Abstract
Despite progress in pharmacotherapy in pediatric pulmonary hypertension, real-world patterns of directed pulmonary hypertension therapy have not been studied in the current era. A retrospective observational study of children (≤18 years) with pulmonary hypertension was performed using data from the MarketScan® Commercial and Medicaid claims databases. Associations between etiology of pulmonary hypertension and pharmaceutical regimen were evaluated, as were the associations between subject social and geographic characteristics (insurance-type, race, and/or census region) and regimen. Annualized costs of single- and multi-class regimens were calculated. In total, 873 subjects were studied, of which 94% received phosphodiesterase-5 inhibitors, 31% endothelin receptor antagonist, 9% prostacyclin analogs, and 7% calcium channel blockers. Monotherapy was used in 72% of subjects. Phosphodiesterase-5 inhibitors monotherapy was the most common regimen (93%). Subjects with idiopathic pulmonary hypertension, congenital heart disease, and unclassified pulmonary hypertension receive more than one agent and were more likely to receive both endothelin receptor antagonist and prostacyclin analogs than other forms of pulmonary hypertension. Compared to recipients of public insurance, subjects with commercial insurance were more likely to receive more intense therapy (p = 0.003), which was confirmed in multivariable analysis (OR: 1.4, p = 0.03). Receipt of commercial insurance was also associated with increased annual costs across all subjects (p < 0.001) and for the most common specific regimens. The majority of children with pulmonary hypertension receive phosphodiesterase monotherapy, followed by phosphodiesterase–endothelin receptor antagonist two drug regimens, and finally the addition of prostacyclin analogs for three-drug therapy. However, even after adjustment for measurable confounders, commercial insurance was associated with higher intensity care and higher costs (even within specific classes of pulmonary vasodilators). The effect of these associations on clinical outcome cannot be discerned from the current data set, but patterns of treatment deserve further attention.
Keywords: pediatric cardiology, outcomes research, drug therapy
Introduction
Pulmonary hypertension (PH) affects 2.1–3.7 children per million1–4 and is associated with tremendous morbidity and reduction in life expectancy. 5 A number of pharmaceutical agents have been developed that dilate the pulmonary arterial bed in the hopes of preventing right ventricular failure, reducing symptoms, improving quality of life, and potentially extending lifespan. These drugs include (1) calcium channel blockers (CCB), (2) phosphodiesterase-5 inhibitors (PDE5), (3) prostacyclin analogs (PA), (4) endothelin receptor antagonists (ERA), and (5) soluble guanylate cyclase stimulators. Trials in adults with PH have demonstrated significant benefits in terms of surrogate markers of functional capacity, such as the six-minute walk test distance, as well as middle term mortality. 6 Though pediatric trial data are limited, survival in the era of targeted therapy is significantly better than historical controls with five-year survival between 74–78%.7–9
The optimal pharmacological regimen is not well established. In adults, there is evidence that combination therapy is associated with improved survival,10,11 but there are limited data in children. Retrospective studies have demonstrated an association between multi-class therapeutic regimens and improved survival. 12 To our knowledge, no studies have prospectively evaluated the benefit of single-class versus combination therapy or of progressive intensification of therapy in children with PH. As a result, published guidelines detail the evaluation, classification, and risk stratification of children with PH, but there is limited evidence defining the optimal regimen for directed therapy.13,14 The Tracking Outcomes and Practice in Pediatric Pulmonary Hypertension Registry reported the patterns of treatment at their member institutions. 15 However, this multinational registry may have limited generalizability as it drew from specialized PH referral centers. To our knowledge, the real-world utilization of pharmacotherapy in pediatric patients with PH in the current era has not been studied.
We utilized an insurance claims database to study the use of pulmonary vasodilators in United States children and adolescents with PH. We sought to describe the prevalent prescribing patterns of PH-directed therapy, explore whether disease etiology and other patient characteristics were associated with different patterns of therapy, and calculate estimated annual costs for the most common treatment regimens. Real-world data on drug utilization is an important step in establishing equipoise for future clinical efficacy studies.
Methods
Data source
The Truven Health MarketScan® Claims and Encounter Database (Truven Health Analytics, IBM Watson Health™, Ann Arbor, MI, USA) contains insurance claims data. 16 For this project, modules containing data from both commercial insurers and Medicaid programs were used, as described in previously published studies.17–22 This database provides in-depth, cross-sectional, and longitudinal patient-level data. It is a large convenience sample that is geographically representative of the population within the United States, spanning the entire nation. The databases used included data about recipients of commercial insurance and their families as well as United States public insurance (Medicaid). The commercial database includes data from both large and small employers with some degree of over-representation of larger employers, 16 though there was no reason prior to analysis to expect that this would bias results in a pediatric PH population. Data are de-identified, and, as a result, the study was judged to be exempt from review by The Children's Hospital of Philadelphia Institutional Review Board according to the Common Rule. Subject-level data and methods will not be published or made publicly available, because this was prohibited by our data use agreement.
Study population
Subjects eligible for inclusion were 0–18 years of age with a diagnosis of PH, receiving directed therapy, and with data in the MarketScan® Commercial and/or Medicaid databases between 1 January 2013 and 31 December 2016. Subjects were identified by screening all encounters in the database for encounters with an International Classification of Diseases (ICD) v9 or v10 code that reflected PH (Supplementary Table 1). These codes are not perfectly accurate in identifying true PH in a population. However, to maximize the accuracy of cohort identification, study subjects were included only if they (1) had ≥ 2 encounters in a 90-day span with a PH diagnosis code and (2) if over the study period they had ≥3 encounters with a PH diagnosis code. These additional criteria were used to improve the specificity of identifying PH and excluding persistent PH of the newborn and other misclassification (suspected PH that was later ruled out) from the study sample. Characteristics of potential subjects receiving pulmonary vasodilators and those without were compared (Supplementary Table 2).
Study measures
The primary outcome of interest in this study was the relative likelihood of prescribing one or more of the following classes of medication: PDE5, CCB, PA, and ERA. These were identified using National Drug Codes (NDC). The MarketScan Database has a look-up table connecting generic and trade names of pharmaceuticals with their NDC. To insure this captured all NDC of interest, the RxNorm (US National Library of Medicine) and United States Food and Drug Administration look-up tables were cross-referenced. The indication for therapy is not ascertainable, so misclassification of therapy is possible (e.g. CCB for dysrhythmia or for hypertension). Though insurance claims data are a standard method for measuring outpatient utilization of pharmaceuticals, there is evidence that these data are not completely sensitive. 23 We hoped to ameliorate this risk by looking at prescriptions over the course of the coverage (reducing the risk of missed prescriptions during shorter time periods) and by evaluating the distribution of prescriptions in those who did receive pulmonary vasodilator medications. Another potential issue is that many PH patients receive medications either from specialty pharmacies or through cost-sharing/reducing programs, which might be filled outside of their conventional prescription benefit. This could result in missing data for individual subjects and a potential source of error.
Subject age and sex were taken directly from the database. Etiology of PH was identified using ICD-9 and 10 codes based on the World Health Organization classification system (Supplementary Table 1). Importantly, it is not possible to differentiate familial and inherited forms of PH from idiopathic PH (IPAH). Also, race and ethnicity data were available as a single variable, but only in recipients of Medicaid. Census region data were only available for recipients of commercial insurance. For analytic purposes for these variables, a category identifying subjects in which the data were not provided in the database was created to allow for inclusion and to avoid bias. All data were extracted directly.
To quantify the economic impact of treatments with pulmonary vasodilators, outpatient pharmacy costs were extracted from the database. The impact of pharmacotherapy on the health system at large was the focus, co-payment and reimbursement rates were not measured. All costs were adjusted to 2016 United States Dollars using the consumer price index for medical goods and services from the United States Bureau of Labor Statistics (www.bls.gov/cpi/). Wholesale prices were used because there are not, to our knowledge, established methodologies to convert from these prices to true cost and, to the best of our knowledge, these prices are independent of pharmacy and insurance payer. For each individual subject, prices were collected for pre-identified pulmonary vasodilator prescriptions over the period between the subjects first and last outpatient pharmacy visit and normalized to a per year cost (by dividing the total by the duration of time of days supplied).
Statistical analysis
Descriptive statistics were calculated in order to describe the proportion of the entire cohort and of diagnosis-specific strata that were treated with each of the pulmonary vasodilators and their classes.
The primary goal of the analysis was to study the association between subject medical (etiology of PH) and social (commercial versus Medicaid insurance, race and/or ethnicity, and census region) factors on the propensity for (1) treatment with PDE5 agents as monotherapy versus all other classes and combinations of classes and (2) treatment with PA versus all other agents and combinations of agents. Exceeding PDE5 monotherapy and use of PA were chosen as outcomes that represented escalating intensity of pharmacologic regimen.
The primary hypotheses of this analysis were that (1) PH associated with congenital heart disease (APAH-CHD) would be associated with a lower proportion of subjects with PH-directed therapy in excess of PDE5 monotherapy than subjects with IPAH or unclassified PH and (2) that there would not be differences in treatment based on other etiologies of PH. We also hypothesized that there would not be significant differences in prescribing patterns based on region, insurance type (commercial insurance versus Medicaid), and (within the Medicaid population) race. These were evaluated without adjustment using Chi-square test. For each of these hypotheses, multivariable logistic regression models for each of the two outcomes were calculated adjusting for sex, age (divided into age categories as defined previously24–27), race, and census region. Because of the incomplete data for race and census region, models were first calculated without these covariates. Subsequent models introducing race and census region were calculated separately. Covariates were chosen prior to analysis. No model refinement was performed. No formal power calculations were performed, but the assumption given the number of subjects was that the study was, if anything, over-powered for most outcomes.
As a secondary analysis, the economic burden of pulmonary vasodilator therapy was explored. Descriptive statistics were calculated for the entire cohort. Annualized costs were calculated for different regimens. Next, comparisons in the costs of regimen were compared based on insurance status. This was done based on observed costs using Wilcoxon ranksum test.
All analyses were performed using SAS v9.4 (SAS Institute, Cary, NC). The only field for which missing data were seen was race and census region. Race was missing in >20% of subjects with Medicaid insurance. Census region was also missing at a lower rate (<5% for each subcategory). It was not thought that either variable could be imputed from other data so these subjects were given a “missing” classification to avoid bias as described previously.24,28–32 The threshold for significance was set at p < 0.05. Primary and secondary outcomes were established prior to analysis, and no adjustment for multiple comparisons was made.
Results
Study population
Ultimately, 873 children and adolescents of median age 4 (IQR: 1–10) were studied. The study population was 49% male and comprised of 12% IPAH, 47% APAH-CHD, 21% PH associated with pulmonary conditions, 2% associated with cardiomyopathy, and 19% with PH whose etiology is otherwise unclassified by ICD code. Medicaid recipients comprised 62% of the study population with the remainder receiving commercial insurance. Baseline characteristics of subjects stratified by etiology of PH are depicted in Table 1.
Table 1.
Study population.
| IPAH N = 105 | APAH-CHD N = 405 | Respiratory disease N = 183 | Cardiomyopathy N = 13 | Other/unclassified N = 162 | p | |
|---|---|---|---|---|---|---|
| Female sex | 52% (55) | 55% (221) | 41% (75) | 69% (9) | 53% (86) | 0.02 |
| Age (years) | 1 (IQR: 1–3) | 5 (IQR: 2–11) | 2 (IQR: 1–4) | 15 (IQR: 3–15) | 8 (IQR: 3–15) | <0.001 |
| Age categories | <0.001 | |||||
| ≤1 year | 61% (64) | 17% (70) | 31% (57) | 8% (1) | 20% (32) | |
| 2–6 years | 22% (23) | 43% (174) | 52% (96) | 31% (4) | 21% (34) | |
| 7–12 years | 8% (8) | 19% (77) | 10% (18) | 8% (1) | 25% (39) | |
| 13–18 years | 10% (10) | 21% (84) | 7% (12) | 54% (7) | 35% (57) | |
| Insurance payer | ||||||
| Medicaid | 62% (64) | 60% (243) | 66% (121) | 46% (6) | 64% (103) | 0.47 |
| Commercial | 38% (40) | 40% (162) | 34% (62) | 54% (7) | 36% (59) | |
| Race/ethnicity (Medicaid only) | N = 64 | N = 243 | N = 121 | N = 6 | N = 103 | <0.001 |
| White | 25% (16) | 47% (114) | 31% (37) | 33% (2) | 45% (46) | |
| Black | 19% (12) | 16% (38) | 36% (44) | 33% (2) | 24% (25) | |
| Hispanic | 6% (4) | 8% (20) | 3% (4) | 17% (1) | 4% (4) | |
| Other | 8% (5) | 2% (6) | 2% (3) | 17% (1) | 3% (3) | |
| Missing | 42% (27) | 27% (65) | 27% (33) | 0 (0) | 24% (25) | |
| Region (commercial only) | N = 40 | N = 155 | N = 61 | N = 7 | N = 58 | 0.82 |
| Northeast | 13% (5) | 16% (25) | 15% (9) | 29% (2) | 12% (7) | |
| Midwest | 23% (9) | 29% (45) | 23% (14) | 43% (3) | 24% (14) | |
| South | 43% (17) | 30% (47) | 39% (24) | 29% (2) | 38% (22) | |
| West | 23% (9) | 25% (38) | 23% (14) | 0% (0) | 26% (15) | |
| Missing | 0% (0) | 4% (7) | 2% (1) | 0% (0) | 2% (1) | |
IPAH: idiopathic pulmonary hypertension; APAH-CHD: pulmonary hypertension associated with congenital heart disease.
Distribution of directed PH therapy
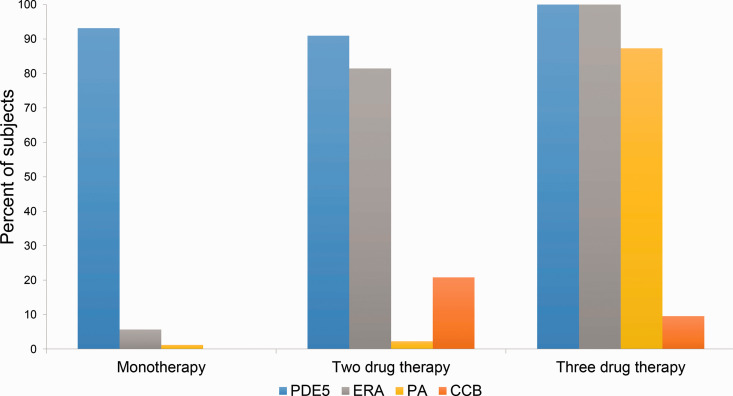
The distribution of pharmaceutical treatments is depicted in Fig. 1. Over the study population, 71% were receiving a regimen with a single class of pulmonary vasodilation, 22% were receiving two classes, 6.5% three classes, and 0.5% four classes. PDE5 were the predominant therapy in each regimen (93% of monotherapy, 91% of two-class regimens, and 100% of three-drug regimens), followed by ERA (6% of monotherapy, 81% of two-class regiments, and 100% of three-drug regimens) with PA and CCB making up the difference. Of the total study population, 94% received PDE5, 31% ERA, 9% PA, and 7% CCB.
Fig. 1.
Distribution of pulmonary vasodilator drug classes in different regimens.
The distribution of pulmonary vasodilator drug classes received by subjects on one, two, and three-drug regimens are depicted. Phosphodiesterase 5 inhibitors (PDE5) are depicted in blue, endothelin receptor antagonists (ERA) in blue, prostacyclin analogs (PA) in yellow, and calcium channel blockers (CCB) in orange.
There were small but statistically significant differences in the distribution of these classes of medication among different etiologies for PH (Supplementary Table 3). A higher proportion of APAH-CHD, IPAH, and other/unclassified subjects received PDE5, ERA, and PA (p = 0.04, 0.001, 0.001, respectively) compared to respiratory disease and cardiomyopathy, while a higher proportion of subjects with cardiomyopathy received CCB (p = 0.007) compared to all other etiologies of PH. In terms of the number of classes of agents used, a larger proportion of subjects with respiratory disease as the cause of their PH received monotherapy (79%), while IPAH, APAH-CHD, and other/unclassified etiologies of PH were more likely to have three or more agents (6%, 7%, and 11%, respectively, p < 0.0001, Supplementary Table 4).
In terms of insurance type, recipients of commercial insurance were more likely to receive ERA (40% versus 27%, p < 0.001) and CCB (9% versus 5%, p = 0.01, Supplementary Table 5) than recipients of Medicaid. There were no significant differences in the likelihood of receiving PDE5 or PA. Recipients of commercial insurance were similarly more likely than recipients of Medicaid to receive combination therapy with 30% receiving two classes of pulmonary vasodilator and 10% receiving three or more classes (as opposed to 22% and 6% in patients with Medicaid, p = 0.003, Supplementary Table 6).
In multivariable models to measure the association between patient characteristics and likelihood of receiving PDE5 monotherapy, no significant difference was seen between IPAH, APAH-CHD, and other/unclassified PH (Table 2). PH in pulmonary disease was associated with a higher likelihood of receiving PDE5 monotherapy (OR: 2.00, 95% CI: 1.11–3.60, p = 0.02). Commercial insurance was associated with a decreased likelihood of PDE5 monotherapy (OR: 0.71, 95% CI: 0.52–0.97, p = 0.03). Compared to school-aged children (age 7–12 years), infants (OR: 6.64, 95% CI: 4.00–11.00, p < 0.0001) and children aged 2–6 years were associated with a higher likelihood of PDE5 monotherapy (OR: 3.24, 95% CI: 2.13–4.94, p < 0.0001). The point estimate for adolescent subjects suggested a lower likelihood of PDE5 monotherapy (OR: 0.65), but the association was not significant (p = 0.07). In secondary analyses evaluating the association between race (Supplementary Table 7) and census region (Supplementary Table 8) with likelihood of receiving PDE5 monotherapy, no significant association was seen.
Table 2.
Association of patient characteristics and the likelihood of phosphodiesterase-5 monotherapy.
| OR | 95% CI | p | |
|---|---|---|---|
| Etiology of PH | |||
| IPAH | 1 | n/a | n/a |
| APAH-CHD | 1.44 | 0.85–2.42 | 0.17 |
| Respiratory | 2.00 | 1.11–3.60 | 0.02 |
| Cardiomyopathy | 2.03 | 0.55–7.47 | 0.29 |
| Other unclassified | 1.22 | 0.68–2.18 | 0.51 |
| Commercial insurance (vs. Medicaid) | 0.71 | 0.52–0.97 | 0.03 |
| Female sex | 0.80 | 0.60–1.10 | 0.17 |
| Age category | |||
| ≤1 year | 6.64 | 4.00–11.00 | <0.0001 |
| 2–6 | 3.24 | 2.13–4.94 | <0.0001 |
| 7–12 | 1 | n/a | n/a |
| 13–18 | 0.65 | 0.41–1.04 | 0.07 |
APAH-CHD: pulmonary hypertension associated with congenital heart disease; CI: confidence interval; IPAH: idiopathic pulmonary hypertension; OR: odds ratio; PH: pulmonary hypertension.
In analogous models for the likelihood of receiving PA, a similar pattern was seen (Table 3). IPAH, APAH-CHD, and other/unclassified PH did not differ significantly in the odds of receiving PA. The point estimate for the association between PH with pulmonary disease and receiving PA suggested decreased odds (OR: 0.39) but the association was not significant (p = 0.10). No association was seen between commercial insurance and odds of receiving PA (OR: 1.06, p = 0.82). Female sex had a point estimate consistent with increased odds of receiving PA (OR: 1.59), but this association was not significant (p = 0.07). As with previous model, younger age was associated with lower intensity of therapy, in this case, a lower odds of PA in infants (OR: 0.04, p < 0.0001) and subjects aged 2–6 years (OR: 0.23, p < 0.0001). The point estimate for adolescents was consistent with increased odds of receiving PA (OR: 1.41, p = 0.25). As with models for PDE monotherapy, no significant association was seen between race (Supplementary Table 9) or census region (Supplementary Table 10) with the likelihood of receiving PA.
Table 3.
Association of patient characteristics and the likelihood of receipt of prostacyclin analogs.
| OR | 95% CI | p | |
|---|---|---|---|
| Etiology of PH | |||
| IPAH | 1 | n/a | n/a |
| APAH-CHD | 0.50 | 0.21–1.22 | 0.13 |
| Respiratory | 0.39 | 0.13–1.19 | 0.10 |
| Cardiomyopathy | 0.25 | 0.03–2.37 | 0.23 |
| Other unclassified | 0.83 | 0.33–2.10 | 0.69 |
| Commercial insurance (vs. Medicaid) | 1.06 | 0.65–1.74 | 0.82 |
| Female sex | 1.59 | 0.96–2.61 | 0.07 |
| Age category | |||
| ≤1 year | 0.04 | 0.01–0.17 | <0.0001 |
| 2–6 | 0.23 | 0.12–0.48 | <0.0001 |
| 7–12 | 1 | n/a | n/a |
| 13–18 | 1.41 | 0.80–2.48 | 0.25 |
APAH-CHD: pulmonary hypertension associated with congenital heart disease; CI: confidence interval; IPAH: idiopathic pulmonary hypertension; OR: odds ratio; PH: pulmonary hypertension.
Economic impact of pharmacotherapy
Annualized costs are described for specific regimens in Table 4. The annual costs of PDE monotherapy, PDE/ERA two-drug-class therapy, and PDE/ERA/PA three-drug-class therapy regimens were all significantly higher for recipients of commercial insurance than those receiving Medicaid (p < 0.0001, 0.001, and 0.04, respectively). Of note, the annualized cost of sildenafil (median: $24,982, IQR: $8719–66,195) was not significantly different than tadalafil (median: $21,106, IQR: $14,160–31,539). The median cost of ERA monotherapy in commercial subjects (median: $95,765, IQR: $64,408–105,096) was larger than in Medicaid subjects (median: $59,393, IQR: $42,160–$88,634) but the association was not significant (p = 0.09), but the analysis was limited by the very small number of subjects receiving monotherapy (n = 38 and 17, respectively). Duration of therapy and the number of prescriptions over follow-up time were not significantly different for any strata (data not shown).
Table 4.
Costs of specific drug regimens by insurance type.
| Medicaid | Commercial | p | |
|---|---|---|---|
| PDE | N = 383 18,493 (IQR: 6025–49,374) | N = 200 41,095 (IQR: 18,610–74,404) | <0.0001 |
| ERA | N = 17 59,393 (IQR: 42,160–88,634) | N = 20 95,765 (IQR: 64,408–105,096) | 0.09 |
| PDE + ERA | N = 92 92,095 (IQR: 59,438–116,278) | N = 72 115,420 (IQR: 76,880–143,798) | 0.001 |
| PDE + ERA + PA | N = 32 197,197 (IQR: 177,923–263,226) | N = 30 278,664 (IQR: 183,157–346,577) | 0.04 |
Comparisons for PDE + CCB and PDE + PA were not possible because the sample size was too small.
ERA: endothelin receptor antagonist; IQR: interquartile range; PA: prostacyclin analog; PDE: phosphodiesterase 5 inhibitor.
Discussion
Using insurance claims data, this study provides a novel opportunity to study the pharmacological regimens of a non-selected sample of children with PH. The majority of patients received PDE5 monotherapy, followed by two-drug-class therapy with PDE5 and ERA, and finally three-drug-class-therapy with PDE5, ERA, and PA. Children with PH attributed to pulmonary conditions were less likely to receive more than one class of agents and agents other than PDE5, while APAH-CHD, IPAH, and otherwise unclassified PH patients received similar regimens. An unexpected finding was that, in both raw and adjusted analyses, commercial insurance was associated with higher proportion of patients receiving more intense regimens. Though clinical severity is not ascertainable in the current dataset, it seems improbable that disease severity would be disproportionately present in the recipients of commercial insurance. Additionally, even stratified by individual treatment regimen, annual costs were higher for subjects with commercial insurance compared to Medicaid recipients with the same classes of drugs. At this time, it is not possible to determine the mechanisms underlying these differences, nor is it possible to determine whether these differences are associated with differences in outcome. Further research is necessary, but there is potentially significant difference in the pharmacotherapy of subjects with commercial and Medicaid insurance.
Identifying distributions of therapy is important to establish real-world equipoise for future research. Though some trials in adults have evaluated the benefit of combination therapy with ERA and PDE5,10,11 there are only limited pediatric data from observational studies demonstrating better outcomes with two- and three-drug-class regimens.12,33 To our knowledge, no such trials (randomized or pragmatic) have been performed in children. Current treatment guidelines suggest consideration of early combination therapy in high-risk patients, 34 but there is no pediatric-specific data to guide this decision. In the current study, clinical data that could be used to measure severity of PH are not available, and therefore there is no way to determine if regimens were escalated in conjunction with severity of disease.
Retrospective studies from single centers 5 and clinical registries15,35 have described the treatment regimens of children with PH. However, these studies arise from specialized PH referral centers with limited generalizability to broad practice. Children with IPAH and those with more severe disease are also over-represented in these studies.4,36–39 Studies based in relatively unselected populations have shown that the burden of disease in children with congenital heart disease is underappreciated in PH registries.25,27,40,41 Because of coding conventions, the prevalence of PH associated with pulmonary conditions (especially survivors of premature birth with chronic lung disease) may be under-reported to an even greater degree. 42 As a result, the treatment strategies of both APAH-CHD and PH associated with pulmonary disease have not received the same attention as IPAH. Optimal treatment of these subgroups may differ significantly from patients with IPAH. To our knowledge, one previous study has studied the epidemiology of PH in a generalized population including PH-directed therapy between 2007 and 2013. 43 The current study expands on the findings of this initial study not only by including recipients of public insurance who represent a sizable minority of children with chronic medical conditions, but also including granular detail about different etiologies of PH and their influence on treatment regimen. In addition, the current study evaluates potential determinants of these regimens, which was not part of the prior study.
The observation that commercial insurance was associated with more intensive pharmaceutical regimens was unexpected. To our knowledge, previous registry studies have not evaluated this association. This highlights the advantage of the current study design. One possible explanation for the difference in observation between this study and previous registry studies is in the constitution of the study population. Care at the highest volume PH programs (who contribute to registries) is unlikely to be influenced by insurance status. However, differences in socioeconomic status (in this case represented by insurance type) may influence access to specialized care for families outside the immediate catchment areas of specialized centers. These would not be reflected in center-based registry studies. We acknowledge that it is not possible to determine the center or type of center at which subjects in this study were seen to evaluate this possible explanation.
Though differences in treatment regimens between recipients of commercial insurance and Medicaid are provocative, care should be taken in evaluating differences in patterns of treatment with social factors. To the best of our ability, we adjusted for measurable confounders. It is unlikely that disease severity or other clinically relevant factors should introduce differential bias to our analysis. However, we acknowledge the possibility of unmeasured confounding. Moreover, care must be taken to address the complicated relationship between socioeconomic status, insurance, access to care (especially specialized care), and race/ethnicity. The structure of the MarketScan database makes it impossible to evaluate these factors. With that in mind, we would contend that it was important to explore these issues to the best of our ability. Identifying disparities in pharmacotherapy based on insurance is a first step in addressing these issues. If these associations are verified in additional studies, it is also important to work to understand whether disparities reflect differential access to care, differences in coverage, or differences in prescribing. The remedies for each of these are different.
Another important issue to address is that ICD-9 and 10 codes for PH diagnoses are not consistent with either Panama or WHO classification of PH,25,27,42 nor are they perfectly accurate at identifying true PH. We can identify subjects with congenital heart disease, cardiomyopathy, and concomitant pulmonary disease, but differentiating other forms of PH is challenging. Additionally, in the ninth version of ICD coding, a large proportion of patients were described as being unclassified/other etiology. A previous study of adults with PH demonstrated that the transition to ICD-10 coding was accompanied by an increase in the prevalence of IPAH cases, 44 suggesting that IPAH cases were misclassified by ICD-9 coding. We cannot be certain in children a similar pattern of misclassification is seen, but in general, it appears that the children with PH classified as other/unknown had similar characteristics and treatment as those with IPAH.
The use of CCB appears more common in the study population than expected. The proportion is likely inflated by CCB that are prescribed for other indications (e.g. systemic hypertension or arrhythmia). As noted, it was not possible to identify the indication for pharmaceuticals. There is no way in this dataset to further investigate whether the prescription of CCB was related to the institution(s) at which the subject received care, severity of illness, or other factors. It was not associated with public versus commercial insurance (data not shown). In previous registry-based studies, the prevalence of PH patients receiving CCB is relatively small, 36 and the reported prevalence of its use in this series is not intended as an endorsement of the practice.
There are additional limitations to this study. We are restricted to a relatively short study period, which makes it impossible to evaluate changes in the use of drugs over time both for the population in general and for individual patients. Restricting our analysis to subjects with longer follow-up and multiple visits confirming the diagnosis of PH, we tried to maximize the accuracy of our cohort identification and to find a cohort in which a stable regimen was identified. A second concern is that several important aspects of patient data are obscured in our database to protect consumer privacy. More detailed information about geographical location of the home, which can be used to estimate both socioeconomic status (e.g. median income and education level) and ease of access to care (e.g. urban versus rural and/or distance to a tertiary or quaternary care center), would improve our ability to address these important questions. Overcoming these obstacles is an important step in successfully studying these issues. As with all observational datasets, there is the possibility of miscoding of diagnoses and of unmeasured confounding (specifically race and ethnicity, smoking status, and other exposures) in spite of efforts to be stringent with diagnostic code algorithms. Finally, we acknowledge that in an insurance claims database, the “utilization” of pharmaceuticals that is measured is filled prescriptions. This does not directly measure what medications are prescribed or the degree to which subjects adhere to prescribed regimens, but is the best available estimate.
Conclusions
Despite these limitations, the current study demonstrates the pattern of PH-directed therapy in a representative sample of US children. The intensity of drug therapy was not only associated with patient factors (e.g. diagnosis and age) but also insurance status. Commercial insurance was associated with an increased likelihood of receiving multidrug therapy. Within drug regimens, commercial insurance was also associated with higher annualized costs. Determining whether these differences are associated with differences in outcome is critical and deserves attention.
Supplemental Material
Supplemental material, sj-pdf-1-pul-10.1177_2045894020933083 for Variation in the use of pulmonary vasodilators in children and adolescents with pulmonary hypertension: a study using data from the MarketScan® insurance claims database by Michael L. O'Byrne, Jennifer A. Faerber, Hannah Katcoff, David B. Frank, Alex Davidson, Therese M. Giglia and Catherine M. Avitabile in Pulmonary Circulation
Conflict of interest
Dr Michael L. O’Byrne has received honoraria from Gore Medical (Newark, Delaware). There are no other conflicts of interest to disclose.
Funding
Dr Michael L. O’Byrne received support from the National Institute of Health/National Heart Lung and Blood Institute (K23 HL130420-01) and the Pulmonary Hypertension Association Robyn Barst Grant. Dr Catherine M. Avitabile receives support from the Pediatric Heart Network and is the recipient of an Entelligence Young Investigator Grant. Dr David B. Frank receives support from the National Institute of Health/National Heart, Lung, and Blood Institute (K08-HL140129, the Parker B. Francis Foundation, and the W.W. Smith Charitable Trust. This project received support from the CHOP Pulmonary Hypertension Research Fund. The funding agencies had no role in the planning or execution of the study, nor did they edit the manuscript. The manuscript represents the opinions of the authors alone.
ORCID iD
Michael L. O’Byrne https://orcid.org/0000-0001-6023-1634
Supplemental material
Supplemental material for this article is available online.
References
- 1.Moledina S, Hislop AA, Foster H, et al. Childhood idiopathic pulmonary arterial hypertension: a national cohort study. Heart 2010; 96: 1401–1406. [DOI] [PubMed] [Google Scholar]
- 2.Barst RJ, McGoon MD, Elliott CG, et al. Survival in childhood pulmonary arterial hypertension: insights from the registry to evaluate early and long-term pulmonary arterial hypertension disease management. Circulation 2012; 125: 113–122. [DOI] [PubMed] [Google Scholar]
- 3.Fraisse A, Jais X, Schleich J-M, et al. Characteristics and prospective 2-year follow-up of children with pulmonary arterial hypertension in France. Arch Cardiovasc Dis 2010; 103: 66–74. [DOI] [PubMed] [Google Scholar]
- 4.Van Loon RLE, Roofthooft MTR, Hillege HL, et al. Pediatric pulmonary hypertension in the Netherlands: epidemiology and characterization during the period 1991 to 2005. Circulation 2011; 124: 1755–1764. [DOI] [PubMed] [Google Scholar]
- 5.Mullen MP, Andrus J, Labella MH, et al. Quality of life and parental adjustment in pediatric pulmonary hypertension. Chest 2014; 145: 237–244. [DOI] [PubMed] [Google Scholar]
- 6.Hansmann G, Apitz C. Treatment of children with pulmonary hypertension. Expert consensus statement on the diagnosis and treatment of paediatric pulmonary hypertension. The European Paediatric Pulmonary Vascular Disease Network, endorsed by ISHLT and DGPK. Heart 2016; 102: ii67–ii85. [DOI] [PubMed] [Google Scholar]
- 7.Barst RJ, McGoon MD, Elliott CG, et al. Survival in childhood pulmonary arterial hypertension: insights from the registry to evaluate early and long-term pulmonary arterial hypertension disease management. Circulation 2012; 125: 113–122. [DOI] [PubMed] [Google Scholar]
- 8.Moledina S, Hislop AA, Foster H, et al. Childhood idiopathic pulmonary arterial hypertension: a national cohort study. Heart 2010; 96: 1401–1406. [DOI] [PubMed] [Google Scholar]
- 9.Van Loon RLE, Roofthooft MTR, Delhaas T, et al. Outcome of pediatric patients with pulmonary arterial hypertension in the era of new medical therapies. Am J Cardiol 2010; 106: 117–124. [DOI] [PubMed] [Google Scholar]
- 10.Galiè N, Barberà JA, Frost AE, et al. Initial use of ambrisentan plus tadalafil in pulmonary arterial hypertension. N Engl J Med 2015; 373: 834–844. [DOI] [PubMed] [Google Scholar]
- 11.Sitbon O, Sattler C, Bertoletti L, et al. Initial dual oral combination therapy in pulmonary arterial hypertension. Eur Respir J 2016; 47: 1727–1736. [DOI] [PubMed] [Google Scholar]
- 12.Zijlstra WMH, Douwes JM, Rosenzweig EB, et al. Survival differences in pediatric pulmonary arterial hypertension: clues to a better understanding of outcome and optimal treatment strategies. J Am Coll Cardiol 2014; 63: 2159–2169. [DOI] [PubMed] [Google Scholar]
- 13.Abman SH, Hansmann G, Archer SL, et al. Pediatric pulmonary hypertension: guidelines from the American Heart Association and American Thoracic Society. Circulation 2015; 132: 2037–2099. [DOI] [PubMed] [Google Scholar]
- 14.Hansmann G, Apitz C. Treatment of children with pulmonary hypertension. Expert consensus statement on the diagnosis and treatment of paediatric pulmonary hypertension. The European Paediatric Pulmonary Vascular Disease Network, endorsed by ISHLT and DGPK. Heart 2016; 102: ii67–ii85. [DOI] [PubMed] [Google Scholar]
- 15.Humpl T, Berger RMF, Austin ED, et al. Treatment initiation in paediatric pulmonary hypertension: insights from a multinational registry. Cardiol Young 2017; 27: 1123–1132. [DOI] [PubMed] [Google Scholar]
- 16.The Truven Health MarketScan Databases for Health Services Researchers. truvenhealth.commarketslife-sciencesproductsdata-toolsmarketscan-databases; 1–15 (accessed 5 December 2020).
- 17.Leshem E, Moritz RE, Curns AT, et al. Rotavirus vaccines and health care utilization for diarrhea in the United States (2007-2011). Pediatrics 2014; 134: 15–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thornhill MH, Gibson TB, Cutler E, et al. Antibiotic prophylaxis and incidence of endocarditis before and after the 2007 AHA recommendations. J Am Coll Cardiol 2018; 72: 2443–2454. [DOI] [PubMed] [Google Scholar]
- 19.Graves JM, Rivara FP, Vavilala MS. Health care costs 1 year after pediatric traumatic brain injury. Am J Public Health 2015; 105: e35–e41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Montalbano A, Rodean J, Kangas J, et al. Urgent care and emergency department visits in the pediatric Medicaid population. Pediatrics 2016; 137: e20153100. [DOI] [PubMed] [Google Scholar]
- 21.Jimenez N, Quistberg A, Vavilala MS, et al. Utilization of Mental Health Services After Mild Pediatric Traumatic Brain Injury. Pediatrics 2017; 139: e20162462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Agarwal A, Thombley R, Broberg CS, et al. Age- and lesion-related comorbidity burden among US adults with congenital heart disease: a population-based study. J Amer Heart Assoc 2019; 8: e013450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lauffenburger JC, Balasubramanian A, Farley JF, et al. Completeness of prescription information in US commercial claims databases. Pharmacoepidem Drug Safe 2013; 22: 899–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jayaram N, Spertus JA, O'Byrne ML, et al. Relationship between hospital procedure volume and complications following congenital cardiac catheterization: a report from the IMproving Pediatric and Adult Congenital Treatment (IMPACT) registry. Am Heart J 2017; 183: 118–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.O'Byrne ML, Glatz AC, Hanna BD, et al. Predictors of catastrophic adverse outcomes in children with pulmonary hypertension undergoing cardiac catheterization: a multi-institutional analysis from the pediatric health information systems database. J Am Coll Cardiol 2015; 66: 1261–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.O'Byrne ML, Glatz AC, Shinohara RT, et al. Effect of center catheterization volume on risk of catastrophic adverse event after cardiac catheterization in children. Am Heart J 2015; 169: 823.e5–832.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.O'Byrne ML, Kennedy KF, Kanter JP, et al. Risk factors for major early adverse events related to cardiac catheterization in children and young adults with pulmonary hypertension: an analysis of data from the IMPACT (Improving Adult and Congenital Treatment) registry. J Amer Heart Assoc 2018; 7: e008142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.O'Byrne ML, Glatz AC, Song L, et al. Association between variation in Preoperative Care Before Arterial Switch Operation and Outcomes in Patients With Transposition of the Great Arteries. Circulation 2018; 138: 2119–2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.O'Byrne ML, Millenson ME, Grady CB, et al. Trends in transcatheter and operative closure of patent ductus arteriosus in neonatal intensive care units. Am Heart J 2019; 217: 121–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.O'Byrne ML, Shinohara RT, Grant EK, et al. Increasing propensity to pursue operative closure of atrial septal defects following changes in the instructions for use of the Amplatzer Septal Occluder device: an observational study using data from the Pediatric Health Information Systems database. Am Heart J 2017; 192: 85–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.O'Byrne ML, Gillespie MJ, Shinohara RT, et al. Cost comparison of transcatheter and operative pulmonary valve replacement (from the Pediatric Health Information Systems Database). Am J Cardiol 2016; 117: 121–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.O'Byrne ML, Glatz AC, Faerber JA, et al. Interhospital variation in the costs of pediatric/congenital cardiac catheterization laboratory procedures: analysis of data from the Pediatric Health Information Systems Database. J Amer Heart Assoc 2019; 8: e011543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Douwes JM, Roofthooft MTR, Van Loon RLE, et al. Sildenafil add-on therapy in paediatric pulmonary arterial hypertension, experiences of a national referral centre. Heart 2014; 100: 224–230. [DOI] [PubMed] [Google Scholar]
- 34.Abman SH, Hansmann G, Archer SL, et al. Pediatric pulmonary hypertension: guidelines from the American Heart Association and American Thoracic Society. Circulation 2015; 132: 2037–2099. [DOI] [PubMed] [Google Scholar]
- 35.Berger RMF, Beghetti M, Humpl T, et al. Clinical features of paediatric pulmonary hypertension: a registry study. Lancet 2012; 379: 537–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Douwes JM, Humpl T, Bonnet D, et al. Acute vasodilator response in pediatric pulmonary arterial hypertension: current clinical practice from the TOPP registry. J Am Coll Cardiol 2016; 67: 1312–1323. [DOI] [PubMed] [Google Scholar]
- 37.Beghetti M, Schulze-Neick I, Berger RMF, et al. Haemodynamic characterisation and heart catheterisation complications in children with pulmonary hypertension: insights from the Global TOPP Registry (tracking outcomes and practice in paediatric pulmonary hypertension). Int J Cardiol 2016; 203: 325–330. [DOI] [PubMed] [Google Scholar]
- 38.Beghetti M, Berger RMF, Schulze-Neick I, et al. Diagnostic evaluation of paediatric pulmonary hypertension in current clinical practice. Eur Respir J 2013; 42: 689–700. [DOI] [PubMed] [Google Scholar]
- 39.Berger RMF, Beghetti M, Humpl T, et al. Clinical features of paediatric pulmonary hypertension: a registry study. Lancet 2012; 379: 537–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hill KD, Lim DS, Everett AD, et al. Assessment of pulmonary hypertension in the pediatric catheterization laboratory: current insights from the magic registry. Cathet Cardiovasc Intervent 2010; 76: 865–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Marín MJDC, Rotés AS, Ogando AR, et al. Assessing pulmonary hypertensive vascular disease in childhood. Data from the Spanish registry. Am J Respir Crit Care Med 2014; 190: 1421–1429. [DOI] [PubMed] [Google Scholar]
- 42.Goss KN, Everett AD, Mourani PM, et al. Addressing the challenges of phenotyping pediatric pulmonary vascular disease. Pulm Circ 2017; 7: 7–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li L, Jick S, Breitenstein S, et al. Pulmonary arterial hypertension in the USA: an epidemiological study in a large insured pediatric population. Pulm Circ 2017; 7: 126–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Link J, Glazer C, Torres F, et al. International Classification of Diseases coding changes lead to profound declines in reported idiopathic pulmonary arterial hypertension mortality and hospitalizations implications for database studies. Chest 2011; 139: 497–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-pul-10.1177_2045894020933083 for Variation in the use of pulmonary vasodilators in children and adolescents with pulmonary hypertension: a study using data from the MarketScan® insurance claims database by Michael L. O'Byrne, Jennifer A. Faerber, Hannah Katcoff, David B. Frank, Alex Davidson, Therese M. Giglia and Catherine M. Avitabile in Pulmonary Circulation