Abstract
Background
Rheumatoid arthritis (RA) is a chronic, inflammatory, system disease. It commonly affects the small peripheral joints (such as fingers and wrist). The main goals of intervention for RA are preventing joint deformity, preserving joint function, and reducing inflammation and pain. Transelectrical nerve stimulation (TENS) is a form of electrotherapy and is thought to produce analgesia according to the gate control theory.
Objectives
To determine the efficacy and safety of TENS in the treatment of RA of the hand. The primary outcomes of interest were relief of grip pain and resting pain intensity, relief of joint tenderness, number of tender joints and patient assessment of disease. The secondary objective was to determine the most effective mode of TENS application in pain control.
Search methods
We searched for relevant studies, in English, in the Cochrane field of physical and related therapies, the Cochrane Controlled Trials Register, MEDLINE, EMBASE, HEALTHSTAR, Sports Discus, CINAHL, Current Contents, and the PEDro database, up to October 2002.
Selection criteria
Two independent reviewers selected the trials that met predetermined inclusion criteria.
Data collection and analysis
Study results were extracted by two independent reviewers. Continuous outcomes were analyzed by weighted mean difference (WMD) using a fixed effects model.
Main results
Three RCTs, involving 78 people, were included in this review. AL‐TENS and C‐TENS were compared to placebo and to each other. Administration of 15 minutes of AL‐TENS a week, for 3 weeks, resulted in a significant decrease in rest pain (67% relative benefit, 45 points absolute benefit on 100 mm VAS scale) but not in grip pain compared to placebo. AL‐TENS did result in a clinical beneficial improvement in muscle power scores with a relative difference of 55%, and an absolute benefit of 0.98, compared to placebo. No significant difference was found between one 20‐minute treatment duration of C‐TENS versus AL‐TENS , or C‐TENS versus placebo on decrease in mean scores for rest pain or grip pain, or on the number of tender joints. Results showed a statistically significant reduction in joint tenderness, but no clinical benefit from C‐TENS over placebo in relief of joint tenderness. No statistically significant difference was shown between 15 days of treatment with C‐TENS or AL‐TENS in relief of joint pain, although there was a clinically important benefit of C‐TENS over AL‐TENS on patient assessment of change in disease (risk difference 21%, NNT 5).
Authors' conclusions
There are conflicting effects of TENS on pain outcomes in patients with RA. AL‐TENS is beneficial for reducing pain intensity and improving muscle power scores over placebo while, conversely, C‐TENS resulted in no clinical benefit on pain intensity compared with placebo. However C‐TENS resulted in a clinical benefit on patient assessment of change in disease over AL‐TENS. More well designed studies with a standardized protocol and adequate number of subjects are needed to fully conclude the effect of C‐TENS and AL‐TENS in the treatment of RA of the hand.
Plain language summary
Transelectrical nerve stimulation (TENS) helps decrease hand pain in people with rheumatoid arthritis
There are three main therapeutic methods of administrating TENS. Conventional TENS (C‐TENS) is given at a high stimulation frequency with low intensity. While pain relief is almost immediate, it generally dissipates as soon as the TENS is turned off. A second method is acupuncture‐like TENS (AL‐TENS). This is given at a low frequency and high intensity, close to the person's limit of tolerance. Many people find this method uncomfortable. The third TENS application method is burst TENS, which is high frequency burst impulses at low‐intensity. Results from this Cochrane review indicate that AL‐TENS helps decrease pain and joint tenderness compared to a placebo. No benefit was found on grip pain. More people who received conventional TENS reported a decrease in their disease activity than those who received acupuncture‐like TENS.
Background
Rheumatoid arthritis (RA) is a chronic, systemic, inflammatory disease that mainly affects the synovial membranes of many joints of the body. Although it can occur at any age, the onset of RA is usually occurs during adulthood, between the ages of 20 to 40 years (Schumacher 1993). Many joints of the body can be affected by RA, including the joints of the hand. Joints that are actively involved are usually tender, swollen and likely to be limited in motion (Morgan 1995). Early in RA the synovium is usually the first to be affected by inflammation and edema. As the synovium grows in response to RA, pannus is formed. The appearance of this destructive tissue, along with immunological alterations in the synovial fluid, results in the destruction of all tissues and structures around the joint with RA. These changes result in limited motion and function of the joint as well as disfigurement. It is therefore important to prevent disability, preserve bodily function and reduce pain, inflammation and disfigurement. Pain, discomfort and stiffness may be relieved by a variety of treatments such as medication, hot or cold therapy, rest, exercise and electrotherapy (Luckmann 1990).
Electrotherapy is commonly used in the physical rehabilitation of patients with RA to relieve pain and improve function (Cameron 1999). Transcutaneous electrical nerve stimulation (TENS) is a widely used form of electroanalgesia, with the existence of many clinical reports and studies concerning its use. TENS is thought to produce analgesia according to the gate control theory put forward by Melzack & Wall (Melzack 1965). Its therapeutic application is not standardized or empirical, and there is no consensus on its efficacy in patients with RA, at present. The electrical stimuli delivered by TENS units can be varied to suit patient tolerance, as well as to produce the best efficacy. Amplitude currency, for example, can be set at low, medium or high intensity (for comfort); pulse width or duration from 10 to 1000 milliseconds; and frequency from 0.5‐10 Hz for high intensity, and 80‐100 impulses per second for lower intensity. The positioning of electrodes may also be important in eliciting analgesia (Mannheimer 1986). The placement of electrodes is dependent upon getting optimal stimulation from the mode of TENS being used. According to the gate control theory approach (Melzack 1965), the stimulus from TENS must be transmitted into the central nervous system (CNS). This transfer is enhanced by electrode placement on optimal sites. They may, for example, be placed directly over the painful area, over cutaneous nerves, acupuncture points, or other trigger points (Mannheimer 1986). Another electrode placement site is over the dermatome zone which is most closely related to the area of pain (Belanger 2002). If two or more of these entities are stimulated simultaneously (due to specific placement of electrodes), then greater specificity of the application will be achieved (Mannheimer 1986). The issue of the most appropriate placement of electrodes for TENS administration is, however, still somewhat controversial (Belanger 2002).
There are three main therapeutic methods of administrating TENS (Kaye 2002). Conventional TENS (C‐TENS) is given at a high stimulation frequency (40‐150 Hz), low intensity, and at a current of 10‐30 mA. Pulse duration is short (< 50 microseconds). While pain relief is almost immediate, it generally dissipates as soon as the TENS is turned off, although some people report residual pain relief for a period of time following application. Patients who use this treatment method tend to apply the TENS electrodes, maintain them in place and administer stimuli periodically throughout the day, usually for 30 minute periods. A second method is acupuncture‐like TENS (AL‐TENS). This is given at a low frequency (1‐10 Hz), high intensity, close to the patient's limit of tolerance. Not all patients do tolerate this method, however, as it is reported to be uncomfortable, even though it may be more efficacious than C‐TENS. The third TENS application method is burst TENS, which is high frequency burst impulses at low‐intensity. Bursts are discharged at 1‐2 Hz and are comprised of 100 Hz frequency impulses.
Laboratory research studies have established good physiological evidence on the efficacy of TENS in reducing inflammation‐induced hyperalgesia in animal models of joint inflammation (Sluka 1999, Sluka 1998, Ma 2001). Differing results have been reported with high and low frequency TENS, confirming the importance of the parameters in evaluating the efficacy of TENS for RA (Sluka 2000).
TENS may be effective for relieving musculoskeletal pain (such as joint pain from RA) in people with RA (Kaye 2002, Jette 1997). TENS can be applied by people themselves as needed, conveniently in their own home. Despite the widespread and ongoing use of TENS by therapists and people with several conditions including RA, for the control of pain, the application of this treatment modality in the clinic is largely based on empiric evidence. TENS is suggested as a potential therapy for the treatment of musculoskeletal conditions in the American Physical Therapy Association guidelines (APTA 2001). The Arthritis Society (Clark 1999) also recommends the use of TENS for pain and joint swelling in people with RA.
The literature contains conflicting reports on the effects of using TENS. Some studies report TENS is beneficial for treating pain while others report no benefit (Belanger 2002). Health care professionals must have strong evidence to be able to make informed decisions about treatment options that are both effective and appropriate.
Objectives
The aim of this systematic review was to evaluate the efficacy of TENS in the treatment of people with RA of the hand.
Methods
Criteria for considering studies for this review
Types of studies
Eligible studies included those of Randomized Clinical Trials (RCTs) and Controlled Clinical Trials (CCTs).
Types of participants
Only trials with subjects aged 18 years or more, with clinical and/or radiological confirmation of RA of the hand were included. The diagnosis of RA was defined according to the criteria of the American Rheumatism Association (ARA 1987).
Types of interventions
All types of TENS were eligible for inclusion in this review. Trials that compared different types of TENS intervention and/or placebo were included.
Types of outcome measures
The primary outcome measure was pain (resting and grip pain)
Secondary outcome measures from the potential core set identified by the OMERACT conference on rheumatoid arthritis outcomes (OMERACT 1993) were sought: Number of tender joints per patient Number of swollen joints per patient Physician global assessment Patient global assessment Functional status Range of motion (ROM)
Strength
Other outcomes included change in muscle power and work.
Search methods for identification of studies
We searched publications in English in the Cochrane Field of Physical and Related Therapies Register up to October 2002, Cochrane Musculoskeletal Group Register, Cochrane Controlled Trials Register, MEDLINE, EMBASE, HEALTHSTAR, Sports Discus, CINAHL, Current Contents, and the PEDro database for published clinical trials of TENS for hand RA, up to October 2002. The systematic search strategy for RCTs designed for the Cochrane Collaboration (Dickersin 1994), modified by Haynes (Haynes 1994), was conducted. The references listed in included studies were searched and additional studies were obtained from content experts. Peer‐reviewed abstracts from conference proceedings and specialized journals were also included, as was information from scientific meetings and from personal communication.
The search strategy for MEDLINE database used is in Appendix 1.
Data collection and analysis
The titles and abstracts of trials identified through the search strategy were examined by two independent reviewers [SR, LL] to select trials that met the inclusion criteria. Trials retrieved had been classified as relevant by at least one reviewer. Retrieved articles were then re‐appraised by the second reviewer using a blind manner to verify they met the inclusion criteria.
From the included trials information was collected regarding the trial design, subject characteristics, treatment methods and periods, baseline and study completion outcomes. The results of the studies were extracted by the two independent reviewers [SR, LL] using predeveloped extraction forms. The data were then cross‐checked by a third reviewer [LB]. The extraction forms had been developed and pilot‐tested, based on other forms used by the Cochrane Musculoskeletal Review Group. The extraction form documented specific information about TENS therapy including 1) method (TENS device characteristics, stimulation mode); 2) methods of TENS application such as the electrode placement, total number of electrodes, treatment time per session, schedule of treatment, total number of treatment sessions, and any specific skin preparation and/or safety precautions. Discrepancies in data were agreed by consensus.
The same two independent reviewers assessed the methodological quality of the studies. This included evaluating the extent to which the trial design, data collection and statistical analysis minimized or avoided biases in the treatment comparisons (Moher 1995). The quality assessment was completed using a validated scale (Jadad 1996, Clark 1999). This scale evaluates randomization, appropriateness of blinding, dropouts and withdrawals and follow‐up. Differences in scoring were resolved by consensus with a third reviewer (CL).
Analyses were based on intention‐to‐treat data from the individual trials. Subgroup analyses were conducted to examine the efficacy of TENS adminstered via different application methods and modes (including frequency, mode, treatment schedule and techniques).
Statistical analysis All of the data from the individual trials were entered into a spreadsheet. This spreadsheet provided the data to the Review Manager software (RevMan 4.0.4) which was used for both descriptive and statistical data. Outcomes were continuous in nature (pain, strength, improvement). Outcomes were analyzed by a weighted mean difference (WMD) using a fixed effects model. A statistical approximation derived from the p‐value was used to estimate the standard deviation when not provided. For dichotomous data, relative risks were used.
When applicable, heterogeneity was assessed with a Chi square test on N degrees of freedom where N is the number of studies. Where statistically significant heterogeneity existed, the results were analyzed by a random effects model. Furthermore, the contributions of pre‐determined hypotheses regarding different populations and interventions were examined as possible sources of heterogeneity.
Clinical benefit
For continuous outcomes when data was available the absolute benefit was calculated as the improvement in the treatment group less the improvement in the control group, in the original units. The relative difference in the change from baseline was calculated as the absolute benefit divided by the baseline mean (weighted for the treated and control group). The relative difference in change was used to provide clinically meaningful information about expected improvement relative to the placebo or untreated group with each intervention.
There is some empirical evidence in rheumatology that greater than 20% improvement is viewed by patients as a clinically important difference between two interventions and that this discriminates active from placebo/control in all the RCTs reviewed for the American College of Rheumatology (Felson 1995) A difference of 2 points on the Roland scale (0‐24 scale) is widely used as a minimally important change for back pain, and this amounts to approximately 15% improvement relative to the control group (when considering the usual baseline Roland scores of 11 or 12) (Guyatt 1996). The Philadelphia Panel decided to accept 15% difference between groups as clinically important. Fifiteen percent was used a minimum criteria in this review.
The risk difference and number needed to treat was also calculated and presented when data allowed. The NNT reflects the effort required (or number of patients one would need to treat) to obtain a beneficial outcome with an intervention. If a single study is available and the event rates in the treatment group (pt) and the control group (pc) are provided then the NNT is the reciprocal of the risk difference (absolute risk reduction or ARR) given by 1/(pc‐pt) or, if the outcome is beneficial, by 1/(pt‐pc). Note, when there is no treatment effect the risk difference is 0 and NNT is infinite. The clinical benefit results are provided in the additional tables of this review.
Results
Description of studies
The search strategies identified nine potential articles. Of these three RCTs were included in the systematic review. The reasons for excluding the other six trials were: 1) post‐surgical people (Angulo 1990); 2) no subjects with RA (Herrera‐Lasso 1993); 3) no control group, people were their own controls (Kumar 1982); 4) not RA population, rabbit joints studied (Levy 1987); 5) subjects did not have RA of the upper extremities (Moystad 1990); and 6) there were only two people per group (Bruce 1988).
The included RCTs involved 78 people with RA (Abelson 1983, Langley 1984, Manheimer 1978). Abelson was a single blind; Langley was double blind; and Mannheimer was not blinded. One study examined the effects of high intensity, low frequency acupuncture‐like TENS (AL‐TENS) versus placebo on resting pain intensity and intensity of pain while gripping, as well as grip strength (Abelson 1983). A second RCT compared the effects of low intensity, high frequency conventional TENS (C‐TENS) or AL‐TENS versus placebo on resting pain intensity, intensity of pain while gripping, grip strength and joint tenderness (Langley 1984). The third included RCT compared three different TENS applications: AL‐TENS‐like (70 Hz, high intensity) applied at the wrist under study, C‐TENS‐like (70 Hz but low intensity) applied at the wrist under study, and C‐TENS‐like (70 Hz, low intensity) applied between the shoulder‐blades, on either side of the spinal processes on the subject's back), for effects on intensity of joint pain (Manheimer 1978).
All of the people in the included trials were diagnosed with classic or definite RA based on clinical and/or radiographic evidence, with one or both hands being affected (American Rheumatism Association criteria). Inclusion in the trial required that people had pain in one or both hands, which required pharmaceutical intervention. Although the populations in the included trials appeared to be homogeneous, the TENS application procedures in the trials were markedly diverse. This included different modes of stimulation, stimulus levels, pulse frequencies, electrode placement, length of stimulation time and frequency of TENS application. The results of this review are discussed in relation to these different TENS application methods. Outcomes measured in the studies also varied between trials.
Risk of bias in included studies
Two independent reviewers assessed the quality of the studies. This included evaluating the extent to which the trial design, data collection and statistical analysis minimized or avoided biases in the treatment comparisons (Moher 1995). The quality assessment was completed using a 5‐point validated scale (Jadad 1996, Clark 1999). This scale evaluates (1) randomization (2 points), (2) appropriateness of blinding (2 points), and (3) dropouts and withdrawals (1 point). Differences in scoring were resolved by consensus with a third reviewer as necessary. One study scored 4, one scored 3, while the third study scored 1 out of a possible maximum of 5 points.
Effects of interventions
EFFICACY 1. AL‐TENS compared to placebo (Abelson 1983)
Administration of 15 minutes of AL‐TENS once weekly, over 3 consecutive weeks, improved muscle power scores by a relative difference of 55% and work scores by a relative difference of 5%, absolute benefit of 0.98, in the TENS group compared to placebo at 3 weeks (see graphs and additional tables). Although improvement in the muscle power score was deemed to be of clinically important benefit, the results were not statistically significant for either muscle power scores (Weighted Mean Difference (WMD) = 0.71 W, 95% Confidence Interval (CI): ‐0.33,1.75; p=0.18) or work scores (WMD = 0.29 J, 95% CI: ‐0.39,0.97; p=0.4) when compared to placebo (Abelson 1983). This study also assessed changes in intensity of pain while resting and while gripping. It was found that grip pain scores were not statistically significantly different between the TENS group and placebo group at the end of 3 weeks of treatment (WMD = ‐12.00 VAS 100mm, 95% CI: ‐29.90,5.90; p=0.19), nor did the results demonstrate any clinical benefit of treatment on grip pain. There was, however, a statistically significantly different, clinically relevant benefit of TENS treatment on intensity of pain while resting when compared to placebo (67% relative difference in change from baseline, absolute benefit of 45 points in a 100 mm VAS scale ; (WMD = ‐59.50 VAS 100mm, 95% CI: ‐76.58,‐42.42; p<0.00001 ).
2. C‐TENS and AL‐TENS compared to placebo (Langley 1984)
No significant difference was found between the administration of C‐TENS versus AL‐TENS (data not shown), or C‐TENS application (one treatment of 20 minutes duration) compared with placebo on the decrease in mean scores for intensity of pain while resting (WMD = ‐0.20 VAS 10mm, 95% CI: ‐4.05,3.65; p=0.9) or intensity of pain while gripping (WMD = 0.70 VAS 10mm, 95% CI: ‐4.11,5.51; p=0.8 (Figure 3)) (Langley 1984). There was no significant difference between C‐TENS and placebo on the number of tender joints reported before and after treatment (WMD = 0.58 (number of tender joints over total joints assessed), 95% CI: 0.14,2.48, p=0.5) (data not shown). Finally, joint tenderness scores were also measured. Results showed no clinical benefit from C‐TENS treatment over placebo (relative difference in change from baseline = 0%, Table 2), although there was a statistically significant reduction in joint tenderness scores (WMD = ‐20.00 (22 point score), 95% CI: ‐33.79,‐6.21; p=0.004).
3. C‐TENS compared to AL‐TENS (Manheimer 1978)
The third included trial evaluated the effects of C‐TENS versus AL‐TENS application (Manheimer 1978) on relief of intensity of joint pain, evaluated by measuring loading tests. Treatments were given for 5 minutes, once a day, for 15 days. At the end of 15 days of treatment there was no statistically significant difference (WMD = 6.43 (number of participants improved), 95% CI: 0.67,61.47; p=0.11) between the two types of TENS on patient assessment of change in disease. There was good evidence, however, of a clinically important benefit (21% risk difference, the number needed to treat was approximately 5), of C‐TENS over AL‐TENS on patient assessment of change in disease.
Subgroup Analysis
No subgroup analysis on high (Jadad total score over 3/5) versus low (Jadad total score below or equal to 3/5) quality studies was undertaken as none of the studies examined the same type of TENS or used similar treatment schedules. Due to the small number of trials, the remaining pre‐planned subgroup analyses (treatment duration, type of TENS application, patient characteristics, disease characteristics, and design considerations) were not conducted. Publication bias was not assessed due to the small number of trials.
2. Safety Adverse events were not reported in the included studies.
Discussion
Rheumatoid arthritis (RA) affects 1‐2 percent of the general population, and is an important cause of chronic pain and disability (Morgan 1995). Often, symptoms of pain, discomfort and stiffness in RA are controlled by pharmacologic intervention. people and therapists do, however, often pursue other means of symptom relief, especially to avoid unwanted adverse effects of taking medication. TENS is one non‐pharmacologic modality which has been used to decrease pain in people with RA. There is no consensus, however, on the efficacy of TENS in RA to reduce pain (Belanger 2002). Our objective in this systematic review is to evaluate the efficacy of TENS in the treatment of hand RA.
Several studies have looked at TENS application to relieve pain caused by various disease and other processes (Lewis1994, Jensen 1985, Gersh 1985). Results are controversial, however, with about half showing some significant effects of TENS on pain reduction (Manheimer 1978). Despite these ambiguous results TENS continues to be used as an adjunct to other therapies for the relief of pain. This may be due, in part, to the fact that TENS rarely causes adverse effects, and often may be conveniently self‐administered by the patient in their home environment (Kaye 2002). It is, however, extremely difficult to assess whether, overall, TENS therapy is effective at improving outcomes for people with RA when the three RCTs included in this review do not measure the same disease‐related outcomes.
Confounding variables, such as characteristics of the TENS application, characteristics of the population, characteristics of the disease and methodological considerations may have contributed to the lack of, or ambiguity of, effect of TENS (Carroll 2002) in the studies reviewed. Some of the characteristics of the TENS application that can affect efficacy are: type of TENS (e.g. AL‐TENS or C‐TENS), intensity and mode of stimulus (e.g. burst or wave), position of electrode application (e.g. proximal or distal to pain), duration of the application and schedule of treatment (e.g. 15 minutes, once per week for 3 weeks (Abelson 1983); 20 minutes once only (Langley 1984); daily application for 5 minutes for 15 consecutive days (Manheimer 1978). In the study by Manheimer 1978, a frequency of 70 Hz was used for all three TENS study groups. However the intensity with which TENS was delivered was described as high enough to evoke paresthesia in one group (AL‐TENS‐like) or a lower intensity, enough to elicit a tingling sensation only (C‐TENS‐like). The inconsistency in the delivery of the TENS in the three included studies, and the fact that the parameters used hindered a definitive classification of the modes of TENS being used, may also add to difficulty in describing results and ascribing efficacy to one type of TENS or another. Both animal (Gopalkrishnan 2000) and human (Han 1991) research highly support the importance of the stimulation parameters in TENS analgesia. For instance, changes in frequency would recruit different opioid receptors, supporting the importance of taking into account the parameters that have been used during the TENS treatments (Sluka 1999, Sluka 2000, Belanger 2002).
Population characteristics that should be considered include age (age range was from 18 to 72 in this review) and gender (2 to 4 times as many women in the three RCTs included in this review). Disease duration varied from 1 to 44 years in the studies in this review, which could account for differences in response to therapy. In addition, the total number of subjects included in each study were relatively small (32 in Abelson 1983; 33 in Langley 1984; and 19 in Manheimer 1978), potentially contributing to variation in outcome. Differences in baseline measurement scores should be considered for possible influence on changes achieved following treatment (Guyatt 1993). Resting pain scores, grip pain scores and baseline work scores, for example, were all higher in the placebo group of one study (Abelson 1983); in another study the total number of tender joints and joint tenderness were higher at baseline in two of three treatment groups (Langley 1984); whereas no baseline values are given in the third included study (Manheimer 1978). Finally, there was considerable variation in the length of follow‐up in these studies (3 weeks, 1 ½ hours and 15 days respectively). It is important that such details be addressed in studies of TENS therapy and they must be reported consistently in published studies.
Methodological considerations that may have contributed to the ambiguity of effect are the randomization method (not reported in the studies included in this review), quality of double‐blinding, low sample size that do not allows to reach an ideal statistical power of .80 and selection of outcome measures (Gehlbach 1993). Three RCTs which fulfilled the criteria for inclusion in this review were retrieved from the literature. The validity and reliability of one outcome measure used in an included RCT, which assessed degree of relief from pain after treatment and how long the pain relief persisted following end of treatment (Manheimer 1978), was not mentioned. Standardized or consistent outcome measures and measurement periods should be used to assist the pooling of data from different studies.
Reporting data should ideally also be consistent among the included RCTs. Means and standard deviations of all outcomes should be provided, which was not the case for any of the included trials in this review, other than for baseline values for two of the studies (Abelson 1983, Langley 1984). The use of statistical approximation derived from the p‐value to estimate the standard deviation could affect the conclusion on the efficacy of TENS. Furthermore, some significant results were also contradictory, in that the TENS group in two of the three included studies showed statistically significant improvement in resting pain scores (from baseline) at interim measuring periods while the significance disappeared by end of study and following further treatments. This would suggest that over continuing time and treatment application, TENS loses its beneficial effect (Abelson 1983, Langley 1984). Some studies expressed their results using the difference between baseline values and end of treatment values (Abelson 1983, Langley 1984). It was, therefore, necessary to recalculate the difference between groups at end of treatment. It is possible, however, that when data are modified for pooling and comparison purposes interpretation of the results may change (Philbrick 1985).
The three studies included in this review predate 1985. No English publications reporting studies on TENS use for RA of the hand, and that fulfilled the criteria for inclusion in this review were found since that date. Since this review found no negative outcomes, and indeed some clinical benefit from the use of TENS in the palliative treatment for RA of the hand,further studies are warranted to examine the specific parameters that might be appropriate for the use of TENS (i.e. frequency, intensity, duration).
Authors' conclusions
Implications for practice.
This review has shown that TENS therapy has no negative effects on pain outcomes in people with RA. The reviewers concluded that TENS therapy may be used as required by people with RA of the hands, as analgesic and as an adjunct therapy. Specifically AL‐TENS has a statistically and clinically beneficial effect on pain and a clinical benefit on muscle power scores over placebo while, conversely, C‐TENS resulted in no clinical benefit on pain compared with placebo. However, C‐TENS resulted in a clinical benefit on patient assessment of change in disease over AL‐TENS. These conclusions are limited, however, by the poor methodological quality of the trials available and the large variation in many of the patient and methodological characteristics in the studies included. Our results are in accordance with a review on the effect of TENS for knee osteoarthritis (Osiri 2000), suggesting that our results could be applicable for both arm and leg arthritis.
Implications for research.
A more standardized classification system to describe and categorize modes of TENS therapy is warranted, in order that identification of characteristics of the possible modalities is uniformly agreed upon and applied. Better designed studies are needed to draw substantive conclusions of the efficacy of TENS in the treatment of hand RA. The studies should be randomized, double‐blind, placebo controlled trials, with treatment duration long enough and frequent enough to detect a difference in outcome measures. A standardized study protocol should be designed, which would address type of TENS application, electrode placement, frequency and duration of application of treatment. Outcome measures should also be standardized, using valid and reliable tools, and contain appropriate subjective and objective measures. Once such protocols are in place, the studies will be more easily compared and definitive statements made on the use of TENS in the treatment of RA of the hand.
What's new
| Date | Event | Description |
|---|---|---|
| 10 November 2008 | Amended | Converted to new review format. CMSG ID: C093‐R |
Acknowledgements
The authors are indebted to Shannon Rees, Lucie Lavigne, Catherine Lamothe for their technical support and their help in extraction of data. Special thanks go to Marnie Lamb for the editing of the text. Thanks also to Jessie McGowan for her assistance in searching the literature.
Appendices
Appendix 1. MEDLINE search strategy
1. exp osteoarthritis/ 2. osteoarthritis.tw. 3. osteoarthrosis.tw. 4. degenerative arthritis.tw. 5. exp arthritis, rheumatoid/ 6. rheumatoid arthritis.tw. 7. rheumatism.tw. 8. arthritis, juvenile rheumatoid/ 9. caplan's syndrome.tw. 10. felty's syndrome.tw. 11. rheumatoid.tw. 12. ankylosing spondylitis.tw. 13. arthrosis.tw. 14. sjogren$.tw. 15. or/1‐14 16. exp electric stimulation therapy/ 17. ((electric$ adj nerve) or therapy).tw. 18. electrostimulation.tw. 19. electroanalgesia.tw. 20. (tens or altens).tw. 21. electroacupuncture.tw. 22. (high volt or pulsed or current).tw. 23. (electromagnetic or electrotherap$).tw. 24. clinical trial.pt. 25. randomized controlled trial.pt. 26. tu.fs. 27. dt.fs. 28. random$.tw. 29. placebo$.tw. 30. ((sing$ or doubl$ or tripl$) adj (masked or blind$)). 31. sham.tw. 32. or/24‐31 33. 23 and 32
Data and analyses
Comparison 1. Placebo vs Treatment (end of treatment‐ 3 weeks).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Resting Pain VAS 100mm | 1 | 32 | Mean Difference (IV, Fixed, 95% CI) | ‐59.5 [‐76.58, ‐42.42] |
| 2 Grip pain Vas‐100mm | 1 | 32 | Mean Difference (IV, Fixed, 95% CI) | ‐12.0 [‐29.90, 5.90] |
| 3 Power Score (Watts) | 1 | 32 | Mean Difference (IV, Fixed, 95% CI) | 0.71 [‐0.33, 1.75] |
| 4 Work Score (Joules) | 1 | 32 | Mean Difference (IV, Fixed, 95% CI) | 0.29 [‐0.39, 0.97] |
1.1. Analysis.
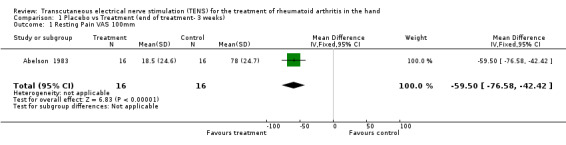
Comparison 1 Placebo vs Treatment (end of treatment‐ 3 weeks), Outcome 1 Resting Pain VAS 100mm.
1.2. Analysis.
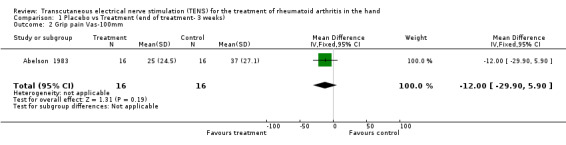
Comparison 1 Placebo vs Treatment (end of treatment‐ 3 weeks), Outcome 2 Grip pain Vas‐100mm.
1.3. Analysis.
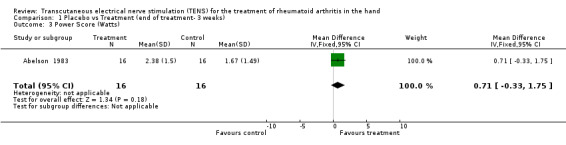
Comparison 1 Placebo vs Treatment (end of treatment‐ 3 weeks), Outcome 3 Power Score (Watts).
1.4. Analysis.
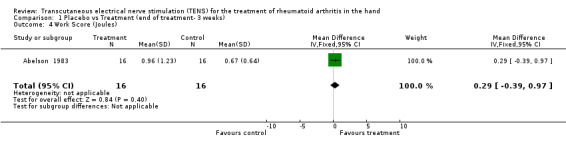
Comparison 1 Placebo vs Treatment (end of treatment‐ 3 weeks), Outcome 4 Work Score (Joules).
Comparison 2. C‐TENS vs Placebo (end of treatment ‐same day).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 *Resting pain scores (VAS) | 1 | 22 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐4.05, 3.65] |
| 2 *Grip pain score (VAS) | 1 | 22 | Mean Difference (IV, Fixed, 95% CI) | 0.70 [‐4.11, 5.51] |
| 3 *Joint Tenderness score (22 pt scale) | 1 | 22 | Mean Difference (IV, Fixed, 95% CI) | ‐20.0 [‐33.79, ‐6.21] |
| 4 No. Tender joints (no tender joints / total joints assessed) | 1 | 30 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.14, 2.48] |
2.1. Analysis.
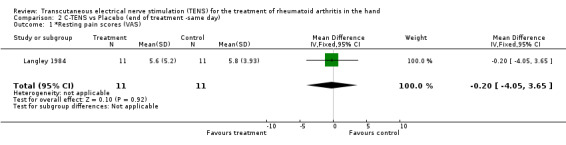
Comparison 2 C‐TENS vs Placebo (end of treatment ‐same day), Outcome 1 *Resting pain scores (VAS).
2.2. Analysis.
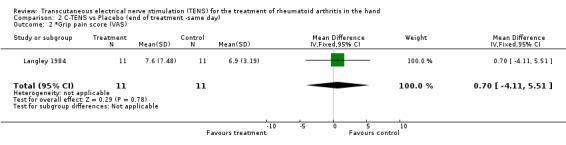
Comparison 2 C‐TENS vs Placebo (end of treatment ‐same day), Outcome 2 *Grip pain score (VAS).
2.3. Analysis.
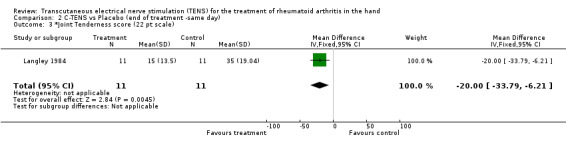
Comparison 2 C‐TENS vs Placebo (end of treatment ‐same day), Outcome 3 *Joint Tenderness score (22 pt scale).
2.4. Analysis.
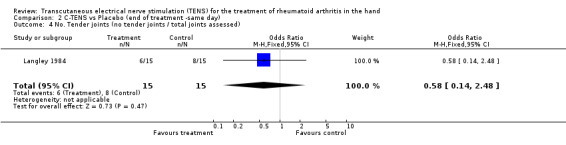
Comparison 2 C‐TENS vs Placebo (end of treatment ‐same day), Outcome 4 No. Tender joints (no tender joints / total joints assessed).
Comparison 3. C‐TENS vs AL‐TENS (head to head ‐end of treatment: 15 days).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of patients improved | 1 | 38 | Odds Ratio (M‐H, Fixed, 95% CI) | 6.43 [0.67, 61.47] |
3.1. Analysis.
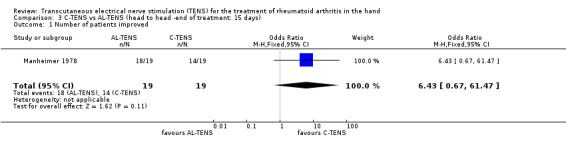
Comparison 3 C‐TENS vs AL‐TENS (head to head ‐end of treatment: 15 days), Outcome 1 Number of patients improved.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Abelson 1983.
| Methods | Randomized, placebo controlled study. Sample size at entry: 26 | |
| Participants | RA (Classical/ definite RA ‐ARA criteria‐ and chronic wrist involvement) Group 1 mean age: 57 SD=8 disease duration: 12 SD=8 Group 2 mean age: 55. Disease duration: 13 SD=6.75 |
|
| Interventions | Treatment gr: 15 min of 70 Hz TENS
Control: 15 min with no stimulation but output signal on. Electrodes applied to the dorsal and ventral aspects of the wrist. |
|
| Outcomes | 1‐ Resting pain score (mm) 2‐ Grip pain (mm) 3‐ Power score (Watts) 4‐ Work score (Joules) | |
| Notes | R=1 B=1 W=0 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Langley 1984.
| Methods | Randomized, Parallel group study. Sample size at entry: 33 | |
| Participants | RA (classical or definite RA ‐ARA criteria‐, chronic hand involvements, pain in one or both hands) Intervention group: mean age: 54.9 SD=15.3 disease duration: 11.3 SD= 7.5 Control group: mean age: 53.4 SD=14.1 disease duration: 10.7 SD=10.7 |
|
| Interventions | Intervention group: 20 mins of high frequency TENS (continuous square wave pulses of 0.2 ms at 100 Hz): monophasic pulses via 2 surface electrodes. Electrodes =wet pad type with surface area 9.08 cm square. Electrodes were placed immediately proximal to the patients wrist, with one electrode on the volar surface and the other on the palmar surface. Control group: 20 mins placebo TENS (no stimulation but output signal on) |
|
| Outcomes | 1‐ Resting pain score 2‐ grip pain score 3‐ joint tenderness score 4‐ No. tender joints | |
| Notes | R=1 B=2 W=1 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Manheimer 1978.
| Methods | A Randomized, cross‐over study. Sample size at entry: 19 | |
| Participants | RA (including spontaneous pain and/or pain on loading from the wrist, the MCP joints and the PIP joints) Age range of sample: 20‐69 Disease duration range: 1‐44 | |
| Interventions | Treatment group: 5 minutes/day for 15 days. Wrist (dorsal and volar) and back (either side of the spinal process) 0‐120 V, 0.2 ms, 70 Hz, conventional electrode size =9 cm square Placebo controlled (electrodes placed on either side of the spinal processes, intensity of stimulation low enought so that only a weak vibration was felt) |
|
| Outcomes | No. of patients improved | |
| Notes | R=1 B=0 W=0 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Angulo 1990 | |
| Bruce 1988 | |
| Herrera‐Lasso 1993 | No patients with RA |
| Kumar 1982 | Subjects are their own controls |
| Levy 1987 | Not RA population ‐rabbit joints |
| Moystad 1990 | Data can not be used |
Contributions of authors
KAY was responsible for writing the manuscript. SR, LL and CL were responsible for extracting and analyzing the data and selecting trials for the initial review. LB was the PI of the project. LB and VR participated in data extraction, updating the reference list, the analysis, and the interpretation of the results. JM developed the search strategy. GW and PT participated in the data analysis and the interpretation of the results MJ contributed to the editing of the text
Sources of support
Internal sources
University of Ottawa, Canada.
External sources
No sources of support supplied
Declarations of interest
This review was initially conducted as part of a guidelines development project that received financial support of The Arthritis Society (Canada). This final review was completed with the support of a research grant obtained from the Ontario Ministry of Health and Long‐Term Care (Canada) and the Ministry of Human Resources (Canada) for the development of Evidence‐Based Clinical Practice Guidelines for physical rehabilitation interventions in the treatment of RA.
Edited (no change to conclusions)
References
References to studies included in this review
Abelson 1983 {published data only}
- Abelson K, Langley GB, Sheppeard H, Vlieg M, Wigley RD. Trascutaneous Electrical Nerve Stimulation in Rheumatoid Arthritis. New Zealand Medical Journal 1983;96:156‐8. [1‐EL] [PubMed] [Google Scholar]
Langley 1984 {published data only}
- Langley GB, Sheppeard H, Johnson M, Wigley RD. The Analgesic Effects of Transcutaneous Electrical Nerve Stimulation and Placebo in Chronic Pain Patients. Rheumatology International 1984;4:119‐23. [14‐EL] [DOI] [PubMed] [Google Scholar]
Manheimer 1978 {published data only}
- Manheimer C, Lund S, Carlsson CA. The Effect of Transcutaneous Electrical Nerve Stimulation (TNS) on Joint Pain in Patients with RA. Scandinavian Journal of Rheumatology 1978;7:13‐16. [18‐EL] [PubMed] [Google Scholar]
References to studies excluded from this review
Angulo 1990 {published data only}
- Angulo DL, Colwell CW. Use of Postoperative TENS and Continuous Passive Motion Following Total Knee Replacement. JOSPT 1990;11(12):599‐604. [DOI] [PubMed] [Google Scholar]
Bruce 1988 {published data only}
- Bruce JR, Riggin CS, Parker JC, Walker SE, Meyer AA, Wellman FE, Kunce J. Pain Management in Rheumatoid Arhtritis: Cognitive Behavior Modification and Transcutaneous Neural Stimulation. Arthritis Care and Research 1988;1(2):78‐84. [Google Scholar]
Herrera‐Lasso 1993 {published data only}
- Herrera‐Lasso I, Mobarak L, Fernandez‐Dominguez L, Cardiel MH, Alarcon‐Segovia D. Comparatice Effectiveness of Packages of Treatment Including Ultrasound or Transcutaneous Electrical Nerve Stimulation in Painful Shoulder Syndrome. Physiotherapy 1993;79(4):251‐3. [11‐EL] [Google Scholar]
Kumar 1982 {published data only}
- Kumar VN, Redford JB. Trancutaneous Nerve Stimulation in Rheumatoid Arthritis. Archives of Physical Medicine and Rehabilitation 1982;63:59‐60. [29‐EL] [PubMed] [Google Scholar]
Levy 1987 {published data only}
- Levy A, Dalith M, Abramovici A, Pinkhas J, Weinberger A. Transcutaneous Electrical Nerve Stimulation in Experimental Acute Arthritis. Archives of Physical Medicine and Rehabilitation 1987;68:75‐8. [5‐EL] [PubMed] [Google Scholar]
Moystad 1990 {published data only}
- Moystad A, Krogstad BS, Larheim TA, Odont. Transcutaneous Nerve Stimulation in a Group of Patients with Rheumatic Disease Involving the Temporomandibular Joint. The Journal of Prosthetic Dentistry 1990;64(5):596‐600. [32‐EL] [DOI] [PubMed] [Google Scholar]
Additional references
Albright 2001
- Albright, J. Allman, R, Bonfiglio, R.P, Conill, A, Dobkin, B, Guccione, A.A, Hasson, S, Russo, R, Shekelle, P, Susman, J.L, Wells, G.A, Tugwell, P, Brosseau, L, Robinson, V.A, Graham, I.D, Shea, B.J, McGowan, J. Philadelphia panel evidence‐based clinical practice guidelines on selected rehabilitation interventions: Overview and methodology. Physical Therapy 2001;81(10):1629‐40. [PubMed] [Google Scholar]
APTA 2001
- American Physical Therapy Association. Guide to Physical Therapist Practice: Part One: A Description of Patient/Clinical Management. Alexandria VA: Springer Netherlands, 2001. [Google Scholar]
ARA 1987
- Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, et al. Revised Criteria for Classification of Rheumatoid Arthritis.. Arthritis Rheumatology. Vol. 31, The American Rheumatism Association, 1987:315‐24. [DOI] [PubMed] [Google Scholar]
Belanger 2002
- Belanger A‐Y. Evidence‐Based Guide to Therapeutic Physical Agents. Philadelphia: Lippincott Williams & Wilkins, 2002. [Google Scholar]
Cameron 1999
- Cameron MH. Physical Agents in Rehabilitation. From Research to Practice. Philadelphia: WB Saunders Company, 1999. [Google Scholar]
Carroll 2002
- Carroll D, Moore RA, McQuay HJ, Fairman F, Tramer M, Leijon G. Transcutaneous electrical nerve stimulation (TENS) for chronic pain. Cochrane Database of Systematic Reviews 2002, Issue 4. [DOI: 10.1002/14651858.CD003222.pub2] [DOI] [PubMed] [Google Scholar]
Clark 1999
- Clark HD, Wells GA, Huet C, McAlister FA, Salmi LR, Ferguson D. Assessing the quality of randomized trials: reliability of the Jadad scale. Controlled Clinical Trials 1999;20(5):448‐52. [DOI] [PubMed] [Google Scholar]
Dickersin 1994
- Dickersin K, Scherer R, Lefebvre C. Identifying relevant studies for systematic reviews. British Medical Journal 1994;309(6964):1286‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Felson 1995
- Felson DT, Anderson JJ, Boers M, Bombardier C, Furst D, Goldsmith C, et al. American‐College‐Of‐Rheumatology Preliminary Definition of Improvement in Rheumatoid‐Arthritis. Arthritis and Rheumatism 1995;38(6):727‐35. [DOI] [PubMed] [Google Scholar]
Gehlbach 1993
- Gehlbach SH. Interpreting the Medical Literature. 3rd Edition. McGraw Hill, 1993. [Google Scholar]
Gersh 1985
- Gersh MR, Wolf SL. Applications of transcutaneous electrical nerve stimulation in the management of patients with pain: State‐of‐the‐art update. Physical Therapy 1985;65(3):314‐22. [DOI] [PubMed] [Google Scholar]
Gopalkrishnan 2000
- Gopalkrishnan P, Sluka KA. Effect of varying frequency, intensity, and pulse duration of transcutaneous electrical nerve stimulation on primary hyperalgesia in inflamed rats.. Archives of Physical Medicine and Rehabilitation 2000;81(7):984‐90. [DOI] [PubMed] [Google Scholar]
Guyatt 1993
- Guyatt GH, Sackett DL, Cook DJ. Users' guide to the medical literature. II. How to use an article about therapy and prevention. Are the results of the study valid?. JAMA 1993;270:2598‐601. [DOI] [PubMed] [Google Scholar]
Guyatt 1996
- Guyatt GH, Sackett DL, Sinclair JC, Hayward R, Cook DJ, Cook RJ. Users' guides to the medical literature. IX. A method for grading health care recommendations. Evidence‐Based Medicine Working Group. JAMA 1996;275(16):1232. [DOI] [PubMed] [Google Scholar]
Han 1991
- Han JS, Chen XH, Sun SL, Xu XJ, Yuan Y, Yan SC 38Han, et al. Effect of Low‐Frequency and High‐Frequency TENS on Met‐Enkephalin‐Arg‐Phe and Dynorphin‐A Immunoreactivity in Human Lumbar CSF. Pain 1991;47:295‐8. [DOI] [PubMed] [Google Scholar]
Haynes 1994
- Haynes RB, Wilczynski N, McKibbon KA, Walker CJ, Sinclair JC. Developing optimal search strategies for detecting clinically sound studies in MEDLINE. Journal of the American Medical Informatics Association 1994;1(6):447‐58. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jadad 1996
- Jadad A, Moore A, Carrol Dea. Assessing the quality of randomized trials: Is blinding necessary?. Controlled Clinical Trials 1996;17:1‐12. [DOI] [PubMed] [Google Scholar]
Jensen 1985
- Jensen JE, Conn RR, Hzelrigg G, Hewett JE. The use of transcutaneous neural stimulation and isokinetic testing in arthroscopic knee surgery. American Journal of Sports Medicine 1985;13(1):27‐33. [DOI] [PubMed] [Google Scholar]
Jette 1997
- Jette AM, Delitto A. Physical Therapy Treatment Choices for Musculoskeletal Impairments. Physical Therapy 1997;77(2):145‐54. [DOI] [PubMed] [Google Scholar]
Kaye 2002 [Computer program]
- Kaye V, Brandstater ME. Transcutaneous Electrical Nerve Stimulation. eMedicine.com, Inc., 2002.
Lewis1994
- Lewis B, Lewis D, Cumming, G. The comparative analgesic efficacy of transcutaneous electricall nerve stimulation and a non‐steroidal anti‐inflammatory drug for painful osteoarthritis. British Journal of Rheumatology 1994;33:455‐60. [DOI] [PubMed] [Google Scholar]
Luckmann 1990
- Luckmann J, Sorensen KR. In: Kay D editor(s). Medical‐Surgical Nursing. 3rd Edition. WB Saunders Company, 1990:1540‐2. [Google Scholar]
Ma 2001
- Ma YT, Sluka KA. Reduction in inflammation‐induced sensitization of dorsal horn neurons by transcutaneous electrical nerve stimulation in anesthetized rats. Experimental Brain Research 2001;137(1):94‐102. [DOI] [PubMed] [Google Scholar]
Mannheimer 1986
- Mannheimer C. In: Banks MA editor(s). International Perspectives in Physical Therapy. Vol. Pain, New York: Churchill Livingstone, 1986:73‐118. [Google Scholar]
Melzack 1965
- Melzack R, Wall PD. Pain mechanisms: a new theory. Science 1965;150(699):971‐9. [DOI] [PubMed] [Google Scholar]
Moher 1995
- Moher D, Jaddad AR, Nichol G, Penman M, Tugwell P, Walsh S. Assessing the quality of randomised controlled trials: an annotated bibliography of scales and checklists. Controlled Clinical Trials 1995;16:62‐73. [DOI] [PubMed] [Google Scholar]
Morgan 1995 [Computer program]
- Morgan B. Rheumatoid Arthritis. Duquesne University, 1995.
OMERACT 1993
- OMERACT. Conference on Outcome Measures in Rheumatoid Arthritis Clinical Trials. Journal of Rheumatology 1993;20:526‐91. [PubMed] [Google Scholar]
Osiri 2000
- Osiri M, Welch V, Brosseau L, Shea B, McGowan J, Tugwell J. Transcutaneous electrical nerve stimulation for knee osteoarthritis. Cochrane Database of Systematic Reviews 2000, Issue 4. [DOI: 10.1002/14651858.CD002823] [DOI] [PubMed] [Google Scholar]
Philbrick 1985
- Philbrick JT, Bracikowski JP. Single‐dose antibiotic treatment for uncomplicated urinary tract infections: Less for less?. Archives of Internal Medicine 1985;145:1672. [PubMed] [Google Scholar]
Schumacher 1993
- Schumacher HR Jr. Primer on the Rheumatic Diseases. Atlanta: Arthritis Foundation, 1993. [Google Scholar]
Sluka 1998
- Sluka KA, Bailey K, Bogush J, Olson R, Ricketts A. Treatment with either high or low frequency TENS reduces the secondary hyperalgesia observed after injection of kaolin and carrageenan into the knee joint. Pain 1998;77(1):97‐102. [DOI] [PubMed] [Google Scholar]
Sluka 1999
- Sluka KA, Deacon M, Stibal A, Strissel S, Terpstra A. Spinal blockade of opioid receptors prevents the analgesia produced by TENS in arthritic rats. Journal of Pharmacololigcal and Experimental Therapeutics 1999;289(2):840‐6. [PubMed] [Google Scholar]
Sluka 2000
- Sluka KA, Judge MA, McColley MM, Reveiz PM, Taylor BM. Low frequency TENS is less effective than high frequency TENS at reducing inflammation‐induced hyperalgesia in morphine‐tolerant rats. European Journal of Pain 2000;4(2):185‐93. [DOI] [PubMed] [Google Scholar]
TAS 1999
- The Arthritis Society. Rheumatoid Arthritis Management Protocol.. In: Lineker S, Wood H editor(s). The Arthritis Society: Consultation and Rehabilitation Service.. Toronto: The Arthritis Society, 1999. [Google Scholar]


