Abstract
Background
A proposal has recently been advanced to change the traditional definition of nonalcoholic fatty liver disease to metabolic-associated fatty liver disease (MAFLD), to reflect the cluster of metabolic abnormalities that may be more closely associated with cardiovascular risk. Long coronavirus disease 2019 (COVID-19) is a smoldering inflammatory condition, characterized by several symptom clusters. This study aims to determine the prevalence of MAFLD in patients with postacute COVID syndrome (PACS) and its association with other PACS-cluster phenotypes.
Methods
We included 235 patients observed at a single university outpatient clinic. The diagnosis of PACS was based on ≥1 cluster of symptoms: respiratory, neurocognitive, musculoskeletal, psychological, sensory, and dermatological. The outcome was prevalence of MAFLD detected by transient elastography during the first postdischarge follow-up outpatient visit. The prevalence of MAFLD at the time of hospital admission was calculated retrospectively using the hepatic steatosis index.
Results
Of 235 patients, 162 (69%) were men (median age 61). The prevalence of MAFLD was 55.3% at follow-up and 37.3% on admission (P < .001). Insulin resistance (odds ratio [OR] = 1.5; 95% confidence interval [CI], 1.14–1.96), body mass index (OR = 1.14; 95% CI, 1.04–1.24), and the metabolic syndrome (OR = 2.54; 95% CI, 1.13–5.68) were independent predictors of MAFLD. The number of PACS clusters was inversely associated with MAFLD (OR = 0.86; 95% CI, .76–0.97). Thirty-one patients (13.2%) had MAFLD with no other associated PACS clusters. All correlations between MAFLD and other PACS clusters were weak.
Conclusions
Metabolic-associated fatty liver disease was highly prevalent after hospital discharge and may represent a specific PACS-cluster phenotype, with potential long-term metabolic and cardiovascular health implications.
Keywords: COVID, 19, metabolic-associated fatty liver disease
It has been proposed that Non-Alcoholic Fatty Liver Disease should be renamed Metabolic Associated Fatty Liver Disease (MAFLD) with potential long-term cardiovascular complications. MAFLD was highly prevalent after hospital discharge in COVID-19 and may represent a specific PACS-cluster phenotype.
Coronavirus disease 2019 (COVID-19) is a complex disease with long-term sequelae after the resolution of the acute phase [1]. Several of the sequalae that may affect the quality of life and increase the risk of death of patients who survived an episode of COVID-19 have been grouped under the umbrella term of postacute COVID syndrome (PACS).
A multitude of signs and symptoms affecting different organ systems have been described in the postacute COVID state, and they have been grouped qualitatively in clusters: neurocognitive (brain fog, dizziness, loss of attention, confusion), autonomic (chest pain, tachycardia, palpitations), gastrointestinal (diarrhea, abdominal pain, vomiting), respiratory (general fatigue, dyspnea, cough, throat pain), musculoskeletal (myalgias, arthralgias), psychological (posttraumatic stress disorder, anxiety, depression, insomnia), sensory (ageusia, anosmia, hearing loss), and dermatological (hair loss, skin rashes). The prevalence of these clusters has been reported to be as high as 50%–80% among survivors up to 3 months after hospital discharge [2–10].
These clusters may carry a significant weight for society. Indeed, a pessimistic view argues that PACS may occur on a scale large enough to overwhelm the existing healthcare capacity and generate a “hidden public health disaster” [11].
Multiple simultaneously interacting and opposing factors are involved in the pathogenesis of PACS orchestrated in a delicate balance of pro- and anti-inflammatory responses [1]. Although few biomarkers, mainly represented by cytokine signaling (the so-called cytokine storm), have been useful to predict outcome during severe COVID-19 pneumonia, in the postacute phase the utility of these biomarkers is less clear. A complex interplay of persistent inflammation, immunosuppression, and high catabolism may identify a set of immune-metabolic biomarkers associated with PACS [12].
Nonalcoholic fatty liver disease has being classically described as a barometer of metabolic health [13, 14] and carries a high risk of cardiovascular complications and mortality. Metabolic (dysfunction)-associated fatty liver disease (MAFLD), a recently proposed renaming of this disease state [15], describes a target organ damage bidirectionally associated with the metabolic syndrome [16]. Recent studies have proposed that MAFLD is a hepatic manifestation of a multisystem disorder, which is heterogeneous in its underlying causes, presentation, course, and outcomes [13, 17, 18]. For the purpose of this article, we will refer to MAFLD exclusively.
Numerous reports highlighted the negative impact of obesity, diabetes mellitus, and MAFLD on the severity of SARS-CoV-2 infection [19–21]. We explored the prevalence of MAFLD in patients with PACS and its association with other PACS-cluster phenotypes.
METHODS
Study Design
This was a cross-sectional, observational, study that included consecutive patients followed at the Modena PACS Clinic (MPC) from July 2020 to April 2021. The MPC is a referral center established after the first COVID-19 pandemic in Italy, where patients who had severe COVID-19 disease are followed after discharge from Modena University hospital. All patients admitted to the hospital for COVID-19 complications, independent of the severity of their condition, were invited for a follow-up visit. This service was offered free of charge to the patients and the majority of them attended their follow-up visit. Patients were screened for signs and symptoms of PACS and metabolic disorders including MAFLD.
Patient Consent Statement
This was a retrospective study conducted using clinical data anonymized in accordance with the requirements of the Italian Personal Data Protection Act. Patients’ consent was deemed unnecessary by the Regional Ethics Committee of Emilia Romagna according to Italy’s Legislative Decree No. 211/2003. The study was conducted according to the guidelines of the Declaration of Helsinki [22].
Inclusion and Exclusion Criteria
We included all consecutive patients ≥18 years of age who were previously admitted to the hospital for severe COVID-19 pneumonia and were then evaluated for MAFLD at MPC.
Covariables
Demographic and anthropometric data, immune-metabolic variables, and PACS signs and symptoms were collected on the day of the MPC visit. Variables collected during hospitalization included weight and body mass index (BMI) on admission and at discharge, standard biochemical variables, C-reactive protein (CRP) on admission and at peak, interleukin 6 (IL-6) on admission, and use of invasive and noninvasive mechanical ventilation. The hepatic steatosis index (HSI) [23] was calculated on admission, at discharge, and during the MPC visit, when data were available. The HSI was calculated using the following formula: HSI = 8 × (ALT/AST ratio) + BMI (+2, if female; +2, if diabetes mellitus). Values >36 were considered suggestive of the presence of liver steatosis.
Treatments for severe-COVID pneumonia were also used as covariables. Glucocorticoids comprised dexamethasone or methylprednisolone. Dexamethasone was used according to standard of care at 6 mg/day for 10 days. Methylprednisolone 2 mg/kg body weight/day was initiated in patients admitted to the intensive care unit for treatment of acute respiratory distress syndrome [24, 25]. Tocilizumab was administered intravenously at 8 mg/kg (up to a maximum of 800 mg) twice daily, 12 hours apart [26]. In addition, all patients were treated with low molecular weight heparin at prophylactic doses as part of standard of care.
The diagnosis of PACS [6] was made if 1 or more of the following clusters of symptoms were present: respiratory (general fatigue, dyspnea, cough), neurocognitive (dizziness, loss of attention, confusion, memory loss), musculoskeletal (myalgias), psychological (insomnia), sensory (ageusia, anosmia, hearing or vision loss), and dermatological clusters (telogen effluvium and skin rash). Only symptoms reported by patients as qualitatively intense were considered for the analyses. The number of reported PACS symptoms was used as a continuous variable.
Diabetes mellitus was defined as (1) fasting serum glucose levels >126 mg/dL or HbA1C >48 mmol/mol or (2) the current use of glucose-lowering drugs. Insulin resistance was defined by homeostasis model assessment of insulin resistance (HOMA-IR) using the following formula: HOMA-IR = [fasting glucose (mg/dL) × fasting insulin (mU/mL)]/405 [27]. Insulin resistance was defined as HOMA-IR score ≥2. Dyslipidemia was defined as elevated total (>239 mg/dL) or low-density lipoprotein (LDL) cholesterol (>130 mg/dL) or low high-density lipoprotein (HDL) cholesterol (<45 mg/dL). Metabolic syndrome was defined using MetS ATPIII classification [28], including 3 or more of the following criteria: waist circumference over 102 cm (men) or 88 cm (women), blood pressure over 130/85 mmHg, fasting triglycerides level >150 mg/dL, fasting HDL cholesterol level <40 mg/dL (men) or <50 mg/dL (women), and fasting blood glucose >100 mg/dL [28]. Physical activity was assessed with the International Physical Activity Questionnaire (IPAQ) and expressed as metabolic equivalent of task (MET) [29]. A MET is the ratio of the rate of energy expended during an activity to the rate of energy expended at rest (1 MET is the rate of energy expenditure while at rest). Level of physical activity was categorized as low (MET score <600) or moderate/intense (MET score >601).
Outcome Measures
The primary outcome of these analyses was prevalence of MAFLD at the time of the first visit in the MPC. Metabolic-associated fatty liver disease was defined as the presence of liver steatosis and at least one of the following criteria [15]: (1) overweight/obesity (defined as BMI ≥25 kg/m2) and (2) type 2 diabetes mellitus (described above). Criteria for type 2 diabetes included the following: lean/normal weight (defined as BMI <25 kg/m2) and at least 2 of the following: (1) waist circumference ≥102/88 cm in men/women; (2) blood pressure ≥130/85 mmHg or specific drug treatment; (3) triglycerides ≥150 mg/dL or specific drug treatment; (4) HDL cholesterol <40/50 mg/dL for men/women; (5) HOMA-IR ≥2.
Hepatic steatosis was defined by controlled attenuation parameter (CAP) using transient elastography (TE) with M probe considering a CAP cutoff value ≥248 dB/m [30]. The TE measurements were performed in the fasting state. People with significant alcohol intake and hepatitis B or C infection were excluded.
The estimated prevalence of MAFLD on hospital admission was calculated using the following formula: prevalence of hepatic steatosis using HSI on hospital admission; prevalence of hepatic steatosis using HSI at follow-up visit = X; prevalence of MAFLD using TE, in which X was prevalence of MAFLD using TE.
Statistical Analysis
Data were expressed as (1) mean ± standard deviation (SD) for normally distributed continuous variables, (2) median and interquartile range (IQR) for nonnormally distributed continuous variables, and (3) frequencies and percentages for categorical variables. Student’s t test was used to identify statistical differences for normally distributed continuous variables, whereas Mann-Whitney and Kruskal-Wallis tests were used for nonnormally distributed continuous variables. The χ2 test was applied for categorical variables.
Multivariable logistic regression models included covariables with a P < .10 in univariable analysis. All univariable and multivariable models were performed in the entire cohort and separately in women and men, including BMI, the metabolic syndrome, HOMA, and the number of reported PACS symptoms.
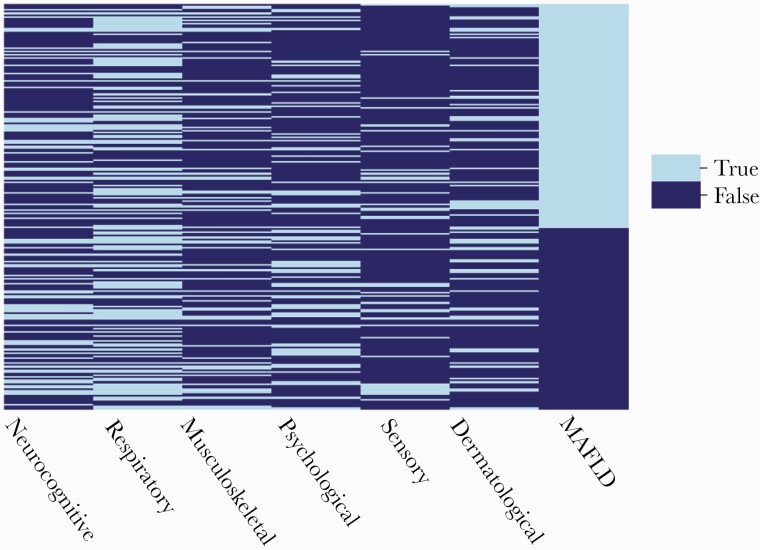
To assess the overlap between MAFLD and PACS symptom clusters, we developed a heatmap for categorical variables. Each line represents a single individual, and a color code was used to identify the presence or absence of a cluster, ranked from the highest to the lowest CAP value that defined MAFLD (Figure 1).
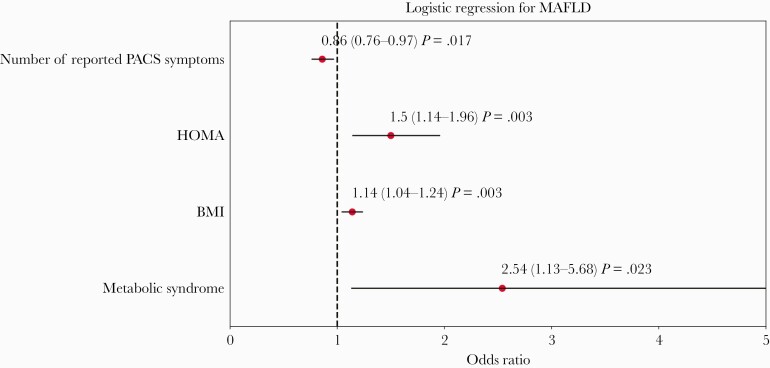
Figure 1.
Multivariate logistic model to identify independent predictors of metabolic associated fatty liver disease (MAFLD). Abbreviations: BMI, body mass index; HOMA, Homeostatic Model Assessment for Insulin Resistance; PACS, post-acute COVID-19 syndrome.
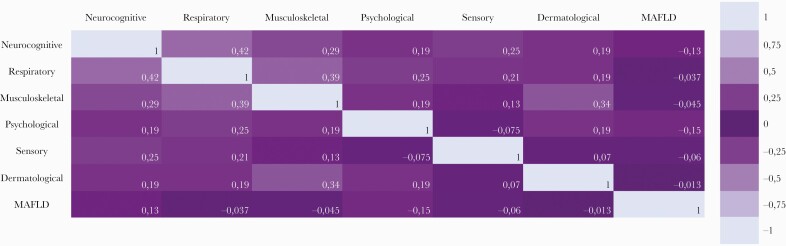
Finally, the Pearson correlation coefficient was used to explore correlations between PACS clusters and MAFLD. Correlations were also visualized by means of a heatmap. The correlation coefficient (r) varies from −1 to + 1. The strength of association was considered very weak if r = 0–0.19, weak if r = 0.2–0.39, moderate if r = 0.40–0.59, strong if r = 0.6–0.79, and very strong if r = 0.8–1 (Figure 2) [31].
Figure 2.
Heatmap of categorical variables intersecting metabolic associated fatty liver disease (MAFLD) and post-acute COVID-19 syndrome (PACS) clusters.
All statistical tests were 2-sided and assumed a significance level of 5%. The open source Python 3.9 was used for statistical analysis and data cleansing.
RESULTS
Between July 2020 and April 2021, 235 patients were included in this study. One hundred sixty-two (69%) were men, and median age was 61 (Q1, Q3: 52.0–72.5) years. Mean duration of hospitalization was 11.7 (SD = 10.5) days, and 45 (19.2%) received invasive or noninvasive mechanical ventilation. The first PACS clinic visit occurred at a median of 143 (IQR, 130–163.5) days from symptoms onset.
One hundred eighty-two (77.5%) patients reported at least 1 PACS cluster at the time of MPC visit. Specifically, neurocognitive cluster was reported by 82 patients (34.9%), respiratory cluster was reported by 125 patients (53.2%), musculoskeletal cluster was reported by 62 patients (26.4%), psychological cluster was reported by 69 patients (29.4%), sensory cluster was reported by 41 patients (17.5%), and dermatological was reported by 59 patients (25.1%).
All patients with liver steatosis were shown to be affected by MAFLD. Metabolic-associated fatty liver disease was present in 130 patients during the MPC visit (55.3% of the study population: 95 men and 35 women). In detail, 123 had a BMI >25 kg/m2, 26 had diabetes mellitus, 24 had both BMI >25 kg/m2 and diabetes mellitus, 1 had a BMI <25 kg/m2 and diabetes mellitus, and 4 had a BMI <25 kg/m2 with no diabetes mellitus but had insulin resistance or dyslipidemia.
The HSI could be calculated on 103 of 235 patients. Its prevalence on admission and discharge was similar (50% vs 48.1%), whereas during the MPC visit it was significantly higher (71.3%). Using proportional estimates between HSI and TE data, we calculated that the prevalence of MAFLD on admission to the hospital would have been 37.3% (vs 55.3% at MPC visit, P < .001) if TE had been used as a diagnostic tool.
Table 1 shows the demographic, anthropometric, and metabolic characteristics during hospitalization and at the time of MPC visit in patients with or without MAFLD. Patients with MAFLD had a higher BMI (30.2 vs 26.8 kg/m2, P < .001), a larger waist circumference (106 vs 97 cm, P < .001), a higher daily caloric intake (1860 vs 1720 Kcal, P = .02), and a higher prevalence of the metabolic syndrome (39.2% vs 13.3%, P < .001), insulin resistance (70.6% vs 31.3%, P < .001), and diabetes mellitus (20% vs 6.7%, P < .006). The weight change during hospitalization (−6 kg [Q1, Q3: −10, −3 kg]) and from hospital discharge to the MPC visit (+5 kg [Q1, Q3: +2, +7 kg]) were the same in patients with and without MAFLD at follow-up.
Table 1.
Demographic, Anthropometric, and Immune-Metabolic Markers in Patients With or Without Metabolic-Associated Fatty Liver Disease (MAFLD)a
| Total | No MAFLD | MAFLD | P | |
|---|---|---|---|---|
| (N = 235) | (N = 105, 44.7%) | (N = 130, 55.3%) | ||
| Demographic and Anthropometric Characteristics | ||||
| Male sex, N (%) | 162 (68.9%) | 67 (63.8%) | 95 (73.1%) | .17 |
| Age, years, median (Q1, Q3) [No.] | 61.0 (52.0–72.5) [235] | 63.0 (52.0–74.0) [105] | 60.0 (52.0–70.0) [130] | .17 |
| Waist circumference, cm, median (Q1, Q3) [No.] | 103.0 (96.0–111.0) [216] | 97.0 (90.0–104.0) [93] | 106.0 (101.0–114.0) [123] | <.001 |
| BMI, kg/m2, median (Q1, Q3) [No.] | 29.0 (26.1–31.9) [227] | 26.8 (24.3–29.4) [100] | 30.7 (28.2–33.5) [127] | <.001 |
| BMI on admission, kg/m2, median (Q1, Q3) [No.] | 29.4 (26.0–32.4) [224] | 26.5 (23.7–30.9) [97] | 30.5 (28.4–34.4) [127] | <.001 |
| BMI at discharge, kg/m2, median (Q1, Q3) [No.] | 27.1 (23.7–30.1) [224] | 24.5 (21.7–27.5) [97] | 28.3 (26.4–31.7) [127] | <.001 |
| Physical activity (moderate/intense), N (%) | 41 (17.5%) | 20 (19.1%) | 21 (16.2%) | .31 |
| Daily calories intake, calories, median (Q1, Q3) [No.] | 1800.0 (1631.3–2000.0) [122] | 1720.0 (1550.0–2000.0) [49] | 1860.0 (1670.0–2000.0) [73] | .02 |
| Involuntary weight loss during prior year, N (%) | 115 (48.9%) | 44 (41.9%) | 71 (54.6%) | .32 |
| Weight change T1-T0, kg, median (Q1, Q3) [No.] | −6.0 (−10.0 to −3.0) [220] | −5.25 (−10.0 to −3.0) [96] | −6.0 (−10.0 to −3.0) [124] | .74 |
| Weight change T2-T1, kg, median (Q1, Q3) [No.] | 5.0 (2.0–7.0) [219] | 5.0 (2.0–8.0) [95] | 4.75 (2.0–7.0) [124] | .69 |
| Hospitalization Data | ||||
| Invasive or noninvasive mechanical ventilation, N (%) | 45 (19.2%) | 21 (20.0%) | 24 (18.5%) | .90 |
| C-reactive protein on admission, mg/dL, median (Q1, Q3) [No.] | 7.1 (3.2–14.4) [232] | 7.2 (3.3–14.2) [104] | 6.9 (3.1–15.2) [128] | .69 |
| C-reactive protein peak during hospitalization, mg/dL, median (Q1, Q3) [No.] | 8.9 (4.3–17.3) [232] | 8.2 (4.6–16.7) [104] | 9.2 (4.2–17.4) [128] | .98 |
| Interleukin-6 on admission, ng/mL, median, (Q1, Q3) [No.] | 126.9 (46.1–334.6) [147] | 165.1 (64.7–354.1) [63] | 111.0 (29.2–298.7) [84] | .79 |
| Use of glucocorticoids, N (%) | 84 (35.7%) | 31 (29.5%) | 53 (40.8%) | .10 |
| Use of tocilizumab, N (%) | 109 (46.4%) | 47 (44.8%) | 62 (47.7%) | .75 |
| Time between symptom initiation and MPC visit, days, median (Q1, Q3) [No.] | 144.0 (130.0–167.5) [223] | 143.5 (131.8–161.3) [100] | 145.0 (129.5–168.0) [123] | .19 |
| Duration of hospitalization, days, mean (SD) [No.] | 11.8 (10.3) [235] | 11.5 (10.2) [105] | 12.1 (10.3) [130] | .61 |
| Hematological and Metabolic Biomarkers at Follow-up Visit | ||||
| Glucose, mg/dL, median (Q1, Q3) [No.] | 94.0 (85.0–106.0) [211] | 93.0 (85.0–101.0) [93] | 98.0 (85.25–120.0) [118] | .002 |
| AST, U/L, median (Q1, Q3) [No.] | 36.0 (27.0–53.0) [149] | 35.0 (27.0–48.0) [57] | 37 (26.8–55.0) [92] | .71 |
| ALT, U/L, median (Q1, Q3) [No.] | 31.0 (21.0–48.0) [234] | 23.0 (19.0–25.0) [105] | 36.0 (23.5–49.5) [129] | .001 |
| Platelets, ×109/L, median (Q1, Q3) [No.] | 214.5 (169.0–272.0) [232] | 199.0 (162.3–258.5) [104] | 230.0 (173.5–286.25) [128] | 0.02 |
| HOMA index, median (Q1, Q3) [No.] | 2.2 (1.4–3.8) [195] | 1.5 (1.2–2.2) [88] | 3.1 (2.0–4.8) [107] | <.001 |
| Total cholesterol, mg/dL, median (Q1, Q3) [No.] | 198.0 (168.0–229.0) [207] | 195.0 (166.5–220.0) [91] | 201.0 (169.8–238.0) [116] | <.001 |
| LDL cholesterol, mg/dL, median (Q1, Q3) [No.] | 129.0 (102.0–153.5) [207] | 122.0 (98.5–147.0) [91] | 132.0 (104.0–164.3) [116] | .03 |
| HDL cholesterol, mg/dL, median (Q1, Q3) [No.] | 52.0 (45.0–60.5) [207] | 53.0 (46.0–60.5) [91] | 51.0 (45.0–60.3) [116] | .45 |
| Total cholesterol to HDL cholesterol ratio, median (Q1, Q3) [No.] | 3.8 (3.0–4.4) [207] | 3.6 (2.9–4.2) [91] | 4.0 (3.1–4.7) [116] | .02 |
| Triglycerides, mg/dL, median (Q1, Q3) [No.] | 115.0 (88.0–160.0) [207] | 103.0 (84.0–135.0) [91] | 127.0 (98.75–185.5) [116] | <.001 |
| ASCVD at follow-up visit, median (Q1, Q3) [No.] | 10.4 (5.1–21.3) [131] | 11.6 (4.5–20.7) [48] | 8.76 (5.7–21.7) [83] | .58 |
| Use of statins, N (%) | 46 (19.6%) | 15 (14.3%) | 31 (23.9%) | .09 |
| Immune-Metabolic Diseases | ||||
| Diabetes mellitus, N (%) | 33 (14.0%) | 7 (6.7%) | 26 (20.0%) | .006 |
| Hypertension, N (%) | 70 (29.8%) | 27 (25.7%) | 43 (33.1%) | .80 |
| Metabolic syndrome, N (%) | 65 (27.7%) | 14 (13.3%) | 51 (39.2%) | <.001 |
| Insulin resistance, N (%) | 85 (36.2%) | 25 (9.4%) | 60 (70.6%) | <.001 |
| PACS | ||||
| Number of reported PACS symptoms, median (Q1, Q3) [No.] | 2.0 (1.0–5.0) [234] | 3.0 (1.0–5.0) [105] | 2.0 (1.0–4.0) [129] | .03 |
| PACS, N (%) | 182 (77.5%) | 83 (79.1%) | 99 (76.2%) | .71 |
| Respiratory cluster, N (%) | 125 (53.2%) | 58 (55.2%) | 67 (51.5%) | .66 |
| Musculoskeletal cluster, N (%) | 62 (26.4%) | 30 (28.6%) | 32 (24.6%) | .59 |
| Neurocognitive cluster, N (%) | 82(34.9%) | 44 (41.9%) | 38 (29.2%) | .06 |
| Psychological cluster, N (%) | 69 (29.4%) | 39 (37.1%) | 30 (23.1%) | .03 |
| Sensory cluster, N (%) | 41 (17.5%) | 21 (20.0%) | 20 (15.4%) | .45 |
| Dermatological cluster, N (%) | 59 (25.1%) | 27 (25.7%) | 32 (24.6%) | .97 |
Abbreviations: ALT, alanine aminotransferase; ASCVD, atherosclerotic cardiovascular disease risk algorithm from American Heart Association/American College of Cardiology; AST, aspartate aminotransferase; BMI, body mass index; HDL, high-density lipoprotein; HOMA, homeostatic model assessment for insulin resistance; LDL, low-density lipoprotein; MAFLD, metabolic-associated fatty liver disease; MPC, Modena PACS Clinic; [No.], number of people in whom the given variable was available; PACS, postacute COVID-19 syndrome; Q1, Q3, lower and upper quartile; SD, standard deviation.
Physical activity was assessed with the International Physical Activity Questionnaire (IPAQ).
Glucocorticoids (40.8% vs 29.5%, P = .10) and tocilizumab (47.7% vs 44.8%, P = .75) during hospitalization were used in a similar proportion of patients with and without MAFLD. Remdesivir was used in 3 patients only; therefore, statistical tests were not performed for this variable.
Supplementary Tables 1 and 2 show the clinical characteristics of men and women with or without MAFLD. Of note, men with MAFLD reported a lower physical activity after discharge (16.8% vs 26.9%, P = .03), whereas women with MAFLD had a higher CRP on admission (6.1 mg/dL vs 4.3 mg/dL, P = .04) and peak CRP during hospitalization (12.2 mg/dL vs 5.4 mg/dL, P = .03).
Table 2 shows the anthropometric, metabolic, and clinical variables collected during hospitalization and PACS symptoms significantly associated in univariable analyses with MAFLD. Supplementary Table 3 shows the anthropometric, metabolic, and clinical variables collected during hospitalization and PACS symptoms significantly associated in univariable analyses with MAFLD in men (Supplementary Table 3A) and women (Supplementary Table 3B).
Table 2.
Univariable Analysis of Factors Associated With MAFLD
| Variables | Odds ratios (95% CI) | P |
|---|---|---|
| Weight at MPC visit | 1.07 (1.05–1.09) | <.001 |
| Weight on admission | 1.05 (1.03–1.08) | <.001 |
| Weight at hospital discharge | 1.05 (1.03–1.08) | <.001 |
| BMI at MPC visit | 1.22 (1.14–1.32) | <.001 |
| BMI on hospital admission | 1.11 (1.06–1.17) | <.001 |
| BMI at hospital discharge | 1.10 (1.05–1.15) | <.001 |
| Waist circumference | 1.07 (1.04–1.10) | <.001 |
| Total cholesterol | 1.01 (1.0–1.01) | .054 |
| LDL cholesterol | 1.01 (1.0–1.01) | .051 |
| Total cholesterol to HDL cholesterol ratio | 1.37 (1.05–1.80) | .02 |
| Triglycerides | 1.01 (1.0–1.02) | <.001 |
| HOMA | 1.98 (1.53–2.55) | <.001 |
| Obesity | 4.67 (2.59–8.41) | <.001 |
| Diabetes mellitus | 3.5 (1.45–8.43) | .005 |
| Metabolic syndrome | 4.19 (2.13–8.23) | <.001 |
| Systolic blood pressure | 1.02 (1.0–1.03) | .08 |
| Diastolic blood pressure | 1.05 (1.02–1.09) | .002 |
| Use of glucocorticoids | 1.64 (0.95–2.84) | .08 |
| Use of statins at MPC visit | 1.88 (0.95–3.71) | .07 |
| Number of reported PACS symptoms | 0.9 (0.82–0.99) | .03 |
| Neurocognitive PACS | 0.57 (0.33–0.98) | .04 |
| Psychological PACS | 0.51 (0.29–0.9) | .02 |
Abbreviations: BMI, body mass index; CI, confidence interval; HDL, high-density lipoprotein; HOMA, homeostatic model assessment for insulin resistance; LDL, low-density lipoprotein; MAFLD, metabolic-associated fatty liver disease; MPC, Modena PACS Clinic; PACS, postacute COVID-19 syndrome.
Multivariable analyses confirmed that independent predictors of MAFLD were HOMA-IR, BMI, and the metabolic syndrome, whereas the number of PACS clusters was inversely associated with MAFLD (Figure 3). Supplementary Figure 1A and B show the results of multivariable analyses conducted in men and women separately, after including only covariables with a P < .10 on univariable analyses. Of note, physical activity was predictive of the absence of MAFLD (odds ratio [OR] = 0.31; 95% confidence interval [CI], 0.1–0.9; P = .032) in men, offsetting the risk brought by HOMA-IR and the metabolic syndrome. In women, the variables associated with MAFLD were the number of PACS clusters (OR = 0.62; 95% CI, 0.46–0.84; P = .002) and HOMA-IR (OR = 1.91; 95% CI, 1.03–3.54; P = .039).
Figure 3.
Correlation between different post-acute COVID-19 syndrome (PACS) clusters and metabolic associated fatty liver disease (MAFLD) explored with Pearson correlation coefficient and shown as a heatmap with values ranging from -1 to 1.
Figure 1 shows the heatmap of categorical variables intersecting MAFLD and PACS clusters. Of note, 31 patients (13.2% of the cohort) had MAFLD with no other associated PACS clusters. Metabolic-associated fatty liver disease was coexisting with the (1) respiratory cluster in 67 patients (28.5%), (2) neurocognitive cluster in 38 patients (16.2%), (3) psychological cluster in 30 patients (12.8%), (4) musculoskeletal cluster in 32 patients (13.6%), (5) sensory cluster in 20 patients (8.5%), and (6) dermatological cluster in 32 patients (13.6%). Twenty-two (9.1%) patients had neither PACS clusters nor MAFLD. Among the 31 patients with MAFLD only, 25 (80.6%) were men, their median age was 61 (Q1, Q3: 56, 70) years, 6 (19.4%) had diabetes mellitus, and 11 (35.5%) were obese.
The correlation between PACS clusters and MAFLD explored with the Pearson coefficient is shown in Figure 2 as a heatmap with values ranging from −1 to +1. All associations were weak or very weak.
DISCUSSION
To the best of our knowledge, this is the first study that describes the prevalence of MAFLD using transient elastography in patients being evaluated for PACS. These data show that MAFLD was a highly prevalent condition at follow-up and may represent an independent PACS-cluster phenotype, with potential metabolic and cardiovascular health consequences in long-COVID-19.
The multisystemic nature of MAFLD and its association with immune-metabolic conditions and adverse outcomes have raised awareness regarding the need for screening for this condition and the identification of patients at risk even outside the hepatology arena. In a recent study, an international panel of experts have detailed the rationale for updating the criteria to describe the liver disease associated with known metabolic dysfunction [15, 32].
Although disagreement still exists regarding the impact and consequences of changing the terminology based on the available evidence [33], the novel definition of MAFLD seems suitable to serve as an excellent barometer of metabolic health.
The MAFLD prevalence of 55.3% of observed in the present study in ambulatory patients with long-COVID-19 is more than double that observed in the general population [34], raising the possibility that this condition may be a new PACS phenotype. Diabetes mellitus and obesity, which contribute to the diagnosis of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, and both are associated with increased mortality [19–21]. In the absence of any data on the presence of MAFLD before COVID-19 in our patients, we cannot exclude a selection bias of metabolically unhealthy individuals being overrepresented in our cohort. The calculated prevalence of MAFLD using the HSI on admission and discharge from the hospital was similar to that reported in the general Italian population [35]. However, it increased from discharge to MPC visit (from 50% to 71.3%). As shown in other observational studies [36], a high prevalence of metabolic impairment was observed at the time of follow-up, suggesting that MAFLD may represent a novel cluster of PACS. We explored the association between MAFLD and the weight changes that occurred during hospitalization (−6 kg) or after discharge (+5 kg). We speculate that the increased prevalence of MAFLD in long-COVID is potentially associated with body composition changes characterized by rapid lean mass loss during the acute phase and liver fat accumulation in the recovery phase.
In a recent publication, Gao et al [37] reported that after adjustment for age, sex, and metabolic comorbidities, an increased serum IL-6 level was associated with higher risk of severe COVID-19 among infected patients with MAFLD, suggesting a pathogenetic link between inflammatory patterns of COVID-19 and this metabolic disorder. We observed an association between MAFLD and CRP levels during hospitalization (both on admission and at its peak) in women, but this signal was not present at the time of ambulatory visit. At that time, the driver for MAFLD may not be represented by an acute inflammatory state but by metabolic impairment.
In this cohort, MAFLD was more prevalent in men, as reported by other studies [38, 39]. Women are at higher risk of MAFLD after menopause, suggesting a protective role of estrogens. Moreover, estrogens may also slow down the progression to liver fibrosis and hepatocellular carcinoma [40]. Sex differences are also observed in the pathophysiological pathways involving the adipose tissue, skeletal muscle, and whole-body metabolism [40]. Last but not least, nonalcoholic steatohepatitis is the second leading cause of liver transplantation overall and the leading cause of liver transplantation in females [41], underscoring the need to separately analyze MAFLD patterns in men and women.
Sedentary lifestyles, low physical activity, and excessive daily caloric intake with an imbalanced proportion of macronutrients are the drivers of increasing burden of MAFLD in the general population [42]. In the absence of any approved pharmacotherapy for MAFLD, it may be worth emphasizing physical activity and weight loss as the first-line intervention in patients with MAFLD and PACS.
Many infections, including viral hepatitis, human immunodeficiency virus, and Helicobacter pylori have been shown to promote or exacerbate MAFLD [43]. Specifically, it has been reported that SARS-CoV-2 may alter fatty acids metabolism. These changes are characterized by alterations of the fatty acid desaturase enzyme that leads to variations in the phospholipids and nonesterified fatty acid profiles [44]. We propose that SARS-CoV-2 may induce or hasten progression of MAFLD towards a more severe disease state, making this condition a true phenotype of PACS.
In our analyses, we further explored the association between PACS clusters and MAFLD (Figure 2). The correlations were weak; however, logistic regression analyses showed that a lower number of reported PACS symptoms was associated with greater odds of MAFLD. This may imply that MAFLD is a separate clinical entity in people with PACS and involves multiple pathogenetic pathways.
A few limitations of the present study should be acknowledged, and some of them are intrinsic to the cross-sectional nature of the study. We cannot exclude the effect of unmeasured confounding and bias, such as genetic differences among our study patients. The main issue relates to the lack of MAFLD data in hospitalized patients owing to the difficulty of performing transient elastography in restricted access COVID-19 wards. We have no data on patients with MAFLD who died of COVID-19 or patients afflicted by COVID-19 who were not admitted to the hospital. This makes a potential selection bias in our cohort a true issue.
Nevertheless, the ability to measure HSI in a subgroup of patients confirmed the hypothesis of an increasing incidence of MAFLD in the postacute phase of the disease. This supports the notion that MAFLD may represent a unique metabolic cluster of PACS.
Furthermore, we did not collect data routinely on gastrointestinal cluster because initially there was very little evidence that patients with long-COVID may be frequently affected by gastrointestinal symptoms [1, 45]. However, recent data showed that SARS-CoV-2 antigen and viral particles can be found in goblet cells and enterocytes of the intestinal tissue up to 7 months after the symptom resolution in asymptomatic patients [46].
The relatively small sample size, limited follow-up time, and lack of a control group did not allow us to collect hard endpoints, including incidence of diabetes mellitus and cardiovascular complications. Further studies should explore the impact of MAFLD on long-term morbidity and mortality. In the future, validated questionnaires and tools may help to establish the presence and standardize the intensity of PACS symptoms, as done in other conditions such as rheumatological disorders.
The strength of our study was the ability to describe metabolic health in PACS and the identification of a MAFLD cluster in the context on an already large number of health conditions in long-COVID. We believe these observations and the true clinical outcomes associated with MAFLD cluster are worthy of being analyzed with longer follow-up studies. At the current stage, attention should be paid to the impact of COVID-19 vaccination to prevent metabolic progression among people at risk.
The natural history of MAFLD in the context of PACS is unknown at this time, and careful follow-up of these patients is needed to understand the clinical implications of this syndrome in the context of long-COVID.
CONCLUSIONS
In conclusion, MAFLD was a highly prevalent condition in our cohort of survivors of hospitalized patients with COVID-19, and we speculate that it may be considered as an independent PACS-cluster phenotype, potentially affecting the metabolic and cardiovascular health of patients with PACS.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
We thank Rossella Fogliani from IT services of Modena University Hospital for technical support.
Author contributions: J. M., G. G., and P. R. contributed to conceptualization. J. M., S. B., L. G., E. B., V. I., G. C., G. D., D. Y., E. A., M. B., M. V., and M. M. contributed to methodology. S. B. contributed to software. J. M., S. B., G. G., and P. R. contributed to validation. J. M. and S. B. contributed to formal analysis. J. M., L. G., D. Y., and V. I. contributed to investigation. J. M. and G. G. contributed to resources. J. M., S. B., L. G., and G. G. contributed to data curation. J. M. and G. G. contributed to writing and original draft preparation. J. M., A. L., C. M., G. S., E. C., A. V., B. B., G. G., and P. R. contributed to writing, review, and editing. J. M. and S. B. contributed to visualization. G. G. and P. R. contributed to supervision. G. G. contributed to project administration. All authors have read and agreed to the published version of the manuscript.
Financial support: G. S. is funded by a Senior Salary Award from Fonds de la Recherche en Santé du Quebéc (No. 296306).
Potential conflicts of interest. G. S. has acted as speaker for Merck, Gilead, Abbvie, Novonordisk, Novartis, Pfizer, served as an advisory board member for Pfizer, Merck, Novartis, Gilead, Allergan and Intercept, and has received unrestricted research funding from Merck and Theratec. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Oronsky B, Larson C, Hammond TC, et al. . A review of persistent post-COVID syndrome (PPCS). Clin Rev Allergy Immunol 2021; 1:9. doi: 10.1007/s12016-021-08848-3. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Carfì A, Bernabei R, Landi F, Group for the GAC-19 P.-ACS. Persistent symptoms in patients after acute COVID-19. JAMA 2020; 324:603–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Garrigues E, Janvier P, Kherabi Y, et al. . Post-discharge persistent symptoms and health-related quality of life after hospitalization for COVID-19. J Infect 2020; 81:e4–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Carvalho-Schneider C, Laurent E, Lemaignen A, et al. . Follow-up of adults with noncritical COVID-19 two months after symptom onset. Clin Microbiol Infect 2021; 27:258–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Arnold DT, Hamilton FW, Milne A, et al. . Patient outcomes after hospitalisation with COVID-19 and implications for follow-up: results from a prospective UK cohort. Thorax 2020; 76:399–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mandal S, Barnett J, Brill SE, et al. . “Long-COVID”: a cross-sectional study of persisting symptoms, biomarker and imaging abnormalities following hospitalisation for COVID-19. Thorax 2020; 76:396–8. doi: 10.1136/thoraxjnl-2020-215818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nehme M, Braillard O, Alcoba G, et al. . COVID-19 symptoms: longitudinal evolution and persistence in outpatient settings. Ann Intern Med 2021; 174:723–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tenforde MW, Kim SS, Lindsell CJ, et al. . Symptom duration and risk factors for delayed return to usual health among outpatients with COVID-19 in a multistate health care systems network - United States, March-June 2020. MMWR Morb Mortal Wkly Rep 2020; 69:993–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Stavem K, Ghanima W, Olsen MK, et al. . Persistent symptoms 1.5-6 months after COVID-19 in non-hospitalised subjects: a population-based cohort study. Thorax 2020; 76:405–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Huang C, Huang L, Wang Y, et al. . 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet 2021; 397:220–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Angus DC. The lingering consequences of sepsis: a hidden public health disaster? JAMA 2010; 304:1833–4. [DOI] [PubMed] [Google Scholar]
- 12. Bergmann CB, Beckmann N, Salyer CE, et al. . Lymphocyte immunosuppression and dysfunction contributing to persistent inflammation, immunosuppression, and catabolism syndrome (PICS). Shock 2021; 55: 723–41. [DOI] [PubMed] [Google Scholar]
- 13. Byrne CD, Targher G.. NAFLD: a multisystem disease. J Hepatol 2015; 62:S47–64. [DOI] [PubMed] [Google Scholar]
- 14. Chitturi S, Farrell GC.. Fatty liver now, diabetes and heart attack later? The liver as a barometer of metabolic health. J Gastroenterol Hepatol 2007; 22:967–9. [DOI] [PubMed] [Google Scholar]
- 15. Eslam M, Newsome PN, Sarin SK, et al. . A new definition for metabolic dysfunction-associated fatty liver disease: an international expert consensus statement. J Hepatol 2020; 73: 202–9. [DOI] [PubMed] [Google Scholar]
- 16. Lonardo A, Leoni S, Alswat KA, Fouad Y.. History of nonalcoholic fatty liver disease. Int J Mol Sci 2020; 21:5888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lonardo A. Renaming NAFLD to MAFLD: could the LDE system assist in this transition? J Clin Med 2021; 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lonardo A, Arab JP, Arrese M.. Perspectives on precision medicine approaches to NAFLD diagnosis and management. Adv Ther 2021; 38:2130–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Targher G, Mantovani A, Wang X-B, et al. . Patients with diabetes are at higher risk for severe illness from COVID-19. Diabet Metab 2020; 46:335–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mantovani A, Byrne CD, Zheng M-H, Targher G.. Diabetes as a risk factor for greater COVID-19 severity and in-hospital death: a meta-analysis of observational studies. Nutr Metab Cardiovasc Dis 2020; 30:1236–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Targher G, Mantovani A, Byrne CD, et al. . Risk of severe illness from COVID-19 in patients with metabolic dysfunction-associated fatty liver disease and increased fibrosis scores. Gut 2020; 69: 1545–7. [DOI] [PubMed] [Google Scholar]
- 22. World Medical Association. World medical association declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA 2013; 310: 2191–4. [DOI] [PubMed] [Google Scholar]
- 23. Lee J-H, Kim D, Kim HJ, et al. . Hepatic steatosis index: a simple screening tool reflecting nonalcoholic fatty liver disease. Dig Liver Dis 2010; 42: 503–8. [DOI] [PubMed] [Google Scholar]
- 24. Meduri GU, Siemieniuk RAC, Ness RA, Seyler SJ.. Prolonged low-dose methylprednisolone treatment is highly effective in reducing duration of mechanical ventilation and mortality in patients with ARDS. J Intensive Care 2018; 6:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Steinberg KP, Hudson LD, Goodman RB, et al. . Efficacy and safety of corticosteroids for persistent acute respiratory distress syndrome. N Engl J Med 2006; 354: 1671–84. [DOI] [PubMed] [Google Scholar]
- 26. Guaraldi G, Meschiari M, Cozzi-Lepri A, et al. . Tocilizumab in patients with severe COVID-19: a retrospective cohort study. Lancet Rheumatol 2020; 2:e474–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Matthews DR, Hosker JP, Rudenski AS, et al. . Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985; 28: 412–9. [DOI] [PubMed] [Google Scholar]
- 28. Grundy SM, Cleeman JI, Daniels SR, et al. . Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation 2005; 112: 2735–52. [DOI] [PubMed] [Google Scholar]
- 29. Craig CL, Marshall AL, Sjostrom M, et al. . International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc 2003; 35: 1381–95. [DOI] [PubMed] [Google Scholar]
- 30. European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J Hepatol 2016; 64:1388–402. [DOI] [PubMed] [Google Scholar]
- 31. Akoglu H. User’s guide to correlation coefficients. Turkish J Emerg Med 2018; 18: 91–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Eslam M, Sanyal AJ, George J.. Toward more accurate nomenclature for fatty liver diseases. Gastroenterology 2019; 157: 590–3. [DOI] [PubMed] [Google Scholar]
- 33. Younossi ZM, Rinella ME, Sanyal AJ, et al. . From NAFLD to MAFLD: implications of a premature change in terminology. Hepatology 2021; 73: 1194–8. [DOI] [PubMed] [Google Scholar]
- 34. Younossi Z, Anstee QM, Marietti M, et al. . Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol 2018; 15: 11–20. [DOI] [PubMed] [Google Scholar]
- 35. Petta S, Di Marco V, Pipitone RM, et al. . Prevalence and severity of nonalcoholic fatty liver disease by transient elastography: genetic and metabolic risk factors in a general population. Liver Int 2018; 38: 2060–8. [DOI] [PubMed] [Google Scholar]
- 36. Montefusco L, Ben Nasr M, D’Addio F, et al. . Acute and long-term disruption of glycometabolic control after SARS-CoV-2 infection. Nat Metab 2021; 3: 774–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gao F, Zheng KI, Yan H-D, et al. . Association and interaction between serum interleukin-6 levels and metabolic dysfunction-associated fatty liver disease in patients with severe coronavirus disease 2019. Front Endocrinol (Lausanne) 2021; 12:604100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Browning JD, Szczepaniak LS, Dobbins R, et al. . Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology 2004; 40: 1387–95. [DOI] [PubMed] [Google Scholar]
- 39. Park SH, Jeon WK, Kim SH, et al. . Prevalence and risk factors of non-alcoholic fatty liver disease among Korean adults. J Gastroenterol Hepatol 2006; 21: 138–43. [DOI] [PubMed] [Google Scholar]
- 40. Lonardo A, Nascimbeni F, Ballestri S, et al. . Sex differences in nonalcoholic fatty liver disease: state of the art and identification of research gaps. Hepatology 2019; 70: 1457–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Noureddin M, Vipani A, Bresee C, et al. . NASH leading cause of liver transplant in women: updated analysis of indications for liver transplant and ethnic and gender variances. Am J Gastroenterol 2018; 113: 1649–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Inoue Y, Qin B, Poti J, et al. . Epidemiology of obesity in adults: latest trends. Curr Obes Rep 2018; 7:276–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Boeckmans J, Rombaut M, Demuyser T, et al. . Infections at the nexus of metabolic-associated fatty liver disease. Arch Toxicol 2021. doi: 10.1007/s00204-021-03069-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Pérez-Torres I, Guarner-Lans V, Soria-Castro E, et al. . Alteration in the lipid profile and the desaturases activity in patients with severe pneumonia by SARS-CoV-2. Front Physiol 2021; 12:667024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Nalbandian A, Sehgal K, Gupta A, et al. . Post-acute COVID-19 syndrome. Nat Med 2021; 27: 601–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Tokuyama M, Ladinsky MS, Jha D, et al. . SARS-CoV-2 persists in intestinal enterocytes up to 7 months after symptom resolution. CROI 2021, March 6–10, 2021. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.