Abstract
Background
Physical activity has anti-inflammatory effects and reduces morbidity and mortality in the general population, but its role in the clinical, CD4/CD8 ratio, and immune activation status of HIV-infected patients has been poorly studied.
Methods
A cross-sectional study was carried out in a cohort of 155 HIV-infected patients on stable antiretroviral therapy (ART) to compare clinical, biochemical, CD4/CD8 ratio, and immune activation status according to their physical activity in the last 2 years (sedentary/low vs moderate/intense) assessed by the iPAQ. A binary logistic regression and mixed analysis of variance were performed to evaluate the impact of levels of physical activity on CD4/CD8 ratio.
Results
In our series, 77 (49.7%) out of 155 patients were sedentary, and 78 (50.3%) practiced moderate/intense physical activity. Moderate/intense physical activity was associated with better metabolic control (lower body mass index, P = .024; glucose, P = .024; and triglyceride, P = .002) and CDC HIV stage (P = .046), lower CD8+ (P = .018), CD4+CD8+ (P = .026), CD4+CD86+ (P = .045), CD4+HLA-DR+ (P = .011), CD8+HLA-DR+ (P = .048) T lymphocytes and CD16+HLA-DR+ natural killer cells (P = .026), and higher CD3+CD4+ T lymphocytes (P = .016) and CD4/CD8 ratio (P = .001). Sedentary lifestyle (odds ratio [OR], 2.12; P = .042), CD4 nadir (OR, 1.005; P < .001), and CD8+CD38+ T cells (OR, 1.27; P = .006) were independently associated with low CD4/CD8 ratio (<0.8). Earlier and more intense CD4/CD8 ratio recovery was observed in patients with higher physical activity in the 2-year follow-up with a significant interaction between these variables: F(2, 124) = 3.31; P = .049; partial η2 = 0.042.
Conclusions
Moderate to high physical activity is associated with beneficial health effects, improvement in metabolic profile, and reduction of chronic inflammation in patients with HIV. Although more studies and clinical trials are needed to confirm these findings, a healthy lifestyle including at least moderate physical activity should be recommended to HIV patients on stable ART.
Keywords: CD4/CD8 ratio, immunoactivation, physical activity
Antiretroviral treatment has been successful in increasing the life expectancy of patients with HIV. Nonetheless, a gap remains for people with HIV compared with people without HIV [1]. Although the exact reasons are unknown, persistent low-level chronic inflammation is one of the mechanisms associated with an increased risk of presenting comorbidities, more non-AIDS events, and higher mortality in these patients [2].
Inversion of CD4/CD8 ratio has proved to be an excellent marker of immune activation and immune senescence states in these patients. It is also an independent predictive parameter of morbidity and mortality in patients receiving antiretroviral therapy (ART) [3–6]. Inversion of CD4/CD8 ratio is especially useful to identify patients who, regardless of whether the total number of CD4+ T cells is >500/µL, would benefit from early ART treatment as they are at higher risk of immune deterioration [4].
Reducing immune activation is an important challenge that can lead to diminishing non-AIDS events in these patients. Currently, most of the treatments used to reduce chronic inflammation in these patients have failed [7, 8]. Only canakinumab (a human anti-interleukin [IL]-1β monoclonal antibody) has been associated with a reduction in immunoinflammatory markers, but its price makes this therapy unaffordable for most health systems [9]. Early ART initiation and treatments with integrase strand transfer inhibitors (INSTIs) or non-nucleoside reverse transcriptase inhibitors (NNRTIs) have been associated with higher probability of CD4/CD8 ratio recovery [4, 10, 11]. However, which factors influence CD4/CD8 ratio recovery remains unknown.
Physical activity is known to have multiple beneficial effects on health. Regular physical exercise has proved to have a positive impact on chronic inflammation in the general population [12, 13]. However, its impact on HIV-infected patients receiving stable ART is unknown [14]. This study has been designed to evaluate the influence of regular moderate- to high-intensity physical activity on CD4/CD8 ratio, immune activation, and immune senescence parameters on patients with HIV on stable ART treatment.
METHODS
Study Design, Participants, Setting, and Eligibility
This cross-sectional observational study was developed in a sample of patients attending a university-based HIV clinical hospital in Murcia (Spain). Patients in this study were included from June 15, 2015, to January 30, 2018. Patients age >18 years were recruited when their physical activity remained stable in the last 2 years and they were on stable ART for at least a year. Comorbidities or other treatments were not exclusion criteria. The study conformed to the principles of the Declaration of Helsinki and the Good Clinical Practice Guidelines and was approved by the local ethics committee (“Comité Ético de Investigación Clínica del Hospital General Universitario Reina Sofía de Murcia”). Written informed consent was obtained from all participants.
Clinical and Laboratory Parameters
Medical records were carefully reviewed, and all subjects underwent a physical examination. Information on gender, age, body mass index (BMI), smoking status, family history of cardiovascular disease, and treatment with antiretroviral drugs was recorded. The presence of arterial hypertension, hypercholesterolemia, and hypertriglyceridemia was defined according to the Adult Treatment Panel III criteria [15]. Dyslipidemia was considered if total cholesterol was ≥200 mg/dL, LDL cholesterol was ≥130 mg/dL, or HDL cholesterol was <40 mg/dL. A sample of fasting venous blood was obtained to determine concentrations of glucose, high-sensitivity C-reactive protein (hsCRP), creatinine, total cholesterol, D-dimer, high-density lipoprotein (HDL) cholesterol, and triglycerides using standard enzymatic methods. Low-density lipoprotein (LDL) cholesterol concentrations were calculated using the Friedewald equation. Plasma viral load was measured using the Cobas TaqMan HIV-1 assay (Roche Diagnostics Systems, Branchburg, NJ, USA). CD4 and CD8 T-cell counts were determined by flow cytometry (Becton Dickinson [BD], San Jose, CA, USA).
Assessment of Physical Activity
Physical activity was evaluated through the International Physical Activity Questionnaire (iPAQ), and results were categorized as follows (https://sites.google.com/site/theipaq/scoring-protocol):
(1) Low: This is the lowest level of physical activity. Those individuals who do not meet criteria for categories 2 or 3 are considered low/inactive.
(2) Moderate: Any 1 of the following 3 criteria: ≥3 days of vigorous activity for ≥20 minutes per day; ≥5 days of moderate-intensity activity or walking for ≥30 minutes per day; or ≥5 days of any combination of walking or moderate-intensity or vigorous-intensity activities achieving a minimum of ≥600 MET-min/wk.
(3) High: Any 1 of the following 2 criteria: vigorous-intensity activity on ≥3 days and accumulating ≥1500 MET-min/wk or 7 days of any combination of walking, moderate-intensity activities, or vigorous-intensity activities achieving a minimum of ≥3000 MET-min/wk.
To simplify the analysis, patients with moderate and high levels of physical activity were unified into a single category (moderate and high) and compared with those with low physical activity and those considered sedentary (sedentary and low).
Flow Cytometric Analysis of Activation and Senescence Biomarkers
EDTA anticoagulated peripheral blood cells were labeled following a lyse/wash protocol with an 8-color/9-monoclonal antibody (mAb) panel including CD3 AmCyam (clone SK7, BD Biosciences), CD4 PECy7 (SK3, BD), CD8 APCCy7 (SK1, BD), CD16 PacBlue (3G8, BD), CD19 PECy7 (SJ25C1, BD), CD28 FICT (CD28.2, BD), CD38 APC (HB7, BD), CD86 PE (IT2.2, BD), and HLA-DR PerCP (L243, BD). Five microliters of each antibody in 100 µL of whole blood was incubated for 15 minutes at room temperature in the dark. Samples were lysed with 3 mL of 1X FACSlysing solution (BD) for 5 minutes and washed with 3 mL of FACSFlow (BD). Half a million cells were immediately acquired in a FACSCanto flow cytometer (BD), daily calibrated using 7-color setup beads (BD), and analyzed with DIVA software (BD) following the gating strategy described in Supplementary Figure 1.
The expression of CD28, CD38, CD86, and HLA-DR activation/senescence markers was evaluated as a percentage or absolute number (cells/µL) of positive cells as well as mean fluorescence intensity (MFI) of the marker on CD3+CD4+, CD3+CD8+, and CD3+CD4+CD8+ T lymphocytes, CD19+ B lymphocytes, CD3-CD19-CD16+ natural killer (NK) lymphocytes, monocytes (CD4+CD86+HLA-DR+ medium side scatter [SSC] cells), granulocytes (CD16++ elevated SSC cells), and eosinophils (elevated SSC auto fluorescent cells).
Statistical Analyses
A descriptive analysis of patients’ characteristics was carried out using frequency tables for categorical variables. Mean and SD were used for continuous variables. Differences in categorical variables between patients with and without subclinical atherosclerosis were assessed using the chi-square test or Fisher test, and Student t tests were used for continuous variables.
A Pearson correlation analysis was conducted between CD4/CD8 ratio and immune activation markers.
A mixed analysis of variance was performed to evaluate the impact of levels of physical activity on CD4/CD8 ratio. Binary logistic regression was used to evaluate the independent variables associated with a CD4/CD8 ratio <0.8. Multivariable models were adjusted for ART type, time on ART, CD4 T-lymphocyte nadir, percentage of CD8+CD38+ T lymphocytes, percentage of CD4+HLA-DR+ T lymphocytes, and physical activity. Significance levels were placed at P < .05. All statistical analyses were performed using SPSS package, version 24.
RESULTS
Clinical and Biological Characteristics of Patients
A total of 155 HIV-infected patients were included in the study. Their basal characteristics are shown in Supplementary Table 1. The average age (SD) was 48.8 (10.54) years. The median CD4+ T-cell count (interquartile range [IQR]) was 721 (466–946) cells/mL. HIV viral load was <20 copies/mL in 93.3% of patients. The median CD4/CD8 ratio (IQR) was 0.82 (0.52–1.1). All patients were on ART: 26% on protease inhibitor (PI)–based regimens, 33.8% on NNRTI-based regimens, and 50.6% on INSTI-based regimens. The median time on ART (IQR) was 4.27 (2.33–9.29) years. Twenty-seven (17.3%) patients suffered hypertension, 3.3% type 2 diabetes, 35.5% dyslipidemia, 19.4% obesity (BMI ≥30 m/kg2), and 52.9% were smokers. The mean waist circumference was 93 ± 12 cm.
According to the questionnaire, 77 patients (49.7%) were classified as sedentary or low physical activity, and 38 (24.5%) and 40 (25.8%) as moderate and intense, respectively. The clinical variables associated with physical activity are represented in Table 1. Patients who practiced moderate or intense physical activity had less severe HIV disease (lower Centers for Disease Control and Prevention HIV stage, P = .046), were treated more frequently with NNRTIs (P = .027), had better metabolic control with lower BMI (P = .024), glucose (P = .024), and triglyceride (P = .002), and had lower probability of having metabolic syndrome (P = .01). Additionally, CD8+ T-lymphocyte absolute counts were lower (P = .018) and the CD4/CD8 ratio was higher (P = .001) than in patients who were sedentary or practiced low physical activity.
Table 1.
Patient Characteristics According to Physical Activity
| Biological Characteristics | Sedentary/Low Intensity (n = 77) | Moderate Intensity (n = 78) | P |
|---|---|---|---|
| Age, mean (SD), y | 50.03 (10.28) | 47.59 (10.72) | .151 |
| Sex, male, No. (%) | 58 (75.3) | 59 (75.6) | 1.000 |
| BMI, median [IQR], kg/m2 | 27.91 [24.34–29.32] | 25.21 [23.48–28.42] | .024 |
| C hepatitis virus antibodies, No. (%) | 18 (23.7) | 12 (16.0) | .327 |
| Parameters related to HIV infection | |||
| Transmission, No. (%) | |||
| Heterosexual | 36 (48.0) | 23 (29.9) | .102 |
| Homosexual/bisexual | 26 (34.7) | 41 (53.2) | |
| Intravenous drug users | 10 (13.3) | 10 (13.0) | |
| Others/unknown | 3 (4.0) | 3 (3.9) | |
| CDC HIV stage, No. (%) | |||
| A | 21 (32.8) | 36 (52.9) | .046 |
| B | 24 (37.5) | 21 (30.9) | |
| C | 19 (29.7) | 11 (16.2) | |
| ART, No. (%) | |||
| Protease inhibitor | 27 (35.1) | 13 (16.9) | .017 |
| NNRTI | 19 (24.7) | 33 (42.9) | .027 |
| Integrase inhibitor | 40 (51.9) | 38 (49.4) | .872 |
| Other ART combinations | 17 (22.4) | 14 (18.2) | .658 |
| Time on ART, median [IQR], y | 3.70 [2.21–9.67] | 4.49 [2.49–8.00] | .744 |
| CD4 nadir, median [IQR], cells/mL | 232.5 [111.5–367.2] | 290.0 [128.5–429.0] | .217 |
| HIV viral load <20 copies/mL, No. (%) | 69 (89.6) | 75 (97.4) | .102 |
| CD4 T cell, median [IQR], cells/µL | 711.0 [433.0–960.0] | 750.0 [549.0–927.0] | .212 |
| CD8 T cell, median [IQR], cells/µL | 947.0 [713.0–1204] | 777.0 [563.0–1064] | .018 |
| CD4/CD8 ratio, median [IQR] | 0.72 [0.42–0.98] | 0.96 [0.64–1.22] | .001 |
| Biochemical parameters | |||
| Glucose, median [IQR], mg/dL | 96.0 [86.0–106.0] | 91.0 [85.0–97.0] | .024 |
| Total cholesterol, median [IQR], mg/dL | 192.0 [164.0–223.0] | 175.0 [159.0–218.0] | .106 |
| LDL cholesterol, median [IQR], mg/dL | 112.0 [88.5–136.5] | 108.0 [85.0–131.0] | .765 |
| HDL cholesterol, median [IQR], mg/dL | 48.00 [40.00–56.00] | 48.0 [41.0–54.0] | .823 |
| Triglycerides, median [IQR], mg/dL | 132.0 [101.0–195.0] | 98.0 [78.0–146.0] | .002 |
| Creatinine, median [IQR], mg/dL | 0.89 [0.81–1.04] | 0.85 [0.77–0.99] | .462 |
| Cardiovascular risk factors | |||
| Type 2 diabetes, No. (%) | 6 (7.8) | 2 (2.6) | .268 |
| Hypertension, No. (%) | 15 (19.5) | 12 (15.4) | .645 |
| Dyslipidemia, No. (%) | 29 (37.7) | 26 (33.3) | .693 |
| Smoker, No. (%) | |||
| Nonsmoker | 30 (39.0) | 40 (51.3) | .088 |
| Current smoker | 44 (57.1) | 38 (48.7) | |
| Previous smoker | 3 (3.9) | 0 (0.0) | |
| Drink alcohol, No. (%) | 36 (46.8) | 42 (53.8) | .66 |
| Coronary heart disease, No. (%) | 5 (6.6) | 1 (1.3) | .200 |
| Stroke, No. (%) | 1 (1.3) | 1 (1.3) | 1.000 |
| Framingham Risk Score, median [IQR] | 3.00 [2.00–4.25] | 2.50 [1.00–4.00] | .380 |
| Metabolic syndrome, No. (%) | 24 (32.4) | 10 (13.3) | .010 |
| HOMA, median [IQR] | 3.01 [1.60–5.38] | 1.93 [1.41–2.54] | .002 |
Abbreviations: ART, antiretroviral therapy; BMI, body mass index; CDC, Centers for Disease Control and Prevention; HDL, high-density lipoprotein; HOMA, homeostatic model assessment; IQR, interquartile range; LDL, low-density lipoprotein; NNRTI, non-nucleoside retroviral transcriptase inhibitor.
Lower Immune Activation Was Associated With Higher Physical Activity
Table 2 represents the association of immunological characteristics according to physical activity. Patients who practiced moderate-intensity physical activity compared with patients who were sedentary or practiced low physical activity showed lower percentages of CD3+CD8+ T lymphocytes (P = .011), CD4+CD8+ double-positive T lymphocytes (P = .026), T lymphocytes expressing immune activation markers such as CD4+CD86+ T cells (P = .045), CD4+HLA-DR+ T cells (P = .011), CD8+HLA-DR+ T cells (P = .048), and CD16+HLA-DR+ NK cells (P = .026), and lower MFI of HLA-DR on CD4+ (P = .001) and CD8+ (P = .048) T lymphocytes. In contrast, patients with higher physical activity showed higher percentages of CD3+CD4+ T lymphocytes (P = .016).
Table 2.
Immunological Characteristics According to Physical Activity
| Sedentary or Low Intensity (n = 77) | Moderate Intensity (n = 78) | P | |
|---|---|---|---|
| Leukocyte subsets, median [IQR] | |||
| Leukocytes, cells/µL | 5700 [4400–7300] | 5600 [3500–6792] | .247 |
| Lymphocytes, % | 32.01 [23.71–37.74] | 28.12 [23.29–36.69] | .324 |
| Monocytes, % | 7.17 [5.88–8.80] | 7.47 [6.10–8.98] | .930 |
| Neutrophils, % | 57.09 [50.58–63.14] | 59.02 [51.34–64.81] | .462 |
| Eosinophils, % | 1.89 [1.26–3.11] | 2.38 [1.26–3.13] | .379 |
| Lymphocyte subsets, median [IQR], % | |||
| B lymphocytes CD19+ | 12.57 [7.57–17.47] | 11.36 [8.84–17.11] | .797 |
| NK lymphocytes CD16+ | 10.33 [5.04–13.77] | 9.29 [6.44–16.47] | .394 |
| T lymphocytes CD3+ | 67.77 [62.63–75.57] | 69.34 [60.72–75.45] | .986 |
| T lymphocytes CD3+CD4+ | 26.37 [18.37–31.85] | 29.14 [24.21–34.18] | .016 |
| T lymphocytes CD3+CD8+ | 39.94 [35.19–46.05] | 35.63 [29.66–43.38] | .011 |
| T lymphocytes CD4+CD8+ | 0.64 [0.36–1.10] | 0.49 [0.31–0.78] | .029 |
| Expression of activation markers, median [IQR], % | |||
| CD4+CD86+ T cells | 2.29 [1.16–6.16] | 1.58 [0.93–3.68] | .045 |
| CD8+CD86+ T cells | 1.87 [1.04–5.62] | 1.42 [0.89–2.57] | .067 |
| CD16+CD86+ NK cells | 2.21 [1.24–5.15] | 1.79 [0.78–3.65] | .086 |
| CD4+HLA-DR+ T cells | 10.89 [6.75–22.16] | 9.26 [4.99–14.00] | .011 |
| CD8+HLA-DR+ T cells | 20.13 [13.15–28.93] | 15.98 [8.62–24.70] | .048 |
| CD16+HLA-DR+ NK cells | 13.04 [6.95–27.55] | 9.63 [4.99–17.01] | .026 |
| Expression of HLA-DR, median [IQR], MFI | |||
| In monocytes | 7045 [4783–10 205] | 6444 [4936–8962] | .444 |
| In CD19+ B lymphocytes | 14 328 [10 057–18 175] | 12 793 [8190–16 947] | .148 |
| In CD4+ T lymphocytes | 599 [406–1083] | 466 [258–673] | .001 |
| In CD8+ T lymphocytes | 542 [327–842] | 400 [242–739] | .048 |
| Expression of CD86, median [IQR], MFI | |||
| In monocytes | 1513 [1252–1989] | 1480 [1279–1950] | .500 |
| In CD4+ T lymphocytes | 119 [75–219] | 90 [34–151] | .014 |
| In CD8+ T lymphocytes | 79 [49–146] | 61 [29–121] | .026 |
No differences in CD28 and CD38 expression were observed in T, B, or NK cells.
Abbreviations: IQR, interquartile range; MFI, mean fluorescence intensity; NK, natural killer.
CD4/CD8 Ratio Was Inversely Correlated With Immune Activation
Pearson correlation analysis between CD4/CD8 ratio and immune activation markers showed a significant correlation with percentages of CD8+CD86+ T cells (r = –0.28; P < .001), CD8+HLA-DR+ T lymphocytes (r = –0.39; P < .001), CD4+HLA-DR+ T lymphocytes (r = –0.23; P = .001), and CD8+CD38+ T lymphocytes (r = –0.27; P < .001) (Supplementary Figure 2).
Moderate/Intense Physical Activity Was an Independent Parameter Associated With CD4/CD8 Ratio
CD4/CD8 ratio was <0.8 in 73 out of 150 patients (48.7%). A binary logistic regression analysis was performed to verify which variables were associated with CD4/CD8 ratio <0.8. This analysis included type of ART, duration of ART, CD4 T-cell nadir, percentage of CD8+CD38+ T lymphocytes, percentage of CD4+DR+ T lymphocytes, and intensity of physical activity. Variables independently associated with CD4/CD8 ratio <0.8 were CD4+ T-cell nadir (odds ratio [OR], 1.005; 95% CI, 1.003–1.007; P < .001), percentage of CD8+CD38+ T lymphocytes (OR, 1.27; 95% CI, 1.07–1.5; P = .006), and sedentary or low physical activity (OR, 2.18; 95% CI, 1.03–4.6; P = .042) (Table 3).
Table 3.
Variables Associated With CD4/CD8 Ratio <0.8
| Multivariable Analysis | |||
|---|---|---|---|
| OR | 95% CI | P | |
| ART type | 2.697 | 0.667–10.91 | .360 |
| Time on ART | 0.980 | 0.929–1.040 | .560 |
| Sedentary or low physical activity | 2.180 | 1.030–4.600 | .042 |
| CD4+ lymphocyte T nadir | 1.005 | 1.003–1.007 | .001 |
| CD8+CD38+ T cells, % | 1.270 | 1.070–1.500 | .006 |
| CD4+DR+ T cells, % | 1.018 | 0.980–1.050 | .355 |
Abbreviations: ART, antiretroviral therapy; OR, odds ratio.
Moderate/Intense Physical Activity Was Associated With Faster Recovery of CD4/CD8 Ratio
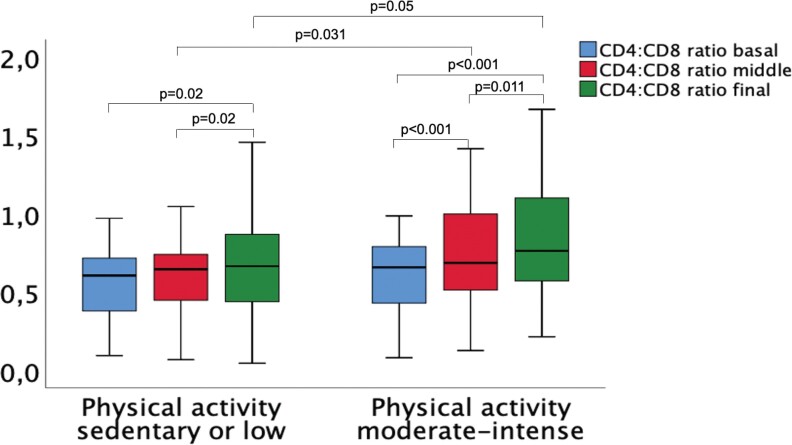
In order to evaluate the impact of physical activity on recovery of CD4/CD8 ratio, we performed a subanalysis in patients with a CD4/CD8 ratio <1.0 who were on stable ART for >2 years. One hundred five patients were included. Figure 1 represents CD4/CD8 ratio according to physical activity at 3 different time points: (1) at baseline or before 2 years; (2) middle term or 1 year before the final evaluation; and (3) final or present. A significant interaction was found between physical activity and CD4/CD8 ratio changes (F (2, 124) = 3.31; P = .049; partial η2 = 0.042). No differences in CD4/CD8 ratio were found between the 2 physical activity groups at baseline. However, differences were found between the means at 1 year of follow-up (mean difference [SE], 0.137 [0.063]; P = .031) and the final moment (mean difference [SE], 0.144 [0.073]; P = .05) in favor of moderate to intense physical activity, compared with sedentary/low physical activity. Therefore, recovery of CD4/CD8 ratio was more intense in patients with moderate or intense physical activity.
Figure 1.
CD4/CD8 ratio recovery according to physical activity. Box plots showing the evolution of CD4/CD8 ratio according to intensity of physical activity at 3 different moments: 2 years before the final evaluation (baseline), 1 year before the final evaluation (middle), and final . Statistical analysis was performed with mixed analysis of variance.
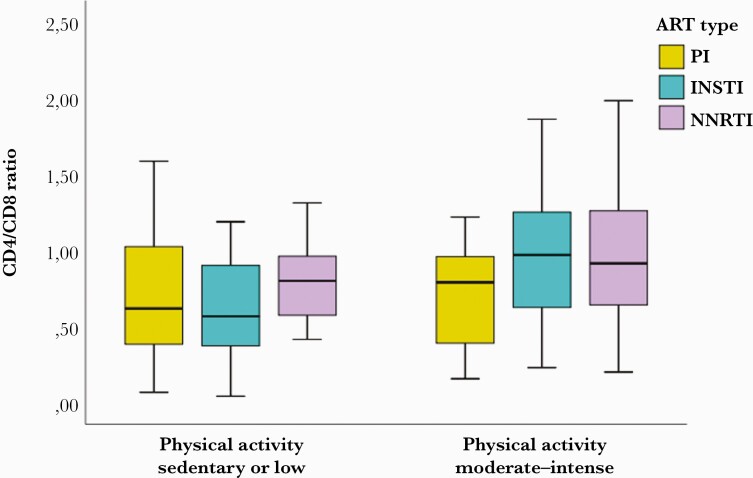
Although the results were not significant, a clear trend was observed that INSTI- and NNRTI-based ART treatments could favor CD4/CD8 ratio recovery when they are associated with moderate/intense physical activity (Figure 2).
Figure 2.
INSTI- and NNRTI-based ART regimens could favor CD4/CD8 ratio recovery when associated with moderate/intense physical activity. Box plots show CD4/CD8 ratio for the 3 types of ART treatments: PI-, NNRTI-, and INSTI-based regimens. Statistical analysis was performed with mixed analysis of variance. Abbreviations: ART, antiretroviral therapy; INSTI, integrase strand transfer inhibitor; NNRTI, non-nucleoside retroviral transcriptase inhibitor; PI, protease inhibitor.
Supplementary Table 1 and Supplementary Figures 1 and 2 can be found in the Supplementary Data.
DISCUSSION
This study shows that moderate to intense physical activity is related to earlier and more powerful recovery of CD4/CD8 ratio in patients with HIV on stable ART over a 2-year period. Improved metabolic profile (lower BMI, waist, glucose, triglycerides, and metabolic syndrome) and lower immune activation T-cell markers (CD86 and HLA-DR) were also found in more active subjects.
HIV-infected patients have a higher risk of presenting comorbidities and non-AIDS events than the general population. Persistent chronic inflammation is one of the most related factors, irrespective of the type of ART therapy or the level of viral replication control [2, 4]. Inversion of CD4/CD8 ratio is an excellent subrogate marker of chronic inflammation, and it has been associated with a higher probability of non-AIDS events [3, 4, 16]. Reducing the inflammatory state in these patients is an important challenge to get the frequency of comorbidities closer to that in the general population. So far, no effective treatment has been found that can increase CD4/CD8 ratio without increasing toxicity. INSTI- and NNRTI-based ART treatments could have beneficial effects compared with those based on PIs [17, 18]. Some studies have suggested a role for the integrase inhibitor family, as faster normalization of CD4/CD8 ratio was observed with raltegravir vs efavirenz in a subanalysis of the STRTMRK study [19]. Another study confirmed that an INSTI-based regimen as a first-line therapy was associated with greater recovery of CD4/CD8 ratio compared with NNRTI and PI [17]. In contrast to this result, there was a better outcome with efavirenz vs dolutegravir (DTG) in a subanalysis of the SINGLE study [20]. In line with these results, patients treated with INSTI- and NNRTI-based regimens in our series reached higher CD4/CD8 ratios when associated with higher levels of physical activity, although no significant differences were found with patients treated with PI-based regimens.
Although anti-inflammatory therapy targeting the interleukin-1β innate immunity pathway with canakinumab could reduce recurrent cardiovascular events, the elevated cost and higher incidence of fatal infections hinder its incorporation into routine clinical practice [9]. In contrast, exercise is a risk-free activity that leads the muscles to produce high levels of plasma IL-1 receptor antagonist (IL1-RA), which competitively inhibits IL-1β signaling [21]. In fact, moderate to intense physical activity in our series was an independent factor associated with improved CD4/CD8 ratios and immune activation markers. It is well known that exercising promotes anti-inflammatory and antioxidant states through multiple mechanisms. Although many information gaps exist, exercise-induced beneficial effects may help to ameliorate chronic metabolic diseases, particularly when body fat mass is reduced [12]. Exercise decreases the expression of Toll-like receptor 4 (TLR4) in several tissues and cell types. TLR4 is a key transmembrane receptor on which both infectious and noninfectious stimuli converge to induce proinflammatory responses. TLR4 is also activated by oxidized low-density lipoproteins, and it is involved in obesity-induced insulin resistance, type 2 diabetes, and atherosclerosis [21]. On the other hand, exercise may increase cytotoxic T and NK cells and reduce regulatory T-cell infiltration in tumors, promoting cancer immune surveillance [22].
Physical activity has proven to be beneficial for HIV-infected patients in terms of improving cardiorespiratory fitness, strength, body composition, and quality of life [23]. However, studies evaluating the effect of physical activity on the immune system have shown contradictory findings [14]. In a clinical trial conducted in Italy, 12 weeks of nonlinear resistance training was associated with reduced levels of IL-1β, IL-8, and tumor necrosis factor alpha (TNF-α), but increased levels of IL-10, CD4+, and CD8+ T-cell counts [24]. A clinical trial carried out on 58 HIV patients showed that a 16-week training reduced the levels of IL-5, IL-8, and IL-10 [25]. However, although “no evidence of effect” could be sustained, a systematic review including 23 clinical trials and 1073 participants [14] could not find an association between beneficial effects and biomarker improvements after ≥4 weeks of aerobic or/and resistance exercise. A longer-term study carried out retrospectively in children with HIV who practiced exercise regularly for 2 years showed reduced levels of immune activation markers such as sCD14, TNF-α, interferon-gamma (IFN-γ), and IL-10, and lower expression of CD38 on CD4+ T cells [26]. This latter result is in concordance with the reduction on activation markers expressed in CD4+ and CD8+ T cells and NK cells in patients with higher physical activity from our series.
The improvement in immune activation state observed in patients from our series practicing moderate/intense physical activity is further supported by their lower levels of CD4+CD8+ double-positive T cells. CD4+CD8+ T cells are specialized T cells with high antiviral activity [27]. Expansion of these cells has been observed in HIV, EBV, and HTLV-1 infections [28–31]. Upon exposure to viral antigens, CD4+CD8+ T cells may differentiate into high antiviral effectors producing IFN-γ and TNF-α that exceed the activity of their single-positive counterparts [28–32]. Circulating CD4+CD8+ T-cell levels correlate with viral kinetics in animal models [27], suggesting that the higher numbers of CD4+CD8+ T cells observed in patients with lower physical activity would be indicative of higher virus reactivation. In fact, double-positive T cells from patients with symptomatic HIV disease have increased activation and exhaustion signs, compared with asymptomatic subjects and with single-positive T cells from the same subjects [33].
The limitations of our study include the number of patients, which restricted the significance of the results, and its cross-sectional nature, which made it difficult to assign causality to the observed associations. On the other hand, directionality cannot be inferred, as it is possible that relatively poor health contributed to low physical activity, and it is yet to be shown that introduction of physical activity would improve the immune profile in sedentary individuals.
CONCLUSIONS
Our results show that moderate to high physical activity is associated with beneficial health effects, improvement in metabolic profile, and reduction in chronic inflammation in patients with HIV. Although more studies and clinical trials are needed to confirm these findings, a healthy lifestyle including at least moderate physical activity should be recommended to HIV patients on stable ART.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
This study would not have been possible without the collaboration of all the patients, medical and nursery staff, and data managers who took part in the project.
Financial support. This study was funded by Instituto de Salud Carlos III through the project “PI17/01545” (Co-funded by the European Regional Development Fund/European Social Fund, “Investing in Your Future”) and AMIEI (“Asociación Murciana para la Investigación en Enfermedades Infecciosas”).
Potential conflicts of interest. All authors: no reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Author contributions. E.B. and A.M. conceptualized the study; E.B., M.M., and A.C. analyzed the data, generated the figures, and wrote the first draft of the manuscript; E.B., J.A.C., G.P., and C.B. contributed to writing and figure generation; E.B. and A.M. supervised the statistical analysis; E.B., C.T., A.J., M.J.A., A.A., A.M., E.O., A.T., I.M., A.C., and A.M. contributed to data mining. All authors revised and approved the final manuscript.
Patient consent. The patient’s written consent was obtained. The design of the work has been approved by local ethical committees.
References
- 1. Marcus JL, Chao CR, Leyden WA, et al. Narrowing the gap in life expectancy between HIV-infected and HIV-uninfected individuals with access to care. J Acquir Immune Defic Syndr 2016; 73:39–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hunt PW. HIV and inflammation: mechanisms and consequences. Curr HIV/AIDS Rep 2012; 9:139–47. [DOI] [PubMed] [Google Scholar]
- 3. Bernal Morell E, Serrano Cabeza J, Muñoz A, et al. The CD4/CD8 ratio is inversely associated with carotid intima-media thickness progression in human immunodeficiency virus-infected patients on antiretroviral treatment. AIDS Res Hum Retroviruses 2016; 32:648–53. [DOI] [PubMed] [Google Scholar]
- 4. Serrano-Villar S, Sainz T, Lee SA, et al. HIV-infected individuals with low CD4/CD8 ratio despite effective antiretroviral therapy exhibit altered T cell subsets, heightened CD8+ T cell activation, and increased risk of non-AIDS morbidity and mortality. PLoS Pathog 2014; 10:e1004078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Serrano-Villar S, Pérez-Elías MJ, Dronda F, et al. Increased risk of serious non-AIDS-related events in HIV-infected subjects on antiretroviral therapy associated with a low CD4/CD8 ratio. PLoS One 2014; 9:e85798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sainz T, Serrano-Villar S, Díaz L, et al. The CD4/CD8 ratio as a marker T-cell activation, senescence and activation/exhaustion in treated HIV-infected children and young adults. AIDS 2013; 27:1513–6. [DOI] [PubMed] [Google Scholar]
- 7. Hsue PY, Ribaudo HJ, Deeks SG, et al. Safety and impact of low-dose methotrexate on endothelial function and inflammation in individuals with treated human immunodeficiency virus: AIDS Clinical Trials Group Study A5314. Clin Infect Dis 2019; 68:1877–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dubé MP, Chan ES, Lake JE, et al. A randomized, double-blinded, placebo-controlled trial of sitagliptin for reducing inflammation and immune activation in treated and suppressed human immunodeficiency virus infection. Clin Infect Dis 2019; 69:1165–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ridker PM, Everett BM, Thuren T, et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med 2017; 377:1119–31. [DOI] [PubMed] [Google Scholar]
- 10. Mussini C, Lorenzini P, Cozzi-Lepri A, et al. CD4/CD8 ratio normalisation and non-AIDS-related events in individuals with HIV who achieve viral load suppression with antiretroviral therapy: an observational cohort study. Lancet HIV 2015; 2:e98–106. [DOI] [PubMed] [Google Scholar]
- 11. Thornhill J, Inshaw J, Oomeer S, et al. Enhanced normalisation of CD4/CD8 ratio with early antiretroviral therapy in primary HIV infection. J Int AIDS Soc 2014; 17(4 Suppl 3):19480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nieman DC, Wentz LM.. The compelling link between physical activity and the body’s defense system. J Sport Health Sci 2019; 8:201–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Beavers KM, Brinkley TE, Nicklas BJ.. Effect of exercise training on chronic inflammation. Clin Chim Acta 2010; 411:785–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ibeneme SC, Irem FO, Iloanusi NI, et al. Impact of physical exercises on immune function, bone mineral density, and quality of life in people living with HIV/AIDS: a systematic review with meta-analysis. BMC Infect Dis 2019; 19:340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive summary of the third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA 2001; 285:2486–97. [DOI] [PubMed] [Google Scholar]
- 16. Bernal E, Martinez M, Torres A, et al. T cell senescence predicts subclinical atherosclerosis in HIV-infected patients similarly to traditional cardiovascular risk factors. Antiviral Res 2019; 162:163–70. [DOI] [PubMed] [Google Scholar]
- 17. Serrano-Villar S, Martínez-Sanz J, Ron R, et al. Effects of first-line antiretroviral therapy on the CD4/CD8 ratio and CD8 cell counts in CoRIS: a prospective multicentre cohort study. Lancet HIV 2020; 7:e565–73. [DOI] [PubMed] [Google Scholar]
- 18. Masiá M, Padilla S, Barber X, et al. Comparative impact of suppressive antiretroviral regimens on the CD4/CD8 T-cell ratio: a cohort study. Medicine 2016; 95:e3108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Serrano-Villar S, Zhou Y, Rodgers AJ, Moreno S.. Different impact of raltegravir versus efavirenz on CD4/CD8 ratio recovery in HIV-infected patients. J Antimicrob Chemother 2017; 72:235–9. [DOI] [PubMed] [Google Scholar]
- 20. Blanco JR, Alejos B, Moreno S.. Impact of dolutegravir and efavirenz on immune recovery markers: results from a randomized clinical trial. Clin Microbiol Infect 2018; 24:900–7. [DOI] [PubMed] [Google Scholar]
- 21. Lancaster GI, Febbraio MA.. The immunomodulating role of exercise in metabolic disease. Trends Immunol 2014; 35:262–9. [DOI] [PubMed] [Google Scholar]
- 22. Valacchi G, Virgili F, Cervellati C, Pecorelli A.. OxInflammation: from subclinical condition to pathological biomarker. Front Physiol 2018; 9:858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. O’Brien KK, Tynan A-M, Nixon SA, Glazier RH.. Effectiveness of aerobic exercise for adults living with HIV: systematic review and meta-analysis using the Cochrane Collaboration Protocol. BMC Infect Dis 2016; 16:182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zanetti HR, Cruz L, Lourenço CLM, Neves F de F, Silva-Vergara ML, Mendes EL.. Non-linear resistance training reduces inflammatory biomarkers in persons living with HIV: a randomized controlled trial. Eur J Sport Sci 2016; 16:1232–9. [DOI] [PubMed] [Google Scholar]
- 25. Pedro RE, Candido N, Guariglia DA, et al. Exercise improves cytokine profile in HIV-infected people: a randomized clinical trial. Cytokine 2017; 99:18–23. [DOI] [PubMed] [Google Scholar]
- 26. Gopalan BP, Dias M, Arumugam K, et al. Effect of structured physical activity on inflammation and immune activation profile of antiretroviral therapy-experienced children living with HIV. Pediatr Exerc Sci 2020; 32:73–80. [DOI] [PubMed] [Google Scholar]
- 27. Nascimbeni M, Shin E-C, Chiriboga L, Kleiner DE, Rehermann B.. Peripheral CD4(+)CD8(+) T cells are differentiated effector memory cells with antiviral functions. Blood 2004; 104:478–86. [DOI] [PubMed] [Google Scholar]
- 28. Frahm MA, Picking RA, Kuruc JD, et al. CD4+CD8+ T cells represent a significant portion of the anti-HIV T cell response to acute HIV infection. J Immunol 2012; 188:4289–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Weiss L, Roux A, Garcia S, et al. Persistent expansion, in a human immunodeficiency virus-infected person, of V beta-restricted CD4+CD8+ T lymphocytes that express cytotoxicity-associated molecules and are committed to produce interferon-gamma and tumor necrosis factor-alpha. J Infect Dis 1998; 178:1158–62. [DOI] [PubMed] [Google Scholar]
- 30. Ortolani C, Forti E, Radin E, Cibin R, Cossarizza A.. Cytofluorimetric identification of two populations of double positive (CD4+,CD8+) T lymphocytes in human peripheral blood. Biochem Biophys Res Commun 1993; 191:601–9. [DOI] [PubMed] [Google Scholar]
- 31. Macchi B, Graziani G, Zhang J, Mastino A.. Emergence of double-positive CD4/CD8 cells from adult peripheral blood mononuclear cells infected with human T cell leukemia virus type I (HTLV-I). Cell Immunol 1993; 149:376–89. [DOI] [PubMed] [Google Scholar]
- 32. Kitchen SG, Whitmire JK, Jones NR, et al. The CD4 molecule on CD8+ T lymphocytes directly enhances the immune response to viral and cellular antigens. Proc Natl Acad Sci U S A 2005; 102:3794–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chauhan NK, Vajpayee M, Mojumdar K, Singh R, Singh A.. Study of CD4+CD8+ double positive T-lymphocyte phenotype and function in Indian patients infected with HIV-1. J Med Virol 2012; 84:845–56. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.