Abstract
Treprostinil is a prostacyclin analogue approved for the treatment of pulmonary arterial hypertension. Apart from the inhaled formulation, there is neither a target dose nor a ceiling dose to guide clinicians using treprostinil; doses are individualized for each patient based upon tolerability and clinical improvement. Using combined data from the pivotal subcutaneous and oral treprostinil studies, we evaluated the effect of treprostinil dose on hospitalization and exercise capacity to better define the treprostinil dose–response relationship. Data from the pivotal subcutaneous and oral treprostinil studies were combined by converting oral doses to weight-based continuous doses (ng/kg/min) accounting for patient weight and bioavailability. Patients were divided into dose tertiles (lowest, middle, highest 33%) and retrospectively analyzed. Analysis 1 assessed the effect of dose on pulmonary arterial hypertension-related and all-cause hospitalizations. Analysis 2 evaluated the effects of dose on six-minute walk distance, Borg dyspnea score, and World Health Organization functional class. Results showed that, in Analysis 1, higher doses of treprostinil were associated with significantly longer times to first pulmonary arterial hypertension-related and all-cause hospitalization. In Analysis 2, there was a trend toward improvements in six-minute walk distance with higher doses. In patients with pulmonary arterial hypertension on systemic treprostinil therapy, higher doses were associated with significantly longer time to first pulmonary arterial hypertension-related and all-cause hospitalization. There was a trend toward improvements in six-minute walk distance. Collectively, these results underscore the importance of managing prostacyclin adverse events in order to achieve appropriate dose titration. Further studies are required to confirm these findings and to better characterize the dose–response relationship of treprostinil.
Keywords: prostacyclin, pulmonary arterial hypertension (PAH), six-minute walk distance, dose–response
Introduction
Pulmonary arterial hypertension (PAH) is a progressive and severely disabling disorder characterized by luminal narrowing in the small- and medium-sized pulmonary arteries, which leads to an increase in pulmonary vascular resistance and may culminate in right ventricular failure and premature death. 1 There are currently four therapeutic drug classes targeting three distinct molecular pathways approved for the treatment of PAH: endothelin receptor antagonists (ERAs), phosphodiesterase type 5 (PDE-5) inhibitors and soluble guanylate cyclase (sGC) stimulators, and prostacyclin analogues/receptor agonists. 2
Evidence suggests that in patients with PAH, there are abnormalities in prostacyclin metabolic pathways within the pulmonary vasculature which lead to vasoconstriction of pulmonary arteries and endothelial cell proliferation. 3 Treprostinil is a prostacyclin analogue that reduces pulmonary arterial pressure through direct vasodilation of the pulmonary and systemic arterial vascular beds. Additional effects include inhibition of platelet aggregation and in vitro reversal of pulmonary artery remodeling via reduction in smooth muscle cell proliferation.4–7 Emerging pre-clinical data suggest anti-fibrotic properties as well.8–10 These mechanisms lead to improvements in pulmonary gas exchange, systemic oxygen transport, and cardiac output with minimal alteration to heart rate. 11
As established by United States Food and Drug Administration and International Conference on Harmonization (ICH) guidelines on studying dose–response relationships, a well-controlled dose–response study would ideally evaluate patients randomized to different doses. Associations can then be drawn between dose and measures of efficacy or safety.12,13 With systemically administered treprostinil, this ideal trial design is challenging because there is no known dose target and treprostinil doses are individualized based on tolerability and clinical improvement. Therefore, patients cannot be randomized to different drug exposures without considering the ethical implications of prolonged use of a possibly sub-therapeutic treprostinil dose for a life-threatening condition such as PAH. Low doses of prostacyclin therapy have been shown to acutely improve hemodynamics, 14 but in clinical practice, patients are not maintained at a low dose. Instead, they are gradually titrated to the highest dose that balances clinical metrics, patient symptomatology, and manageable adverse events. Therefore, it would also not be clinically appropriate to randomize a patient to a treprostinil dose which may be supratherapeutic and harmful for the purposes of a dose–response study. Finally, treprostinil is a very potent vasodilator with potential for significant adverse events and inherent route-specific complications; achieving adequate doses to derive dose–response relationships cannot be safely or ethically administered to healthy volunteers.
As these obstacles preclude the formal study of treprostinil dose–response in a prospective, randomized manner, physicians have derived a large portion of their understanding from clinical experience. Traditional PAH clinical trials have utilized the six-minute walk distance (6MWD) as the primary endpoint and more recent trials have employed composite endpoints evaluating time to clinical worsening, with results largely driven by hospitalization and disease progression.15–18 Both of these endpoints are clinically relevant and warrant evaluation. In this paper, we present two novel analyses that combine data from patients enrolled in the subcutaneous (SC) treprostinil registration study with data from patients from the FREEDOM-M registration study for oral treprostinil.19–22 Both pivotal studies were 12 weeks long, had the same primary endpoint of 6MWD, studied systemically administered treprostinil monotherapy, and titrated treprostinil to the maximum tolerated dose.19,20 Using these combined data, we evaluate the effects of higher treprostinil doses on both hospitalization rates and functional capacity in patients with PAH.
Methods
Analysis 1: effect of dose on hospitalization
Patients were included in the analysis if they completed the randomized, pivotal oral or SC treprostinil studies, and entered the open-label studies.21,22 Inhaled treprostinil was not included in the analysis because unlike parenteral and oral treprostinil, the manufacturer recommends a maximum target dose. 23 The manufacturer's global drug safety database was retrospectively analyzed for hospitalizations reported per severe adverse events associated with the open-label extension studies. Hospitalizations were adjudicated for relatedness to PAH by physicians employed by the drug manufacturer who are familiar with treprostinil's safety profile. Hospitalizations were considered unrelated to PAH if the patient was treated for another underlying etiology or experienced decompensation due to acute medical problems. To combine the dosing data, oral doses were converted to weight-based continuous doses (ng/kg/min), accounting for patient weight and bioavailability using the formula in the manufacturer package insert. Patients were grouped into tertiles based on last known oral or SC treprostinil dose (i.e. lowest 33% “low”, middle 33% “medium”, highest 33% “high”) in the open-label extension studies. Pairwise comparisons were made between dose groups to evaluate difference in time to first PAH-related and all-cause hospitalizations. Cox proportional-hazards model was used to compare risk of PAH-related and all-cause hospitalizations between dose groups. Kaplan–Meier curves and log-rank tests were used to compare dose groups. An adjusted analysis using a Cox proportional model was repeated to control for confounding differences in baseline characteristics known to impact hospitalizations in PAH. 24 All statistical calculations were completed using SAS® version 9.2 (SAS Institute, Inc. Cary, NC).
Analysis 2: effect of dose on 6MWD
Data from patients in the active arm (i.e. not placebo) in the SC and oral treprostinil pivotal studies were combined.19,20 Dosing data were combined as described in the methods for Analysis 1. Patients were grouped into tertiles based on dose at Week 12 of the clinical studies (i.e. lowest 33% “low”, middle 33% “medium”, highest 33% “high”). Last observation carried forward was used to impute last observations of 6MWD, Borg dyspnea score (BDS), World Health Organization functional class (WHO FC), and dose. One-way analysis of variance and Jonckheere–Terpstra tests were used to assess differences and linear trends in 6MWD, BDS, and WHO FC based on treprostinil dose received. An adjusted analysis using multiple linear regression was repeated to control for confounding differences in baseline characteristics known to impact 6MWD in PAH. 25 All statistical calculations were completed using SAS® version 9.2 (SAS Institute, Inc. Cary, NC).
Results
Analysis 1: effect of dose on hospitalization
A total of 1619 patients were included in this analysis (SC, n = 860; oral, n = 759). Baseline demographics between the three dose groups were similar with the exception of age, WHO FC, PAH etiology, and time on treprostinil (Table 1). Patients were primarily female with a mean age of 46.3 years. Most patients had WHO FC III symptoms at baseline with a median baseline 6MWD of 363 m. After grouping the study population into dose tertiles, the median doses in the low ( <12.5 ng/kg/min, n = 537), medium (12.5–33.3 ng/kg/min, n = 543), and high ( >33.3 ng/kg/min, n = 539) dose groups were 4.5, 22.5, and 50.0 ng/kg/min.
Table 1.
Analysis 1—baseline patient characteristics.
| Baseline characteristics a | Total population | Low dose (n = 537) | Medium dose (n = 543) | High dose (n = 539) | p-Values |
|---|---|---|---|---|---|
| Oral treprostinil, n (%) | 759 | 197 (36.7) | 264 (48.6) | 298 (55.3) | – |
| Subcutaneous treprostinil, n (%) | 860 | 340 (63.3) | 279 (51.4) | 241 (44.7) | – |
| Mean age, years (SD) | 46.3 (14.9) | 46.3 (15.0) | 48.0 (14.7) | 44.4 (14.9) | <0.001 |
| Age < 18 years old, n (%) | 49 (3.0) | 13 (2.4) | 12 (2.2) | 24 (4.5) | 0.060 |
| Female, % | 76.5 | 75.0 | 75.9 | 78.5 | 0.383 |
| PAH etiology, % Idiopathic or familial Connective tissue disease Other | 59.5 21.3 19.2 | 53.4 22.7 23.8 | 57.8 23.2 19.0 | 67.2 18.0 14.8 | <0.001 |
| WHO FC, % I II III IV | 0.7 23.7 70.2 5.4 | 0.0 20.5 71.9 7.6 | 0.9 26.2 67.2 5.7 | 1.3 24.3 71.6 2.8 | 0.0007 |
| Median time since diagnosis, years (IQR) | 0.9 (0.4–2.1) | 0.8 (0.4–2.2) | 0.9 (0.4–2.2) | 0.8 (0.3–2.0) | 0.138 |
| Median 6MWD, m (IQR) | 363 (297–400) | 365 (294–404) | 362 (302–398) | 361 (295–397) | 0.876 |
| Median time on oral treprostinil, days (IQR) | 677 (274–1219) | 281 (100–664) | 713 (357–1219) | 1088 (633–1506) | <0.001 |
SD: standard deviation; WHO FC: World Health Organization functional class; PAH: pulmonary arterial hypertension; IQR: inter-quartile range.
Defined as start of active therapy in the pivotal or open-label extension studies.
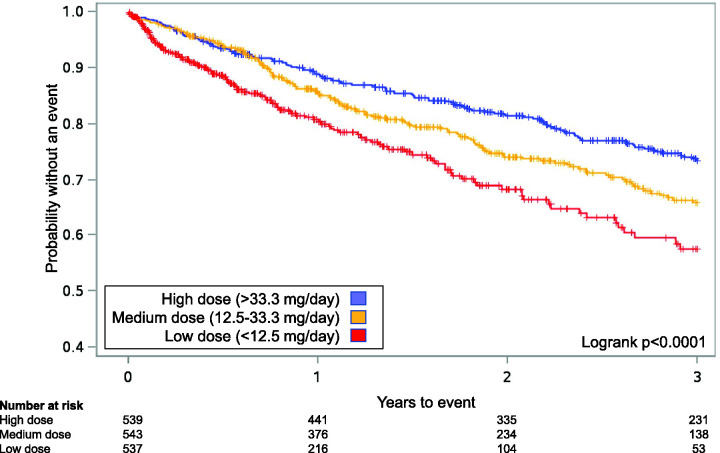
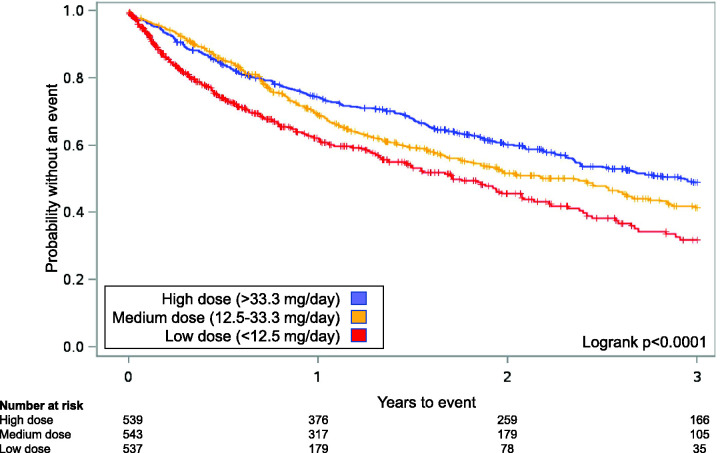
Of the 1619 patients, 730 patients (45.1%) experienced a hospitalization, including 396 (24.4%) due to PAH. Median time to first PAH-related hospitalization for low, medium, and high dose groups was 209, 378, and 529 days (p <0.001 low/med, p = 0.041 med/high, p <0.001 low/high). Median time to first all-cause hospitalization was 163, 322, and 380 days (p <0.001 low/med, p = 0.203 med/high, p <0.001 low/high). Higher doses were associated with a decreased risk of PAH-related and all-cause hospitalization (p <0.05, all comparisons; Table 2). There was a statistically significant difference in Kaplan–Meier estimates between dose groups for PAH-related hospitalization (logrank p <0.0001; Fig. 1) and for all-cause hospitalizations (logrank p <0.0001; Fig. 2).
Table 2.
Analysis 1—hazard ratios for PAH-related and all-cause hospitalizations between dose groups.
| Medium vs. low dose HR (95% CI) | p-Value for medium vs. low dose | High vs. medium dose HR (95% CI) | p-Value for high vs. medium dose | High vs. low dose HR (95% CI) | p-Value for high vs. low dose | |
|---|---|---|---|---|---|---|
| PAH-related hospitalization | ||||||
| Unadjusted | 0.72 (0.56–0.92) | 0.009 | 0.74 (0.58–0.95) | 0.02 | 0.53 (0.41–0.69) | <0.0001 |
| Adjusted for baseline WHO FC | 0.71 (0.55–0.91) | 0.006 | 0.76 (0.59–0.97) | 0.03 | 0.54 (0.41–0.69) | <0.0001 |
| All-cause hospitalization | ||||||
| Unadjusted | 0.74 (0.62, 0.89) | 0.001 | 0.82 (0.69–0.98) | 0.03 | 0.61 (0.51–0.73) | <0.0001 |
| Adjusted for baseline WHO FC | 0.73 (0.61, 0.87) | 0.0007 | 0.84 (0.71–1.01) | 0.06 | 0.61 (0.51–0.74) | <0.0001 |
PAH: pulmonary arterial hypertension; HR: hazard ratio; CI: confidence interval; WHO FC: World Health Organization functional class.
Fig. 1.
Kaplan–Meier curves for Analysis 1 for time to first pulmonary arterial hypertension (PAH)-related hospitalization for patients receiving oral treprostinil and subcutaneous (SC) treprostinil in the open-label extension studies after the randomized registration studies.21,22 Patients were grouped into tertiles based on the last known dose (i.e. lowest 33% “low”, middle 33% “medium”, highest 33% “high”). In the combined analysis for oral and SC treprostinil, a significant treatment effect in favor of higher doses was observed (logrank p < 0.0001, both).
Source: reproduced with permission from Barst et al., 2006 21 and White et al., 2013. 22
Fig. 2.
Kaplan–Meier curves for Analysis 1 for time to first all-cause hospitalizations for patients receiving oral treprostinil and subcutaneous (SC) treprostinil in the open-label extension studies after the randomized registration studies.21,22 Patients were grouped into tertiles based on the last known dose (i.e. lowest 33% “low”, middle 33% “medium”, highest 33% “high”). In the combined analysis for oral and SC treprostinil, a significant treatment effect in favor of higher doses was observed (logrank p < 0.0001, both).
Source: reproduced with permission from Barst et al., 2006 21 and White et al., 2013. 22
Noting that WHO FC was significantly different between dose groups at baseline, we performed an adjusted analysis based on published literature suggesting disease severity may influence hospitalization. 24 Disease severity reflected by 6MWD, age <18 years old, and comorbidities were also considered as confounding variables. Baseline 6MWD and age <18 years were balanced between dose groups. Baseline comorbidities could not be corrected for because these data were not available from the parent studies used in Analysis 1. In the adjusted analysis controlling for WHO FC, higher doses remained associated with significant decreases in risk of PAH-related hospitalization (p = 0.006 low/medium, p = 0.03 medium/high, p <0.0001 low/high) but not all-cause hospitalization (p = 0.0007 low/medium, p = 0.06 medium/high, p <0.0001 low/high) (Table 2).
To evaluate whether results differed by treprostinil route of administration, SC and oral dose data were evaluated separately. Patients on low, medium, and high doses of oral treprostinil had a median time to first PAH-related hospitalization of 339, 412, and 658 days, respectively (p = 0.049 medium/high, p <0.001 low/high). Median time to first all-cause hospitalization was 293, 400, and 552 days (p = 0.003 low/medium, p <0.001 low/high). There was a statistically significant difference in Kaplan–Meier estimates between dose groups for PAH-related hospitalization (logrank p = 0.03) but not for all-cause hospitalizations (logrank p = 0.23). Patients on low, medium, and high doses of SC treprostinil had a median time to first PAH-related hospitalization of 83, 318, and 428 days, respectively (p <0.001 low/medium and low/high). Median time to first all-cause hospitalization was 95, 266, and 310 days (p <0.001 low/medium and low/high). There was a statistically significant difference in Kaplan–Meier estimates for both PAH-related and all-cause hospitalization (logrank p = 0.0003 and p <0.0001).
Analysis 2: effect of dose on 6MWD
A total of 466 patients were included in this analysis (SC, n = 233; oral, n = 233). Most patients had WHO FC III symptoms at baseline with a median baseline 6MWD of 335 m. Patients were primarily female with a mean age of 44.8 years. There were differences between the dose groups in a several baseline demographics such as age, sex, and body mass index (BMI) but baseline WHO FC, 6MWD, and BDS were similar (Table 3). The mean SC and oral treprostinil dose at week 12 was 9.3 ng/kg/min and 13.8 ng/kg/min, respectively. The median doses in the low ( <6.3 ng/kg/min, n = 151), medium (6.3–13.4 ng/kg/min, n = 159), and high dose ( >13.5 ng/kg/min, n = 156) groups were 3.7, 9.1, and 18.5 ng/kg/min, respectively (Table 4).
Table 3.
Analysis 2—baseline patient characteristics.
| Baseline characteristics | Total population | Low dose (n = 151) | Medium dose (n = 159) | High dose (n = 156) | p-Values |
|---|---|---|---|---|---|
| Subcutaneous treprostinil, n (%) | 233 (50%) | 92 (60.9) | 86 (54.1) | 55 (35.3) | – |
| Oral treprostinil, n (%) | 233 (50%) | 59 (39.1) | 73 (45.9) | 101 (64.7) | – |
| Mean age, years (SD) | 42.3 (14.5) | 44.8 (12.8) | 45.1 (14.5) | 37.6 (14.6) | <0.001 |
| Female, % | 79.2 | 86.1 | 79.2 | 72.4 | 0.013 |
| Mean weight, kg (SD) | 69.4 (20.4) | 72.5 (20.2) | 71.1 (19.7) | 65.3 (20.7) | 0.001 |
| Mean height, cm (SD) | 162 (9.7) | 161 (8.5) | 162 (10.2) | 163 (10.0) | 0.809 |
| Mean BMI, kg/m2 (SD) | 25.7 (6.9) | 27.2 (7.1) | 26.4 (6.7) | 23.7 (6.6) | <0.001 |
| Mean duration of PAH, months (SD) | 8.5 (19.1) | 8.7 (17.4) | 11.8 (25.6) | 5.0 (10.1) | 0.012 |
| WHO FC, % I II III IV | 1.7 24.5 70.2 3.6 | 1.8 18.8 75.9 3.6 | 1.9 22.7 70.1 5.2 | 1.3 30.5 66.2 1.9 | 0.292 |
| Median 6MWD, m (IQR) | 347 (278–390) | 335 (264–385) | 340 (278–389) | 357 (285–395) | 0.199 |
| Median BDS (IQR) | 4.0 (2.0–5.0) | 4.0 (3.0–5.0) | 3.5 (2.0–5.0) | 3.5 (2.0–5.0) | 0.500 |
SD: standard deviation; BMI: body mass index; PAH: pulmonary arterial hypertension; WHO FC: World Health Organization functional class; 6MWD: six-minute walk distance; IQR: inter-quartile range; BDS: Borg dyspnea score.
Table 4.
Analysis 2—change in 6MWD, BDS, and WHO FC by dose group.
| Low dose (n = 151) | Medium dose (n = 159) | High dose (n = 156) | p-Value 1 (low vs. medium) | p-Value 2 (medium vs. high) | p-Value 3 (low vs. high) | |
|---|---|---|---|---|---|---|
| Median dose at week 12, ng/kg/min (IQR) | 3.7 (2.5, 5.0) | 9.1 (7.5, 11.3) | 18.5 (15.0, 22.5) | <0.001 | <0.001 | <0.001 |
| Median change in 6MWD, m (IQR) | 13 (−18, 55) | 22 (−13, 58) | 30 (−6, 70) | 0.287 | 0.112 | 0.013 |
| Median change in BDS, (IQR) | 0.0 (−1.0, 1.0) | −1.0 (−2.0, 0.0) | −1.0 (−2.0, 0.0) | 0.007 | 0.191 | <0.001 |
| Change in WHO FC Unchanged; worsened, n (%) Improved, n (%) | 57 (50.9) 55 (49.1) | 62 (40.3) 92 (59.7) | 73 (47.4) 81 (52.6) | 0.085 | 0.207 | 0.574 |
| Median change in 6MWD, m (IQR)—oral treprostinil only | 38 (−6, 67) n = 77 | 28.5 (−4, 72) n = 79 | 36 (4, 76) n = 77 | 0.992 | 0.421 | 0.540 |
| Median change in 6MWD, m (IQR)—SC treprostinil only | 12.5 (−17, 42) n = 65 | 6 (−42, 42) n = 83 | 20 (−9, 49) n = 85 | 0.316 | 0.031 | 0.311 |
IQR: inter-quartile range; 6MWD: six-minute walk distance; BDS: Borg dyspnea score; WHO FC: World Health Organization functional class; SC: subcutaneous.
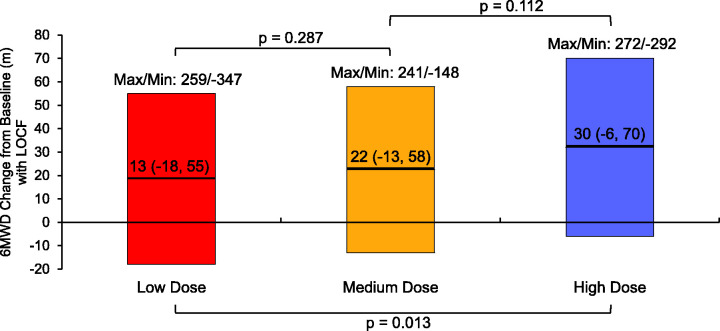
The low, medium, and high dose groups had respective median 6MWD improvements of 13, 22, and 30 m (Fig. 3). There was a statistically significant difference in 6MWD improvement between low/high dose groups (p = 0.013) and a statistically significant linear trend for 6MWD improvement with higher doses (Jonckheere–Terpstra test, one-sided p = 0.0052). Significant differences in BDS were found between low/medium groups (p = 0.007) and low/high groups (p <0.001) with median improvements of 0.0, 1.0, and 1.0 in the low, medium, and high groups, respectively (Table 4). There were differences observed in WHO FC improvements between dose groups but these did not reach statistical significance.
Fig. 3.
Boxplot showing change in exercise capacity by treprostinil dose tertile for Analysis 2 (unadjusted). Numbers within the colored boxes represent median and the interquartile range (IQR) 6MWD change from baseline with last observation carried forward (LOCF) for each dose tertile. Horizontal lines within the boxes represent the median, with maximum and minimum values appearing above the boxes. p-Values for between-group comparisons are denoted using brackets.
6MWD: six-minute walk distance.
Noting that age, gender, and BMI were significantly different between dose groups at baseline, we performed an adjusted analysis based on published literature suggesting this may influence 6MWD. 25 PAH etiology was considered as well but was balanced between the dose groups at baseline. When controlling for age, sex, and BMI, we did not observe any significant differences in 6MWD between dose groups (p = 0.1925).
To evaluate whether 6MWD results differed by treprostinil route of administration, SC and oral dose data were separated and the analysis repeated. For oral treprostinil patients, the low, medium, and high dose groups had respective median 6MWD improvements of 38, 28.5, and 36 m (p = Nonsignificant (NS), all). For SC treprostinil patients, the low, medium, and high dose groups had respective median 6MWD improvements of 12.5, 6, and 20 m (low/medium and low/high p = NS, med/high p = 0.031).
Discussion
Until recently, 6MWD has been used as the primary endpoint in nearly all pivotal Phase 3 PAH clinical trials. Much about what is currently understood about treprostinil dose–response is derived from increased exercise capacity associated with escalating doses. Although the validity of the 6MWD as a surrogate endpoint is often questioned, findings from European PAH registries have confirmed that 6MWD at both baseline and at follow-up is a strong clinical prognosticator.26,27 Recent clinical trials have emphasized the importance of preventing clinical worsening and PAH-related hospitalizations.15–18 In the contemporary era, both endpoints are highly relevant. In these novel analyses, we have summarized how patients achieving higher treprostinil doses demonstrate lower risk of hospitalization and a trend toward improved exercise tolerance.
In Analysis 1, the high and medium dose Kaplan–Meier curves for PAH-related and all-cause hospitalization separate from the low dose curve early on, and there was not much separation between the high and medium dose groups until month 9. This could be due to the dose cutoffs used in this study which were based on unbiased tertiles; it is possible that early on in the course of therapy, certain dose thresholds are needed to achieve noticeable benefits and our cutoffs do not adequately reflect these thresholds. In our adjusted analysis controlling for baseline differences in WHO FC, the high dose group still demonstrated significant reductions in risk when compared to the low dose group. Results from evaluating SC and oral treprostinil patients separately suggest that both routes of administration independently demonstrate a dose–response relationship with regard to hospitalization.
The improvements in 6MWD observed in Analysis 2 are relatively modest but it is important to note that the treprostinil doses analyzed are low compared to those routinely used in contemporary clinical practice. Furthermore, many factors can impact 6MWD and when controlling for differences in baseline demographics between dose groups, we did not observe a significant difference in the Week 12 6MWD associated with dose. It is possible that 6MWD may not be a sensitive marker of response to treprostinil in the way that hospitalization may be, or that doses utilized in this study were too low to demonstrate a statistically significant improvement.
Our findings are consistent with other empiric data from prior studies to suggest that a dose–response relationship for treprostinil exists (Table 5). In the pivotal SC treprostinil study, the authors observed a dose–response relationship after stratifying patients into quartiles based on their week 12 dose. 20 In two separate retrospective reviews, there were survival and mortality benefits observed with increasing doses of parenteral treprostinil.28,29 These results should be interpreted with caution because dose–response was not a pre-planned endpoint and a few analyses of SC treprostinil have not demonstrated a dose–response relationship. Benza and colleagues did not find a correlation between the week 12 SC treprostinil dose with improved survival in a retrospective review of 811 patients enrolled in three SC treprostinil studies. 28 The authors hypothesize that this was due to suboptimal dosing achieved at week 12 in the parent studies. Grünig and colleagues performed an open-label study of SC treprostinil which utilized a rapid uptitration dosing regimen in 39 patients with PAH. 30 The authors did not observe an association with higher doses of SC treprostinil and longer 6MWD. The majority of patients were on dual background therapy of a PDE-5 inhibitor and ERA (n = 35, 90%) and the lack of a dose–response finding could be explained by a diminished response in a heavily pre-treated population. Despite these two negative dose–response findings, there are data to suggest that as parenteral treprostinil is adequately titrated to therapeutic effect, patients may benefit from increased doses in the setting of proper adverse event management and clinical follow-up.
Table 5.
Summary of dose–response findings from studies of SC/IV and oral treprostinil.
| Study | Study description | Duration | Total sample size a | Analysis | Dose information | Dose–response outcomes |
|---|---|---|---|---|---|---|
| Simonneau et al., 2002. (SC treprostinil registration trial) 20 | Randomized, double-blind, placebo-controlled trial | 12 weeks | 233 | Week 12 dose assessed by quartiles | < 5 ng/kg/min (n = 45) 5–8.1 ng/kg/min (n = 55) 8.2–13.8 ng/kg/min (n = 49) >13.8 ng/kg/min (n = 53) | Mean 6MWD Δ + 3.3 ± 10 m Mean 6MWD Δ + 1.4 ± 9 m Mean 6MWD Δ + 20 ± 8 m Mean 6MWD Δ + 36.1 ± 10 m Patients in the highest quartile had the greatest improvement in 6MWD while those in the lowest two quartiles had significantly less improvement (p = 0.03). |
| Benza et al., 2011. 28 | Retrospective review of three SC treprostinil extension studies | 3 years | 811 | Multivariate analysis to identify prognostic factors associated with survival | ≥40 ng/kg/min (n = 230 (28%)) | Associated with improved-long survival compared to lower doses (HR for death: 0.29 (95% CI: 0.20–0.44), p < 0.001) |
| Every 10 ng/kg/min increase in dose | Associated with a 34% decrease in the hazard of all-cause death (n = 811, HR: 0.66 (95% CI: 0.48–0.90), p = 0.009) | |||||
| Dose achieved at week 12 | Not associated with improved long-term survival, likely due to the relatively low average dose at week 12 of 8.3 ± 5.9 ng/kg/min. | |||||
| Preston and Farber, 2013. 29 | Retrospective review of specialty pharmacy data of all patients in the United States started on SC and IV treprostinil | 2 years | 1877 | Cox proportional hazard model used to assess the effect of dose on survival | Every 10 ng/kg/min increase in dose | Associated with a 7.7% decrease in the hazard of all-cause death (HR for death: 0.992, p < 0.0001). The highest risk of death was in patients on newly initiated treprostinil therapy, especially at < 20 ng/kg/min. |
| Grünig et al., 2016. 30 | Open-label study of SC treprostinil | 16 weeks | 39 | Median dose assessed at weeks 4, 8, 12, and 16 | 17.2 ng/kg/min (n = 39) 26.8 ng/kg/min (n = 34) 32.6 ng/kg/min (n = 34) 35.7 ng/kg/min (n = 32) | Median 6MWD Δ + 10 m Median 6MWD Δ + 9 m Median 6MWD Δ + 14 m Median 6MWD Δ + 11.5 m Although a statistically significant improvement in 6MWD was observed at week 16 (p = 0.0086), higher doses of SC treprostinil were not associated with increased changes in 6MWD. |
| Kumar et al., 2013.31,32 | Post-hoc analysis of FREEDOM-M data (oral treprostinil) | 12 weeks | 233 | Response to therapy defined as combined ranking of change in 6MWD and Borg dyspnea score at week 12 | Time above total daily dose of 8 mg | Associated with increased response to therapy (Spearman's correlation, ρ = 0.208, p < 0.001) |
| Cumulative oral treprostinil dose (∑(day 1 dose, day 2 dose…)/# days) | Positively correlated with response to therapy (Spearman's correlation, ρ = 0.294, p < 0.001). | |||||
| Every 1 mg increase in cumulative dose | Associated with approximately 20% increased odds of achieving a meaningful response to therapy (defined as >23 m Δ6MWD which was the median between-treatment group improvement in the primary analysis population) | |||||
| Center for Drug Evaluation and Research: Clinical Pharmacology and Biopharmaceutics Reviews, 2011. 33 | FDA Independent exploratory analysis of FREEDOM-M (oral treprostinil) | Linear trend estimation | Every 100 mg increase in cumulative dose | Associated with a 2.5% change from baseline in 6MWD. Significant non-zero slope for the relationship between cumulative treprostinil dose and 6MWD at week 12. | ||
| Every 0.01 mg/kg increase in week 12 dose (mITT population) | Associated with a 1.23% change from baseline in 6MWD. | |||||
| White and Rao, 2016. 34 | Retrospective analysis of FREEDOM-C and C2 (oral treprostinil) | 16 weeks | 263 | Week 16 dose was assessed by tertiles | < 2 mg BID (low, n = 95) 2–3.5 mg BID (med, n = 81) >3.5 mg BID (high, n = 87) | In both analyses conducted by the authors, the median change in 6MWD at Week 16 for the high-dose group was 34 m or greater and was significantly different than the low-dose group (p ≤ 0.006, both). |
| White et al., 2019. 35 | A priori analysis of FREEDOM-EV (oral treprostinil) | Event-driven study | 339 | Week 24 dose assessed | < 3 mg TID (n = 153) ≥ 3 mg TID (n = 186) | Median 6MWD Δ + 1 m NT-proBNP: –21% from baseline WHO FC b : 12%, 69%, 19% BDS b : 30%, 33%, 37% Median 6MWD Δ + 14 m NT-proBNP: –35% from baseline WHO FC b : 17%, 77%, 5% BDS b : 44%, 34%, 22% |
SC: subcutaneous; IV: intravenous; 6MWD: six-minute walk distance; HR: hazard ratio; CI: confidence interval; FDA: United States Food and Drug Administration; mITT: modified intention to treat; BID: twice daily; TID: thrice daily.
For studies that utilized placebo, only patients in the active arms are counted in the study's sample size.
% improved, no change, deteriorated.
Similar findings have been observed for oral treprostinil (Table 5). Several post-hoc analyses suggest a dose–response relationship of oral treprostinil monotherapy in which higher doses correspond to greater improvements in functional capacity.31–33 In another analysis of oral treprostinil used as combination therapy, White and Rao found a significant difference in 6MWD change from baseline between the low and high dose groups at week 16. 34 More recently, longer term dose–response findings in the FREEDOM-EV study were published showing that patients achieving ≥ 3 mg thrice daily (TID) at week 24 had greater improvements in 6MWD, N-terminal pro-brain natriuretic peptide (NT PRO BNP), WHO FC, and BDS compared to the <3 mg TID group while experiencing a similar rate of adverse events. 35
There are limitations to these present analyses. Although combining SC and oral data is a novel concept supported by the systemic nature of drug exposure for both treprostinil formulations, there is evidence that parenteral and oral routes are different. 36 Analysis 1 was retrospective in nature, and hospitalizations were adjudicated by physicians employed by the drug manufacturer rather than treating clinician. Results may be confounded by changes or addition of background PAH therapy, which were unavailable for assessment. Dose data were extrapolated from last known dose in the open-label study and the actual dose for patients may be different. Analysis 2 was retrospective in nature and dose groups varied with respect to age, sex, weight, and duration of PAH at baseline. The doses used in Analysis 2 were relatively low which may limit the applicability of the findings; for instance, the median dose in the high dose group was 18.5 ng/kg/min, which corresponds to an oral treprostinil regimen of approximately 3 mg TID in a 70 kg patient. There are also limitations to using 6MWD as an endpoint, as improvements in this clinical measure may be influenced by numerous parameters other than dose. 25 Finally, there were no significant 6MWD findings when SC and oral treprostinil doses were evaluated separately, likely due to the small sizes of each group.
Prostacyclin dose–response relationships are also confounded by the time it takes to titrate patients to clinically appropriate doses. This is especially apparent when considering the mean dose of 26 ng/kg/min at one year in the long-term SC treprostinil study used in Analysis 1, which is low compared to the mean dose of 55 ng/kg/min achieved at one year in contemporary clinical practice.21,37,38 It is possible that longer exposure time rather than increased dose is responsible for the improved clinical benefit over time; in a post-hoc study of patients on oral treprostinil, Kumar and colleagues found that the time a patient spent above a total daily dose of 8 mg was associated with an increased response to therapy.31,32 This suggests that there may be a temporal component to the dose–response relationship of treprostinil.
Along with epoprostenol, parenteral and oral treprostinil therapies are unique in PAH as they are the only drugs without a ceiling dose. In patients who achieve clinical improvement and stability, incremental prostacyclin dose increases are often required over time. Whether the need for dose titration arises from disease progression or drug tolerance is uncertain. The benefit of the dose–response relationship for systemically administered prostacyclins is that drug is titratable with no ceiling dose, allowing this drug class to remain a viable long-term treatment option. In effect, dose titration can outpace disease progression. In contrast, ERAs, PDE-5 inhibitors, sGC stimulators, and prostacyclin receptor agonists are employed with dose limits and when a patient experiences disease progression on these therapies, additional treatment with drugs from other therapeutic classes or conversion to prostacyclins is required.
There are potential risks associated with progressively higher doses of prostacyclins. These have been best characterized with parenteral epoprostenol. A high cardiac output state has been described with higher doses of epoprostenol. 39 High output heart failure should be considered in previously stable patients at high doses who develop worsening flushing, gastrointestinal symptoms, and palpitations. Right heart catheterization often reveals a markedly elevated cardiac output and symptoms subside with dose reduction without worsening of PAH symptoms. High output heart failure has not been well described with oral treprostinil. Increased incidence of adverse events such as headache, nausea, flushing, and jaw pain may also be associated with higher doses of prostacyclins, so proper follow-up remains important as patients uptitrate on dose.
These two novel post-hoc analyses demonstrate that increased treprostinil dose is associated with improvements in 6MWD and significantly longer time to hospitalization. Despite the differences between the oral and parenteral formulations of treprostinil and the heterogeneity of the doses achieved in clinical trials, the significantly longer time to first PAH-related and all-cause hospitalization associated with higher, clinically relevant doses of treprostinil supports the importance of aggressive dose titration. The exact nature of the dose–response relationship (e.g. linear vs. non-linear) remains difficult to characterize owing to the background progression of disease, and whether this relationship continues to exist at very high doses is unknown. It is also unclear whether the dose–response relationship exists for certain PAH phenotypes that are more aggressive in nature and less responsive to pharmacotherapy. In addition, these results should be interpreted cautiously as the use of higher treprostinil doses is dependent on a patient's PAH symptomatology as well as drug tolerability. Nonetheless, these unique properties of treprostinil distinguish it from other non-prostacyclin therapies. Further research into the dose–response relationship may focus on the relationship between side effects and therapeutic benefits, and better understanding the physiologic and biochemical mechanisms that lead to clinical improvement with higher treprostinil doses.
Acknowledgements
We would like to thank Erick Borg, PharmD for his work on Analysis 1 and his guidance with data presentation. Preliminary data were presented at ATS 2018 International Conference, 18–21 May 2018, San Diego, CA; and at the CHEST Annual Meeting 2018, 6–10 October 2018, San Antonio, TX.
Guarantor
Dr Gautam Ramani
Contributorship
GR and SC collected data, drafted manuscript, directed statistical analyses, and incorporated author feedback for the final submission. ES, MB, and AW assembled data tables, drafted methods section, and responded to author queries for data. MB and AN collected data and offered critical feedback. QS aggregated data and performed statistical analyses as directed by authors. GR, SC, ES, and MB guarantee the integrity of the work as a whole.
Conflict of interest
The University of Maryland contracts with United Therapeutics Corporation and Actelion Pharmaceuticals Ltd. for GR to perform research. SC and AW do not have any conflicts of interest to declare and does not have affiliations with institutions, organizations, or companies mentioned in the manuscript or whose products or services are discussed. ES, MB, QS, and AN are employees or contractors of United Therapeutics Corporation.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
ORCID iDs
Steven Cassady https://orcid.org/0000-0002-8621-9497
Eric Shen https://orcid.org/0000-0001-5621-6570
References
- 1.Mclaughlin VV, Archer SL, Badesch DB, et al. ACCF/AHA 2009 expert consensus document on pulmonary hypertension: a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association developed in collaboration with the American College of Chest Physicians; American Thoracic Society, Inc.; and the Pulmonary Hypertension Association. J Am Coll Cardiol 2009; 53: 1573–1619. [DOI] [PubMed] [Google Scholar]
- 2.Galiè N, Humbert M, Vachiery JL, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: the Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J 2016; 37: 67–119. [DOI] [PubMed] [Google Scholar]
- 3.Galiè N, Manes A, Branzi A. Prostanoids for pulmonary arterial hypertension. Am J Respir Med 2003; 2: 123–137. [DOI] [PubMed] [Google Scholar]
- 4.Clapp LH, Finney P, Turcato S, et al. Differential effects of stable prostacyclin analogs on smooth muscle proliferation and cyclic AMP generation in human pulmonary artery. Am J Respir Cell Mol Biol 2002; 26: 194–201. [DOI] [PubMed] [Google Scholar]
- 5.Orenitram® (package insert). Research Triangle Park, NC: United Therapeutics Corporation, 2017.
- 6.Falcetti E, Hall SM, Phillips PG, et al. Smooth muscle proliferation and role of the prostacyclin (IP) receptor in idiopathic pulmonary arterial hypertension. Am J Respir Crit Care Med 2010; 182: 1161–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Patel J, Shen L, Hall S, et al. EP2 receptors play a key role in mediating the anti-proliferative activity of treprostinil in smooth muscle cells derived from the lungs of pulmonary hypertensive patients. Am J Respir Crit Care Med 2015; 191: A5954. [Google Scholar]
- 8.Ali FY, Egan K, Fitzgerald GA, et al. Role of prostacyclin versus peroxisome proliferator-activated receptor beta receptors in prostacyclin sensing by lung fibroblasts. Am J Respir Cell Mol Biol 2006; 34: 242–246. [DOI] [PubMed] [Google Scholar]
- 9.Manitsopoulos N, Kotanidou A, Magkou C, et al. Treprostinil administration attenuates bleomycin-induced lung fibrosis in mice. In: Poster 3837 presented at European Respiratory Society International Congress 2015, Amsterdam, Kingdom of the Netherlands, 26–30 September, 2015.
- 10.Lambers C, Feng Z, Jaksch P, et al. Treprostinil effectively inhibits PDGF and TGF-β1 induced extracellular matrix composition by IPF fibroblasts. Eur Respir J 2015; 46: PA3893. [Google Scholar]
- 11.Simonneau G, Barst RJ, Galiè N, et al. Continuous subcutaneous infusion of treprostinil, a prostacyclin analogue, in patients with pulmonary arterial hypertension: a double-blind, randomized, placebo-controlled trial. Am J Respir Crit Care Med 2002; 165: 800–804. [DOI] [PubMed] [Google Scholar]
- 12.Food and Drug Administration. Guidance for industry: exposure-response relationships – study design, data analysis, and regulatory applications, www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm072109.pdf (April 2003, accessed 6 October 2017).
- 13.International Conference on Harmonisation. ICH Harmonised Tripartite Guideline: Dose-Response Information to Support Drug Registration (E4) Geneva, Switzerland: International Conference on Harmonisation; 10 March 1994. [Google Scholar]
- 14.Vanderpool RR, Desai AA, Knapp SM, et al. How prostacyclin therapy improves right ventricular function in pulmonary arterial hypertension. Eur Respir J 2017; 50: 1700764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Galiè N, Barberà JA, Frost AE, et al. Initial use of ambrisentan plus tadalafil in pulmonary arterial hypertension. N Engl J Med 2015; 373: 834–844. [DOI] [PubMed] [Google Scholar]
- 16.Sitbon O, Channick R, Chin KM, et al. Selexipag for the treatment of pulmonary arterial hypertension. N Engl J Med 2015; 373: 2522–2533. [DOI] [PubMed] [Google Scholar]
- 17.Pulido T, Adzerikho I, Channick RN, et al. Macitentan and morbidity and mortality in pulmonary arterial hypertension. N Engl J Med 2013; 369: 809–818. [DOI] [PubMed] [Google Scholar]
- 18.White RJ, Jerjes-sanchez C, Bohns meyer GM, et al. Combination therapy with oral treprostinil for pulmonary arterial hypertension: a double-blind, placebo-controlled study. Am J Respir Crit Care Med 2020; 201: 707–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jing ZC, Parikh K, Pulido T, et al. Efficacy and safety of oral treprostinil monotherapy for the treatment of pulmonary arterial hypertension: a randomized, controlled trial. Circulation 2013; 127: 624–633. [DOI] [PubMed] [Google Scholar]
- 20.Simonneau G, Barst RJ, Galiè N, et al. Continuous subcutaneous infusion of treprostinil, a prostacyclin analogue, in patients with pulmonary arterial hypertension: a double-blind, randomized, placebo-controlled trial. Am J Respir Crit Care Med 2002; 165: 800–804. [DOI] [PubMed] [Google Scholar]
- 21.Barst RJ, Galie N, Naeije R, et al. Long-term outcome in pulmonary arterial hypertension patients treated with subcutaneous treprostinil. Eur Respir J 2006; 28: 1195–1203. [DOI] [PubMed] [Google Scholar]
- 22.White RJ, Jing Z, Parikh K, et al. An open-label extension trial of oral treprostinil in subjects with pulmonary arterial hypertension. Am J Respir Crit Care Med 2013; 187: A3270. [Google Scholar]
- 23.Tyvaso® (package insert). Research Triangle Park, NC: United Therapeutics Corporation, 2017.
- 24.Burger CD, Long PK, Shah MR, et al. Characterization of first-time hospitalizations in patients with newly diagnosed pulmonary arterial hypertension in the REVEAL registry. Chest 2014; 146: 1263–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med 2002; 166: 111–117. [DOI] [PubMed] [Google Scholar]
- 26.Hoeper MM, Kramer T, Pan Z, et al. Mortality in pulmonary arterial hypertension: prediction by the 2015 European pulmonary hypertension guidelines risk stratification model. Eur Respir J 2017; 50: 1700740. [DOI] [PubMed] [Google Scholar]
- 27.Boucly A, Weatherald J, Savale L, et al. Risk assessment, prognosis and guideline implementation in pulmonary arterial hypertension. Eur Respir J 2017; 50: 1700889. [DOI] [PubMed] [Google Scholar]
- 28.Benza RL, Gomberg-Maitland M, Naeije R, et al. Prognostic factors associated with increased survival in patients with pulmonary arterial hypertension treated with subcutaneous treprostinil in randomized, placebo-controlled trials. J Heart Lung Transplant 2011; 30: 982–989. [DOI] [PubMed] [Google Scholar]
- 29.Preston IR, Farber HW. Impact of parenteral treprostinil dosing in pulmonary arterial hypertension (Abstract 156). J Heart Lung Transplant 2013; 32: S64–S65. [Google Scholar]
- 30.Grünig E, Benjamin N, Lange TJ, et al. Safety, tolerability and clinical effects of a rapid dose titration of subcutaneous treprostinil therapy in pulmonary arterial hypertension: a prospective multi-centre trial. Respiration 2016; 92: 362–370. [DOI] [PubMed] [Google Scholar]
- 31.Kumar P, Arneson C, Laliberte K, et al. Dose–response relationship of oral treprostinil diolamine (TRE) in patients with pulmonary arterial hypertension. Am J Respir Crit Care Med 2013; 187: A3271. [Google Scholar]
- 32.Kumar P, Arneson C, Laliberte K, et al. Dose–response relationship of oral treprostinil in patients with pulmonary arterial hypertension (PAH). In: Poster (No. 40197) presented at American Thoracic Society 2013 International Conference, Philadelphia, PA, 17–22 May 2013.
- 33.Center for Drug Evaluation and Research. Clinical Pharmacology and Biopharmaceutics reviews – application number 203496Orig1s000, www.accessdata.fda.gov/drugsatfda_docs/nda/2013/203496Orig1s000ClinPharmR.pdf (Dec 2011, accessed 9 April 2018).
- 34.White RJ, Rao Y. Novel analysis of the oral treprostinil combination therapy trial data. Am J Respir Crit Care Med 2016; 193: 1434–1436. [DOI] [PubMed] [Google Scholar]
- 35.White RJ, Grünig E, Jerjes-Sanchez C, et al. Dose–response relationship of oral treprostinil for secondary endpoints in the FREEDOM-EV study. Eur Respir J 2019; 54: PA5462. [Google Scholar]
- 36.Maestas T, Hansen LM, Vanderpool RR, et al. Right ventricular afterload predicts long-term transition from parenteral to oral treprostinil in pulmonary arterial hypertension. Pulm Circ 2018; 8: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Balasubramanian V, Melendres-Groves L, Safdar Z, et al. Real-world dosing characteristics of parenteral treprostinil. Am J Respir Crit Care Med 2019; 199: A5066. [Google Scholar]
- 38.Balasubramanian V, Melendres-Groves L, Safdar Z, et al. Real-world dosing characteristics of parenteral treprostinil. In: Poster (No. 1142) presented at American Thoracic Society 2019 international conference, Dallas, TX, 17–22 May 2019.
- 39.Rich S, Mclaughlin VV. The effects of chronic prostacyclin therapy on cardiac output and symptoms in primary pulmonary hypertension. J Am Coll Cardiol 1999; 34: 1184–1187. [DOI] [PubMed] [Google Scholar]