Abstract
The crystallization of calcium carbonate is shown to be dictated by the Ostwald rule of stages (ORS), for high relative initial supersaturations (), under sweet (carbon dioxide saturated) and anoxic (oxygen depleted) solution conditions. Rhombohedral calcite crystals emerge after the sequential crystallization and dissolution of the metastable polymorphs: vaterite (snowflake-shaped) and aragonite (needle-shaped). However, the presence of certain cations, which can form trigonal carbonates (e.g. Fe2+ and Ni2+), in concentrations as low as 1.5 mM, triggers the emergence of calcite crystals, with a star-shaped crystal habit, first. These star-shaped crystals dissolve to yield needle-shaped aragonite crystals, which in turn dissolve to give the rhombohedral calcite crystals. The star-shaped crystals, formed at high SCaCO3, possess higher surface free energy (therefore higher apparent solubility) than their rhombohedral counterparts. This sequence of dissolution and recrystallization demonstrates that the ORS does not only drive the crystal towards its thermodynamically most stable polymorph but also towards its most stable crystal habit.
Keywords: calcium carbonate, crystal habit, Ostwald rule of stages, polymorphism, surface energy
1. Introduction
In 1897 [1,2], the Nobel Laureate Wilhelm Ostwald wrote in relation to the crystallization of salts:
When leaving a given state and in transforming to another state, the state which is sought out is not the thermodynamically stable one, but the state nearest in stability to the original state.
This statement, the backbone of the Ostwald rule of stages (ORS), describes the polymorphic transformations occurring during crystallization at high supersaturations (). It has been widely reported that the formation of the most thermodynamically stable polymorph is the outcome of a sequential process, where the pathway is comprised of crystallization and dissolution of metastable polymorphs [3–7]. All polymorphic forms should have the same nominal solubility; equal to the solubility of the most stable polymorph. Metastable, i.e. less thermodynamically stable, polymorphs can demonstrate a solubility higher than their nominal one. As such, apparent solubility is defined to describe this phenomenon: apparent solubility is the maximum solubility that can be observed for a certain polymorph, under discrete conditions. The existence of apparent solubility is a consequence of the lower energetic penalty required for the solvation of crystalline molecules at the solid–liquid interface for some metastable polymorphs as compared to counterparts. Therefore, it can be suggested that the discrepancy between the apparent solubilities of either two metastable polymorphs, or a metastable polymorph and the corresponding thermodynamically stable polymorph [8], is driving the transformations described by the ORS.
One could then query how the apparent solubility of two different crystals of the same polymorph, where one has the most stable crystal habit (i.e. a crystal habit which minimizes its surface free energy), would also have distinct apparent solubilities. If the hypothesis is extended, the crystal with the most stable habit should exhibit a lower apparent solubility as compared to crystals formed with less stable habits. This occurs because the crystals contain different facets, which will each contribute to the solvation energies for the crystal. This is akin to the different surface energy contributions from individual facets to the crystal as a whole [9,10]. Thus, a deviation from the most stable crystal habit will result in crystals with a metastable crystal habit, where the total solvation energy requirements will be higher. In a recent paper, the authors demonstrate this possibility in the context of two different crystal habits of calcite: the established rhombohedral one and a star-shaped one. Using a combination of thermogravimetric analysis, surface energy measurements and thermodynamic calculations, they demonstrated that the particles of a stable polymorph, with a morphology of sufficiently high surface energy, may be, in fact, metastable with respect to particles of a presumably less stable polymorph, if the latter have obtained their most stable crystal habit [11].
Herein, this phenomenon and experimental evidence establishing this relationship between crystal habit and the ORS are further elucidated. This is achieved using a model system: calcium carbonate (CaCO3), under anoxic (oxygen depleted) and sweet (carbon dioxide saturated) solution conditions, with cationic additives. CaCO3 has three polymorphs [12]: calcite, the thermodynamically most stable polymorph with a trigonal crystal structure; aragonite, the second most stable polymorph with an orthorhombic crystal structure [13]; and vaterite, the least stable polymorph with a hexagonal crystal structure [14]. Moreover, with the use of cationic additives, it is possible to achieve a range of crystal habits within individual polymorphs. In this context, the ‘prematureearly’ nucleation of calcite, in the presence of certain additves, is demonstrated as the trigger for a cascade of transformations, commencing with a star-shaped calcite crystal, moving to aragonite and subsequently to rhombohedral calcite.
Calcium carbonate is widespread, from geological formations to living organisms [15]. It is also of tremendous industrial importance, contributing to scale formation in process equipment [16,17], especially those operating under sweet conditions (i.e. in the presence of carbon dioxide saturated flows). Industrially, it is not uncommon for the nucleation and growth of calcium carbonate to take place in the presence of different cationic species. Understanding the mechanisms by which these cations influence the crystallization of CaCO3 is crucial for the development of scale mitigation strategies.
The role of cationic additives in the crystallization of CaCO3 has been a subject of intense investigation [18,–20]. The majority of these studies focus on low SCaCO3 conditions, most commonly aqueous solutions containing small concentrations of carbonate and bicarbonate anions. The role of ion adsorption in crystallization of CaCO3 has been probed, where influence in the nucleation step has been noted. In a calcite-forming system, when specific cations are introduced not only does aragonite emerge but also it presents itself as the stable alternative both to calcite and cocrystals (containing Ca2+ and the cation additive) [21,22]. Therefore, in the presence of such cations, the formation of aragonite will be favoured. Despite the plethora of investigations on the role of cationic additives in the formation of CaCO3, to our knowledge, there has been a rather small number of studies on the role of Fe2+ and Fe3+ ions, in particular. One such report highlighted that the cations adsorb on the surfaces of the seeds, thereby inhibiting crystal (CaCO3) growth [22]. Other published literature [23,24] has also reported the addition of Fe2+ and Fe3+ can serve to be an effective inhibitor of calcite nucleation. This behaviour has been attributed to a decrease in SCaCO3 in the presence of these cations, as compared to a neat solution.
Fewer studies are dedicated to the crystallization of CaCO3 at very high SCaCO3, in sweet and anoxic solution conditions. These conditions, while less popular in academic literature, are highly relevant both industrially and towards understanding natural systems.
Notably, CO2 saturated flows are commonly encountered in the oil and gas industry; deployment of CO2 capture and storage has further increased the prevalence of these conditions. Additionally, naturally occurring relevant systems include existing CO2 rich springs and prehistoric environments (during oceanic formation) where Earth's atmosphere was far richer in CO2. As such these CaCO3 formation conditions, CO2 rich and O2 lean aqueous solutions, have broad reaching implications in understanding of carbonates on early Earth [25].
Herein, an experimental investigation considers the crystallization of calcium carbonate, as a function of SCaCO3, in the absence of any additives, in deaerated, sweet solutions, at high supersaturations. Subsequently, the influence of 1.5 mM of Fe2+ in CaCO3 supersaturated solutions is considered. Industrially, this mimics a scenario where corrosion has released Fe2+ into the system. Not only is the impact notable, but the findings enable us to draw important conclusions on the role of additives under these conditions. At the same time, a mechanism enabling the isolation of a metastable star-shaped habit of calcite crystals is observed. The study was then expanded to investigate the influence of other cations: Ba2+, Fe3+, Li+, Ni2+ and Zn2+.
2. Experimental
All the experiments were conducted in round bottom flasks containing 250 ml of deionized water at 80°C, which were de-aerated for 3 h with a N2 stream, at 200 ml min−1 (1 bar). Then, CO2 was introduced at 200 ml min−1 (0.5 bar) for 12 h. The pH of the solution was adjusted to around 7.2 using 1.05 g of NaHCO3 (Sigma Aldrich). The required SCaCO3 was achieved with the addition of CaCl2·2H2O (Sigma Aldrich). The pH meters were conducted using the InLab Expert Pro (Mettler Toledo). The concentration of calcium chloride used at different experiments is shown in table 1, along with the concentrations of the different compounds, which have been used in order to investigate the effects of cationic additives. Purging with CO2 was continued throughout the experiment at 200 ml min−1; thus the solution remains oxygen free. At the conclusion of each experiment, the solution was filtered to separate solids greater than 3 µm. The dried solid products were assessed using X-ray powder diffraction (XRPD). Measurements reported in this paper were all conducted using an X'Pert PRO diffractometer using Cu-Kα radiation (Malvern PANalytical) and scanning electron microscope (SEM) images were taken using a Hitachi TM-100 table-top SEM (Hitachi Ltd). The XRPD measurements were conducted at a 2θ range from 5° to 80°, with a step size of 0.01° (2θ) and a count time of one second. A back-loaded sample holder was used. As the material was relatively free flowing and fine, no mechanical force was exerted on it, to assist in the packing. Thanks to the fine nature of the particles, the surface of the sample can be considered flat with respect to the sample holder. For the crystals produced at 5 min, in the presence of Fe2+ and Ni2+, the material was unloaded from the sample holder, mixed with the mother batch and then a new sample was taken for a second measurement. The same procedure was repeated once again. The three measurements were identical.
Table 1.
Summary of the experiments conducted in this paper.
| Experiment | CaCl2 (mg ml−1) | 1.5 mM additive |
|---|---|---|
| A1 | 2.00 (SCaCO3 ca 2500) [13,14] | — |
| A2 | 8.00 (SCaCO3 ca 10 000) [13,14] | — |
| A3 | 24.00 (SCaCO3 ca 30 000) [13,14] | — |
| B1 | 8.00 | 0.20 mg ml−1 of FeCl2 |
| B2 | 2.00 | 0.20 mg ml−1 of FeCl2 |
| B3 | 3.00 | 0.20 mg ml−1 of FeCl2 |
| B4 | 4.00 | 0.20 mg ml−1 of FeCl2 |
| B5 | 16.00 | 0.20 mg ml−1 of FeCl2 |
| B6 | 24.00 | 0.20 mg ml−1 of FeCl2 |
| B7 | 44.00 | 0.20 mg ml−1 of FeCl2 |
| C1 | 8.00 | 0.34 mg ml−1 of BaCl2 |
| C2 | 8.00 | 0.26 mg ml−1 of FeCl3 |
| C3 | 8.00 | 0.07 mg ml−1 of LiCl |
| C4 | 8.00 | 0.21 mg ml−1 of NiCl2 |
| C5 | 8.00 | 0.22 mg ml−1 of ZnCl2 |
3. Results
(a) . Crystallization of calcium carbonate at high SCaCO3
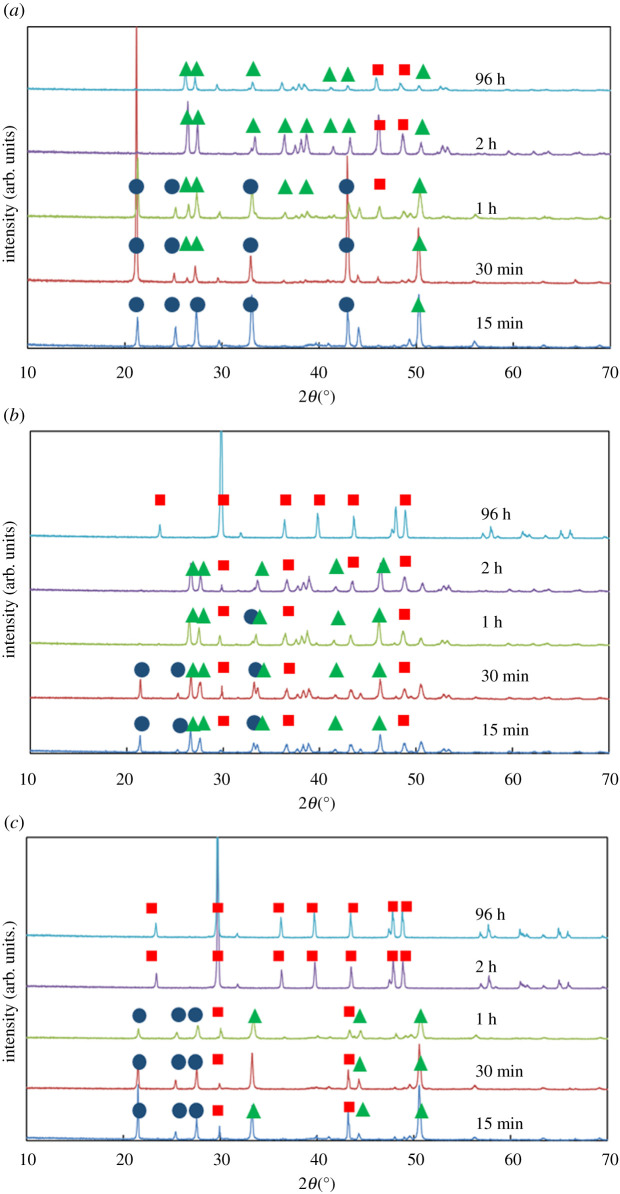
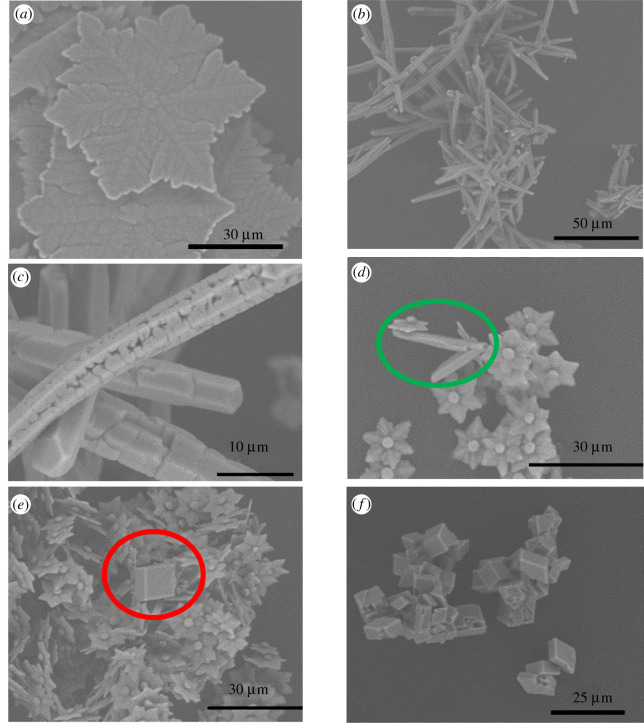
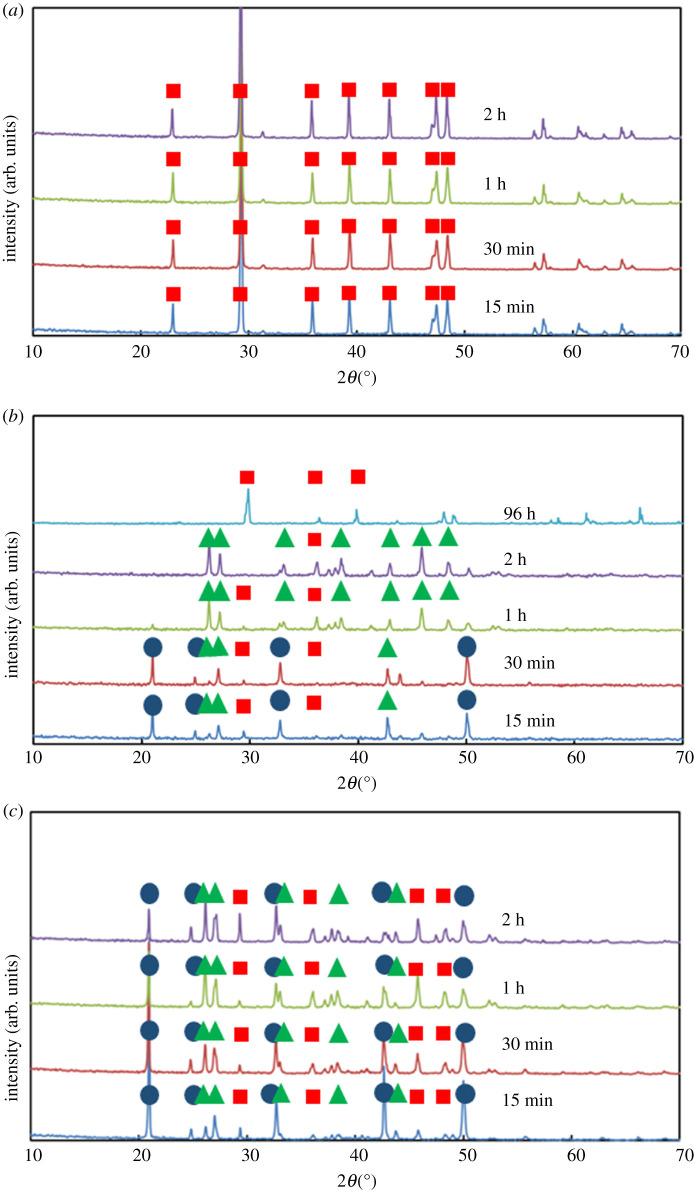
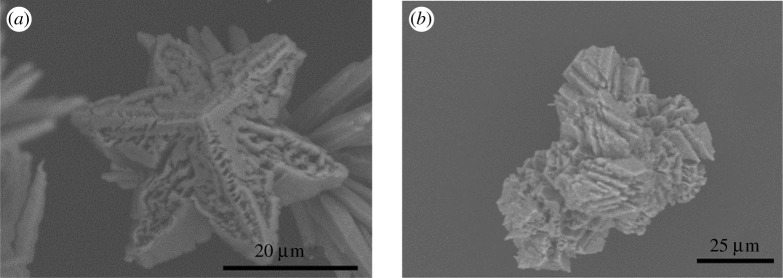
Three baseline experiments were conducted (table 1, A1–A3), using CaCl2·2H2O: 2 (SCaCO3 ca 2500), 8 (SCaCO3 ca 10 000) and 24 mg ml−1 (SCaCO3 ca 30 000) [14,15]. In all three cases, vaterite emerges first; it then gradually dissolves coincidentally with aragonite formation (figure 1). The presence of calcite is not observed under all conditions tested. At the lowest supersaturation considered, SCaCO3 ca 2500, vaterite crystals with a snowflake shape are apparent at 15 min (figure 2a). The dissolution of aragonite needles is still observed at 96 h (figure 2c).
Figure 1.
The XRPD patterns for the powder obtained from the crystallization of calcium carbonate at different durations (shown in the legend) at different concentrations of calcium chloride: (a) 2 mg ml−1, (b) 8 mg ml−1, (c) 24 mg ml−1. Blue circles indicate the characteristic peaks of vaterite, green triangles the characteristic peaks of aragonite and red squares the characteristic peaks of calcite. (Online version in colour.)
Figure 2.
SEM images obtained from crystallization experiments conducted at: (a) a calcium chloride concentration of 2 mg ml−1 for 15 min, (b,c) a calcium chloride concentration of 2 mg ml−1 for 96 h, (d,e) a calcium chloride concentration of 8 mg ml−1 after 1 h (the green circle indicates needle-shaped aragonite crystals and the red circle indicates a rhombohedral calcite crystal) and (f) a calcium chloride concentration of 8 mg ml−1 after 96 h. (Online version in colour.)
By contrast, upon increasing the SCaCO3 to ca 10 000 (corresponding to a calcium chloride concentration of 8 mg ml−1), needle-shaped aragonite crystals begin to appear in 1 h, alongside flower-shaped vaterite crystals (figure 2d); simultaneously, a rhombohedral calcite crystal is also observed, along with flower-shaped vaterite crystals as well (figure 2e). The appearance of calcite signals that the system has reached the solubility limit for CaCO3. A further increase in SCaCO3, to ca 30 000, demonstrates a completed polymorphic transformation to the stable calcite polymorph within 96 h (figure 2f), reaching the end of the ORS.
(b) . Crystallization of calcium carbonate in the presence of 1.5 mM of Fe2+
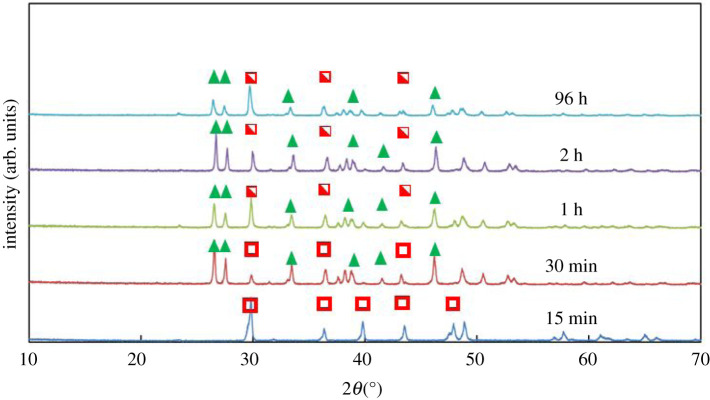
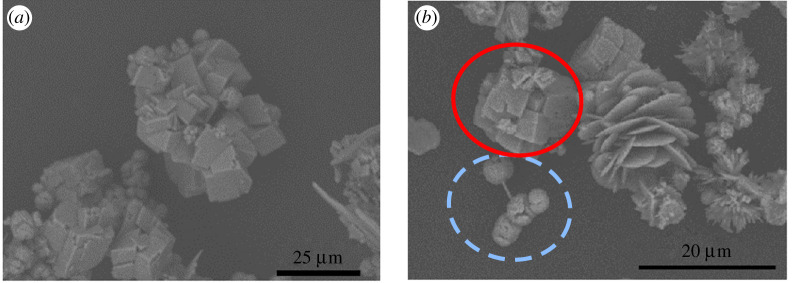
Comparable experiments were conducted with the addition of 1.5 mM Fe2+ (table 1, B1–B7). For 8 mg l−1 with 1.5 mM of Fe2+ (SCaCO3 ca 10 000 with no Fe2+) only calcite is identified at 15 min (figure 3), whereas the predominant polymorph at the same time without the addition of Fe2+ was vaterite. At this early stage, the calcite polymorph has a distinct star-shaped habit (figure 4a). The formation, i.e. ‘premature’, star-shaped crystals has been observed (figure 4b). During the growth of these star-shaped crystals, elucidated from SEM images and XRPD patterns (figure 3) for times less than 15 min, no evidence of any other crystal habits or polymorphs has been identified.
Figure 3.
XRPD patterns obtained from crystallization experiments at a calcium chloride concentration of 8 mg ml−1, in the presence of 1.5 mM of Fe2+, at different times. The green triangles indicate the characteristic peaks of aragonite, the open red squares demarcate the characteristic peaks of calcite for the cases where the SEM images suggest the presence of star-shaped calcite crystals and the half-filled red squares represent the peaks of calcite for the cases where SEM images suggest the coexistence of star-shaped and rhombohedral calcite crystals. (Online version in colour.)
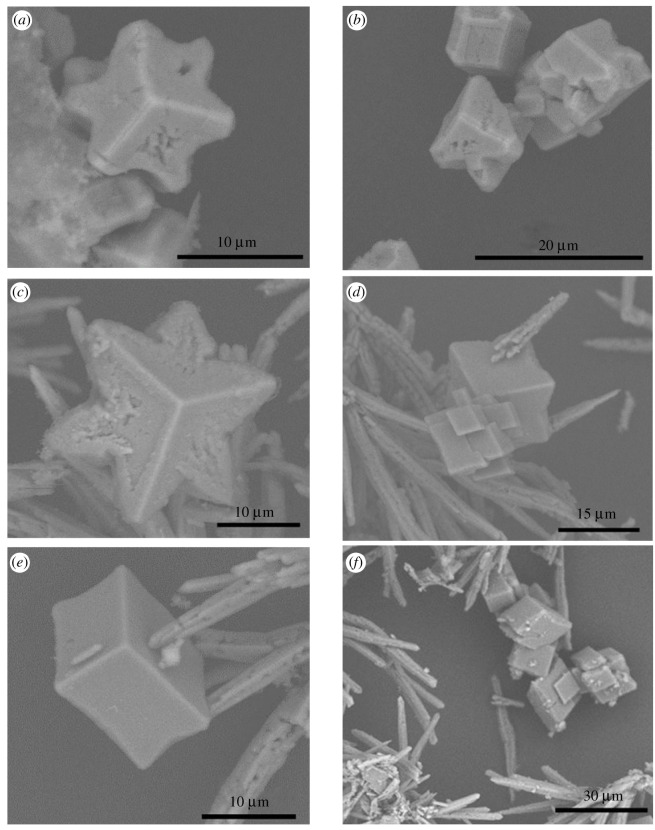
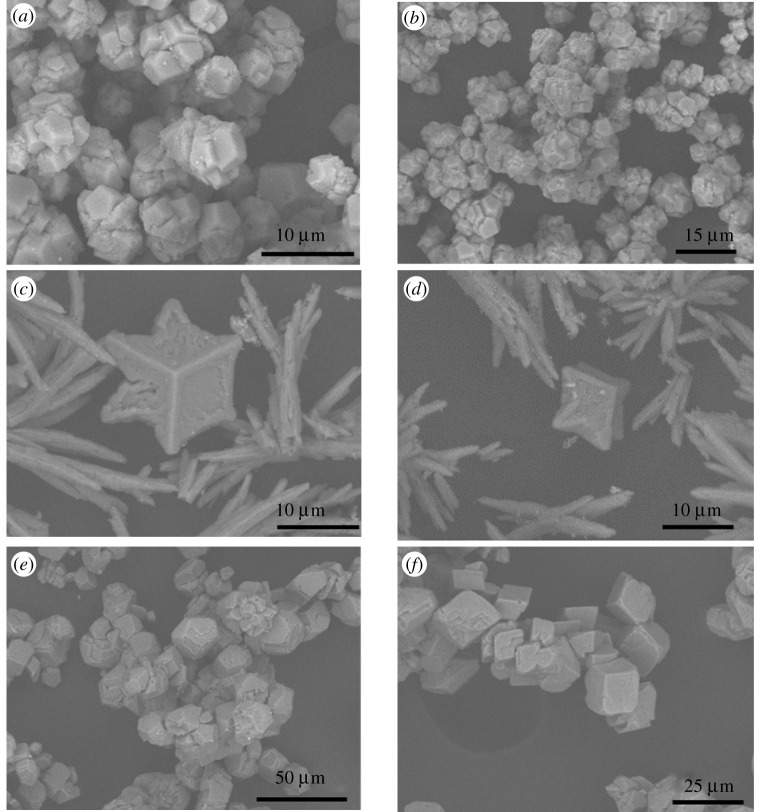
Figure 4.
SEM images of crystals obtained from crystallization experiments at (a,b) calcium chloride concentration of 8 mg ml−1, in the presence of 1.5 mM of Fe2+, after 15 min, (c) calcium chloride concentration of 8 mg ml−1, in the presence of 1.5 mM of Fe2+, after 1 h, and (d–f) calcium chloride concentration of 24 mg ml−1, in the presence of 1.5 mM of Fe2+, after 48 h. (Online version in colour.)
With increasing time, aragonite, with a needle-like crystal habit, emerges as the dominant polymorph. Qualitatively this is observed in SEM images, as well as semi-quantitatively in the XRPD patterns (figure 3). During the relevant time-window (30 min–2 h), an increasingly strong aragonite signal is observed (e.g. 2θ of 26.25° and 27.25°). During this period (notably at 1 h), the simultaneous apparent dissolution of the star-shaped calcite crystals commences (figure 4c).
To accelerate the progression of the ORS, an increased concentration of Ca2+, with 1.5 mM Fe2+, was studied. As can be seen in figure 4d, after 48 h, both star-shaped calcite crystals and rhombohedral calcite crystals coexist; the star-shaped calcite crystals are dissolving and the rhombohedral calcite crystals are growing. In some instances, rhombohedral crystals (figure 4d,e) appear to retain curved edges, inconsistent with the traditional formation of this calcite habit, suggesting that the transition from star-shaped to rhombohedral crystals does not necessarily require the complete dissolution of star-shaped crystals. Elsewhere (figure 4f), the calcite crystals seem to have the well-known rhombohedral shape. This thereby suggests that the star-shaped crystals dissolve either completely or partially. For the latter, the dissolution would yield multifaceted entities, which regrow into rhombohedral-shaped crystals. It must also be noted that no FeCO3, commonly known as siderite, was observed by either XRPD or SEM.
(c) . Crystallization of calcium carbonate in the presence of other cations
Experiments were performed, as described above, with 1.5 mM of other cation additives (table 1, C1–C5): Ba2+, Fe3+, Li+, Ni2+, Zn2+. The changes to CaCO3 supersaturation are assumed to be small, with the exception of Ba2+ [26]. In the presence of Ba2+, crystallization does not follow the ORS. Rhombohedral calcite emerges as the only polymorph after the very rapid onset of crystallization in less than 15 min (figure 5a).
Figure 5.
The XRPD patterns for the powder obtained from the crystallization of calcium carbonate at different durations (shown in the legend) at 8 mg ml−1 of calcium chloride in the presence of 1.5 mM of: (a) Ba2+, (b) Fe3+, (c) Li+. Blue circles indicate the characteristic peaks of vaterite, green triangles the characteristic peaks of aragonite and red squares the characteristic peaks of calcite. (Online version in colour.)
With the addition of either Fe3+ or Li+, the polymorphism evolves as was observed in the absence of any other cations: vaterite to aragonite, and then to rhombohedral calcite (figure 5b,c).
Neither BaCO3 nor LiCO3 (nor any other carbonate-containing products) are detected in the experiments when either Ba2+ or Li+ is present, respectively. However, a red precipitate, attributed to amorphous Fe(OH)3, was observed when FeCl3 was used. This side reaction reduces the concentration of Fe3+ in solution. Therefore, the change in chemical potential of the solution, caused by the addition of Fe3+, is small, and the SCaCO3 remains high.
The formation of an additional product is notable because it likely influences a more rapid progression through the ORS. As such, the polymorphic transformations are, therefore, faster in the presence of Fe3+ rather than in the presence of Li+. The progression of crystalline species is traced through XRPD patterns at different times (figure 5b,c). In contrast to the case with Li+ which has vaterite crystals after 2 h, in the presence of Fe3+, after 2 h, the transformation to calcite is complete.
It should be noted, however, that the polymorphic transformations in the presence of either Fe3+ or Li+ are significantly slower than the comparable cases in the absence of the cations (at the same concentrations of Ca2+).
The addition of Ni2+ is similar to that of Fe2+. That is, star-shaped calcite crystals (figure 6a) emerge first, followed by aragonite crystals. The metastability of these stars can be clearly evidenced in imaging showing them in the process of dissolution (figure 6b). The dissolution pattern of the star-shaped calcite crystals clearly demonstrates that the evolution is not directly to rhombohedral calcite.
Figure 6.
SEM images of crystals obtained from crystallization experiments with calcium chloride concentration of 8 mg ml−1, in the presence of 1.5 mM of Ni2+, after (a) 15 min and (b) 30 min.
In the presence of Zn2+, a breadth of species is observed. Early on, poorly specified (i.e. non-rhombohedral) calcite emerges as an aggregate of particles (figure 7a). Alongside calcite, cauliflower-shaped aragonite, vaterite and spherulitic particles of smithsonite (ZnCO3) (in the dotted circle in figure 7b) [27] are also observed. With longer crystallization times, rhombohedral calcite crystals are evident (figure 7b). Other changes in the crystal structures are also observed: vaterite crystals start as platelets and later assemble into flower-shaped aggregates (figure 7b). This crystal habit has been previously identified in the presence of highly charged amphiphilic molecules or ions [20]. Smithsonite also appeared on the corresponding XRPD pattern and spherulitic particles have been previously reported [28].
Figure 7.
SEM images of crystals obtained from crystallization experiments with calcium chloride concentration of 8 mg ml−1, in the presence of 1.5 mM of Zn2+, after (a) 15 min and (b) 1 h. The red circle indicates rhombohedral calcite crystals, while the dotted brown circle indicates spherulites of smithsonite (ZnCO3). (Online version in colour.)
4. Discussion
Nucleation kinetics have been used to explain the high supersaturation prerequisite required to trigger the ORS [29,30]. The magnitude of supersaturation required (to trigger the ORS) strongly depends on the nature of the compound.
Herein, very high supersaturations (SCaCO3 ≥ 2500) were, initially, explored through the exemplar case of calcium carbonate crystallization in the absence of any additives. Under these conditions, crystallization was observed to evolve through a series of polymorphic transformations. The most metastable polymorph (i.e. least stable), vaterite, emerged first as snowflake-shaped crystals. This unusual crystal habit has only been previously obtained through double diffusion experiments [31]. As the crystallization proceeds, the solution SCaCO3 decreases.
As SCaCO3 decreases, eventually [Ca2+]· [CO2−3] < Ksp'Aragonite (where Ksp'Aragonite stands for the solubility product of aragonite) resulting in the dissolution of vaterite crystals, as described by the ORS. Subsequently, needle-shaped aragonite crystals emerge. In the same motif, as the crystallization continues, the SCaCO3 decreases further. Eventually, [Ca2+]·[CO2−3] < Ksp'Calcite (where Ksp′Calcite stands for the solubility product of calcite). This leads, in turn, to the dissolution of aragonite crystals and the formation of rhombohedral calcite crystals—the most thermodynamically stable polymorph.
The rate of the ORS events can be controlled by modifying the initial SCaCO3. An increase in SCaCO3 increases the nucleation rate. This increase of the SCaCO3 increases the rate of the polymorphic transitions, which in turn allows for the faster appearance of the stable rhombohedral calcite crystals.
The implications of increasing supersaturation, i.e. speeding-up the appearance of stable rhombohedral calcite crystals, can be considered in the context of the relative magnitude of the SCaCO3. Firstly are the cases with very low SCaCO3 (which can be driven both by the low concentration of Ca2+ and CO2−3, but also from the presence of cationic additives). There, calcite is the sole polymorph that will emerge as the conditions for ORS will not be met and therefore the phenomenon will not be triggered. Secondly, there are the cases with intermediate magnitude of SCaCO3, where the conditions of ORS are met. Under these conditions, ORS does occur, but at a very slow rate. Therefore, stable rhombohedral calcite crystals will not be observable during a reasonable time frame, such as less than 48 h; although they should be observed with increased timescales. During an extended period of time, such as more than 48 h, aragonite crystals will be, initially, predominant with increasing presence of rhombohedral calcite crystals. Thirdly, for cases with elevated SCaCO3, the ORS phenomenon does occur very quickly. As the rate of transformations will be really fast, stable rhombohedral calcite crystals emerge relatively quickly. These scenaria are all supported by the previously presented experimental results in figure 1. In short, the highest and lowest SCaCO3 give stable rhombohedral calcite crystals, whereas the intermediate SCaCO3 produces primarily aragonite crystals.
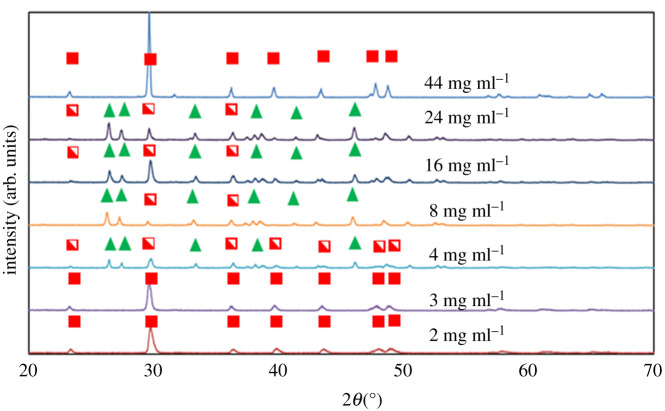
The three cases, of different relative values of SCaCO3, were further explored using a range of Ca2+ concentrations with the addition of 1.5 mM of Fe2+ (table 1, B1–B7). Experiments were conducted where Ni2+ was used instead of Fe2+ with very similar outcomes. For both the lowest (2 and 3 mg ml−1) and highest (44 mg ml−1) concentrations of CaCl2, rhombohedral calcite crystals predominated after 48 h (figure 8). By contrast, crystals obtained from solutions with intermediate concentrations (4–44 mg ml−1) were a mixture comprising needle-shaped aragonite crystals, star-shaped calcite crystals and rhombohedral calcite crystals (figure 8). Under the investigated conditions, the presence of Fe2+ does not seem to promote the formation of aragonite via inhibition of the nucleation and/or growth of calcite crystals.
Figure 8.
XRPD patterns obtained from crystallization experiments conducted using the concentrations of calcium chloride shown in the legend, in the presence of 1.5 mM of Fe2+, for 48 h. Green triangles stand for the characteristic peaks of aragonite. Filled red squares stand for the characteristic peaks of calcite, in cases where SEM images suggest the existence of rhombohedral calcite crystals only. The half-filled red squares represent the peaks of calcite for the cases where SEM images suggest the coexistence of star-shaped crystals with rhombohedral crystals. (Online version in colour.)
The observations strongly support the supersaturation-dependent triggering of the ORS. At the very low concentrations (2 and 3 mg ml−1), the ORS was not triggered and only rhombohedral calcite crystals are observed (figure 9a,b) with no relics of any other polymorph. At high supersaturations (44 mg ml−1), the polymorphic transformations have proceeded quickly towards stable rhombohedral calcite crystals (figure 9e,f). At intermediate supersaturations, both aragonite needles and star-shaped calcite crystals are observed at 48 h (figure 9c,d). To confirm the explanation for the failure to trigger ORS at low concentrations, additional experiments (calcium chloride ≤ 3 mg ml−1) were carried at 15, 30, 60 and 120 min. The results confirmed that no star-shaped calcite crystals emerge. Similarly, crystallization at high concentrations (calcium chloride at 44 mg ml−1), i.e. undergoing rapid polymorphic transformation, was similarly probed at short times (15 and 30 min). The emergence of star-shaped crystals (which eventually dissolve at the expense of rhombohedral calcite crystals) was confirmed. Another interesting observation has to do with the fact that Ba2+ seems to prevent completely the occurrence of ORS.
Figure 9.
SEM images of crystals obtained from crystallization experiments with: (a,b) 2 mg ml−1 of calcium chloride, in the presence of 1.5 mM of Fe2+, after 48 h, (c,d) 16 mg ml−1 of calcium chloride, in the presence of 1.5 mM of Fe2+, after 48 h and (e,f) 44 mg ml−1 of calcium chloride, in the presence of 1.5 mM of Fe2+, after 48 h.
In a recent paper, Barlow et al. elaborated, starting from a mathematical description of the transformations occurring during ORS, on the conditions required for the ORS to be observable [32]. Their findings suggest that for the ORS cascade of transformations to be observable, the rate constant of the first phase transition needs to be the dominant/fastest one. Applying the notions developed in that work in the current paper, some important conclusions can be deduced. At low SCaCO3, in the absence of any additives, the kinetics of vaterite and aragonite formation are way too slow. Similarly, the presence of Ba2+ seems to reduce SCaCO3 so much that again the kinetics for vaterite and aragonite are diminished.
The observation of star-shaped calcite crystals emerging first, at particular values of SCaCO3 in the presence of Fe2+ ions, and their subsequent dissolution, in favour of aragonite crystals, is a key outcome of this work. The dissolution of the star-shaped calcite crystals, at the expense of aragonite, is indicative that this crystal habit, of calcite, must have a higher apparent solubility than needle-shaped aragonite. This unexpected outcome is the result of a higher apparent solubility for the star-shaped calcite crystals, which is attributed to the higher surface energy of their crystal facets. The authors have already demonstrated this difference in surface energy, in a recent paper [33]. As such, the distinction between the two habits of calcite suggest that the star-shaped calcite crystals should be classified as metastable. This classification is with respect to not only the needle-shaped aragonite crystals, but also the rhombohedral calcite crystals. This observation complicates the supposition that under ORS, crystals are driven towards the thermodynamically stable polymorph. Previously, no regard for the crystal habit has been included. The evidence herein suggests that the ORS drives the crystyallization towards not only the most stable polymorph but also towards the most stable crystal habit.
The Fe2+ ions, in the presence of CO2-derived species, have the potential of forming ferrous carbonate, siderite. Notably, siderite exhibits the same crystal structure as calcite (and gaspeite, in the case of Ni2+, which also has a trigonal crystal structure like calcite). Previous crystallization studies, at much lower supersaturations, have suggested that the presence of such cations favours the formation of aragonite. The experiments conducted herein suggest that this behaviour is condition-specific, as these same results were not observed in this work. Rather, herein star-shaped calcite crystals emerge before aragonite. For the calcite crystals obtained in the presence of 1.5 mM of Fe2+ after 15 min of crystallization, no traces of aragonite have been detected by means of XRPD, SEM or Fourier transform infrared (FTIR) spectroscopy [11].
In previous studies, it has been suggested that the presence of Fe2+ ions (as well as of Fe3+), at low supersaturations, leads to the inhibition of the growth of calcite seeds. In those low supersatutations [22,23,34], the inhibition required only a few micromoles of Fe2+; the inhibitive properties are observed to be further enhanced in the presence of dissolved oxygen. Those works suggest that the presence of Fe3+ ions severely inhibits the growth of calcite, by means of adsorption on specific crystal facets. The findings of this paper show that this effect decreases with increasing SCaCO3 (provided that the concentration of Fe3+ remains constant).
Evidence presented in this work (including XRPD patterns from figure 1b and figure 5b, as well as SEM images) only identifies small differences in the crystals (polymorphism, crystal habit and size) produced using 8 mg ml−1 of CaCl2, in the presence and the absence of 1.5 mM of Fe3+ ions. This occurs because Fe3+ ions precipitate in solution, creating an amorphous hydroxide (Fe(OH)3). The Fe3+ ions need to be present in the solution in the form of free ions, in order to contribute to the kinetics of the ORS. The fact that a large number of Fe3+ ions are consumed in the formation of the hydroxide means that Fe3+ ions are not available to contribute to the ORS. By contrast, Li+ ions remain in solution; no precipitation of a lithium salt is observed. Therefore, they modify the chemical potential of the solution, changing the kinetics of the ORS. It was demonstrated that cations, only in their free form, decrease the SCaCO3 and slow the kinetics of the ORS. This behaviour is consistent with existing theory based on similar phenomena involving different cationic species. It should be noted that various factors, including the size and the charge of the cations, determine influence the chemical potential differently; an a priori prediction of chemical potential, for different arbitrary systems, is not yet possible. Therefore, extrapolation of the phenomena reported in the previous study to the conditions herein appears to be inapplicable.
Star-shaped calcite crystals emerge in the presence of Fe2+ and Ni2+ cations, both of which have the ability to form carbonates with the same crystal structure as calcite [33]. In both cases, the star-shaped crystals emerge before aragonite.
It can be argued that Fe2+ and Ni2+ inhibit the nucleation and/or growth of aragonite, early on. Thus, calcite emerges as the dominant polymorph and the star-shaped morphology can then be attributed to preferential adsorption of Fe2+ ions on specific facets. However, this explanation is not consistent with other reports from the literature which suggest that the presence of Fe2+ and Ni2+ ions suppresses the formation of calcite altogether, favouring aragonite formation instead. Furthermore, it fails to explain why the star-shaped calcite crystals dissolve and later rhombohedral calcite crystals grow, instead of growing to rhombohedral calcite crystals at the outset.
An alternative explanation is that the star-shaped crystals are, in fact, cocrystals/solid solutions of Ca2+ with either Fe2+ or Ni2+. The star-shaped cocrystals are metastable with respect to aragonite and as the crystallization occurs through the ORS they emerge first. This explanation is not fully satisfactory, as no experimental evidence (neither XRPD patterns nor FTIR spectra) have suggested the formation of such cocrystals. Furthermore, vapour pressure measurements [33] did not provide definitive evidence that the higher apparent solubility of the star-shaped calcite crystals is because of the formation of solid solutions/cocrystals. Besides, an added challenge is that no star-shaped calcite crystals or any other high apparent solubility calcite crystal habits are observed for other case studies (such as with Zn2+or Mg2+) where the literature has previously indicated a penchant for the formation of cocrystals in relevant Ca2+ solutions.
A final possible explanation is that the presence of either Fe2+ or Ni2+ cations may promote the nucleation of calcite, at the expense of aragonite, under some conditions. As most literature has focused on lower supersaturation solutions, the observation of alternative crystal habits is unique to these conditions. Moreover, the formation of the star-shaped calcite crystals occurs where the supersaturation in the bulk solution is very high. And consistent with the literature, the formation of rhombohedral calcite crystals has been observed when the supersaturation in the bulk solution was reduced, to levels more consistent with the literature. As such, a metastable crystal habit can emerge thanks to the high supersaturation.
Putting the findings from Fe2+ and Ni2+, suggesting the occurrence of star-shaped calcite crystals, in the scrutiny of the work by Barlow et al. there seem to be some interesting implications [31]. The presence of these additives could favour the kinetics of the formation of star-shaped calcite or cocrystal. This may be done by means of lowering the energetic barrier for formation of calcite or of the hypothetical cocrystal. Template-assisted nucleation is well known to provide access to certain polymorphs by similar means.
It has been noted, both in the Results section and briefly in the Discussion, that the lifecycle of the crystals observed in the experiments conducted herein shows a pattern of dissolution and formation [35]. That is, the emergence of a stable crystal habit is from a sequence of dissolution (complete or partial) and formation steps. This is in direct contrast to the alternative behaviour whereby changes in crystal habit, towards the metastable form, result from facet specific growth. This distinction verifies that the surface properties are a crucial descriptive factor in the determination of the identity of individual particles. Surface properties, stemming from the differences in crystal habit, should be considered when the dissolution properties are a matter of concern. Thus, it will be particularly interesting to re-examine, in the light of the findings of this work, the findings of experiments conducted to assess the dissolution performance and the stability of crystalline materials [36].
However, the most noteworthy finding of this work is the role of the ‘early’ nucleation in the occurrence star-shaped calcite crystals. This phenomenon triggers the cascade of transformations, described in detail in this paper, for the first time. This feature is controlled by the additives and it can be correlated with the ability of certain additives to form carbonates with the same crystal structure as calcite: trigonal.
In areas like the pharmaceutical industry, where particle morphology modifications can be used as a vehicle to improve the solubility of hydrophobic active pharmaceutical ingredients, this finding can allow the design and production of particles with exotic morphologies, debottlenecking development activities. This could be done in synergy with the already established field of nucleation control; especially considering more recent advances in the development of soft templates.
5. Conclusion
The crystallization of CaCO3 under sweet and anoxic conditions and elevated SCaCO3 obeys the ORS. The introduction of cations which can form carbonates with a trigonal crystal structure, such as Fe2+ and Ni2+, may facilitate the nucleation of calcite, the trigonal polymorph of calcium carbonate, prior to the emergence of aragonite.
Notably, the ‘early’ nucleation of calcite and the subsequent growth under high supersaturation lead to the formation of calcite crystals with high surface energy, star-shaped, crystal habits, which are newly observed in this work. This capability, to use additives for the generation of stable polymorphs with metastable crystal habits, could be harnessed for particle engineering operations.
Supplementary Material
Data accessibility
Raw data for the XRD patterns can be found in the electronic supplementary material [37].
Authors' contributions
E.H.: conceptualization, data curation, formal analysis, investigation, methodology, validation, writing—original draft, writing—review and editing; S.M.V.: conceptualization, funding acquisition, methodology, project administration, supervision, writing—review and editing; J.D.L.: validation, writing—review and editing; K.L.S.C.: funding acquisition, investigation, methodology, supervision, writing—original draft, writing—review and editing.
All authors gave final approval for publication and agreed to be held accountable for the work performed therein.
Competing interests
We declare we have no competing interests.
Funding
The authors would like to acknowledge the funding and technical support from BP through the BP International Center for Advanced Materials (BP-ICAM), which made this research possible. This work was also partly funded by the EPSRC through a Prosperity Partnership grant (EP/R00496X/1).
References
- 1.Ostwald W. 1897. Studien über die Bildung und Umwandlung fester Körper. Z. Phys. Chem. 22U, 289-330. ( 10.1515/zpch-1897-2233) [DOI] [Google Scholar]
- 2.Threlfall T. 2003. Structural and thermodynamic explanations of Ostwald's Rule. Org. Process Res. Dev. 7, 1017-1027. ( 10.1021/op030026l) [DOI] [Google Scholar]
- 3.Vrcelj RM, Gallagher HG, Sherwood JN. 2001. Polymorphism in 2,4,6-trinitrotoluene crystallized from solution. J. Am. Chem. Soc. 123, 2291-2295. ( 10.1021/ja0031422) [DOI] [PubMed] [Google Scholar]
- 4.Hammond RB, Pencheva K, Roberts KJ. 2005. Simulation of energetic stability of facetted L-glutamic acid nanocrystalline clusters in relation to their polymorphic phase stability as a function of crystal size. J. Phys. Chem. B 109, 19 550-19 552. ( 10.1021/jp053546m) [DOI] [PubMed] [Google Scholar]
- 5.Bhattachar SN, Deschenes LA, Wesley JA. 2006. Solubility: it's not just for physical chemists. Drug Discov. Today 11, 1012-1018. ( 10.1016/j.drudis.2006.09.002) [DOI] [PubMed] [Google Scholar]
- 6.Burley JC, Duer MJ, Stein RS, Vrcelj RM. 2007. Enforcing Ostwald's rule of stages: isolation of paracetamol forms III and II. Eur. J. Pharm. Sci. 31, 271-276. ( 10.1016/j.ejps.2007.04.002) [DOI] [PubMed] [Google Scholar]
- 7.Chung S-Y, Kim Y-M, Kim J-G, Kim Y-J. 2009. Multiphase transformation and Ostwald's rule of stages during crystallization of a metal phosphate. Nat. Phys. 5, 68-73. ( 10.1038/nphys1148) [DOI] [Google Scholar]
- 8.Hall VJ, Simpson GJ. 2010. Direct observation of transient Ostwald crystallization ordering from racemic serine solutions. J. Am. Chem. Soc. 132, 13 598-13 599. ( 10.1021/ja106728c) [DOI] [PubMed] [Google Scholar]
- 9.Li J, Tilbury CJ, Joswiak MN, Peters B, Doherty MF. 2016. Rate expressions for kink attachment and detachment during crystal growth. Cryst. Growth Des. 16, 3313-3322. ( 10.1021/acs.cgd.6b00292) [DOI] [Google Scholar]
- 10.Tilbury CJ, Green DA, Marshall WJ, Doherty MF. 2016. Predicting the effect of solvent on the crystal habit of small organic molecules. Cryst. Growth Des. 16, 2590-2604. ( 10.1021/acs.cgd.5b01660) [DOI] [Google Scholar]
- 11.Hadjittofis E, Vargas SM, Litster JD, Campbell KLS. 2021. The role of surface energy in the apparent solubility of two different calcite crystal habits. Proc. R. Soc. A 477, 20210200. ( 10.1098/rspa.2021.0200) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bragg WL. 1924. The refractive indices of calcite and aragonite. Proc. R. Soc. Lond. A 105, 370-386. ( 10.1098/rspa.1924.0026) [DOI] [Google Scholar]
- 13.Kamhi HJ. 1963. On the structure of vaterite CaCO3. Acta Crystallogr. 16, 770-772. ( 10.1107/S0365110X63002000) [DOI] [Google Scholar]
- 14.Zachara JM, Cowan CE, Resch CT. 1991. Sorption of divalent metals on calcite. Geochim. Cosmochim. Acta 55, 1549-1562. ( 10.1016/0016-7037(91)90127-Q) [DOI] [Google Scholar]
- 15.Gebauer D, Volkel A, Colfen H. 2008. Stable prenucleation calcium carbonate clusters. Science 322, 1819-1822. ( 10.1126/science.1164271) [DOI] [PubMed] [Google Scholar]
- 16.Hasson D, Averiel M, Resnick W, Rozemann T, Winderich S. 1968. Mechanism of calcium carbonate scale deposition on heat-transfer surfaces. Ind. Eng. Chem. Fundam. 7, 59-65. ( 10.1021/i160025a011) [DOI] [Google Scholar]
- 17.Gal J, Bollinger J, Tolosa H, Gache N. 1996. Calcium carbonate solubility: a reappraisal of scale formation and inhibition. Talanta 43, 1497-1509. ( 10.1016/0039-9140(96)01925-X) [DOI] [PubMed] [Google Scholar]
- 18.Plummer LN, Busenberg E. 1982. The solubilities of calcite, aragonite and vaterite in CO2-H2O solutions between 0 and 90°C, and an evaluation of the aqueous model for the system CaCO3-CO2-H2O. Geochim. Cosmochim. Acta 46, 1011-1040. ( 10.1016/0016-7037(82)90056-4) [DOI] [Google Scholar]
- 19.Crocket JH, Winchester JW. 1966. Coprecipitation of zinc with calcium carbonate. Geochim. Cosmochim. Acta 30, 1093-1109. ( 10.1016/0016-7037(66)90119-0) [DOI] [Google Scholar]
- 20.Brecevic L, Nothing-Laslo V, Kralj D, Popovic S. 1996. Effect of divalent cations on the formation and structure of calcium carbonate polymorphs. J. Chem. Soc., Faraday Trans. 92, 1017-1022. ( 10.1039/FT9969201017) [DOI] [Google Scholar]
- 21.Wada N, Yamashita K, Umegaki T. 1995. Effects of divalent cations upon nucleation, growth and transformation of calcium carbonate polymorphs under conditions of double diffusion. J. Cryst. Growth 148, 297-304. ( 10.1016/0022-0248(94)00880-9) [DOI] [Google Scholar]
- 22.Davis JA, Fuller CC, Cook AD. 1987. A model for trace metal sorption processes at the calcite surface: adsorption of Cd2+ and subsequent solid solution formation. Geochim. Cosmochim. Acta 51, 1477-1490. ( 10.1016/0016-7037(87)90330-9) [DOI] [Google Scholar]
- 23.Chakraborty S, Bardelli F, Charlet L. 2010. Reactivities of Fe(II) on calcite: selenium reduction. Environ. Sci. Technol. 44, 1288-1294. ( 10.1021/es903037s) [DOI] [PubMed] [Google Scholar]
- 24.Mejri W, Ben Salah I, Tlili MM. 2015. Speciation of Fe(II) and Fe(III) effect on CaCO3 crystallization. Cryst. Res. Technol. 50, 236-243. ( 10.1002/crat.201400444) [DOI] [Google Scholar]
- 25.Kasting JF. 1993. Earth's early atmosphere. Science 259, 920-926. ( 10.1126/science.11536547) [DOI] [PubMed] [Google Scholar]
- 26.Hodkin J, Stewart DI, Graham JT, Cibin G, Burke IT. 2018. Enhanced crystallographic incorporation of strontium(II) ions into calcite via preferential adsorption at obtuse growth steps. Cryst. Growth Des. 18, 2836-2843. ( 10.1021/acs.cgd.7b01614) [DOI] [Google Scholar]
- 27.Fricke M, Volkmer D, Krill CE, Kellermann M, Hirsch A. 2006. Vaterite polymorph switching controlled by surface charge density of an amphiphilic dendron-calix[4]arene. Cryst. Growth Des. 6, 1120-1123. ( 10.1021/cg050534h) [DOI] [Google Scholar]
- 28.Chen X-F, Qie L, Zhang L-L, Zhang W-X, Huang Y-H. 2013. Self-templated synthesis of hollow porous submicron ZnMn2O4 sphere as anode for lithium-ion batteries. J. Alloys Compd. 559, 5-10. ( 10.1016/j.jallcom.2013.01.036) [DOI] [Google Scholar]
- 29.Feenstra TP, de Bruyn PL. 1981. The Ostwald rule of stages in precipitation from highly supersaturated solutions: a model and its application to the formation of the nonstoichiometric amorphous calcium phosphate precursor phase. J. Colloid Interface Sci. 84, 66-72. ( 10.1016/0021-9797(81)90260-5) [DOI] [Google Scholar]
- 30.Van Straten HA, Holtkamp BTW, De Bruyn PL. 1984. Precipitation from supersaturated aluminate solutions. J. Colloid Interface Sci. 98, 342-362. ( 10.1016/0021-9797(84)90159-0) [DOI] [Google Scholar]
- 31.Wang H, Han YS, Li JH. 2013. Dominant role of compromise between diffusion and reaction in the formation of snow-shaped vaterite. Cryst. Growth Des. 13, 1820-1825. ( 10.1021/cg301241s) [DOI] [Google Scholar]
- 32.Barlow DA, Pantha B. 2021. Kinetic model for Ostwald's rule of stages with applications to Boc-diphenylalanine self-assembly. Int. J. Chem. Kinet. 54, 42-49. ( 10.1002/kin.21539) [DOI] [Google Scholar]
- 33.Pertlik F. 1986. Structures of hydrothermally synthesized cobalt(II) carbonate and nickel(II) carbonate. Acta Crystallogr., Sect. C: Cryst. Struct. Commun. C42, 4-5. ( 10.1107/S0108270186097524) [DOI] [Google Scholar]
- 34.Katz JL, Reick MR, Herzog RE, Parsiegla KI. 1993. Calcite growth inhibition by iron. Langmuir 9, 1423-1430. ( 10.1021/la00029a043) [DOI] [Google Scholar]
- 35.Zhu LY, Zhao QR, Zheng XW, Xie Y. 2006. Formation of star-shaped calcite crystals with Mg2+ inorganic mineralizer without organic template. J. Solid State Chem. 179, 1247-1252. ( 10.1016/j.jssc.2006.01.036) [DOI] [Google Scholar]
- 36.Hadjittofis E, Isbell MA, Karde V, Varghese S, Ghoroi C, Heng JYY. 2018. Influences of crystal anisotropy in pharmaceutical process development. Pharm. Res. 35, 100. ( 10.1007/s11095-018-2374-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hadjittofis E, Vargas SM, Litster JD, Campbell KLS. 2022. Exploring the role of crystal habit in the Ostwald rule of stages. Figshare. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Hadjittofis E, Vargas SM, Litster JD, Campbell KLS. 2022. Exploring the role of crystal habit in the Ostwald rule of stages. Figshare. [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
Raw data for the XRD patterns can be found in the electronic supplementary material [37].