Abstract
Background and purposes
Minimal hepatic encephalopathy (MHE) has no recognizable clinical symptoms, but patients have cognitive and psychomotor deficits. Hyperammonemia along with neuroinflammation lead to microstructural changes in cerebral parenchyma. Changes at conventional imaging are detected usually at the overt clinical stage, but microstructural alterations by advanced magnetic resonance imaging techniques can be detected at an early stage.
Materials and methods
Whole brain diffusion kurtosis imaging (DKI) data acquired at 3T was analyzed to investigate microstructural parenchymal changes in 15 patients with MHE and compared with 15 age- and sex-matched controls. DKI parametric maps, namely kurtosis fractional anisotropy (kFA), mean kurtosis (MK), axial kurtosis (AK) and radial kurtosis (RK), were evaluated at 64 white matter (WM) and gray matter (GM) regions of interest (ROIs) in the whole brain and correlated with the psychometric hepatic encephalopathy score (PHES).
Results
The MHE group showed a decrease in kFA and AK across the whole brain, whereas MK and RK decreased in WM ROIs but increased in several cortical and deep GM ROIs. These alterations were consistent with brain regions involved in cognitive function. Significant moderate to strong correlations (–0.52 to –0.66; 0.56) between RK, MK and kFA kurtosis metrics and PHES were observed.
Conclusion
DKI parameters show extensive microstructural brain abnormalities in MHE with minor correlation between the severity of tissue damage and psychometric scores.
Keywords: Hepatic encephalopathy, magnetic resonance imaging, diffusion kurtosis imaging
Introduction
Minimal hepatic encephalopathy (MHE), a potentially reversible “subclinical” form, is seen in up to 80% of patients with cirrhosis.1–3 MHE is the earliest form of hepatic encephalopathy, defined by a psychometric hepatic encephalopathy score (PHES) of less than –5. MHE has a severe detrimental impact on quality of life of patients by causing cognitive dysfunctions in early stages. 4 Patients with MHE have lack of attention, visuomotor incoordination, frequent falls, psychometric slowing and loss of mental skills, and show early changes in memory and learning. MHE is difficult to diagnose as there is often a lack of insight amongst patients and caregivers about cerebral changes from liver failure. Patients with late and undiagnosed MHE may convert to clinically manifest hepatic encephalopathy over time, which leads to an irreversible stage resulting in increased morbidity and mortality. For early diagnosis, many tests are employed (Table 1); however, consistency and reproducibility of a test is essential before it can be used in a clinical scenario. Psychometric tests are often used in a clinical setting but are time consuming and influenced by age, gender and education level of patients. Moreover, the application of these clinical tests depends on local expertise and resources. A learning effect may occur on repeated usage of these tests and therefore more objective and repeatable tests need to be formulated. Brain imaging methods such as morphometry, spectroscopy, perfusion and functional magnetic resonance imaging (MRI) have shown promising surrogate markers in diagnosing MHE and good correlations with clinical psychometric scores. Diffusion tensor imaging (DTI) has emerged as a powerful and widely used technique to measure microstructural changes in the brain for a variety of diseases. Diffusion kurtosis imaging (DKI) is a more improved method that addresses shortcomings of DTI and arrives at more detailed measures of tissue microstructure. 5 In this study, we acquired DKI data on the whole brain to investigate parenchymal microstructural abnormalities throughout the cerebrum and cerebellum and evaluated correlations of DKI measures with psychometric scores in MHE patients.
Table 1.
Tests for hepatic encephalopathy.
| Clinical tests for grading of hepatic encephalopathy | |
| 1. West Haven criteria (WHC) 2. Hepatic encephalopathy scoring algorithm (HESA) 3. Modified orientation log (MO-Log) 4. Clinical hepatic encephalopathy staging scale (CHESS)5. Glasgow coma scale (GCS) | |
| Neuropsychometric tests | |
| A. Paper and pencil | 1. Psychometric hepatic encephalopathy score (PHES)2. Number connection test (NCT) A/B3. Digit symbol test (DST)4. Repeatable battery for assessment of neuropsychological status (RBANS) |
| B. Computerized | 1. Inhibitory control test2. EncephalApp Stroop test3. Scan test |
| C. Neurophysiological | 1. Electroencephalography2. Evoked potentials3. Critical flicker frequency |
| Imaging techniques | |
| 1. Conventional MRI2. Volumetric MRI3. MR spectroscopy4. Functional MRI including perfusion5. Magnetization transfer imaging6. Diffusion-weighted imaging 7. Diffusion tensor imaging8. Diffusion kurtosis imaging | |
| Nuclear medicine imaging techniques | |
| 1. Positron emission tomography2. Single photon emission computed tomography | |
MR: magnetic resonance; MRI magnetic resonance imaging.
Methods
Study design, enrollment and patient selection
This was an institutional ethics committee approved, single center prospective study. Between July 2018 and November 2019, 15 cirrhotic patients with MHE (PHES ≤ –5) and 15 age- and sex-matched healthy controls underwent MRI. Complete demographic information is given in Table 2. Patients with overt hepatic encephalopathy, history of upper gastrointestinal bleed, spontaneous bacterial peritonitis, known neuropsychiatric disease or history of consumption of psychoactive drugs/antibiotic usage were excluded. Patients with any malignancy or history of previous transjugular intrahepatic portosystemic shunt or shunt surgery were also excluded from the study.Consecutive MHE patients who fulfilled the above inclusion and exclusion criteria were enrolled into the study after informed consent was obtained.
Table 2.
Subject demographics.
| Control | MHE patients | p-value | |
|---|---|---|---|
| Age (mean ± SD) | 51.73 ± 4.17 | 51.33 ± 9.11 | 0.88 |
| Sex | 11 M / 4 F | 11 M / 4 F | |
| Etiology | |||
| Alcohol | NA | 7 (46.7%) | |
| HBV | NA | 5 (33.3%) | |
| HCV | NA | 3 (20.0%) |
F: female; HBV: hepatitis B virus; HCV: hepatitis C virus; M: male; MHE: minimal hepatic encephalopathy; NA: not applicable; SD: standard deviation.
Neuropsychometric testing was performed using a PHES battery for both patients and controls and the battery comprised five tests: number connection test (NCT) A, digit symbol test (DST), finger connection test (FCT) A (Indian version replacing NCT B 6 ), serial dotting test and line drawing test. Test values for controls was obtained in a randomized way for time (t) and error (e) and PHES for patients with cirrhosis were expressed as z scores (i.e. standard deviation between observed and expected scores for given age and education).
Imaging protocol
All subjects were scanned using a 3.0 Tesla MRI scanner (Siemens Healthcare, Erlangen, Germany) with a 32-channel head coil. DKI data were acquired using a single-shot twice-refocussed spin-echo imaging sequence with TR/TE 11500/98 ms, 3 b-values (0, 1000, 2000 s/mm2) along 30 diffusion encoding directions, field-of-view 222 mm × 222 mm, acquisition matrix 74 × 100, slice thickness of 3 mm with no gaps, 54 slices, in-plane resolution of 3 × 3 mm, and an acquisition time of 7 min. Data from conventional T1-weighted, T2-weighted and fluid-attenuated inversion recovery sequences were also obtained to rule out other structural abnormalities.
Data processing and image analysis
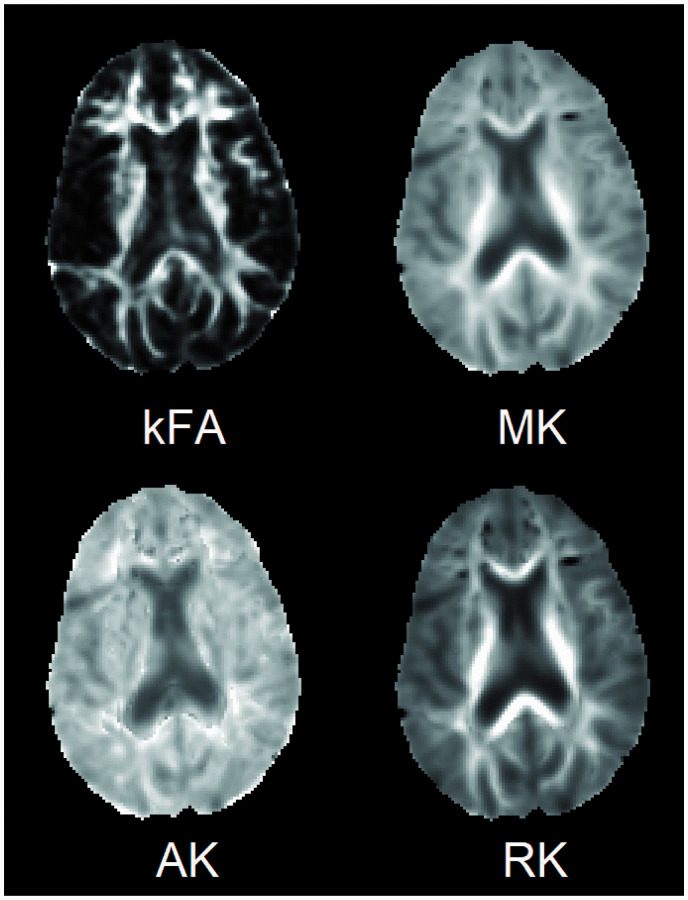
Diffusion-weighted imaging (DWI) data was processed on a 64-bit workstation using Diffusional Kurtosis Estimator (DKE) software. 7 Pre-processing steps embedded within DKE correct the images for eddy current distortions using a 12-parameter affine transformation. Automated skull stripping was also performed on the b0 image using BET skull-strip tool from the MRIcro software. DKE then calculates the kurtosis metrics comprising mean kurtosis (MK), axial kurtosis (AK), radial kurtosis (RK) and kurtosis fractional anisotropy (kFA), as shown in Figure 1. These metrics were evaluated at specific regions of interest (ROIs) in the brain using the JHU-MNI-SS type 2 atlas which contains 189 extensively segmented and labeled ROIs drawn on the “Eve” brain template in MNI space. The atlas was registered from the template to each subject space using a non-linear large deformation diffeomorphic metric mapping transformation with dual contrast (fractional anisotropy (FA) and b0). 8 This atlas-based approach has several advantages over voxel-based or white matter skeleton-based (e.g. tract-based spatial statistics 9 ) analytical methods by capturing the mean value from all the voxels in an ROI, improving measurement accuracy and increasing the effect size to detect changes. 10 Finally, the number of ROIs is reduced by removing irrelevant regions (e.g. cerebrospinal fluid), merging smaller subdivisions into single macro ROIs, and combining left and right brain ROIs. This process reduced the number of ROIs to 64 (Table S1 in the supplementary file). The Indian version of the PHES was used in which the NCT B was replaced with the FCT A. 6
Figure 1.
Diffusion kurtosis imaging parametric maps showing kurtosis fractional anisotropy (kFA), mean kurtosis (MK), axial kurtosis (AK) and radial kurtosis (RK) obtained from an axial slice of the brain of a minimal hepatic encephalopathy subject.
Statistical analysis
All statistical tests were implemented with R software. Diffusion parameters were first checked for normality by the Shapiro–Wilk method. Data outliers were also removed using standard z score cut-off values (z < –3; z > 3). For each ROI, group-wise comparisons between controls and MHE were performed using Student’s t-test. Finally, a Pearson test was used to find correlations between the PHES and DKI parameters. Results were considered as significant for a p < 0.05, corrected for multiple comparisons using the false discovery rate method.
Results
Patient characteristics
A total of 15 cases and 15 controls were age and sex matched, with mean age of 51.5 ± 6.6 years and 11 male (73.3%) subjects in each group. Etiology of cirrhosis was alcohol related in 7 patients, hepatitis B in 5 patients and hepatitis C in 3 patients. A PHES of –6 was seen in 9 patients, –7 in 3 patients, –10 in 2 patients and –9 in 1 patient.
DKI parameters
Consistent decrease in kFA was recorded in the MHE group throughout the brain in both white matter (WM) and gray matter (GM) regions (Figure 2) with significant decreases in 15 ROIs (Table S2 in the supplementary file). AK showed a trend of decrease in multiple regions of the brain (Figure 3), with 14 ROIs showing significant change (Table S3 in the supplementary file). Changes in MK values (Figure 4) and RK values (Figure 5) were less impactful. MK showed an overall decreasing trend in the WM with significant changes in 4 ROIs but no significant changes in cortical and deep GM areas (Table S4 in the supplementary file). RK values were mostly increasing in cortical and deep GM but decreasing in WM with significant changes in 6 ROIs (Table S5 in the supplementary file). Those regions in the brain with significant between-group differences are shown in Figure 6 on coronal MRI reformatted slices. The PHES showed a positive correlation with kFA values in the superior longitudinal fasciculus (r = 0.559, p = 0.03) and negative correlations with MK in the lateral fronto-orbital gyrus (r = –0.562, p = 0.029) and with RK in the lateral fronto-orbital gyrus (r = –0.66, p = 0.007), caudate (r = –0.524, p = 0.045) and thalamus (r = –0.515, p = 0.049). Figure 7 shows the above correlations with PHES. The DKI parameters of the remaining ROIs did not show significant correlations with the PHES.
Figure 2.
Bar plot showing group differences in kurtosis fractional anisotropy (kFA) between the control and minimal hepatic encephalopathy (MHE) groups at 64 brain regions of interest (ROIs). The bottom plot shows deep white matter (WM) ROIs and the top plot shows ROIs in the cortical areas, deep gray matter (GM) and the cerebellum. Asterisks indicate ROIs with a significant between-group difference (p < 0.05, false discovery rate (FDR)). Expansions for the abbreviations used for indicating the brain anatomical ROIs are provided in Table S1 (in the supplementary file).
Figure 3.
Bar plot showing group differences in axial kurtosis (AK) between the control and minimal hepatic encephalopathy (MHE) groups at 64 brain regions of interest (ROIs). The bottom plot shows deep white matter (WM) ROIs and the top plot shows ROIs in the cortical areas, deep gray matter (GM) and the cerebellum. Asterisks indicate ROIs with a significant between-group difference (p < 0.05, false discovery rate (FDR)). Expansions for the abbreviations used for indicating the brain anatomical ROIs are provided in Table S1 (in the supplementary file).
Figure 4.
Bar plot showing group differences in mean kurtosis (MK) between the control and minimal hepatic encephalopathy (MHE) groups at 64 brain regions of interest (ROIs). The bottom plot shows deep white matter (WM) ROIs and the top plot shows ROIs in the cortical areas, deep gray matter (GM) and the cerebellum. Asterisks indicate ROIs with a significant between-group difference (p < 0.05, false discovery rate (FDR)). Expansions for the abbreviations used for indicating the brain anatomical ROIs are provided in Table S1 (in the supplementary file).
Figure 5.
Bar plot showing group differences in radial kurtosis (RK) between the control and minimal hepatic encephalopathy (MHE) groups at 64 brain regions of interest (ROIs). The bottom plot shows deep white matter (WM) ROIs and the top plot shows ROIs in the cortical areas, deep gray matter (GM) and the cerebellum. Asterisks indicate ROIs with a significant between-group difference (p < 0.05, false discovery rate (FDR)). Expansions for the abbreviations used for indicating the brain anatomical ROIs are provided in Table S1 (in the supplementary file).
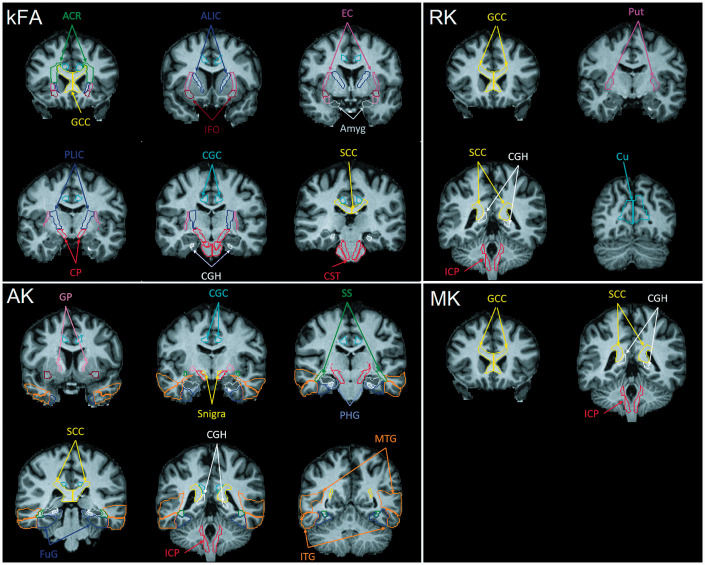
Figure 6.
Coronal slices of T1 template image in MNI space showing the location of all the ROIs where significant structural changes in MHE patients were seen using each of the 4 DKI metrics: kurtosis fractional anisotropy (kFA) top left, axial kurtosis (AK) bottom left, radial kurtosis (RK) top right, and mean kurtosis (MK) bottom right. With kFA and AK all the regions of interest (ROIs) highlighted throughout the brain had a significant decrease. White matter ROIs highlighted for MK and RK also had a significant decrease, whereas gray matter ROIs (i.e. putamen (Put) and Cuneus gyrus (Cu)) had an increase in RK. Expansions for the abbreviations used for indicating the brain anatomical ROIs are provided in Table S1 (in the supplementary file).
Figure 7.
Scatter plots showing Pearson correlations between psychometric hepatic encephalopathy score (PHES) and diffusion kurtosis imaging metrics. Psychometric scores had positive correlation with kurtosis fractional anisotropy (kFA) in the superior longitudinal fasciculus (r = 0.559, p = 0.03), negative correlation with mean kurtosis (MK) in the lateral fronto-orbital gyrus (r = –0.562, p = 0.029), and negative correlation with radial kurtosis (RK) in the lateral fronto-orbital gyrus (r = –0.66, p = 0.007), caudate (r = –0.524, p = 0.045), and thalamus (r = –0.515, p = 0.049).
Discussion
Hepatic encephalopathy is a devastating disease with morbid consequences. PHES is the commonly used clinical test for identification of MHE that is performed in an outpatient setting within 20–30 min; however, it is not specific for MHE due to an increase in false positives in testing patients with a low pre-test likelihood of the disease. Objective quantification of tissue structural, metabolic and physiological alterations due to MHE in involved brain anatomical regions using advanced brain imaging methods may help us to solve the diagnostic conundrum. Various mechanisms described for cerebral changes in hepatic encephalopathy include an osmotic effect 11 due to ammonia after crossing the blood–brain barrier directly along with hyponatremia, dysregulation of amino acid metabolism, changes in extracellular concentration of neurotransmitters 12 and free radical damage from pro-inflammatory state. MRI techniques have been used to evaluate the structural and functional changes in hepatic encephalopathy. Besides conventional MRI, magnetization transfer imaging has shown a decrease in magnetization transfer ratio from increased cerebral water content in MHE patients. 13 Failure of autoregulation and its resultant changes in cerebral perfusion have been reproduced by magnetic resonance perfusion techniques 14 with studies showing an increase in putaminal blood flow. 15 T2*-based and susceptibility-weighted imaging have shown abnormal iron deposition in the frontal-basal ganglia-thalamocortical circuit which has been linked with neurocognitive decline in these patients. 16 Metabolic alterations from conversion of cerebral ammonia (glutathione synthase activity of astrocytes) produce a characteristic triad of increased glutamine, decreased myo-inositol (usually the earliest and most consistent finding) and decreased choline on magnetic resonance spectroscopy. 13 Advanced imaging techniques such as blood-oxygenation-level-dependent functional MRI have also been used to assess functional changes in spatial working memory. Involvement of bilateral prefrontal cortices, bilateral premotor areas, supplementary motor areas and bilateral parietal areas in MHE has been documented previously. 17 Resting-state functional MRI studies have also shown weaker functional connectivity of bilateral putamen, pallidum and thalami (subcortical areas). 18
Diffusion MRI techniques assess the increased cerebral water content which alters the diffusion properties of water molecules such as decreased FA and increased mean diffusivity and axial diffusivity on DTI studies in MHE patients. 13 A counter-regulatory volume effect occurs with a resultant increase in extracellular water to combat the astrocytic swelling. Because biological tissues are composed of both hindered and restricted components, a more advanced diffusion model than DWI and DTI (i.e. kurtosis imaging) is essential. DKI assesses the degree of non-Gaussian diffusion with higher order description of the water diffusion process in the cerebral microstructure. The water molecules in the brain microstructures show non-monoexponential b-value dependency at higher b-values (b > 1000 s/mm2), 19 which can be completely characterized by a 2nd order three-dimensional (3D) diffusivity tensor (as in conventional DTI) along with a 4th order 3D kurtosis tensor used in DKI. DKI has shown promising results in a variety of central nervous system pathologies (e.g. Parkinson’s disease 20 and Alzheimer’s dementia 21 ). Therefore, we used DKI in this study to investigate microstructural alterations in the brain of patients with MHE.
The most commonly used kurtosis metrics are AK, MK, RK and kFA. AK is the directional kurtosis parameter parallel to the principal diffusion direction and reflects axonal integrity. MK is the overall average apparent diffusion kurtosis coefficient and reflects the microstructural complexity of brain tissues. RK is the average apparent kurtosis coefficient measured in equatorial plane. kFA is a measure of anisotropy (similar to conventional FA) but uses the kurtosis tensor for calculation, making it less prone to errors from complex WM fiber arrangements such as crossing fibers. In our study, there was a mean decrease in kFA and AK in the whole brain, an increase in GM MK and RK, and a decrease in WM MK and RK in MHE group versus control group in most ROIs. The most affected WM areas were the corpus callosum, cingulum, cerebral peduncle, corticospinal tract, corona radiata, internal capsule and fronto-occipital fasciculus. The decrease in kFA in those regions shows clear signs of WM axonal damage, suggesting definite involvement of these areas in MHE. In cortical regions and in deep GM we see most often the significant alterations in the temporal gyrus, amygdala, hippocampus, globus pallidus and putamen, all showing a consistent decrease in kFA and AK. The corpus callosum is one of the major interhemispheric connections and a crucial component of the cognitive nervous pathway. Impairment in corpus callosum affects the interhemispheric functional interactions. Similarly, involvement of superior longitudinal and inferior fronto-occipital fasciculi is commonly associated with cognitive dysfunction and executive, visuospatial-visuoconstructive impairments. 22 , 23 These findings elucidate the role of these important structures in neurocognitive decline in MHE.
The reduction in AK indicates impairment of the axon function in WM. Li et al. 24 have shown reduced AK in the anterior thalamic radiation, decreased MK in left thalamus and body of corpus callosum and reduced RK in the body and splenium of corpus callosum in MHE patients. Sun et al. 25 showed reduced AK in the left cingulum, right caudate nucleus, hippocampus, inferior temporal gyrus, fusiform gyrus and right thalamus. Chen et al. 26 reported reductions of all kurtosis metrics connoting decrease in microstructural complexity in GM and WM in MHE patients. However, in our study, some structures showed an opposite pattern of change in MK and RK with predominant increase in mean values from most GM ROIs. DKI-derived metrics change in any disease with time. Whether these changes are attributable to alterations in cortical synaptic plasticity in early stages of hepatic encephalopathy 27 or from difference in neurotransmitter effects in different brain areas or from higher reactive astrogliosis 28 is debatable and a matter of further research. Longitudinal follow up to document further changes with pattern of normalization followed by decline in MK and RK values will confirm the DKI metric, one of the major limitations of our study.
Correlation of DKI metrics with clinical PHES showed positive correlation with kFA in the superior longitudinal fasciculus, suggesting that severe PHES (lower scores) correlate with more severe axonal damage (lower kFA). We also note negative correlations with MK in the lateral fronto-orbital gyrus, and negative correlation with RK in the lateral fronto-orbital gyrus, caudate and thalamus. In the study by Chen et al., 26 the average AK, MK and RK values in GM and WM were correlated with the PHES rather than individual ROIs.
An interesting finding in our study which incites further research is the possibility of DKI to assess derangements in cerebral microarchitecture to the level of aquaporin-4 channels, 29 which regulate blood brain barrier permeability. We found significant DKI parametric changes in the ventricles in our study on MHE patients which was not present in controls. This has also been reported previously in patients with Alzheimer’s dementia 30 and sheds light on the potential role of DKI in assessing, quantifying and understanding pathophysiology of cerebral edema in MHE patients.
Our study has a few limitations. First, due to the small sample size, there is a limit to the statistical power of our study for group comparisons, and to our correlation analysis as few MHE patients have severely low psychometric scores (PHES < –8). Second, we did not evaluate the effects of different etiologies of cirrhosis on DKI metrics and cerebral microstructure, as the final pathway for hepatic encephalopathy is nearly similar in all etiologies. As previously discussed, considering the disease progression of MHE, a longitudinal study is necessary to further explore the pathogenic mechanisms and neuroimaging features. Further, cirrhotic patients without MHE also present with brain structural changes. However, we ruled out the involvement of these microstructural changes by elaborating the new areas which are involved in MHE by a careful review of literature. The areas of involvement in our study did not corroborate with those due to cirrhosis alone and thus represent true involved areas in MHE.
In conclusion, the practice to perform neuropsychological functions for evaluation of MHE gives little information about the involvement of brain structures and its severity. This study elucidates the utility of DKI, a newer technique to show the microstructural involvement in MHE. The correlation of DKI and neurocognitive abnormalities may provide early and more accurate localization of the brain involvement and may help in predicting prognosis and neuropsychological sequelae.
Supplemental Material
Supplemental material, sj-pdf-1-neu-10.1177_19714009211026924 for Whole brain atlas-based diffusion kurtosis imaging parameters for evaluation of minimal hepatic encephalopathy by Prateek Gupta, Sameer Vyas, Teddy Salan, Chirag Jain, Sunil Taneja, RK Dhiman, Paramjeet Singh, Chirag K Ahuja, Nirmalya Ray and Varan Govind in The Neuroradiology Journal
Acknowledgement
Gaurav Garg for DKI data analysis.
Conflict of interest: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Ethical approval: All procedures performed in the studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent: Informed consent was obtained from all individual participants included in the study.
ORCID iDs: Sameer Vyas https://orcid.org/0000-0002-0113-0486
Chirag Jain https://orcid.org/0000-0001-9674-9371
Supplemental material: Supplementary material for this article is available online.
References
- 1.Acharya C, Bajaj JS. Current management of hepatic encephalopathy. Am J Gastroenterol 2018; 113: 1600–1612. [DOI] [PubMed] [Google Scholar]
- 2.Maldonado-Garza HJ, Vázquez-Elizondo G, Gaytán-Torres JO, et al. Prevalence of minimal hepatic encephalopathy in cirrhotic patients. Ann Hepatol 2011; 10 Suppl 2: S40–44. [PubMed] [Google Scholar]
- 3.Dhiman RK, Saraswat VA, Sharma BK, et al. Minimal hepatic encephalopathy: consensus statement of a working party of the Indian National Association for Study of the Liver. J Gastroenterol Hepatol 2010; 25: 1029–1041. [DOI] [PubMed] [Google Scholar]
- 4.Stepanova M, Mishra A, Venkatesan C, et al. In-hospital mortality and economic burden associated with hepatic encephalopathy in the United States from 2005 to 2009. Clin Gastroenterol Hepatol 2012; 10: 1034–1041.e1. [DOI] [PubMed] [Google Scholar]
- 5.Jensen JH, Helpern JA, Ramani A, et al. Diffusional kurtosis imaging: the quantification of non-Gaussian water diffusion by means of magnetic resonance imaging. Magn Reson Med 2005; 53: 1432–1440. [DOI] [PubMed] [Google Scholar]
- 6.Dhiman RK, Kurmi R, Thumburu KK, et al. Diagnosis and prognostic significance of minimal hepatic encephalopathy in patients with cirrhosis of liver. Dig Dis Sci 2010; 55: 2381–2390. [DOI] [PubMed] [Google Scholar]
- 7.Tabesh A, Jensen JH, Ardekani BA, et al. Estimation of tensors and tensor-derived measures in diffusional kurtosis imaging. Mag Reson Med 2011; 65: 823–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ceritoglu C, Oishi K, Li X, et al. Multi-contrast large deformation diffeomorphic metric mapping for diffusion tensor imaging. Neuroimage 2009; 47: 618–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mishra VR, Sreenivasan KR, Zhuang X, et al. Influence of analytic techniques on comparing DTI-derived measurements in early stage Parkinson’s disease. Heliyon 2019; 5: e01481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hunter LE, Lubin N, Glassman NR, et al. Comparing region of interest versus voxel-wise diffusion tensor imaging analytic methods in mild and moderate traumatic brain injury: a systematic review and meta-analysis. J Neurotrauma 2019; 36: 1222–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shah NJ, Neeb H, Kircheis G, et al. Quantitative cerebral water content mapping in hepatic encephalopathy. Neuroimage 2008; 41: 706–717. [DOI] [PubMed] [Google Scholar]
- 12.Felipo V. Hepatic encephalopathy: effects of liver failure on brain function. Nat Rev Neurosci 2013; 14: 851–858. [DOI] [PubMed] [Google Scholar]
- 13.Zhang X-D, Zhang L-J, Wu S-Y, et al. Multimodality magnetic resonance imaging in hepatic encephalopathy: an update. World J Gastroenterol 2014; 20: 11262–11272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li T, Li X, Zhou W, et al. Dynamic susceptibility contrast-enhanced first-pass perfusion MR imaging in patients with subclinical hepatic encephalopathy. J Neuroradiol 2012; 39: 290–294. [DOI] [PubMed] [Google Scholar]
- 15.Li Y, Liu H, Yang J, et al. Combining arterial-spin labeling with functional magnetic resonance imaging measurement for characterizing patients with minimal hepatic encephalopathy. Hepatol Res 2017; 47: 862–871. [DOI] [PubMed] [Google Scholar]
- 16.Liu J-Y, Ding J, Lin D, et al. T2* MRI of minimal hepatic encephalopathy and cognitive correlates in vivo. J Magn Reson Imaging 2013; 37: 179–186. [DOI] [PubMed] [Google Scholar]
- 17.Liao L-M, Zhou L-X, Le H-B, et al. Spatial working memory dysfunction in minimal hepatic encephalopathy: an ethology and BOLD-fMRI study. Brain Res 2012; 1445: 62–72. [DOI] [PubMed] [Google Scholar]
- 18.Qi R, Zhang LJ, Zhong J, et al. Disrupted thalamic resting-state functional connectivity in patients with minimal hepatic encephalopathy. Eur J Radiol 2013; 82: 850–856. [DOI] [PubMed] [Google Scholar]
- 19.Cheung MM, Hui ES, Chan KC, et al. Does diffusion kurtosis imaging lead to better neural tissue characterization? A rodent brain maturation study. Neuroimage 2009; 45: 386–392. [DOI] [PubMed] [Google Scholar]
- 20.Wang J-J, Lin W-Y, Lu C-S, et al. Parkinson disease: diagnostic utility of diffusion kurtosis imaging. Radiology 2011; 261: 210–217. [DOI] [PubMed] [Google Scholar]
- 21.Gong N-J, Wong C-S, Chan C-C, et al. Correlations between microstructural alterations and severity of cognitive deficiency in Alzheimer’s disease and mild cognitive impairment: a diffusional kurtosis imaging study. Magn Reson Imaging 2013; 31: 688–694. [DOI] [PubMed] [Google Scholar]
- 22.Gómez-Ansón B, Román E, de Bobadilla RF, et al. Alterations in cerebral white matter and neuropsychology in patients with cirrhosis and falls. PLoS One 2015; 10: e0118930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hoeft F, Barnea-Goraly N, Haas BW, et al. More is not always better: increased fractional anisotropy of superior longitudinal fasciculus associated with poor visuospatial abilities in Williams syndrome. J Neurosci 2007; 27: 11960–11965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li J-L, Jiang H, Zhang X-D, et al. Microstructural brain abnormalities correlate with neurocognitive dysfunction in minimal hepatic encephalopathy: a diffusion kurtosis imaging study. Neuroradiology 2019; 61: 685–694. [DOI] [PubMed] [Google Scholar]
- 25.Sun Q, Fan W, Liu Y, et al. Characterization of brain microstructural abnormalities in cirrhotic patients without overt hepatic encephalopathy using diffusion kurtosis imaging. Brain Imaging Behav 2020; 14: 627–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen H-J, Liu P-F, Chen Q-F, Shi H-B. Brain microstructural abnormalities in patients with cirrhosis without overt hepatic encephalopathy: a voxel-based diffusion kurtosis imaging study. AJR Am J Roentgenol 2017; 209: 1128–1135. [DOI] [PubMed] [Google Scholar]
- 27.Golaszewski S, Langthaler PB, Schwenker K, et al. Abnormal cortical synaptic plasticity in minimal hepatic encephalopathy. Brain Res Bull 2016; 125: 200–204. [DOI] [PubMed] [Google Scholar]
- 28.Zhuo J, Xu S, Proctor JL, et al. Diffusion kurtosis as an in vivo imaging marker for reactive astrogliosis in traumatic brain injury. Neuroimage 2012; 59: 467–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wright G, Soper R, Brooks HF, et al. Role of aquaporin-4 in the development of brain oedema in liver failure. J Hepatol 2010; 53: 91–97. [DOI] [PubMed] [Google Scholar]
- 30.Xue Y, Zhang Z, Wen C, et al. Characterization of Alzheimer’s disease using ultra-high b-values apparent diffusion coefficient and diffusion kurtosis imaging. Aging Dis 2019; 10: 1026–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-neu-10.1177_19714009211026924 for Whole brain atlas-based diffusion kurtosis imaging parameters for evaluation of minimal hepatic encephalopathy by Prateek Gupta, Sameer Vyas, Teddy Salan, Chirag Jain, Sunil Taneja, RK Dhiman, Paramjeet Singh, Chirag K Ahuja, Nirmalya Ray and Varan Govind in The Neuroradiology Journal