Abstract
Purpose
The trend of atherosclerotic plaque feature evolution is unclear in stroke patients with and without recurrence. We aimed to use three-dimensional whole-brain magnetic resonance vessel wall imaging to quantify the morphological changes of causative lesions during medical therapy in patients with symptomatic intracranial atherosclerotic disease.
Methods
Patients with acute ischemic stroke attributed to intracranial atherosclerotic disease were retrospectively enrolled if they underwent both baseline and follow-up magnetic resonance vessel wall imaging. The morphological features of the causative plaque, including plaque volume, peak normalized wall index, maximum wall thickness, degree of stenosis, pre-contrast plaque-wall contrast ratio, and post-contrast plaque enhancement ratio, were quantified and compared between the non-recurrent and recurrent groups (defined as the recurrence of a vascular event within 18 months of stroke).
Results
Twenty-nine patients were included in the final analysis. No significant differences were found in plaque features in the baseline scan between the non-recurrent (n = 22) and recurrent groups (n = 7). The changes in maximum wall thickness (–13.32% vs. 8.93%, P = 0.026), plaque-wall contrast ratio (–0.82% vs. 3.42%, P = 0.005) and plaque enhancement ratio (–11.03% vs. 9.75%, P = 0.019) were significantly different between the non-recurrent and recurrent groups. Univariable logistic regression showed that the increase in plaque-wall contrast ratio (odds ratio 3.22, 95% confidence interval 1.55–9.98, P = 0.003) was related to stroke recurrence.
Conclusion
Morphological changes of plaque features on magnetic resonance vessel wall imaging demonstrated distinct trends in symptomatic intracranial atherosclerotic disease patients with and without stroke recurrence.
Keywords: Intracranial atherosclerosis, stroke, plaque, vessel wall imaging, follow-up study
Introduction
Intracranial atherosclerotic disease (ICAD) represents one of the most common causes of ischemic stroke worldwide. 1 Despite intensive medical management, the rate of stroke recurrence is 13% in the first year. 2 Therefore, actively monitoring the therapeutic response in patients with symptomatic ICAD may be helpful for the success of secondary stroke prevention.
Currently, clinical follow-up of these patients relies on assessments of functional abilities and mobility, clinical risk factors, and the severity of luminal stenosis on computed tomography angiography (CTA) or magnetic resonance angiography (MRA). 3 While the latter imaging-based approach is lesion oriented, the degree of stenosis alone may not be a reliable indicator of plaque severity.3,4 An atherosclerotic plaque, although lacking progression in the degree of stenosis, may rupture or extend over the ostia of perforator arteries and cause recurrent stroke. 5 In such a scenario, it is plausible that non-luminal (i.e. vessel wall-related) changes within individual ICAD lesions occur and precede recurrent clinical events. We hypothesize that a non-invasive imaging approach that can quantify the lesion-specific morphological changes may help identify medically refractory patients early and further tailor stroke therapies based on such additional surrogate markers of disease progression.
Magnetic resonance vessel wall imaging (MR-VWI) is a no-invasive imaging method that can directly characterize ICAD lesions in terms of their morphological aspects.5–9 In ICAD patients, the following plaque features on MR-VWI have been linked to causative lesions: large plaque burden,10,11 high signal on T1-weighted images (highly suggestive of fresh or recent intraplaque hemorrhage),12–16 and contrast enhancement (a marker of inflammation and neovascularization).16–19 Three-dimensional (3D) turbo spin-echo with variable refocusing flip angles is increasingly becoming a method of choice for intracranial MR-VWI because of its large spatial coverage, high spatial resolution and signal-to-noise ratio, and flexibility in image visualization.20–22 The method is reproducible in vessel wall quantitative analysis,20,23 and in particular a recently proposed 3D whole-brain MR-VWI technique performs reliably in both healthy and patient cohorts.24–27 It is thus possible to utilize the method to carry out longitudinal studies, such as assessing new therapeutics or developing personalized treatment paradigms. The feasibility of using MR-VWI to detect morphological changes of plaques during medical treatment has been explored in previous studies with small sample sizes or limited qualitative features.19,28–30 However, tracking a more complete set of quantitative plaque features simultaneously and investigating their temporal changes and links to the incidence of recurrence using 3D MR-VWI have not been performed.
Our study aimed to use 3D whole-brain MR-VWI to quantify the morphological changes of causative lesions during preventive therapy, and determine the trend of plaque feature evolution in symptomatic ICAD patients with and without stroke recurrence in 18 months.
Methods
Study population
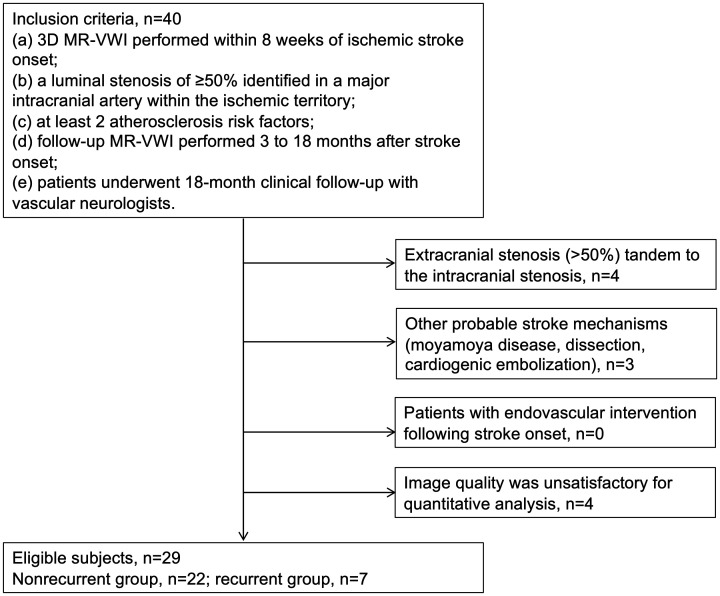
Local institutional review boards approved and waived the requirement for written informed consent for this retrospective study. We searched cases collected between September 2015 and September 2019 in the clinical picture archiving and communication system (PACS) of whole-brain vessel wall imaging in stroke patients (WISP) study participating sites. The WISP study enrolled adult patients who had ischemic stroke within 8 weeks to undergo MR-VWI during their diagnostic work-up. At the select WISP participating sites, MR-VWI is part of a ‘MRA with contrast’ imaging protocol that is routinely prescribed both for diagnostic work-up in ischemic stroke patients and for treatment follow-up. Patients were included if they met the following criteria: (a) 3D MR-VWI performed within 8 weeks of ischemic stroke onset; (b) a luminal stenosis of 50% or greater (as confirmed by CTA or MRA) identified in a major intracranial artery within the ischemic territory; (c) at least two atherosclerosis risk factors (hypertension, diabetes mellitus, dyslipidemia, obesity, smoking, coronary artery disease, age >50 years for men or >60 years for women); (d) follow-up MR-VWI performed 3–18 months after stroke onset; (e) patients underwent 18-month clinical follow-up with vascular neurologists. The exclusion criteria included: (a) extracranial stenosis (>50%) tandem to the intracranial stenosis as confirmed by ultrasound, CTA or MRA; (b) other probable stroke mechanisms (moyamoya disease, dissection, cardiogenic embolization); (c) any endovascular intervention following stroke onset; (d) image quality was unsatisfactory for quantitative analysis. A flow chart for subject recruitment is presented in Figure 1.
Figure 1.
Flow chart of the study population.
Magnetic resonance imaging protocol
In each subject, both baseline and follow-up magnetic resonance examinations were acquired at the same 3T system (Siemens Healthineers, Erlangen, Germany). The imaging protocol consisted of 3D time-of-flight (TOF) MRA, diffusion weighted imaging (DWI), and pre and post-contrast 3D T1-weighted whole-brain MR-VWI.24,25 Imaging parameters for MR-VWI were as follows: sagittal orientation; TR/TE 900/15 ms; field of view (FOV) 170 × 170 mm2 or 170 × 210 mm2; 224 or 240 slices; voxel size isotropic 0.53 or 0.55 mm; echo train length 52; GRAPPA factor 2; scan time 8 minutes. Post-contrast MR-VWI was performed 5 minutes after the injection of a single dose (0.1 mmol/kg of body weight) of contrast agent (Magnevist from Schering or Gadavist from Bayer HealthCare Pharmaceuticals).
Image analysis
A causative plaque was defined as the most stenotic or the only plaque within the vascular territory of the stroke. 17 The MR-VWI images were reviewed in consensus by a neuroradiologist (JX, with 5 years of experience) and a vascular neurologist (KHS, with 11 years of experience) who had access to clinical history and conventional brain and vascular magnetic resonance studies to decide the causative plaque. The probable stroke mechanism in each subject was determined using previously reported methods. 31 Large intracranial blood vessels, including the intracranial internal carotid artery, the A1 segment of the anterior cerebral artery, the M1 and M2 segments of the middle cerebral artery, the V4 segment of the vertebral artery, the basilar artery, and the P1 segment of the posterior cerebral artery, were evaluated.
The plaque geometry of the causative lesion was quantified on pre-contrast MR-VWI images. A custom-designed software package, intracranial vessel analysis (IVA), 32 was used by a neuroradiologist (5 years of experience in MR-VWI) who was blinded to clinical information to reconstruct contiguous cross-sectional slices from causative lesions with a semi-automatic centerline tracking functionality and segment vessel wall in each slice with a deep learning-based algorithm. After manual adjustment, when necessary, for outer and inner vessel wall contours, vessel wall and lumen areas of individual slices, peak normalized wall index (pNWI) (maximal ratio of wall area to vessel area), and plaque volume were quantified. The maximum wall thickness was measured manually at the most thickened location of the plaque. The degree of stenosis was quantified as (1– lesion lumen area/adjacent normal lumen area) × 100% on pre-contrast MR-VWI images to minimize the impact of signal loss from TOF-MRA saturation effects.33,34
Signal intensity-based features of causative lesions were quantified on paired pre and post-contrast MR-VWI images. In particular, the mean signal intensity was quantified with manually drawn regions of interest in the brightest region of the plaque, entire lesion, grey matter, and adjacent normal vessel wall on pre-contrast images, and also in the entire lesion and grey matter on post-contrast images. The plaque-wall contrast ratio (CR) was calculated as the signal intensity ratio between the brightest region within the plaque and the reference vessel wall from pre-contrast images, and the plaque enhancement ratio (ER) was calculated as the signal intensity ratio between the post and pre-contrast plaque normalized by adjacent grey matter.6,16
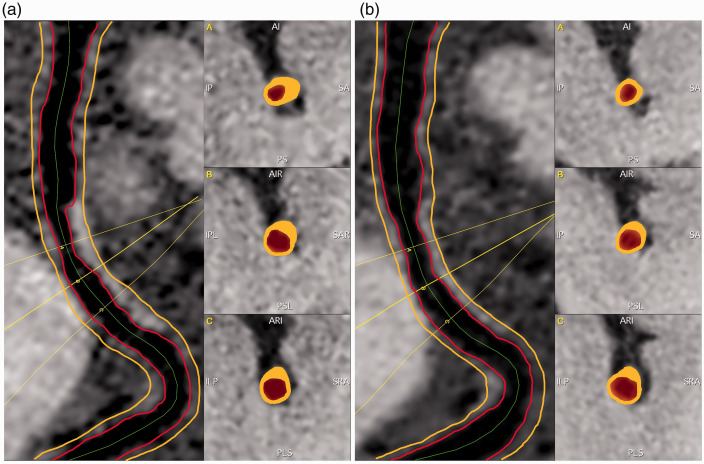
Using the same software, the images from the follow-up MR-VWI examination were spatially registered to those from the baseline, and the same centerline path and slice range encompassing the causative lesion were applied to generate location matched contiguous cross-sectional slices (Figure 2). Geometric and signal features were quantified using the methods described above.
Figure 2.
Magnetic resonance vessel wall imaging (MR-VWI) was measured using a custom-designed intracranial vessel analysis software package. In the baseline examination (a), contiguous cross-sectional slices from causative lesions were reconstructed with a semi-automatic centerline tracking functionality, and vessel wall was segmented in each slice with a deep learning-based algorithm. The images from the follow-up examination (b) were spatially registered to those from the baseline, and the same centerline path and slice range involving the original causative lesion were applied to generate location-matched contiguous cross-sectional slices.
Clinical follow-up
All patients were prescribed antiplatelet and intensive lipid-lowering therapy. They were followed up with either a telephone interview or in-person clinic visit unless they had already experienced a second cerebrovascular event (new stroke, transient ischemic attack (TIA)) on medical records. The patients who reported a neurological symptom or sign suggesting new cerebrovascular events were further evaluated in the neurology clinic. Patients who experienced a second clinical event in the vascular territory of the originally causative lesion within 18 months after stroke onset were categorized into the recurrent group, whereas those who experienced no clinical event within 18 months were categorized into the non-recurrent group. Patients who experienced a clinical event, but not in the territory of the originally causative lesion were excluded.
Statistical analysis
Quantitative variables are expressed as means ± standard deviations and qualitative variables are described with relative frequency (percentage). Descriptive measures were presented for all variables. The assumptions of normality and homogeneity of variance were verified by the Shapiro–Francia test and Levene test, respectively. Welch’s t-test and Brunner–Munzel’s t-test were applied to compare continuous variables, while Fisher’s exact test was applied to compare categorical variables between stroke status. Odds ratios of recurrent stroke with their respective 95% confidence intervals (CIs) were assessed using univariable logistic regression with bias correction. P values were adjusted for multiple comparisons using the Benjamin and Hochberg correction for false discovery rate. A P value of less than 0.05 indicated statistical significance. All statistical analyses were performed by using R version 4.0.2.
Results
A total of 40 patients was included, of which four were excluded due to coexisting stenosis at extracranial carotid arteries, three were excluded due to non-atherosclerotic intracranial arterial diseases and four were excluded because of motion artefacts affecting vessel wall assessment. Twenty-nine patients (22 men; age 43.0 ± 11.6 years) were eligible for the final analysis, with 22 classified into the non-recurrent group and seven into the recurrent group. The median time interval between stroke onset and the baseline MR-VWI examination was 8 days (1–44 days). The median time interval between the baseline and follow-up examinations was 8 months (3–15 months). In the recurrent group, one patient had recurrent TIA 2 months after the follow-up scan; two patients had recurrent stroke 2 and 6 months after follow-up scans; four patients had recurrent stroke within 5 to 10 months of the index stroke and underwent follow-up scans 1–4 days after recurrence. The baseline clinical characteristics of the non-recurrent and recurrent groups are shown in Table 1. No significant differences were found in the baseline clinical characteristics between the two groups.
Table 1.
Clinical characteristics of the non-recurrent (n = 22) and recurrent group (n = 7).
| Characteristics | Non-recurrent group | Recurrent group | P value |
|---|---|---|---|
| Age, year, mean (range) | 41.7 (29–65) | 48.7 (39–66) | 0.171 |
| No. of men, n (%) | 17 (77.27) | 5 (71.43) | 1 |
| Hypertension, n (%) | 13 (59.09) | 5 (71.43) | 0.677 |
| Diabetes mellitus, n (%) | 4 (18.18) | 1 (14.29) | 1 |
| Dyslipidemia, n (%) | 12 (54.55) | 3 (42.86) | 0.682 |
| Obesity, n (%) | 7 (31.82) | 2 (28.57) | 1 |
| History of smoking, n (%) | 7 (31.82) | 3 (42.86) | 0.665 |
| Coronary artery disease, n (%) | 2 (9.09) | 1 (14.29) | 1 |
| Age >50 years (men) or >60 years (women), n (%) | 6 (27.27) | 2 (28.57) | 1 |
| Medications at baseline | |||
| Antiplatelet, n (%) | 7 (31.82) | 2 (28.57) | 1 |
| Statins, n (%) | 6 (27.27) | 2 (28.57) | 1 |
| Onset to baseline MR-VWI scan, days, mean (range) | 8 (1–44) | 7 (1–35) | 0.369 |
| Interval between baseline and follow-up scan, months, mean (range) | 9 (3–15) | 6 (3–10) | 0.054 |
| Probable stroke mechanisms at baseline, n (%) | |||
| Parent artery atherosclerosis occluding penetrating artery | 2 (9.09) | 0 (0.00) | 0.408 |
| Artery-to-artery embolism | 4 (18.18) | 2 (28.57) | 0.554 |
| Hypoperfusion | 6 (27.27) | 2 (28.57) | 0.947 |
| Mixed mechanisms | 10 (45.45) | 3 (42.86) | 0.904 |
MR-VWI: magnetic resonance vessel wall imaging.
Among the 29 participants, all interrogated plaque features exhibited changes during the course of medical therapy. Absolute percentage changes were the largest in maximum wall thickness (22.86% ± 18.62%) and the least in pNWI (5.60% ± 6.20%). In addition, plaque volume (14.24% ± 11.69%), plaque-wall CR (15.36% ± 10.69%), and plaque ER (19.35% ± 11.73%) showed higher absolute percentage changes than the degree of stenosis (10.06% ± 10.30%).
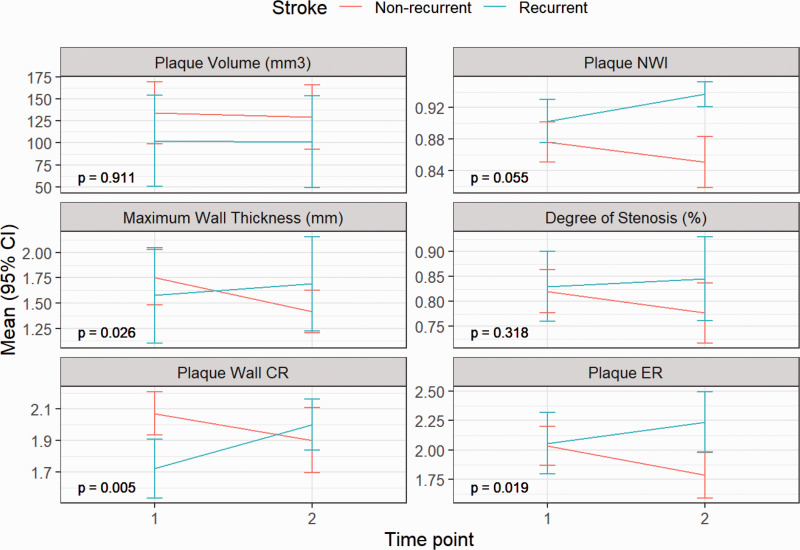
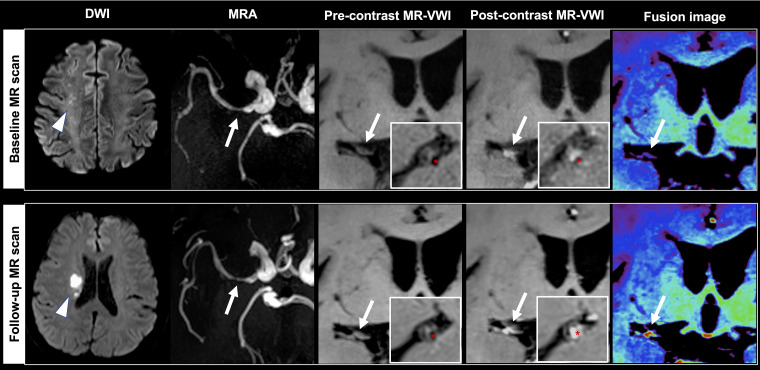
No significant differences were found in plaque features in the baseline scan between the two groups (Table 2). In the non-recurrent group, all features decreased at follow-up scans. However, in the recurrent group, most plaque features showed varying degrees of increase over the treatment period (Figure 3). When comparing the two groups, the changes in maximum wall thickness (–13.32% vs. 8.93%, P = 0.026), plaque-wall CR (–8.21% vs. 17.19%, P = 0.005) and plaque ER (–11.03% vs. 9.75%, P = 0.019) showed significant differences (Table 2). Representative cases from the two groups are shown in Figure 4 and Figure 5, respectively.
Table 2.
Comparison of quantitative measurements between the non-recurrent (n = 22) and recurrent group (n = 7).
| Plaque features | Baseline scan, mean (SD) |
Follow-up scan, mean (SD) |
Changes (%), mean (SD) |
|||||
|---|---|---|---|---|---|---|---|---|
| Non-recurrent group | Recurrent group | P valuea | Non-recurrent group | Recurrent group | Non-recurrent group | Recurrent group | P valuea | |
| Plaque volume, mm3 | 134.27 (80.10) | 102.40 (69.49) | 0.799 | 129.11 (58.9) | 101.17 (70.51) | –1.57 (25.01) | –0.45 (12.71) | 0.911 |
| pNWI | 0.87 (0.06) | 0.90 (0.04) | 0.616 | 0.85 (0.08) | 0.94 (0.02) | –2.69 (8.52) | 4.01 (6.19) | 0.055 |
| Maximum wall thickness, mm | 1.75 (0.66) | 1.58 (0.64) | 0.616 | 1.42 (0.51) | 1.69 (0.63) | –13.32 (29.93) | 8.93 (16.14) | 0.026 |
| Degree of stenosis, % | 81.99 (10.34) | 82.94 (9.50) | 0.884 | 77.67 (14.47) | 84.58 (11.26) | –4.98 (15.28) | 1.94 (8.08) | 0.318 |
| Plaque-wall CR | 2.07 (0.33) | 1.72 (0.25) | 0.093 | 1.90 (0.49) | 2.00 (0.22) | –8.21 (16.51) | 17.19 (11.42) | 0.005 |
| Plaque ER | 2.03 (0.40) | 2.06 (0.35) | 0.884 | 1.78 (0.46) | 2.24 (0.35) | –11.03 (22.26) | 9.75 (12.61) | 0.019 |
SD: standard deviation; pNWI: peak normalized wall index; CR: contrast ratio; ER: enhancement ratio.
P value adjusted using the Benjamini and Hochberg correction.
Figure 3.
Quantitative variables are expressed as means ± 1.96 standard error. All plaque features showed a trend towards improvement in the non-recurrent group (n=22) while in the recurrent group (n = 7), most plaque features showed varying degrees of increase over the treatment period. The changes in maximum wall thickness (P = 0.026), plaque-wall contrast ratio (CR) (P = 0.005) and plaque enhancement ratio (ER) (P = 0.019) showed significant differences between the two groups. P values were adjusted for multiple comparisons using the Benjamin and Hochberg correction for false discovery rate. pNWI: peak normalized wall index.
Figure 4.
A 32-year-old female non-recurrent patient received baseline magnetic resonance (MR) 9 days after stroke (arrowhead) and follow-up MR at 9 months. The causative plaque (arrows) at the left middle cerebral artery positively responded to medical therapy with smaller volume (−20.70%), maximum wall thickness (−43.18%), lower plaque-wall contrast ratio (CR) (−16.83%) and plaque enhancement ratio (ER) (−9.61%).
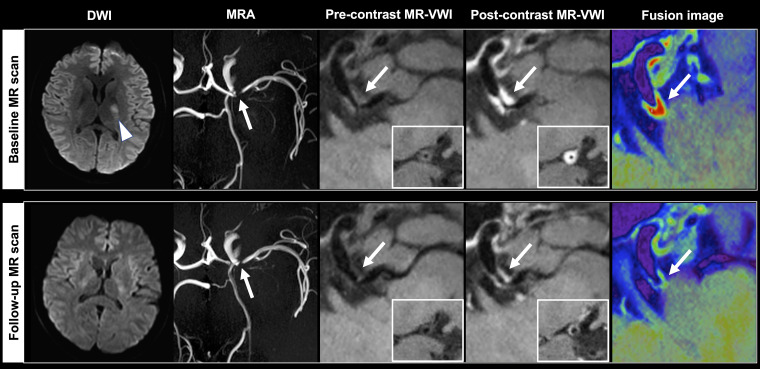
Figure 5.
A 61-year-old male patient received baseline magnetic resonance imaging (MRI) 4 days after stroke and follow-up MRI one day after recurrent stroke (arrowheads) at 10 months. The causative plaque (arrows) at the right middle cerebral artery deteriorated with increases in plaque volume (16.18%), peak normalized wall index (pNWI) (6.82%), plaque-wall contrast ratio (CR) (23.78%) and plaque enhancement ratio (ER) (26.65%). Asterisks indicate the lumen.
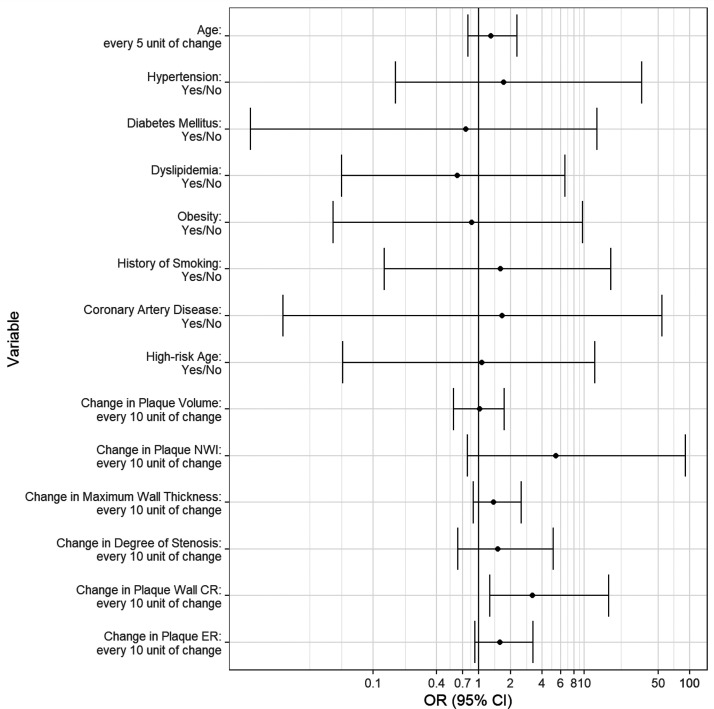
Univariable logistic regression showed that a 10% increase in the plaque-wall CR led to an odds ratio of 3.22 for stroke recurrence (95% CI 1.55–9.98, P = 0.003). Although statistical significance was not achieved, 10% increases in pNWI and plaque ER led to an odds ratio of 5.38 (95% CI 1.19–38.92, P = 0.053) and 1.59 (95% CI 1.05–2.65, P = 0.053), respectively, for stroke recurrence (Figure 6).
Figure 6.
Odds ratio (OR) and 95% confidence interval (CI) of recurrent group versus non-recurrent group on the basis of univariable logistic regression for each feature. A 10% increase in the plaque-wall contrast ratio (CR) led to an OR of 3.22 for stroke recurrence (95% CI 1.55–9.98, P = 0.003). Although statistical significance was not achieved, 10% increases in peak normalized wall index (pNWI) and plaque enhancement ratio (ER) led to an OR of 5.38 (95% CI 1.19–38.92, P = 0.053) and 1.59 (95% CI 1.05–2.65, P = 0.053), respectively, for stroke recurrence.
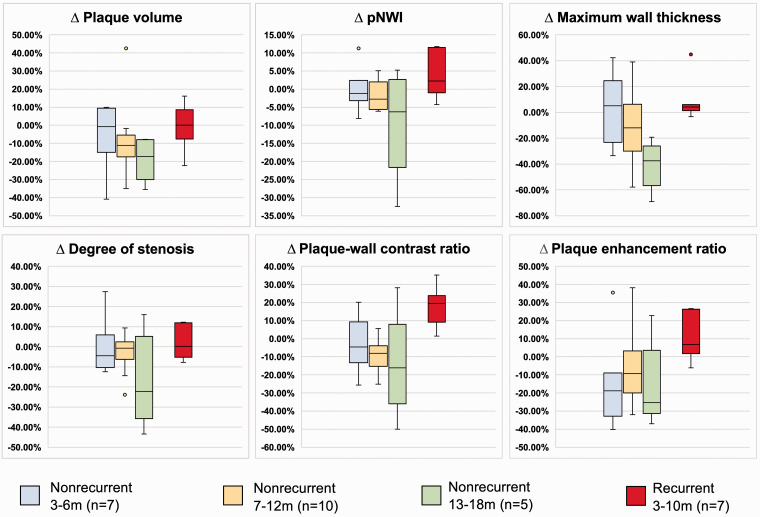
Patients in the non-recurrent group were further divided into three subgroups according to the time interval between two MR-VWI examinations: 3–6 months (n = 7), 7–12 months (n = 10), and 13–18 months (n = 5). Percentage changes in plaque features among the non-recurrent subgroups and the recurrent group are illustrated in Figure 7. After 6 months, opposite trends between the two groups were observed in all plaque features.
Figure 7.
Comparison of percentage changes in the non-recurrent subgroups and recurrent group. Data are presented as median and interquartile range.
Discussion
In this study, using 3D MR-VWI, we quantified morphological changes of plaque features in stroke patients who were taking preventive therapy. Six plaque morphological features were analyzed to characterize causative plaques in terms of luminal stenotic severity (degree of stenosis), plaque burden (plaque volume, pNWI, and maximum wall thickness), intraplaque hemorrhage (plaque-wall CR), and inflammatory status (plaque ER). Most of them have been associated with acute cerebrovascular events according to previous studies.16,17,35 We identified a trend towards improvement in all features in patients without recurrent vascular events during 18 months of clinical follow-up, but a trend towards worsening in most of the features in patients with recurrence. Our study showed that the increases in plaque-wall CR were related to stroke recurrence with statistical significance, and increases in plaque ER and pNWI were related to stroke recurrence at the margin of statistical significance. It suggests that quantitative assessment at the level of atherosclerotic plaques in stroke patients who were taking preventive therapy is clinically feasible with 3D MR-VWI, and the derived feature changes may provide important information predictive of long-term treatment outcomes.
ICAD is a dynamic disease. 36 Individual patients, despite their similar plaque features at the commencement of treatment, may demonstrate disparate responses due to differential pharmacodynamics and treatment compliance. 37 Hence, the morphological changes of plaque features are likely to be more indicative than a baseline assessment. This is corroborated by a previous transcranial Doppler study in which the risk of recurrent stroke was associated with the serial change of stenosis grading instead of the initial or 6-month grading alone. 38 In the baseline scan, there were no significant differences in plaque features between the two groups, which indicates a potential difficulty in differentiating the two groups based on baseline MR-VWI alone. A similar result was obtained in previous MR-VWI studies in which baseline plaque features did not predict recurrent stroke in ICAD patients.29,39 However, following a period of medical therapy, the non-recurrent and recurrent groups showed disparate trends in each of the plaque features, suggesting that 3D MR-VWI may provide quantitative, dynamic information that could be highly sensitive to plaque progression or regression and thus may be more predictive of long-term outcomes.
Our results support the necessity of assessing vessel wall-related features in addition to the degree of stenosis. Previous longitudinal studies focused on the severity of luminal stenosis only.38,40–42 However, two previous transcranial Doppler studies had conflicting results as to whether stroke recurrence is associated with the change of stenosis grading.43,44 Regarding vessel wall-related plaque features, a recent qualitative two-dimensional (2D) MR-VWI study reported that change in plaque enhancement grade was an independent factor for stroke recurrence. 29 The authors also found that change in stenosis grade was significantly correlated with stroke recurrence. In our study, several plaque features demonstrated larger absolute changes than the degree of stenosis. In the comparison between the two groups, changes of causative lesions exhibited significant differences in maximum wall thickness, plaque-wall CR and plaque ER but not in the degree of stenosis. Our results showed that only the increase in plaque-wall CR was related to stroke recurrence with statistical significance. Therefore, vessel wall-related plaque features and luminal stenosis are likely to be complementary to each other in the detection of plaque responses to preventive therapy that may manifest themselves in stenotic severity and/or plaque burden, neovascularity, and inflammatory status.
Early identification of patients who are not responsive to a prescribed medical therapy would be highly useful for therapeutic decision making. Physicians typically follow up patients within 1–3 months after the initiation of medical therapy but often face the question as to whether intensive medical management should be extended or an alternative therapy be initiated thereafter.2,45 In our study, although the sample size is not enough to reach a solid conclusion, we observed that beyond the 6-month window, all features showed distinct reductions in the non-recurrent group. These quantitative and lesion-specific markers may be complementary to systemic serum biomarkers for the evaluation of stroke patients taking preventive therapy. As expected, a best imaging follow-up window needs to be determined for each specific medical management strategy and with a well-controlled clinical study.
It is beyond the scope of this study to identify an imaging biomarker to predict the prognosis of stroke. The study was intended to demonstrate the quantifiable plaque-specific changes with MR-VWI. These changes could be the results of several factors such as medication efficacy, patient pharmacodynamics, and compliance. Hence, our results may not be directly generalized to any specific medical therapy, and any trial on the efficacy of a therapeutic strategy has to be performed with complete knowledge of patient compliance.
This study has some limitations. First, the limited sample size and the uneven distribution of non-recurrent and recurrent patients caused by the rate of stroke recurrence constrain our capacity to take other confounding covariates into account and determine any independent association of each plaque feature with prognosis. Second, due to the nature of its retrospective design, the heterogeneity in imaging time intervals between the two MR-VWI scans may contribute to a relatively large variance in quantification. Some of the follow-up examinations were performed after symptom recurrence. The vessel wall contrast enhancement we observed might be transient and related to local inflammatory responses to the recurrent event. A prospective, controlled large-scale study is needed to define the role of serial MR-VWI in monitoring medical treatment response.
Conclusions
Morphological changes of plaque features on 3D whole-brain MR-VWI demonstrated disparate trends in symptomatic ICAD patients with and without stroke recurrence in 18 months. This may be useful for the prediction of clinical outcome and decision making for medical management.
Acknowledgements
Not applicable.
Footnotes
Conflict of interest: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: SSS, MMM and ZF received salary support from the National Institutes of Health (grant NIH/NHLBI R01HL147355).
ORCID iD: Jiayu Xiao https://orcid.org/0000-0001-7478-6534
References
- 1.Holmstedt CA, Turan TN, Chimowitz MI. Atherosclerotic intracranial arterial stenosis: risk factors, diagnosis, and treatment. Lancet Neurol 2013; 12: 1106–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Derdeyn CP, Chimowitz MI, Lynn MJ, et al. Aggressive medical treatment with or without stenting in high-risk patients with intracranial artery stenosis (SAMMPRIS): the final results of a randomised trial. Lancet 2014; 383: 333–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leng X, Wong KS, Liebeskind DS. Evaluating intracranial atherosclerosis rather than intracranial stenosis. Stroke 2014; 45: 645–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kasner SE, Chimowitz MI, Lynn MJ, et al. Predictors of ischemic stroke in the territory of a symptomatic intracranial arterial stenosis. Circulation 2006; 113: 555–563. [DOI] [PubMed] [Google Scholar]
- 5.Bodle JD, Feldmann E, Swartz RH, et al. High-resolution magnetic resonance imaging: an emerging tool for evaluating intracranial arterial disease. Stroke 2013; 44: 287–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mandell DM, Mossa-Basha M, Qiao Y, et al. Intracranial vessel wall MRI: principles and expert consensus recommendations of the American society of neuroradiology. AJNR Am J Neuroradiol 2017; 38: 218–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McNally JS, Havenon A, Kim SE, et al. Rabbit models of intracranial atherosclerotic disease for pathological validation of vessel wall MRI. Neuroradiol J 2020; 34: 193--199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Song JW, Pavlou A, Xiao J, et al. Vessel wall magnetic resonance imaging biomarkers of symptomatic intracranial atherosclerosis: a meta-analysis. Stroke 2021; 52: 193–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Song JW, Moon BF, Burke MP, et al. MR intracranial vessel wall Imaging: a systematic review. J Neuroimaging 2020; 30: 428–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu WH, Li ML, Gao S, et al. In vivo high-resolution MR imaging of symptomatic and asymptomatic middle cerebral artery atherosclerotic stenosis. Atherosclerosis 2010; 212: 507–511. [DOI] [PubMed] [Google Scholar]
- 11.Teng Z, Peng W, Zhan Q, et al. An assessment on the incremental value of high-resolution magnetic resonance imaging to identify culprit plaques in atherosclerotic disease of the middle cerebral artery. Eur Radiol 2016; 26: 2206–2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu WH, Li ML, Gao S, et al. Middle cerebral artery intraplaque hemorrhage: prevalence and clinical relevance. Ann Neurol 2012; 71: 195–198. [DOI] [PubMed] [Google Scholar]
- 13.Zhu C, Tian X, Degnan AJ, et al. Clinical significance of intraplaque hemorrhage in low- and high-grade basilar artery stenosis on high-resolution MRI. AJNR Am J Neuroradiol 2018; 39: 1286–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yu JH, Kwak HS, Chung GH, et al. Association of intraplaque hemorrhage and acute infarction in patients with basilar artery plaque. Stroke 2015; 46: 2768–2772. [DOI] [PubMed] [Google Scholar]
- 15.Wu F, Song H, Ma Q, et al. Hyperintense plaque on intracranial vessel wall magnetic resonance imaging as a predictor of artery-to-artery embolic infarction. Stroke 2018; 49: 905–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu F, Ma Q, Song H, et al. Differential features of culprit intracranial atherosclerotic lesions: a whole-brain vessel wall imaging study in patients with acute ischemic stroke. J Am Heart Assoc 2018; 7: e009705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Qiao Y, Zeiler SR, Mirbagheri S, et al. Intracranial plaque enhancement in patients with cerebrovascular events on high-spatial-resolution MR images. Radiology 2014; 271: 534–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang M, Wu F, Yang Y, et al. Quantitative assessment of symptomatic intracranial atherosclerosis and lenticulostriate arteries in recent stroke patients using whole-brain high-resolution cardiovascular magnetic resonance imaging. J Cardiovasc Magn Reson 2018; 20: 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Skarpathiotakis M, Mandell DM, Swartz RH, et al. Intracranial atherosclerotic plaque enhancement in patients with ischemic stroke. Am J Neuroradiol 2013; 34: 299–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Qiao Y, Steinman DA, Qin Q, et al. Intracranial arterial wall imaging using three-dimensional high isotropic resolution black blood MRI at 3.0 Tesla. J Magn Reson Imaging 2011; 34: 22–30. [DOI] [PubMed] [Google Scholar]
- 21.Zhang L, Zhang N, Wu J, et al. High resolution three dimensional intracranial arterial wall imaging at 3 T using T1 weighted SPACE. Magn Reson Imaging 2015; 33: 1026–1034. [DOI] [PubMed] [Google Scholar]
- 22.van der Kolk AG, Zwanenburg JJ, Brundel M, et al. Intracranial vessel wall imaging at 7.0-T MRI. Stroke 2011; 42: 2478–2484. [DOI] [PubMed] [Google Scholar]
- 23.Qiao Y, Guallar E, Suri FK, et al. MR imaging measures of intracranial atherosclerosis in a population-based study. Radiology 2016; 280: 860–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fan Z, Yang Q, Deng Z, et al. Whole-brain intracranial vessel wall imaging at 3 Tesla using cerebrospinal fluid-attenuated T1-weighted 3D turbo spin echo. Magn Reson Med 2017; 77: 1142–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang Q, Deng Z, Bi X, et al. Whole-brain vessel wall MRI: a parameter tune-up solution to improve the scan efficiency of three-dimensional variable flip-angle turbo spin-echo. J Magn Reson Imaging 2017; 46: 751–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang N, Zhang F, Deng Z, et al. 3D Whole-brain vessel wall cardiovascular magnetic resonance imaging: a study on the reliability in the quantification of intracranial vessel dimensions. J Cardiovasc Magn Reson 2018; 20: 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang N, Liu X, Xiao J, et al. Plaque morphologic quantification reliability of 3D whole-brain vessel wall imaging in patients with intracranial atherosclerotic disease: a comparison with conventional 3D targeted vessel wall imaging. J Magn Reson Imaging 2021; 54: 166--174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xu WH, Li ML, Gao S. Intracranial plaque regression after intensive medical treatments: a high-resolution MRI observation. Ann Transl Med 2014; 2: 82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang X, Chen L, Li S, et al. Enhancement characteristics of middle cerebral arterial atherosclerotic plaques over time and their correlation with stroke recurrence. J Magn Reson Imaging 2021; 53: 953--962. [DOI] [PubMed] [Google Scholar]
- 30.Fan Z, Yang Q, Guo X, et al. Quantitatively monitoring regression or progression in intracranial atherosclerotic plaques using 3D vessel wall imaging. In: Society for Magnetic Resonance Angiography. 2017 Annual Meeting, Stellenbosch, South Africa, 4--6 October 2017.
- 31.Feng X, Chan KL, Lan L, et al. Stroke mechanisms in symptomatic intracranial atherosclerotic disease: classification and clinical implications. Stroke 2019; 50: 2692–2699. [DOI] [PubMed] [Google Scholar]
- 32.Shi F, Yang Q, Guo X, et al. Vessel wall segmentation using convolutional neural networks. IEEE Trans Biomed Eng 2019; 66: 2840–2847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chung JW, Hwang J, Lee MJ, et al. Previous statin use and high-resolution magnetic resonance imaging characteristics of intracranial atherosclerotic plaque: the intensive statin treatment in acute ischemic stroke patients with intracranial atherosclerosis study. Stroke 2016; 47: 1789–1796. [DOI] [PubMed] [Google Scholar]
- 34.Morelli JN, Gerdes CM, Schmitt P, et al. Technical considerations in MR angiography: an image-based guide. J Magn Reson Imaging 2013; 37: 1326–1341. [DOI] [PubMed] [Google Scholar]
- 35.Larson AS, Brinjikji W, Savastano L, et al. Left-sided carotid arteries have a higher prevalence of intraplaque hemorrhage than right-sided: an asymmetric conundrum. Neuroradiol J 2020; 33: 494–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Famakin BM, Chimowitz MI, Lynn MJ, et al. Causes and severity of ischemic stroke in patients with symptomatic intracranial arterial stenosis. Stroke 2009; 40: 1999–2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rocca B, Petrucci G. Variability in the responsiveness to low-dose aspirin: pharmacological and disease-related mechanisms. Thrombosis 2012; 2012: 376721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wong K, Li H, Lam W, et al. Progression of middle cerebral artery occlusive disease and its relationship with further vascular events after stroke. Stroke 2002; 33: 532–536. [DOI] [PubMed] [Google Scholar]
- 39.Lyu J, Ma N, Tian C, et al. Perfusion and plaque evaluation to predict recurrent stroke in symptomatic middle cerebral artery stenosis. Stroke Vasc Neurol 2019; 4: 129–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kwon SU, Cho Y-J, Koo J-S, et al. Cilostazol prevents the progression of the symptomatic intracranial arterial stenosis the multicenter double-blind placebo-controlled trial of cilostazol in symptomatic intracranial arterial stenosis. Stroke 2005; 36: 782–786. [DOI] [PubMed] [Google Scholar]
- 41.Ryu WS, Park SS, Kim YS, et al. Long-term natural history of intracranial arterial stenosis: an MRA follow-up study. Cerebrovasc Dis 2014; 38: 290–296. [DOI] [PubMed] [Google Scholar]
- 42.Leung TW, Wang L, Soo YO, et al. Evolution of intracranial atherosclerotic disease under modern medical therapy. Ann Neurol 2015; 77: 478–486. [DOI] [PubMed] [Google Scholar]
- 43.Arenillas JF, Molina CA, Montaner J, et al. Progression and clinical recurrence of symptomatic middle cerebral artery stenosis: a long-term follow-up transcranial Doppler ultrasound study. Stroke 2001; 32: 2898–2904. [DOI] [PubMed] [Google Scholar]
- 44.Caliandro P, Reale G, Demchuk AM, et al. Symptomatic intracranial atherosclerotic disease: an ultrasound 2-year follow-up pilot study. Neurol Sci 2018; 39: 1955–1959. [DOI] [PubMed] [Google Scholar]
- 45.Johnston SC, Easton JD, Farrant M, et al. Clopidogrel and aspirin in acute ischemic stroke and high-risk TIA. N Engl J Med 2018; 379: 215–225. [DOI] [PMC free article] [PubMed] [Google Scholar]