at higher levels. A4 flies also overexpress the UDP-glucuronosyltransferase genes Ugt86Dd, while A3 harbours a mutation in this gene that significantly reduces nicotine resistance [19]. Ugt86Dd is located in a quantitative trait locus (QTL) that contributes 50.3% of the broad-sense heritability in nicotine resistance of DSPR lines, while a QTL containing Cyp28d1 and Cyp28d2 accounts for 5% [18,20]. The contributions of these three genes to nicotine resistance have been confirmed using gene knockout [21]. Previous QTL and expression-QTL studies did not report evidence for TSI or toxin-binding proteins in A3 or A4 lines. While these mechanisms could exist, variation in metabolic enzymes appears to underlie the major difference between A3 and A4 nicotine resistance.
2. Results and discussion
We first quantified A3 and A4 nicotine resistance by estimating the median lethal concentration (LC50) of nicotine (figure 1). Because A4 flies had low viability in general, to compare LC50 between strains for this assay we normalized percentage survival by the maximum survival of each line on control food (see the electronic supplementary material for non-normalized values). The A4 LC50 was nearly twice that of A3 (LC50A4 = 1.9 ± 0.3 mM (mean ± s.d.), LC50A3 = 1.1 ± 0.2 mM; figure 1). While A3 survival decreased significantly at 0.5 mM nicotine, A4 survival was not significantly impacted until 1.75 mM. We proceeded to use an intermediate level of 1.25 mM nicotine for subsequent experiments.
Figure 1.
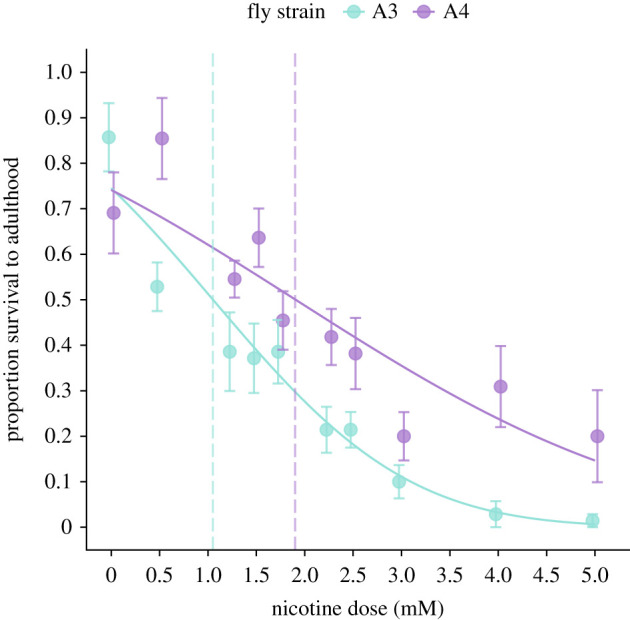
(a) Nicotine concentration–survival curve for DSPR A3 and A4 Drosophila melanogaster. Data are normalized by maximum survival of each strain on control food. Vertical dashed lines represent LC50 of each strain.
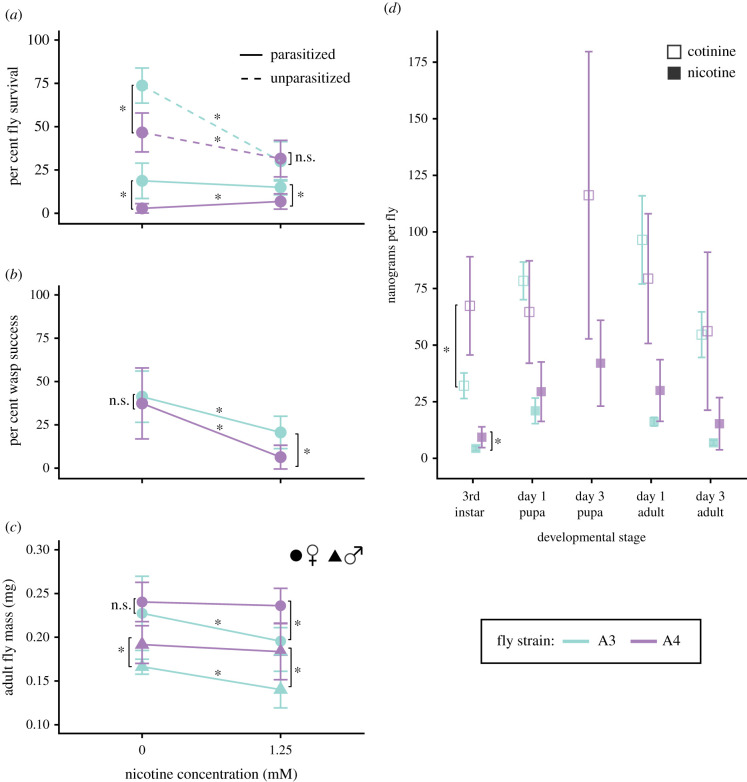
We next assessed whether ingesting 1.25 mM nicotine after parasitism by the figitid wasp Leptopilina heterotoma increased D. melanogaster survival. Leptopilina heterotoma oviposits into the haemocoel of developing fly larvae, and actively suppresses the drosophilid defensive immune response against endoparasites [22]. Thus, developing parasites are exposed to host haemolymph and, presumably, to circulating toxins consumed by fly larvae. In the control-fed, parasitized treatment, 2.8 ± 2.7% of A4 larvae survived to adulthood, while in the nicotine-fed, parasitized treatment, A4 survival increased significantly to 6.8 ± 4.4% (p = 0.03, Z = −2.2; figure 2a). Correspondingly, L. heterotoma developmental success decreased five-fold from 37 ± 20% to 6.4 ± 6.8% (p < 0.0001, Z = 7.0; figure 2b). Thus, nicotine consumption increased A4 fly survival against parasitism.
Figure 2.
(a) Nicotine consumption significantly decreased survival in unparasitized A3 and A4 Drosophila melanogaster flies. Nicotine consumption increased survival of parasitized A4 but not A3 flies. (b) Nicotine consumption by A4 and A3 flies significantly decreased Leptopilina heterotoma developmental success. (c) Nicotine consumption reduced A3 but not A4 adult body mass. (d) Nicotine-fed A3 and A4 flies accumulated nicotine and its metabolic byproduct cotinine across developmental stages. Asterisks indicate significant differences; n.s., not significant.
By contrast, the survival of parasitized, nicotine-fed A3 larvae (15 ± 4.4%) was the same as that of parasitized, control-fed A3 larvae (19 ± 10%; p = 0.36, Z = 0.92; figure 2a). However, wasp developmental success on A3 flies halved from 41 ± 15% to 21 ± 9.3% when A3 flies consumed nicotine (p = 0.0001, Z = 4; figure 2b). This suggests nicotine consumption partially alleviated A3 parasitism-induced mortality. Nicotine consumption decreased unparasitized A3 fly survival by 44% (p < 0.0001, Z = 7.6), while nicotine consumption decreased parasitized A3 survival by only a tenth as much: 3.5%. The comparatively insignificant effect of nicotine consumption on parasitized A3 flies paired with an approximately 50% decrease in wasp success suggests that nicotine may have offset parasitism-induced mortality for A3 flies, although to a lesser degree compared with A4 flies.
Next, we quantified nicotine accumulation in whole bodies of nicotine-fed larvae and adult flies. After 24 h ± 2.5 h on nicotine medium, third-instar A4 larvae contained twice as much nicotine as A3 larvae (9.3 ± 4.6 versus 4.3 ± 1.0 ng nicotine, p = 0.016, W = 1; figure 2d). Nicotine continued to accumulate until pupation and persisted through metamorphosis in both strains (figure 2d; also observed with ouabain [6]), suggesting that nicotine remained after the meconium was shed and may provide a defensive advantage into adulthood. The greater amount of nicotine in A4 could underlie the stronger effect of nicotine on parasite success in A4 versus A3 individuals (figure 2b). Although nicotine-fed A3 adults are approximately 20% smaller than nicotine-fed A4 adults (figure 2c), this difference cannot explain the two-fold difference observed in nicotine accumulation between strains. The developmental rate of nicotine-fed A3 and A4 flies did not differ significantly at 1.25 mM nicotine and is also unlikely to underlie differences in nicotine accumulation (electronic supplementary material, figure S1).
Our finding that A4 larvae accumulated more nicotine than A3 defies genotypic expectations, as A4 flies have genetic variants that are expected to increase nicotine breakdown [19,21]. To better understand this pattern, we compared relative amounts of cotinine, a metabolic byproduct of nicotine (figure 2d) between strains. A4 larvae contained significantly higher levels of cotinine compared with A3 individuals (figure 2d). Intriguingly, 1-day-old and 3-day-old A3 flies had significantly higher cotinine to nicotine ratios than A4, suggesting that A4 larvae have a distinct metabolic detoxification pathway compared with A3 (p1-day-old = 0.031, W1-day-old = 23, p3-day-old = 0.008, W3-day-old = 25 [13]). This result matches expectations based on genotype, as the largest QTL underlying resistance in A4 contains several UDP-glucuronosyltransferases (UGTs), which convert nicotine to glucuronides instead of cotinine [18].
The higher nicotine levels in A4 flies suggested that A3 flies are unable to survive high toxin loads, and thus might consume less to avoid nicotine accumulation. To quantify differences in feeding, we compared A3 and A4 adult body mass when reared on control versus nicotine food. While nicotine consumption significantly reduced A3 adult body mass, A4 mass remained unaffected (figure 2c), indicating that nicotine sensitivity constrained A3 food intake. The tobacco hornworm, Manduca sexta, employs a more extreme version of this pattern: nicotine exposure activates xenobiotic enzymes, which further stimulates feeding [23]. Thus, perhaps unexpectedly, increased metabolic detoxification may promote rather than preclude toxin accumulation via increased feeding.
Intriguingly, while nicotine consumption increased A4 fly survival against parasitism, A4 flies under all but the nicotine-fed, unparasitized condition had lower viability than A3 flies (figure 2a). Thus, in a hypothetical population made only of A3 and A4 flies and exposed to L. heterotoma and nicotine, natural selection may be unlikely to favour A4 individuals. In this scenario, the evolutionary outcome would depend partly on whether antagonistic pleiotropy exists among loci determining metabolic resistance and viability. One general viability QTL has been identified in DSPR strains, but this QTL does not contain detoxification genes. Moreover, A4 and A3 flies seem to share the same allele at this QTL [15]. Furthermore, while A4 survival was generally lower than A3, A3 (and not A4) female body mass was reduced by nicotine consumption. Body mass is correlated with fecundity in D. melanogaster, and thus nicotine-fed A4 flies may have greater reproductive success than A3 [24], which would potentially offset the cost of lower survival.
To our knowledge, D. melanogaster does not possess active nicotine sequestration mechanisms. Some drosophilids, such as Drosophila sechellia, are known to acquire chemical defences from toxic food sources [25], and D. melanogaster uses ethanol to self-medicate against parasitoids [26]. However, other drosophilids that consume toxins have not been evaluated for chemical defences [15,16]. Our finding that flies can use nicotine for defence without active sequestration mechanisms suggests that other organisms that tolerate toxin consumption could receive a transient defensive advantage, too. The biochemical properties and metabolic context of each toxin should affect their propensity to bioaccumulate. For example, non-toxic glucosinolates (GLS) rapidly break down into toxic mustard oils; thus, GLS-sequestration requires adaptations that interrupt this process [16]. Many organisms sequester toxic steroids or alkaloids [4,27,28], perhaps because these more readily diffuse or are transported across tissues. Here we find that in addition to having increased nicotine metabolism, A4 D. melanogaster flies also likely consume much higher quantities of nicotine than A3 flies (figure 2c). We hypothesize that higher intake may allow relatively more nicotine to escape metabolism and permeate into the haemolymph of A4 flies, affecting L. heterotoma development to a greater degree than in A3 flies. This pattern could be verified with future studies that compare nicotine abundance in different tissues of A4 and A3 flies.
In conclusion, we find that elevated resistance increases passive toxin accumulation. Further, this accumulation produces a toxin-mediated fitness advantage against natural enemies, in animals without identified sequestration mechanisms. Reliance on metabolic detoxification is likely the ancestral character state for organisms with acquired chemical defences, and variation in toxin metabolism is common [29]. We, therefore, propose that one of the first steps in the evolution of chemical defence may paradoxically be natural selection for increased toxin metabolism.
3. Methods
(a) . Fly and wasp stocks
Flies were maintained at room temperature on molasses medium from the Fly Food Facility at UCB; survival and parasitism experiments used Ward's Instant Drosophila medium to facilitate toxin dosing.
Wasps were maintained at room temperature on W118 D. melanogaster and 70%-honey water. Experiments used wasps within two weeks of eclosion.
(b) . Generation of fly larvae
Approximately 1000 flies were allowed to lay eggs for 3 days in three replicate resealable plastic containers with a layer of molasses-agar smeared with yeast paste. Larvae were then pooled from each container, and second-instar larvae (L2) were selected based on morphology under a dissection microscope. Flies were not sorted by sex.
(c) . Nicotine-resistance experiment
Twenty A4 and A3 L2 larvae were transferred one-by-one from egg-laying chambers into five replicate vials containing medium treated with the following nicotine concentrations: 0, 0.5, 1.25, 1.75, 2.25, 2.50, 3.00, 4.00 and 5.00 mM. Vials were checked daily for new pupae and eclosed flies, and daily counts were used to calculate developmental rate across nicotine doses (electronic supplementary material, figure S1).
(d) . Parasitism experiment
For each fly strain, 400 L2 were transferred into six replicate plastic containers containing molasses-agar. Forty female and 20 male wasps were added to three containers (‘wasp’ treatment) while the other three were left unmanipulated (‘no-wasp’ treatment); all containers were left for 24 h. One ‘no-wasp’ container contained only 80 L2s. The L2s were then counted individually (to avoid batch bias) into 40 vials containing either control or 1.25 mM nicotine medium. We pooled data on A4 flies from two separate runs of this experiment (average survival was not significantly different between runs). In run 1 (A4 only), we added 20 larvae to each vial. In run 2 (A4 and A3), we added 16 larvae to each vial. Vials were checked every 1–2 days for pupation and emergence. Parasitism was performed prior to nicotine treatment to avoid exposing L. heterotoma adults to nicotine. Therefore, changes in fly and wasp survival reflect the effects of nicotine consumption by D. melanogaster larvae and not any behavioural change by L. heterotoma.
(e) . Nicotine accumulation experiment
One-thousand A4/A3 L2 were distributed one-by-one from egg-laying chambers into five 1.25 mM nicotine-treated vials. At five developmental stages (3rd-instar larva, day-1 pupa, day-3 pupa [A4 only], day-1 adult, day-3 adult), we collected five individuals and washed them individually in glass dissection wells with deionized H2O. Pupae were removed from vials prior to eclosion to avoid contamination of the adult exoskeleton with nicotine. Individuals from each stage for each vial were pooled and frozen at −20°C.
Frozen flies were thawed and soaked with methanol (50 µl) at room temperature for 2–3 days to reach equilibrium. Crude methanolic extracts were transferred to limited volume autosampler vials and injected directly. Gas chromatographic-mass spectrometric conditions were as previously described [30]; full details are given in the electronic supplementary material.
(f) . Body mass measurement
Threee hundred A3/A4 L2 were placed one-by-one from egg-laying chambers into 20 vials containing either control or 1.25 mM nicotine media. Upon pupation, individuals were removed and placed into food-free vials. Adults were starved for 48 h and then weighed.
(g) . Statistical analysis
Statistical analyses were conducted using R v. 4.1.1 [31]. LC50s were calculated using a version of the ‘dose.p’ function from the ‘MASS’ package [32] adapted to a binomial regression model of normalized percentage survival versus nicotine dose generated by the ‘glmer’ function from lme4. Fly survival and wasp success were assessed by applying a least-squared-means test to a binomial regression model of survival as a function of nicotine and (for flies) parasite treatments using the ‘glm’ function from lme4 [33]. Adult fly mass was compared by applying the least-squared-means method described above to a model of average mass per vial as a function of nicotine and sex. Developmental rate and mean nicotine content of flies were compared across strains using Wilcoxon signed-rank tests in base R.
Acknowledgements
We thank Kirsten Verster, Jessica Aguilar, Mariana Karageorgi, Luis Jazo and Max Lambert for input on the experimental design, Noah Whiteman for helpful discussions, Stuart Macdonald for DSPR lines, Todd Schlenke for wasps, and three anonymous reviewers for their input.
Contributor Information
Tyler E. Douglas, Email: tyler.douglas@berkeley.edu.
Rebecca D. Tarvin, Email: rdtarvin@berkeley.edu.
Data accessibility
Raw data files, R script and detailed metadata are available for download from the Dryad Digital Repository: https://doi.org/10.5061/dryad.w3r2280sc [34]. The data are also provided in the electronic supplementary material.
Authors' contributions
T.E.D.: conceptualization, data curation, formal analysis, investigation, methodology, software, validation, visualization, writing—original draft, writing—review and editing; S.G.B.: investigation, methodology, writing—review and editing; C.E.G.: investigation, methodology, writing—review and editing; B.E.N.: investigation, methodology, writing—review and editing; K.E.T.: investigation, writing—review and editing; R.W.F.: data curation, investigation, methodology, resources, supervision, validation, writing—review and editing; R.D.T.: conceptualization, investigation, methodology, resources, supervision, writing—original draft, writing—review and editing. All authors gave final approval for publication and agreed to be held accountable for the work described herein.
Competing interests
We declare we have no competing interests.
Funding
R.D.T. was supported by the Miller Institute for Basic Research in Science, UC Berkeley start-up funds and the Hellman Fellows Program; R.W.F. was supported by NSF CCLI-DUE-0942345 and DEB-1556982.
References
- 1.Daborn PJ, et al. 2002. A single p450 allele associated with insecticide resistance in Drosophila. Science 297, 2253-2256. ( 10.1126/science.1074170) [DOI] [PubMed] [Google Scholar]
- 2.Li X, Berenbaum MR, Schuler MA. 2002. Plant allelochemicals differentially regulate Helicoverpa zea cytochrome P450 genes. Insect Mol. Biol. 11, 343-351. ( 10.1046/j.1365-2583.2002.00341.x) [DOI] [PubMed] [Google Scholar]
- 3.Kumar P, Pandit SS, Steppuhn A, Baldwin IT. 2014. Natural history-driven, plant-mediated RNAi-based study reveals CYP6B46’s role in a nicotine-mediated antipredator herbivore defense. Proc. Natl Acad. Sci. USA 111, 1245-1252. ( 10.1073/pnas.1314848111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Duffey SS. 1980. Sequestration of plant natural products by insects. Annu. Rev. Entomol. 25, 447-477. ( 10.1146/annurev.en.25.010180.002311) [DOI] [Google Scholar]
- 5.Petschenka G, Agrawal AA. 2015. Milkweed butterfly resistance to plant toxins is linked to sequestration, not coping with a toxic diet. Proc. R. Soc. B 282, 20151865. ( 10.1098/rspb.2015.1865) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Karageorgi M, et al. 2019. Genome editing retraces the evolution of toxin resistance in the monarch butterfly. Nature 574, 409-412. ( 10.1038/s41586-019-1610-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crossthwaite AJ, Rendine S, Stenta M, Slater R. 2014. Target-site resistance to neonicotinoids. J. Chem. Biol. 7, 125-128. ( 10.1007/s12154-014-0116-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Caty SN, et al. 2019. Molecular physiology of chemical defenses in a poison frog. J. Exp. Biol. 222, jeb204149. ( 10.1242/jeb.204149) [DOI] [PubMed] [Google Scholar]
- 9.Abderemane-Ali F, et al. 2021. Evidence that toxin resistance in poison birds and frogs is not rooted in sodium channel mutations and may rely on ‘toxin sponge’ proteins. J. Gen. Physiol. 153, e202112872. ( 10.1085/jgp.202112872) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tarvin RD, et al. 2017. Interacting amino acid replacements allow poison frogs to evolve epibatidine resistance. Science 357, 1261-1266. ( 10.1126/science.aan5061) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Whitelaw BL, Cooke IR, Finn J, Zenger K, Strugnell JM. 2019. The evolution and origin of tetrodotoxin acquisition in the blue-ringed octopus (genus Hapalochlaena). Aquat. Toxicol. 206, 114-122. ( 10.1016/j.aquatox.2018.10.012) [DOI] [PubMed] [Google Scholar]
- 12.Dobler S, Dalla S, Wagschal V, Agrawal AA. 2012. Community-wide convergent evolution in insect adaptation to toxic cardenolides by substitutions in the Na,K-ATPase. Proc. Natl Acad. Sci. USA 109, 13 040-13 045. ( 10.1073/pnas.1202111109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.King EG, et al. 2012. Genetic dissection of a model complex trait using the Drosophila synthetic population resource. Genome Res. 22, 1558-1566. ( 10.1101/gr.134031.111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yamamoto JECI. 1999. Nicotinoid insecticides and the nicotinic acetylcholine receptor. Tokyo, Japan: Springer Japan. ( 10.1007/978-4-431-67933-2) [DOI] [Google Scholar]
- 15.Fogleman JC. 2000. Response of Drosophila melanogaster to selection for P450-mediated resistance to isoquinoline alkaloids. Chem. Biol. Interact. 125, 93-105. ( 10.1016/S0009-2797(99)00161-1) [DOI] [PubMed] [Google Scholar]
- 16.Humphrey PT, et al. 2016. Aversion and attraction to harmful plant secondary compounds jointly shape the foraging ecology of a specialist herbivore. Ecol. Evol. 6, 3256-3268. ( 10.1002/ece3.2082) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Addicott GE. 1958. Rhodesian tobacco and world markets. S. Afr. J. Econ. 26, 29-40. ( 10.1111/j.1813-6982.1958.tb01773.x) [DOI] [Google Scholar]
- 18.Marriage TN, King EG, Long AD, Macdonald SJ. 2014. Fine-mapping nicotine resistance loci in Drosophila using a multiparent advanced generation inter-cross population. Genetics 198, 45-57. ( 10.1534/genetics.114.162107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Highfill CA, et al. 2017. Naturally segregating variation at Ugt86Dd contributes to nicotine resistance in Drosophila melanogaster. Genetics 207, 311-325. ( 10.1534/genetics.117.300058) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chakraborty M, Emerson JJ, Macdonald SJ, Long AD. 2019. Structural variants exhibit widespread allelic heterogeneity and shape variation in complex traits. Nat. Commun. 10, 4872. ( 10.1038/s41467-019-12884-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Macdonald SJ, Highfill CA. 2020. A naturally-occurring 22-bp coding deletion in Ugt86Dd reduces nicotine resistance in Drosophila melanogaster. BMC Res. Notes 13, 188. ( 10.1186/s13104-020-05035-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rizki RM, Rizki TM. 1984. Selective destruction of a host blood cell type by a parasitoid wasp. Proc. Natl Acad. Sci. USA 81, 6154-6158. ( 10.1073/pnas.81.19.6154) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Snyder MJ, Glendinning JI. 1996. Causal connection between detoxification enzyme activity and consumption of a toxic plant compound. J. Comp. Physiol. A 179, 255-261. ( 10.1007/BF00222792) [DOI] [PubMed] [Google Scholar]
- 24.Kwang PL, et al. 2008. Lifespan and reproduction in Drosophila: new insights from nutritional geometry. Proc. Natl Acad. Sci. USA 105, 2498-2503. ( 10.1073/pnas.0710787105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Salazar-Jaramillo L, Wertheim B. 2021. Does Drosophila sechellia escape parasitoid attack by feeding on a toxic resource? PeerJ 9, e10528. ( 10.7717/peerj.10528) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Milan NF, Kacsoh BZ, Schlenke TA. 2012. Alcohol consumption as self-medication against blood-borne parasites in the fruit fly. Curr. Biol. 22, 488-493. ( 10.1016/j.cub.2012.01.045) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Groen SC, et al. 2017. Multidrug transporters and organic anion transporting polypeptides protect insects against the toxic effects of cardenolides. Insect Biochem. Mol. Biol. 81, 51-61. ( 10.1016/j.ibmb.2016.12.008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hanifin CT. 2010. The chemical and evolutionary ecology of tetrodotoxin (TTX) toxicity in terrestrial vertebrates. Mar. Drugs 8, 577-593. ( 10.3390/md8030577) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Najarro MA, et al. 2015. Identifying loci contributing to natural variation in xenobiotic resistance in Drosophila. PLoS Genet. 11, e1005663. ( 10.1371/journal.pgen.1005663) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fitch RW, Spande TF, Garraffo HM, Yeh HJC, Daly JW. 2010. Phantasmidine: an epibatidine congener from the Ecuadorian poison frog Epipedobates anthonyi. J. Nat. Prod. 73, 331-337. ( 10.1021/np900727e) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.R Development Core Team. 2021. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. See http://www.R-project.org/. [Google Scholar]
- 32.Venables WN, Ripley BD. 2002. Modern applied statistics with S, 4th edn, p. 193. Cleveland, QL, Australia: Springer: Springer. [Google Scholar]
- 33.Bates D, Mächler M, Bolker B, Walker S. 2015. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1-48. ( 10.18637/jss.v067.i01) [DOI] [Google Scholar]
- 34.Douglas TE, Beskid SG, Gernand CE, Nirtaut BE, Tamsil KE, Fitch RW, Tarvin RD. 2021. Data from: Trade-offs between cost of ingestion and rate of intake drive defensive toxin use. Dryad Digital Repository. ( 10.5061/dryad.w3r2280sc) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Douglas TE, Beskid SG, Gernand CE, Nirtaut BE, Tamsil KE, Fitch RW, Tarvin RD. 2021. Data from: Trade-offs between cost of ingestion and rate of intake drive defensive toxin use. Dryad Digital Repository. ( 10.5061/dryad.w3r2280sc) [DOI] [PMC free article] [PubMed]
Data Availability Statement
Raw data files, R script and detailed metadata are available for download from the Dryad Digital Repository: https://doi.org/10.5061/dryad.w3r2280sc [34]. The data are also provided in the electronic supplementary material.