Abstract
Selective beta-adrenoceptor agonists are worldwide prescribed to manage bronchial obstruction. However, they expose to a potential risk of hyperlactatemia and lactic acidosis even with normal doses. The mechanism still poorly understood and suggested that salbutamol diverts the metabolism of pyruvate acid from Krebs cycle toward lactate formation. We report the case of a 42-year-old patient, admitted to intensive care unit for acute severe asthma. He presented a transient lactic acidosis over the first 48 h, following an excessive use of salbutamol. The metabolic acidosis caused tachypnea, as a compensatory mechanism, leading to respiratory failure. The diagnosis of salbutamol-induced lactic acidosis must be made by elimination and only accepted after deleting the other causes. The main clinical character is the worsening of dyspnea despite regression of bronchospasm. It is transient and usually normalizes within 24–48 h after stopping or decreasing doses of salbutamol.
Keywords: Asthma, salbutamol, lactic acidosis, sevoflurane, ketamine
Introduction
Acute severe asthma is a challenge to the clinician both in terms of recognition and appropriate treatment. About 30% of these episodes need admission to intensive care unit, with a mortality of 8%. 1
Beta adrenergic agents, such as salbutamol, and corticosteroids are the main treatment of this critical situation. 2 However, they expose to a risk of hyperlactatemia and lactic acidosis with a potential risk of worsening dyspnea and respiratory failure requiring mechanical ventilation.
We describe a case of an asthmatic patient admitted for acute severe asthma, who had presented lactic acidosis following excessive salbutamol use.
Case report
A written informed consent was obtained from the patient to publish this observation.
A 42-year-old patient was admitted to emergency department with respiratory distress and wheezing. He reported that over the last 3 days, a runny nose, shortness of breath, dry cough, and audible wheezing. Despite the use of increased doses of inhaled salbutamol at home, he continued to have tightness in chest and trouble breathing.
The patient was known asthmatic since childhood, and his medical history was also marked by a similar episode a year ago.
On admission, the patient could not speak in complete sentences with marked accessory muscles of respiration use. He was afebrile, conscious, and agitated. Pulse rate was 130 beats/min, respiratory rate 45 breaths/min, oxygen saturation 91% on room air and blood pressure was 140/75 mmHg. Chest auscultation revealed bilateral expiratory wheeze. The remaining physical examination was normal.
Arterial blood gas showed a severe acidosis: pH: 7.18; PaCO2: 49 mmHg; PaO2: 65 mmHg; HCO3–: 28; Lactic acid: 1.3 mmol/L.
The chest X-ray showed an enlargement of the intercostal spaces with horizontalization of the ribs. The rest of laboratory investigations were normal (Table 1).
Table 1.
Results of blood tests in our patient.
| White blood cells | 7500/mm3 |
| Hemoglobin | 13.9 g/dl |
| Platelets | 350,000 mm3 |
| Activated protein c | 4.2 mg/L |
| Procalcitonin | 0.01 mg/ml |
| Urea | 0.25 g/L |
| Créatinine | 7 mg/L |
| Troponin | 0.001 mg/ml |
| ALT | 21 IU |
| AST | 15 IU |
ALT: alanine transaminase; AST: aspartate transaminase.
The patient received supplement oxygen with seven doses of nebulized salbutamol (35 mg in total: 5 mg every 20 min) and ipratropium 250 µg as well as intravenous methyl prednisolone 120 mg, deep venous thrombosis, and stress ulcer prophylaxis.
After 2 h, the patient became less responsive to the medical treatment, so a bolus dose of sulfate magnesium 2 g was administered without any clinical improvement: oxygen saturation was 87% on high-flow oxygen with worsening of dyspnea, limited inspiratory flow, and we noticed decreased wheeze.
A second arterial blood gas showed a severe mixed acidosis: pH: 7.09; PaCO2: 63 mmHg; PaO2: 69 mmHg; HCO3–: 38 mmol/L; lactic acid: 15 mmol/L. In the meanwhile, the hemodynamic state was stable (blood pressure 140/75 mmHg; heart beat (HR) 125 beats/min) without any signs of hypoperfusion or sepsis.
While preparing the patient for rapid sequence intubation, we decided to attempt ketamine in nebulization as an ultimate resort, but the patient’s wife did not accept the procedure because of potential side effects.
The patient was intubated and mechanically ventilated at the following settings: control-volume mode, respiratory rate 16 beats/min, tidal volume 400 mL, positive-end expiratory pressure (PEEP) 0 cm H2O, inspiratory flow 90 L/min, and inspiration–expiration ratio at 1 to 4. The airways pressures were high, and peak pressure was at 45 cm H2O.
Salbutamol was stooped as well as corticosteroids were reduced to 60 mg daily. Inhaled sevoflurane 3% was administred for 1 h twice a day, providing a clinical improvement with further decreased wheezes and respiratory pressures (peak pressure: 28 cmH2O).
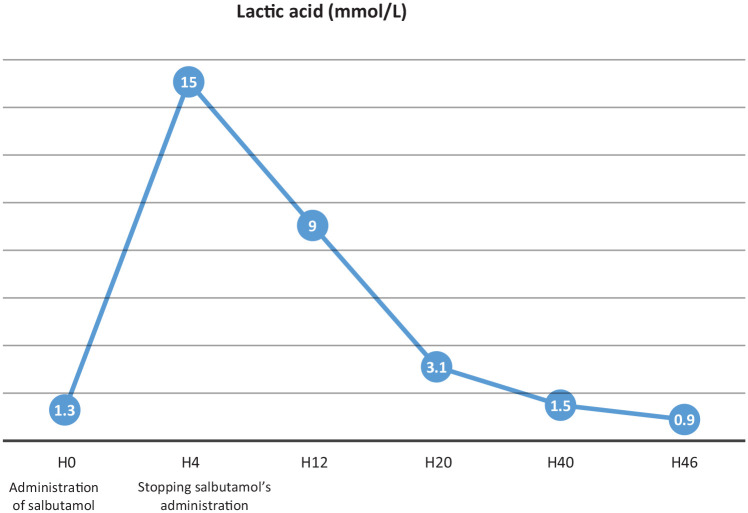
The systemic lactic acidosis persisted during the initial 12 h after intubation but then resolved slowly over the next 48 h, coincident with tapering of salbutamol.
The patient was extubated on the second day and was transferred on the third with a normal acid–base status (Figure 1).
Figure 1.
Evolution of lactic acid blood rate of our patient.
Discussion
Respiratory acidosis is the most important acid–base disturbance in acute severe asthma. It is due to inadequate ventilation, severe airflow limitation, and progressive hypercapnia.
Its early recognition and treatment is important and decisive for the final outcome. 3
Asthma exacerbation can also be associated with several other acid–base disturbances, such as respiratory alkalosis in patients with moderate acute asthma due to hyperventilation leading to hypocapnia and metabolic acidosis. 3
In acute severe asthma, excess blood lactic acid level and lactic acidosis, may result from hypoxemia, hypotension with poor tissue oxygen delivery, overproduction by respiratory muscles performing increased work, metabolic defects caused by liver hypoperfusion, and from drugs such as selective beta-adrenoceptor agonists. 4
In fact, beta-adrenoceptor agonists raise the intracellular concentration of cAMP which allows the activation of glycolysis and lipolysis. They induce a transient increase in blood glucose and high stimulation of lipolysis with increased production of free fatty acids that divert the metabolism of pyruvate from the Krebs cycle toward lactate formation. 5
In a systematic review, Alina G. Liedtke et al. performed a pooled analysis about the prevalence of significant hyperlactatemia in patients receiving a selective ß2-agonist using data retrieved from the selected observational studies and clinical trials. They noted that 30% of asthmatic patients treated with selective ß2-agonist developed hyperlactatemia with a significant relationship between lactate level and intravenous albuterol dose. 4
Given the high prevalence of relevant hyperlactatemia on treatment with beta-adrenoceptor agonists, dyspnea, being a sign of airway obstruction during severe asthmatic crisis, may also occur in case of severe lactic acidosis.
Physical examination, peak flow measurement, and arterial blood gas are advised to determinate the origin of the persisting dyspnea. 6
In our case, we believe that lactic acidosis was induced by salbutamol and was responsible for respiratory failure.
In the other hand, sevoflurane may be used as a part of the treatment of acute asthma with refractory bronchospasm. Sevoflurane reduces airway resistance, decreases the use of the beta2 agonists and as a consequence reducing of lactate production, provides adequate sedation for mechanical ventilation, and improve respiratory status. 7
Ketamine also can be used, as a rescue agent in intensive care unit. It can be administered on bolus, continuous infusion, or via inhaled route through a nebulizer device. The major pathophysiology in asthma comprises of airway hyper reactivity, increased vascular permeability, smooth muscle spasm, and release of inflammatory mediators. 8
Conclusion
Salbutamol is an uncommonly recognized culprit in causing lactic acidosis. It may create a paradoxical situation with improvement of bronchospasm and aggravation of dyspnea. This might lead to misinterpretation with increased use of salbutamol, creating a vicious cycle and leading to respiratory failure.
This negative side of salbutamol must be known in order to adjust therapy accordingly.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical approval: Our institution does not require ethical approval for reporting individual cases or case series.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Informed consent: Written informed consent was obtained from the patient(s) for their anonymized information to be published in this article.
ORCID iDs: Hamza Najout  https://orcid.org/0000-0002-2007-5582.
https://orcid.org/0000-0002-2007-5582.
Mohamed Moutawakil  https://orcid.org/0000-0002-7918-3932.
https://orcid.org/0000-0002-7918-3932.
References
- 1. Kaza V, Bandi V, Guntupalli KK. Acute severe asthma: recent advances. Curr Opin Pulm Med 2007; 13(1): 1–7. [DOI] [PubMed] [Google Scholar]
- 2. Shah R, Saltoun CA. Acute severe asthma (status asthmaticus). Allergy Asthma Proc 2012; 33(Suppl. 1): 47–50. [DOI] [PubMed] [Google Scholar]
- 3. Vasileiadis I, Alevrakis E, Ampelioti S, et al. Acid-base disturbances in patients with asthma: a literature review and comments on their pathophysiology. J Clin Med 2019; 8(4): 563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Liedtke AG, Lava SAG, Milani GP, et al. Selective ß2-adrenoceptor agonists and relevant hyperlactatemia: systematic review and meta-analysis. J Clin Med 2019; 9(1): 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rodrigo GJ, Rodrigo C. Elevated plasma lactate level associated with high dose inhaled albuterol therapy in acute severe asthma. Emerg Med J 2005; 22(6): 404–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tobin A. Intravenous salbutamol: too much of a good thing? Crit Care Resusc 2005; 7(2): 119–127. [PubMed] [Google Scholar]
- 7. Ruszkai Z1, Bokrétás GP, Bartha PT. Sevoflurane therapy for life-threatening acute severe asthma: a case report. Can J Anaesth 2014; 61(10): 943–950. [DOI] [PubMed] [Google Scholar]
- 8. Esmailian M1, Koushkian Esfahani M, Heydari F. The effect of low-dose ketamine in treating acute asthma attack: a randomized clinical trial. Emerg (Tehran) 2018; 6(1): e21. [PMC free article] [PubMed] [Google Scholar]