Abstract
The simultaneous growth of robotic-assisted surgery and telemedicine in recent years has only been accelerated by the recent coronavirus disease 2019 pandemic. Robotic assistance for neurovascular intervention has garnered significant interest due to opportunities for tele-stroke models of care for remote underserved areas. Lessons learned from medical robots in interventional cardiology and neurosurgery have contributed to incremental but vital advances in medical robotics despite important limitations. In this article, we discuss robot types and their clinical justification and ethics, as well as a general overview on available robots in thoracic/abdominal surgery, neurosurgery, and cardiac electrophysiology. We conclude with current clinical research in neuroendovascular intervention and a perspective on future directions.
Keywords: Robotics, neurointerventional, ethics, stroke, telemedicine
Introduction
The coronavirus disease 2019 (COVID-19) pandemic has accelerated the implementation of telehealth networks 1 and prompted the wider adoption of remote healthcare delivery systems along with robotic devices.2–4 Physicians are faced with a mounting complexity in healthcare delivery options and an unprecedented workload,5,6 making technological solutions more pressing. Robotic surgery and telemedicine technologies can address shortages of qualified specialists, as well as improve the quality of diagnosis and treatment.7–9
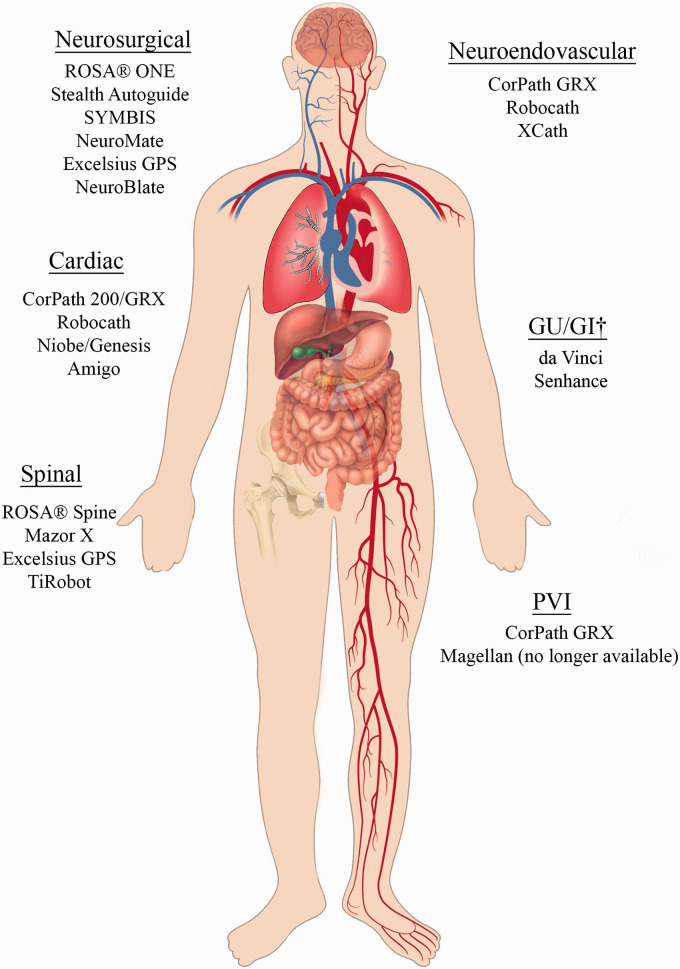
In this article, we review the current state of robotic systems in various surgery specialties (Figure 1 and Table 1), considering their features, areas of application, and available evidence basis. First, we define basic terms and concepts and also justify use of surgical robots. In the second part of the article, we present a brief overview of modern robotic systems in different branches of surgery, including thoracic/abdominal surgery, neurosurgery, cardiac electrophysiology, and neuroendovascular intervention (NVI), with a close look at a representative endovascular robotic system. In the final part of the review, we discuss future perspectives for the development of robotics in the coming years.
Figure 1.
Robotic systems with corresponding applications in various organ systems. See Table 1 for more details on specific robots. GU: genitourinary; GI: gastrointestinal; PVI: peripheral vascular intervention. †Both da Vinci and Senhance have been used in colorectal surgery, hysterectomy, cholecystectomy, prostatectomy, and inguinal hernia repair.
Table 1.
Surgical robots in clinical use.
| Robot | Parent company | FDA approved | Organ system/operations | Features | Drawbacks | References |
|---|---|---|---|---|---|---|
| Surgical robots | ||||||
| da Vinci platforms:X and Xi | Intuitive Surgical (USA) | 2000 | Robot-assisted MIS in various organ systemsa | 6 DoF robotic arms with numerous attachable surgical instrumentsX,Xi: 3 surgical arms, 1 camera arm | Steep learning curveHigh capital costsAccessibility gap | Company website 46 Crew 18 George et al. 47 |
| NeuroMate | Integrated Surgical Systems (USA) acquired by Renishaw (UK) | 1999 | Brain biopsy, DBS, SEEG, neuroendoscopy, radiosurgery | 5 DoF robotic arm with CT or MRI guidance | High costs | Li et al. 23 |
| ROSA® ONE Brain and Spine | Medtech Montpellier (France) Acquired by Zimmer Biomet (USA) | 2018, 2019 | Brain biopsy, DBS, SEEG, ventricular endoscopy, spinal surgery | Image-based preoperative planning and intraoperative CT for bone resection or needle trajectoryFrame-based and frameless stereotactic neurosurgery | Increased surgical timesHigh costsProne to error if patient motion disrupts image reference for robot | Lefranc et al. 24 Paff et al. 25 Chenin et al. 48 |
| Auto Stealth Guide Mazor XRenaissanceSpineAssist | Mazor Robotics (Israel)Acquired by Medtronic (USA) | 2016 | Neurosurgery, spinal surgery | First approved spinal robot; remains most widely used3D preoperative CT planning for screw location/size in spinal surgery | Screw misplacement secondary to tool skiving Prone to improper image registration | D’Souza et al. 49 Khan et al. 50 |
| SYMBISFormerly NeuroArm | IMRIS (Canada) | 2015 | MRI-guided neurosurgical brain tumor resection | 7 + 1 DoF robot with piezoelectric actuationMR conditional robot allowing use in intraoperative MRI suites | High costsUsed only for brain tumors | Sutherland et al. 51 |
| NeuroBlate | Monteris Medical (USA) | 2018 | MRI-guided intracranial laser ablation | 2 DoF cranial robot with remote workstationMR thermometry monitors ablation quasi real-time | Risk of overheating, tissue damage | Sloan 52 |
| Excelsius GPS | Globus Medical (USA) | 2018 | Spinal surgery | 6 DoF robotic arm, first with haptic feedback | Limited data on long-term outcomes | Vardiman 53 |
| Endovascular interventions | ||||||
| CorPath GRXCorPath 200 | Corindus Vascular Robotics (USA)Acquired by Siemens Healthineers (USA) | PCI: 2012 PVI: 2018NVI: CE mark 2018 | PCI, PVI, and NVI | Single-use robotic cassette compatible many endovascular devicescLong distance tele-operated interventions 39 Smart features for endovascular navigation | May be limited by network performance and traffic | Patel et al. 15 , 54 Mahmud et al. 38 Mendes Pereira et al. 43 |
| MagellanSensei X | Hansen Medical (USA)Acquired by Auris Surgical Robotics (USA) | 2012b2009b | Peripheral arterial diseaseEP mappingCardiac ablationAneurysm repair | Electromechanical steerable pull-wire catheter system Remote operator workspace with joystick master-slaveCF sensing feedback technology | FDA approved only for specialized cathetersHuman–robot interface not intuitive Limited widespread adoption due to costs and company acquisition | Bismuth et al. 55 Cochennec et al. 56 Saliba et al. 57 Dello Russo et al. 58 |
| Amigo | Catheter Robotics (USA) | 2012 | EP mappingCardiac ablation | Electromechanical remote steerable catheterIntuitive command interface | Cumbersome design for EP catheterization lab No CF sensing feedback requiring special catheters | Khan et al. 59 Datino et al. 60 Hoffmayer et al. 61 |
| Genesis Niobe ES | Stereotaxis (USA) | Genesis: 2020Niobe: 2013 | EP mappingCardiac ablation | Robotic magnetic navigation of catheter steered with two computer-controlled magnets on each side of patientGenesis is smaller and faster than its Niobe ES predecessor | Requires specialized EP cathetersNiobe encumbered setup No CF sensing technology | Yuan et al. 31 Turagam et al. 62 |
| R-One™ | Robocath (France) | CE Mark 2019 | PCIInvestigating PVI and NVI | Robotic arm with device cassette adaptable to many endovascular devicesMobile radio-protected control station with joystick and interface | Limited clinical evidence | Salimi et al. 63 Cardiovascular News 64 |
| XCath | XCath, Inc. (USA) | N/A | NVI | Robotically controlled steerable smart guidewireHaptic feedback and soft atraumatic tip | Limited clinical evidence | Company website 65 |
aMinimally invasive cardiac, colorectal, gynecologic, head and neck, thoracic, urologic, and general surgeries.
bNo longer commercially available.
c0.014-inch guidewires, rapid-exchange coronary angioplasty balloons, and stent delivery systems.
MIS: minimally invasive surgery; DoF: degrees of freedom; FDA: Food and Drug Administration; CE: Conformité Européene; CT: computed tomography; MRI: magnetic resonance imaging; DBS: deep brain stimulation; SEEG: stereoelectroencephalography; PCI: percutaneous coronary intervention; PVI: peripheral vascular intervention; NVI: neurovascular intervention; EP: electrophysiology; CF: contact force; RMN: robotic magnetic navigation; N/A: not applicable.
Terms and justification
Definitions
Medical robots can be classified into three main types: active, semi-active, and master–slave. 10 Active systems are completely autonomous, capable of performing simple manipulations according to a pre-programmed algorithm, and do not require operator participation. Semi-active systems are also capable of operating autonomously, but allow the participation of an operator, if necessary, to correct the program at any stage. Finally, master–slave systems are completely operator dependent, since they only translate the movements of the operator’s hands into the movements of their mechanical parts. Master–slave robotic systems consist of master manipulators remotely operated by the human operator, controlling the robotic slave manipulators conducting the surgery as an intermediary.
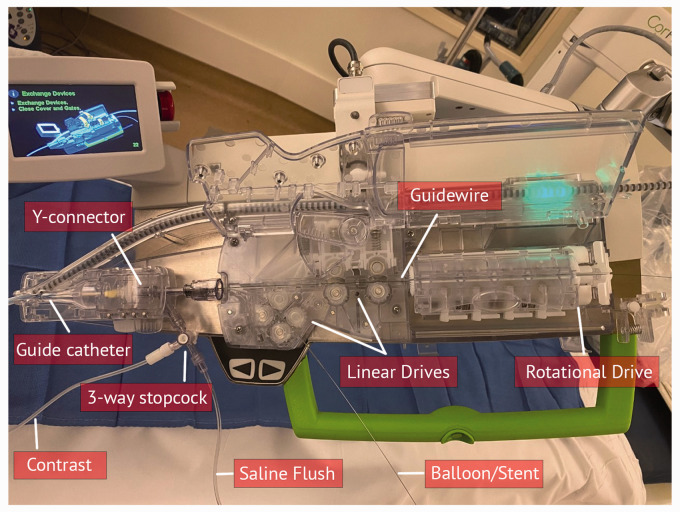
Actuators are key components within a robot. Medical robots depend on actuators to function with a number of degrees of freedom (DoF). Each DoF in robotics refers to a servoed joint that transfers force from a control system (which can be operated by the physician) to perform a desired motion. As an example, a hinge joint can move in one plane and has one DoF. A ball and socket joint can move in x, y, and z planes, possessing at least three degrees of motion, with a fourth degree of motion if the ball and socket joint can rotate clockwise and counterclockwise. Most medical robots are powered by an electromechanical unit that allows linear or rotational movement. Linear actuators convert mechanical energy into unidirectional motion and are usually made from a movable rod installed in a housing (Figure 2). For instance, endovascular robots typically use linear actuators in conjunction with a pinching or clamping device to advance or retract a guidewire or catheter in a straight line. The ability to pinch or grasp itself is a DoF. Rotational actuators are more complex and consist of a gearbox and an electric motor. Gearboxes can vary in size, functioning at specified gear ratios, and can support a wide variety of movements. As the gear ratio of the reducer decreases, the speed of the rotation increases, and consequently the torque or imparted force for movement decreases. For endovascular robots, rotational actuators are typically used in conjunction with a friction roller to press a wire against a capstan and then advance or retract it (Figure 2). Overall, actuators carry out movements in multiple planes with high precision, and therefore may be capable of improving physician performance by controlling movement consistently over a broad range from slow to fast and eliminating or dampening such unfavorable human limitations as tremor.
Figure 2.
Endovascular robotic system, CorPath GRX, with linear and rotational drives to advance/retract/rotate catheters and guidewires that join through the Y-connector.
Stereotaxis is a term that refers to creating a three-dimensional (3D) coordinate system that locates points within the body using an external spatial frame of reference. 11 These techniques are reliant on presurgical 3D isovoxel imaging and external markers. These systems are often used intracranially.
Justification and ethics
Surgical robots have advantages and disadvantages and will be justifiable more readily as they are proven to benefit human health broadly. The first advantage is the potential to improve physician performance. Physicians can become fatigued and inattentive, may have limits to fine motor skill due to tremor, have limited dexterity at very small scale, have limited geometric accuracy, and are difficult to keep sterile. 12 Robots allow physicians to transcend their human limits because they are untiring, stable, and have excellent geometric accuracy.
The second advantage pertains to improved safety and consistency. Occupational health risks of interventionists include exposure to ionizing radiation and orthopedic injury related to radiation protection apparel. Use of surgical robots would enable improved ergonomics and reduce or eliminate these occupational hazards. Recent studies suggest that radiation exposure to the operator and patient is significantly reduced by using robotic systems.13,14 Robotic systems can also limit the occupational spread of infection, such as COVID-19, by limiting staff exposure to the patient during procedures. 3 Robots can also provide mechanical limits to how rapidly or how forcefully an action takes place, or the range of distance over which such actions take place, allowing added measures of protection and safety that are otherwise absent.
The third advantage pertains to improved data collection and transmission in objectively assessing motions and actions, such as is currently done with meticulous multistream measurement of flight data with flight data recording. If data pertaining to the operation are routinely saved and analyzed, improved morbidity and mortality assessments can be made, and best outcomes can be objectively analyzed and planned for. Such data could also be used for optimized surgical simulation by utilizing input variables from cases that succeeded and failed, just as flight simulation currently incorporates multiple failure modes in developing heightened operator expertise. Furthermore, collection of such data and their instantaneous transmission elsewhere not only allows for the possibility of telesurgery in performing a procedure from a remote location, but also permits tele-mentoring in improving both access to subspecialty care and expert tele-training in remote locations. 15
Surgical robots possess disadvantages. First, robots have a constrained and defined ability to adapt to new situations, given the limitations of the specific robotic pedagogy a robot has experienced, and a limited ability to integrate and interpret complex information. This point is especially relevant in light of the advent of novel automated maneuvers that may be possible despite not having been designed in or anticipated at the time of robot certification and distribution. As one such example, the Corindus GRX system gained Food and Drug Administration (FDA) approval and the CE mark in 2018 for two automated maneuvers: rotate on retract (RoR) and “active device fixation.” 16 RoR automatically rotates the guidewire during retraction to facilitate vessel selection, and “active device fixation” is an open-loop control algorithm to maintain constant guidewire position during microcatheter movement. Additional automated maneuvers under development include “spin,” which rotates the guidewire in oscillating fashion to facilitate lesion-crossing, “wiggle,” which rotates the guidewire in reciprocating fashion during catheter advancement, “dotter,” which advances and retracts in stepwise fashion for lesion-crossing, and “constant speed” to select a constant drive speed for accurate lesion measurement. 17 Although these selections seem intuitively valuable to an experienced proceduralist, this is a finite list and does not intrinsically accommodate for additional selections and maneuvers. Even the most technically advanced robotic systems work strictly according to an algorithm and cannot rapidly adapt to the development of a new situation if such a possibility was not provided for by the algorithm. Although multilayer neural networks may allow robotic systems to “learn” and improve performance, currently, humans, after rigorous training, are unmatched in their ability to integrate and interpret large amounts of procedural and clinical data quickly in the interventional suite, sometimes with very limited background or similar experience and information, permitting human intuitive leaps. Second, a major limitation of current robotic endovascular systems is the lack of haptic feedback, and our limited understanding of how much tactile feedback is subconsciously valued or necessary. Tactile feedback provides sensory information to interventionists in addition to that available by imaging. Pressure- and force-sensing capabilities may need to be integrated into next-generation robots and are an area of great investigational interest. Third, robots may represent unjustifiable additional costs in already financially overburdened healthcare systems. As such costs and dependent complexities are considered, robust telecommunications networks will be essential to enabling telesurgery, just as the high cost of consumables and other infrastructure must be considered. One might argue that existing humans currently perform or can be trained to perform the same tasks well enough and with sufficient safety at a much lower cost.
A number of ethical considerations naturally arise when regarding the role of robots in neurointervention. A first concern is autonomy. What level of autonomy should robots be allowed? At one extreme, should only master–slave robots be allowed to operate on patients? A second concern is responsibility. If robots gain progressively increased levels of autonomy over time, and make decisions with diminishing human participation, who bears the liability for the robots’ actions? Will responsibility be allocated and adjusted between the robots and humans, depending on the misstep and consequences? A third concern is dehumanization. The patient–physician relationship has been considered a sacrosanct part of many societies for millennia, where physicians are at their most integrative seen as ministering to body and spirit. Will replacement of humans by robots contribute to the accelerated dehumanization of healthcare? Robots cannot commonly provide emotion, empathy, or warmth—how will this affect our individual patients and ultimately our society? A fourth concern is security. As robots become more complex and begin collecting, storing, and transmitting large amounts of data, the commensurate need for data protection, privacy, and security will need to be addressed. Similarly, unique security concerns apply to robots that are connected to telecommunications systems, as we envision hackers potentially not only pirating data, but actually potentially pirating and hijacking the procedures themselves.
Current applications of surgical robots
In the following sections, we describe robots and robotic systems that are currently clinically utilized for visceral, neurosurgical, endovascular, and neuroendovascular applications.
Visceral
Since FDA clearance in 2000, Intuitive Surgical’s da Vinci robot has been installed in hundreds of hospitals globally. 18 The system consists of a control panel and an operating unit. The operating unit allows a surgeon to visualize the surgical field in an augmented endoscopic manner using an optical system that creates a 3D image with multiple magnifications. At the control panel, the surgeon has two manipulators, one for each hand, which convert hand movements into the movement of specialized surgical instruments. Each surgical instrument is exchanged manually at the operating unit. The operating unit has four actuators, one of which is equipped with a camera, while the other three are equipped with surgical instruments inserted through separate laparoscopic ports. Da Vinci can assist in performing multiple surgeries, including hysterectomy, prostatectomy, and mitral valve repair. 19 Senhance is a related surgical robot made by TransEnterix that was cleared for use by the FDA in 2017–2018. 20 Like the da Vinci robot, the Senhance console has an immersive 3D video screen as part of its control console, and additionally claims several improvements over da Vinci: haptic sensing, eye-tracking camera control, and reusable surgical instruments. Senhance is utilized for inguinal hernia repair, cholecystectomy, hysterectomy, and colorectal surgery.
Neurosurgical
Transcranial
NeuroMate by Integrated Surgical Systems (Davis, CA) is one of the earliest developed medical robotic systems dating back to 1985. 21 It has remained one of the most widely used robots for image-guided stereotactic neurosurgery 22 and received FDA approval in 1999. Since being acquired by Renishaw and along with advances in neurosurgery, it has been applied into both frame-based and frameless procedures, including brain biopsy, stereoelectroencephalography (SEEG), deep brain stimulation (DBS) placement, radiosurgery, and neuroendoscopy. The robot itself has five DoF and has image-guidance with computed tomography (CT) or magnetic resonance imaging (MRI). An early study reported sub-millimeter and millimeter accuracy for frame-based and frameless procedures, respectively. 23
ROSA Brain is another leading neurosurgical robot developed by Medtech (Montpellier, France) that was FDA cleared in 2018. It can be used for transcranial placement of biopsy needles or SEEG electrodes.24,25 The robot aims to improve efficiency and safety by eliminating need for a stereotactic frame and decreasing procedure time. The ROSA robot is unique in that it integrates multiple applications, including spinal procedures and orthopedic surgeries, and is more commonly known as the ROSA One system.
Stealth Autoguide was co-developed by Medtronic (Minneapolis, MN) and the recently acquired Mazor Robotics (Caesarea, Israel) and it is one of the most recently launched cranial robotic platforms. It was FDA cleared in 2019 for transcranial laser catheters (e.g., Visualase), depth electrodes, and biopsy needle placement. 26 In contrast to other navigation systems, its compact structure offers favorable ergonomics in the operating room. The Mazor system has an image-guidance system and a high-speed Midas drill unit, allowing spatial positioning and orientation during continuous real-time navigation.
SYMBIS (IMRIS, Minnetonka, MN), previously known as NeuroArm, and NeuroBlate (Monteris Medical, Minneapolis, MN) differ from other commercial platforms in that these were designed to function solely under MRI guidance and controlled from a remote workstation. 27 SYMBIS is a 7 + 1 DoF teleoperated robot used for stereotactic biopsy and microsurgery, sharing similar characteristics to the da Vinci robot, and it received FDA approval in 2015 for tumor resection. The NeuroBlate system is a 2 DoF robot guiding the position of a proprietary laser probe for ablation procedures that can be monitored in near real time with MR thermometry.
Spinal
Robot-assisted spinal surgery has primarily been used for pedicle screw fixation, and it has evolved considerably over the past two decades to compete with traditional free-hand (FH) spinal procedures. 28 SpineAssist (Medtronic; formerly Mazor Robotics) was the first platform to gain FDA approval in 2004 and was followed by a second-generation robot called Renaissance in 2011. Surgeons planned a screw trajectory from preoperative CT spine images co-registered with intraoperative fluoroscopic images and then utilized a semi-active miniaturized robotic arm mounted onto a frame immediately above a prone patient to guide appropriate screw placement. SpineAssist, however, was limited due to its inability to accommodate for intraoperative movements in real time, and screw placements were commonly complicated by skiving, consequently leading to inaccurate placements. Yet, SpineAssist helped establish a paradigm for the workflow in spinal robotics.
Prominent robotic systems have become integrated across a number of clinical centers, including Mazor X (Medtronic), ROSA Spine (Zimmer Biomet, Warsaw, IN; formerly Medtech), TiRobot (TINAVI Medical, Beijing, P.R. China), and ExcelsiusGPS (Globus Medical, Audubon, PA). 28 These systems, however, departed from the original robotic structure seen with SpineAssist and Renaissance to a larger robotic arm positioned adjacent to surgical bed. In 2016, Mazor X and ROSA Spine were FDA approved, and TiRobot was cleared for use in China only. These were the first to incorporate a linear optic camera for the robotic arm to register its location in 3D space according to a reference marker typically fixed on iliac crest in order to avoid collisions and allow real-time adjustments to movement that may lead to screw misalignment. The workflow for ROSA Spine additionally implemented an intraoperative O-arm scanner for image registration, subsequently allowing a 3D spinal reconstruction on the workstation touchscreen. In 2017, ExcelsiusGPS (Globus Medical) entered the clinical setting and differed from its competitors in that it had automated haptic feedback to alert surgeons during drill skiving or sliding.
Meta-analyses and systematic reviews have demonstrated that robotic-assisted spinal surgery performs equally if not superiorly to FH techniques with clinically acceptable screw placements.29,30 Additionally, although robotic-assistance increases operation times, it reduces radiation exposure to operating staff.
Endovascular
Cardiac electrophysiology
Niobe is a robotic magnetic navigation (RMN) system developed by Stereotaxis (St. Louis, MO) to increase the precision and safety of ablation of arrhythmias and particularly atrial fibrillation during electrophysiological studies. 31 The system was introduced in 2002 and consists of two magnets located on either side of the patient, which generate a constant magnetic field (0.08 or 0.1 T). Niobe uses an atraumatic catheter that is inserted into the patient’s heart cavity and consists of three magnets located in the distal segment of the electrode, allowing it to be navigated remotely using a directed magnetic field (Figure 3(a)). Electrode navigation is achieved by changing the direction of magnetic field vector using a remote workstation. The magnetic field therefore imparts a gentle amount of force on the magnetic elements in the catheter, leading to controlling the degree of catheter curvature and steered deflection. RMN includes a magnetic vector storage function for re-access with automatic catheter guidance. The advancement and traction of the electrode are carried out separately using the joystick to control the Vdrive platform (Figure 3(b)). The system can integrate data from non-fluoroscopic electroanatomic mapping data (e.g., CARTO). 32 Genesis is Stereotaxis’s second-generation RMN system (Figure 3(c)) that places the smaller magnets on flexible robotic arms that rotate along their center of mass, thereby increasing the speed and responsiveness of the system. 33
Figure 3.
Stereotaxis remote magnetic navigation (RMN) systems for cardiac ablations. (a) First-generation Niobe platform in interventional cardiology suite. (b) Closer look at the Vdrive catheter and guidewire platform. (c) Second-generation Genesis RMN with smaller, flexible magnets to aid in suite ergonomics and display using electroanatomic mapping. (Images (a) and (b) from Akca et al. 2014; image (c) courtesy of Stereotaxis). 66
Amigo is an electrophysiological surgical robotic system from Catheter Robotics designed to perform catheter ablation of atrial fibrillation. Since 2012, Amigo has been FDA cleared for diagnostic studies in the right atrium and ventricle. A number of clinical studies have been carried out in which the Amigo robot has shown its effectiveness in ablation of atrioventricular nodal re-entrant tachycardia, TP, ventricular tachycardia, accessory pathways, atrial tachycardia, as well as atrial fibrillation, all while reducing the radiation dose to patients and operators by up to 86%. 34 The Amigo robot includes a robotic arm and a remote-control panel. The control panel is designed to mimic a standard ablation catheter with standard adjustments, allowing the operator to advance/withdraw, flex/deflex, and apply torque to the catheter. Initially, the system requires the direct non-robotic control of a surgeon initially to catheterize the femoral vein and advance the catheter to the inferior vena cava. An important feature of the Amigo robot is the ability to switch to manual operation at any stage of the operation, which can be important in difficult cases. Switching between robotic and manual mode occurs without compromising sterility or catheter positioning.
Neuroendovascular
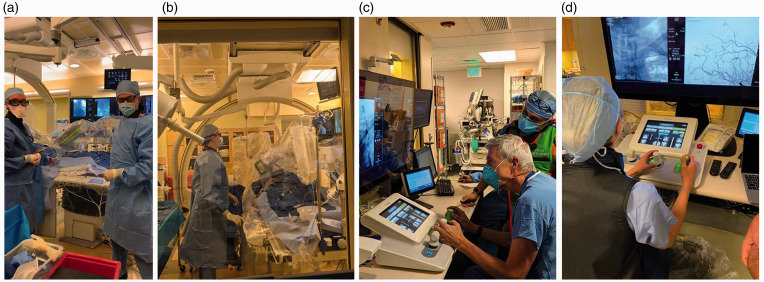
CorPath 200 (Corindus Vascular Robotics, Waltham, MA) was initially launched in 2012 as an interventional cardiology robot. 35 The PRECISE study demonstrated its safety and effectiveness, which reduced the dose of radiation by 95% to the patient and operator. 13 In 2016, Corindus launched its second-generation robot, CorPath GRX. 36 Both systems consist of two main components: the bedside robotic unit and the separate interventional control cockpit with procedural display and available hemodynamic data (Figure 4). GRX integrated a new feature called “active guide management,” allowing control of the guide catheter, and included an auxiliary port for balloon or stent advancement or retraction in controlled 1 mm increments. The GRX system was FDA cleared for percutaneous coronary intervention (PCI) in 2016 and for percutaneous vascular interventions (PVI) in 2018. 37 , 38 Tele-stenting was feasible over a long distance at ∼20 miles between the operator and patient in India 15 using LAN/MAN/WAN connectivity and an advanced audio-visual telecommunications system. Transcontinental telesurgery (∼3000 miles) has also been demonstrated in preclinical models over wired and 5G wireless network connections with clinically negligible latency. 39 Latency times were evaluated in another study in which latencies ≥400 ms were perceptible and appropriate clinically negligible latencies for tele-stenting needed to be ≤250 ms. 40
Figure 4.
(a) and (b) CorPath interventional suite with CorPath GRX robot installed. (c) and (d) Interventional neuroradiologists using control joystick and display to navigate CorPath GRX at a safe distance from radiation exposure.
With its clinical momentum in interventional cardiology, engineers adapted the system for NVI.41,42 The bedside robotic unit contained a rotational drive for rotating the guidewire, a linear drive for advancing/retracting the guidewire, a Y-connector holder, and a bedside touchscreen. While the design has remained broadly unchanged, key modifications for NVI included a Y-connector cover and adaptor to prevent device herniation and added a new driving gear to facilitate smaller diameter devices. CorPath GRX received CE mark for NVI in April 2019, and soon after, a first-in-human study was published showing safe robot-assisted coiling of a large basilar aneurysm. 43 Two research studies followed, demonstrating safe diagnostic angiography and carotid angioplasty.44,45 Its application eventually broadened to treat all vascular beds in the 33 countries that accept CE mark and holds implications for tele-stroke therapy, particularly for under-resourced settings. CorPath GRX has not yet been cleared for neuroendovascular interventions by the FDA.
Corindus also developed novel algorithms for efficient endovascular device navigation. The robot’s five automatic movement features (“Rotate on retract,” “wiggle,” “spin,” “dotter,” and “constant speed”) were FDA cleared in December 2020 for PCI and PVI procedures. Rotate on retract features automatic rotation of the guidewire when the guidewire is retracted by the operator to facilitate instrumentation and catheterization of targeted branch vessels. A “precise measurement feature” allows calculation of a length along the course of the target vessel in order to select optimal stent length.
Conclusion
Robots are playing an increasing role in modern medicine. In recent years, multiple new robotic systems have been cleared, and the COVID-19 pandemic has accelerated the adoption of telemedicine. Robotic-assisted intervention can contribute to the growth of telemedicine and delivery of care by expanding networks of care globally while building upon the unique advantages conferred by robotic systems related to performance, precision, efficiency, and safety when compared with traditional non-robotic manual interventions. Improvements in haptic feedback, triaxial compatibility, machine learning, and rapid and secure telecommunication will be needed to realize the potential of robotic assistance for NVIs.
Footnotes
Conflict of interest: KM is an employee of Siemens Healthineers. The authors declare no other potential conflicts of interest with respect to the research, authorship, and/or publication of this article. AMN reports personal fees from Penumbra Inc. and Stryker Inc. as a scientific advisor as well as stock with Boston Imaging Core Laboratories, none of which affect the submitted work.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The authors received funding support from NIH NIBIB R01EB012031 (SWH, KHN), NIH NINDS U54 NS065705 (KHN).
ORCID iD: Kazim H Narsinh https://orcid.org/0000-0002-2019-5461
References
- 1.Koonin LM. Trends in the use of telehealth during the emergence of the COVID-19 pandemic—United States, January–March 2020. MMWR Morb Mortal Wkly Rep 2020; 69: 1595–1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hollander JE, Carr BG. Virtually perfect? Telemedicine for Covid-19. N Engl J Med 2020; 382: 1679–1681. [DOI] [PubMed] [Google Scholar]
- 3.Tabaza L, Virk HUH, Janzer S, et al. Robotic‐assisted percutaneous coronary intervention in a COVID‐19 patient. Catheter Cardiovasc Interv 2021; 97: E343–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhaskar S, Bradley S, Sakhamuri S, et al. Designing futuristic telemedicine using artificial intelligence and robotics in the COVID-19 era. Front Public Health 2020; 8: 556789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Michtalik HJ, Yeh H-C, Pronovost PJ, et al. Impact of attending physician workload on patient care: a survey of hospitalists. JAMA Intern Med 2013; 173: 375. [DOI] [PubMed] [Google Scholar]
- 6.West CP Dyrbye LN, andShanafelt TD.. Physician burnout: contributors, consequences and solutions. J Intern Med 2018; 283: 516–529. [DOI] [PubMed] [Google Scholar]
- 7.Elad M, Eleid MF, Gulati R, et al. Current and future use of robotic devices to perform percutaneous coronary interventions: a review. J Am Heart Assoc 2017; 6: e006239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Muaddi H, Hafid ME, Choi WJ, et al. Clinical outcomes of robotic surgery compared to conventional surgical approaches (laparoscopic or open): a systematic overview of reviews. Ann Surg 2021; 273: 467–473. [DOI] [PubMed] [Google Scholar]
- 9.Avgousti S, Christoforou EG, Panayides AS, et al. Medical telerobotic systems: current status and future trends. Biomed Eng OnLine 2016; 15: 96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lane T. A short history of robotic surgery. Ann R Coll Surg Engl 2018; 100: 5–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Faria C, Erlhagen W, Rito M, et al. Review of robotic technology for stereotactic neurosurgery. IEEE Rev Biomed Eng 2015; 8: 125–137. [DOI] [PubMed] [Google Scholar]
- 12.Fargen KM Turner RD, andSpiotta AM.. Factors that affect physiologic tremor and dexterity during surgery: a primer for neurosurgeons. World Neurosurg 2016; 86: 384–389. [DOI] [PubMed] [Google Scholar]
- 13.Weisz G, Metzger DC, Caputo RP, et al. Safety and feasibility of robotic percutaneous coronary intervention: PRECISE (Percutaneous Robotically-Enhanced Coronary Intervention) Study. J Am Coll Cardiol 2013; 61 :1596–1600. [DOI] [PubMed] [Google Scholar]
- 14.Patel Tejas M, Shah Sanjay C, Soni Yash Y, et al. Comparison of robotic percutaneous coronary intervention with traditional percutaneous coronary intervention. Circ Cardiovasc Interv 2020; 13: e008888. [DOI] [PubMed] [Google Scholar]
- 15.Patel TM Shah SC, andPancholy SB.. Long distance tele-robotic-assisted percutaneous coronary intervention: a report of first-in-human experience. EClinicalMedicine 2019; 14: 53–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Corindus, A Siemens Healthineers Company, https://www.corindus.com/news-events/press-releases/corindus-announces-global-launch-of-techniq (accessed 3 March 2021).
- 17.technIQ—smart procedural automation, https://www.corindus.com/corpath-grx/techniq (accessed 3 March 2021).
- 18.Crew B. Worth the cost? A closer look at the da Vinci robot’s impact on prostate cancer surgery. Nature 2020; 580: S5–S7. [Google Scholar]
- 19.Leung T, Vyas D. Robotic surgery: applications. Am J Robot Surg 2014; 1 :1–64. [PMC free article] [PubMed] [Google Scholar]
- 20.Samalavicius NE, Janusonis V, Siaulys R, et al. Robotic surgery using Senhance® robotic platform: single center experience with first 100 cases. J Robot Surg 2020; 14: 371–376. [DOI] [PubMed] [Google Scholar]
- 21.Hockstein NG, Gourin CG, Faust RA, et al. A history of robots: from science fiction to surgical robotics. J Robot Surg 2007; 1: 113–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smith JA, Jivraj J, Wong R, et al. 30 years of neurosurgical robots: review and trends for manipulators and associated navigational systems. Ann Biomed Eng 2016; 44: 836–846. [DOI] [PubMed] [Google Scholar]
- 23.Li QH, Zamorano L, Pandya A, et al. The application accuracy of the NeuroMate robot—a quantitative comparison with frameless and frame-based surgical localization systems. Comput Aided Surg 2002; 7: 90–98. [DOI] [PubMed] [Google Scholar]
- 24.Lefranc M, Capel C, Pruvot-Occean A-S, et al. Frameless robotic stereotactic biopsies: a consecutive series of 100 cases. J Neurosurg 2015; 122: 342–352. [DOI] [PubMed] [Google Scholar]
- 25.Paff M, Wang AS, Phielipp N, et al. Two-year clinical outcomes associated with robotic-assisted subthalamic lead implantation in patients with Parkinson’s disease. J Robot Surg 2020; 14: 559–565. [DOI] [PubMed] [Google Scholar]
- 26.Bernardo A. The changing face of technologically integrated neurosurgery: today’s high-tech operating room. World Neurosurg 2017; 106: 1001–1014. [DOI] [PubMed] [Google Scholar]
- 27.Guo Z, Leong MC-W, Su H, et al. Techniques for stereotactic neurosurgery: beyond the frame, toward the intraoperative magnetic resonance imaging–guided and robot-assisted approaches. World Neurosurg 2018; 116: 77–87. [DOI] [PubMed] [Google Scholar]
- 28.D’Souza M, Gendreau J, Feng A, et al. Robotic-assisted spine surgery: history, efficacy, cost, and future trends. Robot Surg Res Rev 2019; 6: 9–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peng Y-N, Tsai L-C, Hsu H-C, et al. Accuracy of robot-assisted versus conventional freehand pedicle screw placement in spine surgery: a systematic review and meta-analysis of randomized controlled trials. Ann Transl Med 2020; 8: 824–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fatima N, Massaad E, Hadzipasic M, et al. Safety and accuracy of robot-assisted placement of pedicle screws compared to conventional free-hand technique: a systematic review and meta-analysis. Spine J 2021; 21: 181–192. [DOI] [PubMed] [Google Scholar]
- 31.Yuan S, Holmqvist F, Kongstad O, et al. Long-term outcomes of the current remote magnetic catheter navigation technique for ablation of atrial fibrillation. Scand Cardiovasc J 2017; 51: 308–315. [DOI] [PubMed] [Google Scholar]
- 32.Rolf S, Hindricks G, Sommer P, et al. Electroanatomical mapping of atrial fibrillation: review of the current techniques and advances. J Atr Fibrillation 2014; 7: 1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stereotaxis earns FDA clearance and announces U.S. launch of Genesis robotic magnetic navigation system, https://www.cathlabdigest.com/content/stereotaxis-earns-fda-clearance-and-announces-us-launch-genesis-robotic-magnetic-navigation-system (accessed 16 October 2020).
- 34.Shaikh Z Eilenberg M, andCohen T.. The Amigo™ remote catheter system: from concept to bedside. J Innov Card Rhythm Manag 2017; 8: 2795–2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Granada JF, Delgado JA, Uribe MP, et al. First-in-human evaluation of a novel robotic-assisted coronary angioplasty system. JACC Cardiovasc Interv 2011; 4: 460–465. [DOI] [PubMed] [Google Scholar]
- 36.Smitson CC, Ang L, Pourdjabbar A, et al. Safety and feasibility of a novel, second-generation robotic-assisted system for percutaneous coronary intervention: first-in-human report. J Invasive Cardiol 2018; 30: 152–156. [PubMed] [Google Scholar]
- 37.Mahmud E, Naghi J, Ang L, et al. Demonstration of the safety and feasibility of robotically assisted percutaneous coronary intervention in complex coronary lesions. JACC Cardiovasc Interv 2017; 10: 1320–1327. [DOI] [PubMed] [Google Scholar]
- 38.Mahmud E, Schmid F, Kalmar P, et al. Feasibility and safety of robotic peripheral vascular interventions. JACC Cardiovasc Interv 2016; 9: 2058–2064. [DOI] [PubMed] [Google Scholar]
- 39.Madder RD, Vanoosterhout S, Parker J, et al. Robotic telestenting performance in transcontinental and regional pre‐clinical models. Catheter Cardiovasc Interv. Epub ahead of print 25 June 2020. DOI: 10.1002/ccd.29115. [DOI] [PubMed]
- 40.Madder RD, Vanoosterhout S, Mulder A, et al. Network latency and long‐distance robotic telestenting: exploring the potential impact of network delays on telestenting performance. Catheter Cardiovasc Interv 2020; 95: 914–919. [DOI] [PubMed] [Google Scholar]
- 41.Britz GW, Panesar SS, Falb P, et al. Neuroendovascular-specific engineering modifications to the CorPath GRX Robotic System. J Neurosurg 2020; 133: 1830–1836. [DOI] [PubMed] [Google Scholar]
- 42.Britz GW Tomas J, andLumsden A.. Feasibility of robotic-assisted neurovascular interventions: initial experience in flow model and porcine model. Neurosurgery 2020; 86: 309–314. [DOI] [PubMed] [Google Scholar]
- 43.Mendes Pereira V, Cancelliere NM, Nicholson P, et al. First-in-human, robotic-assisted neuroendovascular intervention. J NeuroInterventional Surg 2020; 12: 338–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sajja KC, Sweid A, Al Saiegh F, et al. Endovascular robotic: feasibility and proof of principle for diagnostic cerebral angiography and carotid artery stenting. J NeuroInterventional Surg 2020; 12: 345–349. [DOI] [PubMed] [Google Scholar]
- 45.Nogueira RG, Sachdeva R, Al-Bayati AR, et al. Robotic assisted carotid artery stenting for the treatment of symptomatic carotid disease: technical feasibility and preliminary results. J NeuroInterventional Surg 2020; 12: 341–344. [DOI] [PubMed] [Google Scholar]
- 46.Intuitive. Robotic-assisted surgery Da Vinci surgical system, https://www.intuitive.com/en-us (accessed 18 October 2020).
- 47.George EI, Brand TC, LaPorta A, et al. Origins of robotic surgery: from skepticism to standard of care. JSLS 2018; 22: e2018.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chenin L Peltier J, andLefranc M.. Minimally invasive transforaminal lumbar interbody fusion with the ROSATM Spine robot and intraoperative flat-panel CT guidance. Acta Neurochir 2016; 158: 1125–1128. [DOI] [PubMed] [Google Scholar]
- 49.D’Souza M, Gendreau J, Feng A, et al. Robotic-assisted spine surgery: history, efficacy, cost, and future trends. Robot Surg Res Rev 2019; 6: 9–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Khan A, Meyers JE, Siasios I, et al. Next-generation robotic spine surgery: first report on feasibility, safety, and learning curve. Oper Neurosurg 2019; 17: 61–69. [DOI] [PubMed] [Google Scholar]
- 51.Sutherland GR Latour I, andGreer AD.. Integrating an image-guided robot with intraoperative MRI. IEEE Eng Med Biol Mag 2008; 27: 59–65. [DOI] [PubMed] [Google Scholar]
- 52.Sloan AE, Ahluwalia MS, Valerio-Pascua J, et al. Results of the NeuroBlate system first-in-humans Phase I clinical trial for recurrent glioblastoma: clinical article. J Neurosurg 2013; 118: 1202–1219. [DOI] [PubMed] [Google Scholar]
- 53.Vardiman AB, Wallace DJ, Crawford NR, et al. Pedicle screw accuracy in clinical utilization of minimally invasive navigated robot-assisted spine surgery. J Robot Surg 2020; 14: 409–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Patel TM, Shah SC, Soni YY, et al. Comparison of robotic percutaneous coronary intervention with traditional percutaneous coronary intervention: a propensity score–matched analysis of a large cohort. Circ Cardiovasc Interv 2020; 13: e008888. [DOI] [PubMed] [Google Scholar]
- 55.Bismuth J, Duran C, Stankovic M, et al. A first-in-man study of the role of flexible robotics in overcoming navigation challenges in the iliofemoral arteries. J Vasc Surg 2013; 57: 14S–19S. [DOI] [PubMed] [Google Scholar]
- 56.Cochennec F, Kobeiter H, Gohel M, et al. Feasibility and safety of renal and visceral target vessel cannulation using robotically steerable catheters during complex endovascular aortic procedures. J Endovasc Ther 2015; 22: 187–193. [DOI] [PubMed] [Google Scholar]
- 57.Saliba W, Reddy VY, Wazni O, et al. Atrial fibrillation ablation using a robotic catheter remote control system: initial human experience and long-term follow-up results. J Am Coll Cardiol 2008; 51: 2407–2411. [DOI] [PubMed] [Google Scholar]
- 58.Dello Russo A, Fassini G, Conti S, et al. Analysis of catheter contact force during atrial fibrillation ablation using the robotic navigation system: results from a randomized study. J Interv Card Electrophysiol 2016; 46: 97–103. [DOI] [PubMed] [Google Scholar]
- 59.Khan EM, Frumkin W, Ng GA, et al. First experience with a novel robotic remote catheter system: Amigo™ mapping trial. J Interv Card Electrophysiol 2013; 37: 121–129. [DOI] [PubMed] [Google Scholar]
- 60.Datino T, Arenal A, Pelliza M, et al. Comparison of the safety and feasibility of arrhythmia ablation using the Amigo robotic remote catheter system versus manual ablation. Am J Cardiol 2014; 113: 827–831. [DOI] [PubMed] [Google Scholar]
- 61.Hoffmayer KS, Krainski F, Shah S, et al. Randomized controlled trial of Amigo® robotically controlled versus manually controlled ablation of the cavo-tricuspid isthmus using a contact force ablation catheter. J Interv Card Electrophysiol 2018; 51: 125–132. [DOI] [PubMed] [Google Scholar]
- 62.Turagam MK, Atkins D, Tung R, et al. A meta-analysis of manual versus remote magnetic navigation for ventricular tachycardia ablation. J Interv Card Electrophysiol 2017; 49: 227–235. [DOI] [PubMed] [Google Scholar]
- 63.Salimi A, Ramezanifar A, Mohammadpour J, et al. ROBOCATH: a patient-mounted parallel robot to position and orient surgical catheters. ASME 2012 5th Annual Dynamic Systems and Control Conference joint with the JSME 2012 11th Motion and Vibration Conference, October 17–19, 2012, Fort Lauderdale, FL.
- 64.First remote robotic-assisted PCI completed in Europe using R-One platform, https://cardiovascularnews.com/first-remote-robotic-assisted-pci-completed-in-europe-using-r-one-platform/ (accessed 6 March 2021).
- 65.Xcath, Inc., https://www.xcathinc.com (accessed 6 March 2021).
- 66.Akca F Dabiri L, andSzili-Torok T.. Robotic ablation in electrophysiology. In: Kibos AS, Knight BP, Essebag V, et al. (eds) Cardiac arrhythmias: from basic mechanism to state-of-the-art management. London: Springer, 2014, pp.533–541. [Google Scholar]