Abstract
Background
Respiratory syncytial virus (RSV) is the leading cause of acute respiratory infections (ARIs) in hospitalized children. Although prematurity and underlying medical conditions are known risk factors, most of these children are healthy, and factors including RSV load and subgroups may contribute to severity. Therefore, we aimed to evaluate the role of RSV in ARI severity and determine factors associated with increased RSV-ARI severity in young children.
Methods
Children aged <5 years with fever and/or ARI symptoms were recruited from the emergency department (ED) or inpatient settings at Vanderbilt Children’s Hospital. Nasal and/or throat swabs were tested using quantitative reverse-transcription polymerase chain reaction for common respiratory viruses, including RSV. A severity score was calculated for RSV-positive children.
Results
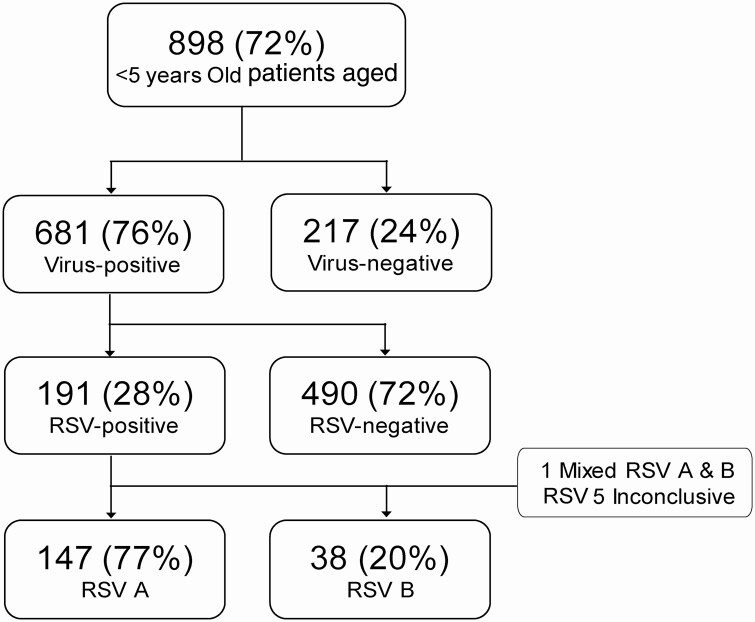
From November 2015 through July 2016, 898 participants were enrolled, and 681 (76%) had at least 1 virus detected, with 191 (28%) testing positive for RSV. RSV-positive children were more likely to be hospitalized, require intensive care unit admission, and receive oxygen compared with children positive for other viruses. Higher viral load, White race, younger age, and higher severity score were independently associated with hospitalization in RSV-positive children. No differences in disease severity were noted between RSV A and RSV B.
Conclusions
RSV was associated with increased ARI severity in young children enrolled from the ED and inpatient settings, but no differences in disease severity were noted between RSV A and RSV B. These findings emphasize the need for antiviral therapy and/or preventive measures such as vaccines against RSV in young children.
Keywords: respiratory syncytial virus, acute respiratory infections, respiratory syncytial virus subgroups, severity score, children
Respiratory syncytial virus (RSV) is associated with increased severity compared to other viruses in young children. Differences in RSV A and RSV B clinical presentation, but not severity, were noted. A severity score at presentation predicted intensive care unit admission.
Respiratory syncytial virus (RSV) is the leading cause of acute respiratory infections (ARIs) in children aged <5 years, contributing to 57 000 hospitalizations and an estimated economic burden of $400 million annually in the United States [1–4]. Although prematurity, young age, and underlying medical conditions are important risk factors for hospitalization, most children hospitalized with RSV are known to have been previously healthy [2, 5]. Moreover, the vast majority of healthcare visits due to RSV are in the outpatient setting, with an estimated 1.5 million outpatient visits attributable to RSV infections in young children. Additionally, the actual burden of RSV disease associated with emergency department (ED) visits is also underrecognized due to limited or inconsistent routine clinical RSV testing, plausibly originating from the variability in laboratory resources in different settings and regions, and limited data from nonpediatric hospitals and children beyond infancy [2, 3, 6].
Many recent studies have investigated the role of RSV viral load in disease severity. An accepted and widely used method of estimating viral load is the cycle threshold (Ct), which is inversely related to viral load [7–12]. Although most studies, including those of RSV and other respiratory viruses infections, report that a higher viral load correlates with disease severity, recent reports noted opposite results [7–12].
Variations in disease severity may also be attributed to the specific circulating RSV subgroup. RSV has 1 serotype, and molecular analyses have revealed 2 antigenic subgroups (A and B). These 2 subgroups co-circulate simultaneously in each season, but usually, 1 predominates [13, 14]. Antigenic subgroup A has been reported to be associated with greater clinical severity compared with RSV B [15, 16]; however, a few reports have documented increased disease severity with subgroup B [17, 18]. Many of these studies were limited by small sample sizes and only included hospitalized children. Therefore, in this study, our aim was to evaluate the role of RSV in ARI severity and determine factors associated with increased RSV-ARI severity. We also explored the utility of an objective severity score in predicting RSV disease severity.
METHODS
Study Design and Participants
In a prospective respiratory viral surveillance study, children aged <5 years who presented with fever and/or respiratory symptoms within 14 days of symptom onset to the ED and/or who were admitted to Monroe Carell Jr Children’s Hospital at Vanderbilt University in Nashville, Tennessee, were eligible for enrollment. Children were excluded if they had been previously enrolled in the last 7 days, were newborns who had never been discharged from the hospital, were neutropenic (absolute neutrophil count <500 × 103/µL), had been admitted >48 hours for this illness, and/or had a known nonrespiratory cause for their symptoms.
Children were included if they resided in 1 of the 9 county regions of Middle Tennessee: Davidson, Williamson, Cheatham, Dickson, Wilson, Rutherford, Montgomery, Robertson, and Sumner. Children were recruited 4 days a week (8-hour shifts) from the ED and at least 5 days a week from the inpatient setting.
The Vanderbilt University Institutional Review Board approved this study, and written informed consent was obtained from parents or legal guardians.
Data and Specimen Collection
After obtaining informed consent, trained research staff obtained nasal and throat swabs. Parents/guardians were interviewed by the research staff using a standardized case report form that collected demographic information, medical history, and clinical data about the child’s current illness. Medical records were reviewed to collect data including underlying medical conditions (UMCs), prematurity (ie, gestational age less than 37 weeks), oxygen use, hospital admission, hospital length of stay, need for intensive care unit (ICU) admission, and mechanical ventilation. Children were considered to have UMCs if they had any of the following: asthma/reactive airway disease, bronchopulmonary dysplasia, cancer, cerebral palsy, cystic fibrosis, diabetes, Down syndrome, eczema, food allergies, gastroesophageal reflux disease, genetic/metabolic disorders, heart disease, immunodeficiency, intellectual disability/developmental delay, kidney disease, liver disease, neurological disorders, other blood disorders, sickle cell disease/trait, seizure disorder, transplant recipient, and/or other conditions.
A severity score was used to determine severity at initial presentation for RSV-positive children (Supplementary Table 1) [12, 20]. In order to calculate the severity score, data were retrospectively abstracted from the medical charts using the first medical encounter for this current illness, including ED and/or clinic visits within 48 hours prior to enrollment. These data included maximum respiratory support, minimum oxygen saturation, cyanosis, nasal flaring, retraction, accessory muscle use, crackles/rhonchi/rales, wheezing, changes in appetite, changes in level of activity, intravenous fluids, and level of respiratory support. All data were compiled into a standardized, secure REDCap (Research Electronic Data Capture, Vanderbilt University, Nashville, TN) database [19].
Laboratory Testing
Nasal and throat swabs were combined in viral transport medium, aliquoted into MagNA Pure Lysis Buffer (Roche), snap-frozen, and then stored at −80°C. All specimens were tested using quantitative reverse-transcription polymerase chain reaction (qRT-PCR) for the following viruses: RSV A and RSV B; human metapneumovirus; human rhinovirus (HRV); influenza A, B, and C; parainfluenza virus 1, 2, 3, and 4; and adenovirus. Cycle threshold (Ct) values were used as a proxy for viral load, with lower Ct values representing higher viral loads.
Data Analyses
Baseline Demographics and Clinical Characteristics
Our analyses included only children aged <5 years with complete qRT-PCR laboratory results. Descriptive statistics were summarized as frequency (percentage), median (interquartile range [IQR]), or mean (standard deviation) where appropriate. RSV A and RSV B were compared using the Pearson χ2 test for categorical variables and a 2-sample t test allowing unequal variances or linear regression with robust standard errors for continuous variables. All analyses had a significance level of 0.05 (2-tailed) and were performed using StataCorp software (version 15.0; College Station, TX).
Regression Analysis Models
Multivariable logistic models with robust standard errors were used to compare the odds of hospital admission and oxygen use across predictors of interest that were selected a priori, including RSV status, age at enrollment, gender, race, ethnicity, breastfeeding, viral codetection, prematurity, and past medical history.
To assess factors associated with increased ARI severity among RSV-positive children, we used logistic regression to compare the odds of hospital admission and linear regression to compare mean severity score across predictors of interest that were selected a priori, which included RSV subgroup, age at enrollment, gender, race, ethnicity, breastfeeding, viral codetection, prematurity, past medical history, severity score at presentation, and RSV Ct values.
As prematurity was only collected for children less that 2 years, for all regression models, we used multiple imputation with chained equations with M = 100 imputation iterations to address missing data. Of the variables in the models, 308 of 898 (34%) children had missing values for prematurity and 13 of 191 (7%) RSV-positive children had missing values for the severity score.
To explore the ability of severity score to predict ICU admission in RSV-positive children, we estimated the area under the receiver operating characteristic (ROC) curve and formed a quantile-based 95% confidence interval (CI) based on 5000 nonparametric bootstrap replicates.
RESULTS
Demographics and Clinical Characteristics
From 16 November 2015 through 14 July 2016, 898 children aged <5 years were enrolled (Figure 1). Overall, 246 (27%) were hospitalized and 652 (73%) were seen in the ED and discharged to home; 53% were male, 57% were White, 35% were Black, and 24% were Hispanic; and 40% were reported by parents to have a preexisting medical condition. The median duration of symptoms before enrollment was 3 days (IQR, 2–5 days), with cough (84%), runny nose (82%), congestion (81%), irritability (80%), fever (77%), and loss of appetite (74%) being the most frequently reported symptoms.
Figure 1.
Study enrollment algorithm and results of nasal swab testing. Abbreviation: RSV, respiratory syncytial virus.
RSV-Positive Children
Of the 898 children, 681 (76%) had at least 1 virus detected; HRV (289, 42.4%) was the most frequently detected respiratory pathogen, followed by RSV (191, 28%; Supplementary Figure 1).
RSV-positive children had lower median age (10 months vs 19.4 months) and were more likely to be White (62% vs 54%) but less likely to have UMCs (34% vs 44%) compared with children positive for other viruses (Table 1). RSV-positive children were more likely to have a longer mean duration of illness and report symptoms of cough, runny nose, nasal congestion, decreased appetite and sleep, irritability, rapid/shallow breathing, nasal flaring/grunting, difficulty breathing, and apnea (P < .05). On physical exam, RSV-positive children were more likely to have wheezing (27% vs 17%), retractions (34% vs 18%), a higher documented maximum respiratory rate, and a lower documented minimum oxygen saturation compared with children positive for other viruses. Furthermore, RSV-positive children were more likely to be admitted to the hospital (34% vs 24%), more likely to require ICU admission (17% vs 7%), and had a higher frequency of oxygen requirement (62% vs 32%) compared with children positive for other viruses (Table 1).
Table 1.
Comparison of Clinical and Demographic Characteristics of Respiratory Syncytial Virus–Positive and Other Virus–Positive Children
| Characteristic | Respiratory Syncytial Virus–Positive, n = 191 | Other Virus–Positive, n = 490 | P Value |
|---|---|---|---|
| Demographics | |||
| Age, median [interquartile range], months | 10 (4.1–21) | 19.4 (9.1–34.7) | <.001 a |
| Male | 96 (50%) | 275 (56%) | .17b |
| Race | .01 b | ||
| White | 119 (62%) | 263 (54%) | |
| Black | 54 (28%) | 197 (40%) | |
| Other/Unknown/Mixed | 18 (9%) | 30 (6%) | |
| Hispanic or Latino | 40 (21%) | 125 (26%) | .21b |
| Breastfeeding | 137 (72%) | 340 (69%) | .55b |
| Smoke exposure | 63/190 (33%) | 158 (32%) | .82b |
| Preschool/School | 61 (32%) | 161/485 (33%) | .75b |
| Prematurityc | 35/148 (24%) | 62/289 (21%) | .60b |
| Underlying medical conditions | 65 (34%) | 220 (44%) | .01 b |
| Signs and symptoms | |||
| Illness duration, days | 4.3 ± 2.2 | 3.7 ± 2.3 | <.001 d |
| Fever | 152 (80%) | 384/487 (79%) | .83b |
| Fever duration, days | 3.3 ± 1.9 | 2.7 ± 1.8 | <.001 d |
| Cough | 187 (98%) | 422 (86%) | <.001 b |
| Runny nose | 176/190 (93%) | 425 (87%) | .03 b |
| Nasal congestion | 180/189 (95%) | 409 (83%) | <.001 b |
| Vomiting | 52 (27%) | 124 /488 (25%) | .62b |
| Diarrhea | 55 (29%) | 147 (30%) | .79b |
| Appetite loss | 156 (82%) | 361/486 (74%) | .04 b |
| Less sleeping | 145 (76%) | 294/489 (60%) | <.001 b |
| Lethargy | 33 (17%) | 75/488 (15%) | .54b |
| Irritability | 169/190 (89%) | 389/488 (80%) | .005 b |
| Loud, noisy breathing | 153 (80%) | 320 (65%) | <.001 b |
| Rapid or shallow breathing | 142 (74%) | 259/488 (53%) | <.001 b |
| Difficulty breathing | 133/189 (70%) | 219/488 (45%) | <.001 b |
| Apnea | 17/187 (9%) | 20/486 (4%) | .01 b |
| Wheezing | 52 (27%) | 85 (17%) | .004 b |
| Retractions | 64 (34%) | 89 (18%) | <.001 b |
| Nasal flaring | 102 (53%) | 187/485 (38%) | .001 b |
| Maximum respiratory rate | 37.3 ± 10.7 | 33.7 ± 11.1 | <.001 d |
| Minimum oxygen saturation | 97.1 ± 3.7 | 98 ± 3.4 | .008 d |
| Laboratory | |||
| Viral codetection | 60 (31%) | 70 (14%) | <.001 b |
| Severity | |||
| Hospital admission | 65 (34%) | 118 (24%) | .009 b |
| Intensive care unit admission | 11/65 (17%) | 8/118 (7%) | .03 b |
| Oxygen use | 40/65 (62%) | 38/118 (32%) | <.001 b |
| Length of hospital stay, days | 2 (1–3) | NA | NA |
Categorial data are in n (%), continuous data are in mean ± standard deviation, median (interquartile range). P values less than .05 are shown in bold.
Abbreviation NA, not available.
aMedian test.
bPearson χ2 test.
cOnly collected from children aged <2 years.
d T test.
Disease Severity
We found sufficient evidence of an association between hospital admission and age, race/ethnicity categories, and previous UMCs (Table 2). In particular, younger age, White race, and previous UMCs were directly associated, while Hispanic ethnicity was inversely associated with hospital admission. Additionally, we found prematurity and RSV infection to be independently associated with oxygen use.
Table 2.
Coefficient Estimates From Logistic Regression Models to Evaluate Association Between Variables Associated With Hospital Admission and Oxygen Use
| Variables | Hospital Admission | Oxygen Usea | ||||
|---|---|---|---|---|---|---|
| Coefficient | 95% CI | P Value | Coefficient | 95% CI | P Value | |
| Respiratory syncytial virus–positive | 0.32 | –.07 to .71 | .11 | 1.46 | .72 to 2.21 | <.001 |
| White race | 0.92 | .50 to 1.34 | <.001 | –0.04 | –.80 to –.73 | .92 |
| Hispanic or Latino | –0.69 | –1.16to –.22 | .004 | –0.06 | –.86 to .74 | .88 |
| Male | 0.01 | –.26 to .46 | .59 | –0.55 | –1.20 to .10 | .10 |
| Underlying medical conditions | 0.51 | .15 to .87 | .006 | –0.07 | –.87 to .74 | .87 |
| Age, months | –0.02 | –.04 to –.01 | <.001 | 0.014 | –.01 to .04 | .28 |
| Prematurity | 0.24 | –.23 to .71 | .32 | 1.07 | .21 to 1.92 | .02 |
| Breastfeeding | 0.29 | –.13 to .71 | .18 | –0.31 | –1.14 to .53 | .47 |
| Viral codetection | –0.32 | –.78 to –.15 | .18 | –0.03 | –.99 to –.93 | .95 |
Adjusted model including all the covariates listed in the table.
Abbreviation: CI, confidence interval.
aAmong hospitalized patients
RSV Subgroups A and B
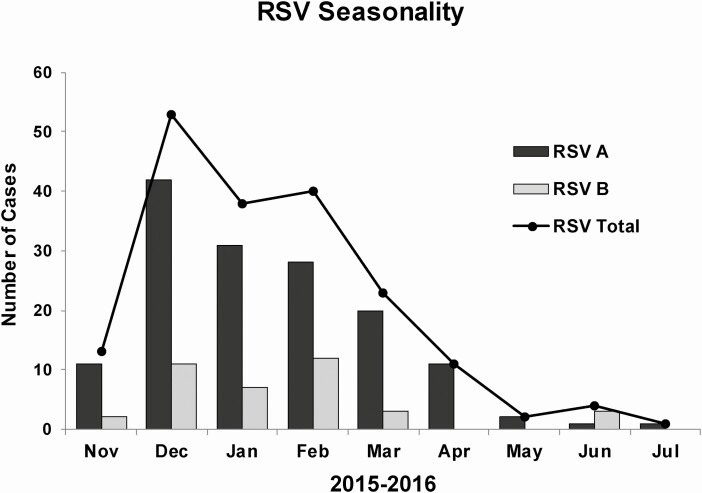
Of the 191 RSV-positive children, 147 (77%) were positive for RSV A, 38 (20%) were positive for RSV B, 1 (0.5%) was coinfected with RSV A and RSV B, and 5 (2.5%) were inconclusive (Figure 1). Figure 2 demonstrates that both RSV A and RSV B co-circulated throughout the study period, both peaking from December through February. A smaller rise was also present for RSV B in June 2016 (Figure 2).
Figure 2.
Seasonal pattern of RSV and RSV subgroups (A and B) during the 2016–2017 season. Abbreviation: RSV, respiratory syncytial virus.
In children infected with either RSV A or RSV B, cough was the most commonly reported symptom followed by nasal congestion and runny nose in both groups (Table 3). However, RSV A–positive children were more likely to have cough, nasal congestion, diarrhea, and lethargy compared with RSV B–positive children (Table 3).
Table 3.
Comparison of Clinical and Demographic Characteristics of Children
| Demographics | RSV A N = 147 | RSV B N = 38 | P Value |
|---|---|---|---|
| Age, median (interquartile range), months | 10 (3.5–20.9) | 10.3 (5.6–22.8) | .97a |
| Male | 74 (50%) | 20 (53%) | .80b |
| Race | .58b | ||
| White | 94 (64%) | 22 (58%) | |
| Black | 38 (26%) | 13 (34%) | |
| Other/Unknown/Mixed | 15 (10%) | 3 (8%) | |
| Hispanic or Latino | 31 (21%) | 7 (18%) | .72b |
| Breastfeeding | 104 (71%) | 29 (76%) | .50b |
| Smoke exposure | 54/146 (37%) | 9 (24%) | .12b |
| Preschool/School | 46 (31%) | 13 (34%) | .73b |
| Prematurityc | 26/113 (23%) | 7/30 (23%) | .97b |
| Underlying medical conditions | 45 (31%) | 17 (45%) | .10b |
| Signs and symptoms | |||
| Illness duration, days | 4.3 ± 2.1 | 4.2 ± 2.5 | .79d |
| Fever | 115 (78%) | 32 (84%) | .48b |
| Fever duration, days | 3.3 ± 1.8 | 3.1 ± 1.6 | .48d |
| Cough | 146 (99%) | 35 (92%) | .006 b |
| Runny nose | 137/146 (94%) | 33 (87%) | 0.15b |
| Nasal congestion | 142/146 (97%) | 33/37 (89%) | .032 b |
| Vomiting | 40 (27%) | 11 (29%) | .83b |
| Diarrhea | 46 (31%) | 5/37 (14%) | .03 b |
| Appetite loss | 119 (81%) | 32 (84%) | .64b |
| Less sleeping | 111 (76%) | 31/37 (84%) | .28b |
| Lethargy | 31 (21%) | 2 (5%) | .02 b |
| Irritability | 131 (89%) | 32/37 (84%) | .65b |
| Loud, noisy breathing | 120 (82%) | 30 (79%) | .71b |
| Rapid or shallow breathing | 112 (76%) | 27 (71%) | .51b |
| Difficulty breathing | 101 (69%) | 29/37 (78%) | .27b |
| Apnea | 12/144 (8%) | 3/8 (8%) | .97b |
| Wheezing | 40 (27%) | 10 (26%) | .91b |
| Retractions | 49 (33%) | 13 (34%) | .92b |
| Nasal flaring | 82 (56%) | 17 (45%) | .40b |
| Maximum respiratory rate | 37.4 ± 10.7 | 36.4 ± 10.8 | .63d |
| Minimum oxygen saturation | 97.1 ± 3.8 | 97.3 ± 3 | .76d |
| Laboratory | |||
| Viral codetection | 46 (31%) | 10 (26%) | .55b |
| Severity | |||
| Hospital admission | 51 (35%) | 11 (29%) | .50b |
| Intensive care unit admission | 11/51 (22%) | 0 | .09b |
| Oxygen use | 29/51 (57%) | 9/11 (82%) | .12b |
| Length of hospital stay, days | 2.7 ± 2.8 | 2.5 ± 1.8 | .8d |
| Severity score | .93b | ||
| Mild | 95 (69%) | 26 (72%) | |
| Moderate | 37 (27%) | 9 (25%) | |
| Severe | 5 (4%) | 1 (3%) |
Categorial data are in n (%), continuous data are in mean ± standard deviation, median (interquartile range). P values less than .05 are shown in bold.
Abbreviation: RSV, respiratory syncytial virus.
aMedian test.
bPearson χ2 test.
cOnly collected from children aged <2 years.
d T test.
Disease Severity in RSV-Positive Children
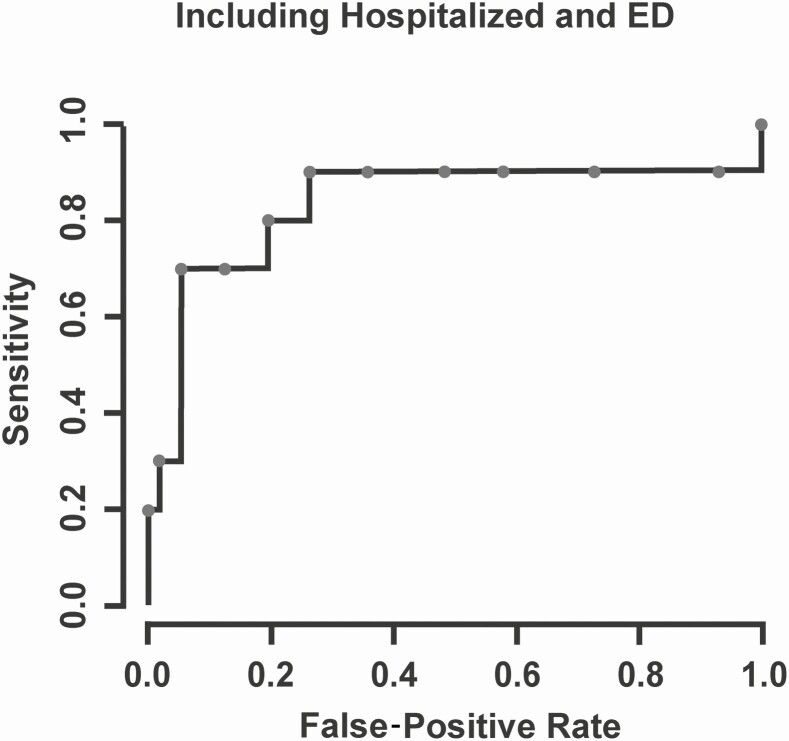
A severity score was calculated for 178 of 191 (93.2%) RSV-positive children at the time of their initial clinical presentation for their medical care. Most RSV-positive children had mild disease (125, 70%), with 47 (26%) and 6 (4%) with moderate and severe disease, respectively. We did not find strong evidence of an association between RSV subgroup category (ie, RSV A vs RSV B) and hospital admission (Table 4). Hospital admission was associated with younger age, lower RSV Ct values, and White race as well as higher severity score at presentation for RSV-positive children (Table 4 and Supplementary Figure 2). The ROC curve in Figure 3 indicates that the child’s severity score at initial clinical presentation predicated ICU admission for RSV-positive patients with an area under the curve of 0.849 (95% CI, .629–.977; P < .001). The only factor associated with increased severity score at presentation for RSV-positive children was lower RSV Ct values, and we did not find sufficient evidence of an association between RSV subgroup category and severity score (Supplementary Table 2).
Table 4.
Coefficient Estimates From Logistic Regression Models to Evaluate Association Between Variables Associated With Hospital Admission, in Respiratory Syncytial Virus–Positive Children
| Hospital Admission | |||
|---|---|---|---|
| Variables | Coefficient | 95% Confidence Interval | P Value |
| RSV A | 0.66 | –.29 to 1.62 | .17 |
| RSV cycle threshold value | –0.11 | –.20 to –.03 | .07 |
| Male | 0.004 | –.84 to .75 | .99 |
| Underlying medical conditions | 0.03 | –.85 to .91 | .95 |
| Age, months | –0.04 | –.08 to –.002 | .04 |
| Prematurity | –0.24 | –1.23 to .76 | .64 |
| White race | 1.01 | .10 to 1.92 | .03 |
| Hispanic or Latino | –0.33 | –1.28 to .62 | .49 |
| Breastfeeding | 0.72 | –.19 to 1.62 | .12 |
| Viral codetection | –0.05 | –.84 to .75 | .91 |
| Severity score | 0.35 | .20 to .50 | <.001 |
Adjusted model including all the covariates listed in the table. P values less than .05 are shown in bold.
Abbreviation: RSV, respiratory syncytial virus.
Figure 3.
Receiver operating characteristic curve characterizing the false-positive rate (1–specificity) and sensitivity for classifying intensive care unit (ICU) admission across various cut-points for the severity threshold. The area under the curve is estimated to be 0.849 (95% confidence interval, .629–.977), signifying strong evidence in favor of the severity score’s predictive ability for ICU admission. Abbreviation: ED, emergency department.
DISCUSSION
In our study, nearly three-quarters of children aged <5 years who presented to the ED or who were admitted to the hospital for fever and/or respiratory symptoms were virus-positive. Compared with children positive for other viruses, RSV-positive children were more likely to have more severe disease evidenced by the fact that they were more likely to be admitted to the hospital and require ICU admission and oxygen. In particular, RSV infection was independently associated with supplemental oxygen use, even after adjusting for other risk factors, including age, prematurity, and UMCs. Likewise, in our previous study in Jordan, which included 3168 hospitalized children aged <2 years who were enrolled over 3 respiratory seasons, RSV-positive children were more likely to require oxygen [5]. These findings are also consistent with other reports from the United States in different seasons and geographic locations [3, 21]. For example, a multicenter study that included children from inpatient and outpatient settings showed that RSV-positive children were more likely to require hospitalization and that young age was independently associated with hospitalization [3]. These results confirm that RSV continues to be a major cause of severe ARI in young children.
We also noted that RSV-positive children were younger and more likely to be White compared with children positive for other viruses. While younger age has been previously shown to be associated with increased RSV infections and severity [3, 22], racial differences in our study may be a reflection of our population and not generalizable to other regions. Nonetheless, after including multiple potential predictors that might have influenced disease severity and thus the need for hospitalization in a multivariable regression model, White race was independently associated with hospital admission. Conversely, Hispanic ethnicity was inversely related to hospital admission in this cohort. These racial and ethnic disparities might be due to inherent genetic differences between races and/or ethnicities that affect RSV disease severity and requires further evaluation. However, neither White race nor Hispanic ethnicity were associated with severity score at admission in an adjusted regression model. Alternatively, hospital admission was based on the discretion of the treating physician. Differences in healthcare-seeking behaviors, insurance status, and/or socioeconomic status may also play a role [3, 23]. For instance, in a retrospective study that included 45 330 infants, Sangaré et al showed that RSV hospitalization rates were highest among White, non-Hispanic, insured infants in California [23]. Additional studies are warranted to elucidate the reasons behind these variances in hospitalization across different racial and ethnic groups, including prospective studies that focus on evaluation of contrasts in healthcare-seeking behaviors, healthcare availability, and disease severities.
Our study documented that RSV A predominated during the study period, but both RSV A and RSV B subgroups co-circulated and peaked during winter months, with a small rise in RSV B in June. Seasonality of RSV and subgroups varies among different geographic locations and in different seasons [5, 24–26]. In the United States, RSV circulation generally occurs between November and March; however, season onset and offset, duration, and peak can have considerable regional and local variability [26]. Additionally, in a study conducted in China that evaluated the epidemiological patterns of RSV subgroups over 3 years among 729 hospitalized children aged <14 years, both RSV A and RSV B peaked in winter and summer months each season [25]. Despite reports that show variances in severity between RSV A and RSV B, we found no evidence of any differences in disease severity between the 2, but we noted some differences in the clinical presentation. Specifically, RSV A–infected children were more likely to have cough, nasal congestion, diarrhea, and lethargy. These findings are in line with a study by Liu et al in which RSV A–infected children were more likely to present with lower respiratory tract infections and gastrointestinal symptoms, while RSV B–infected children were more likely to present with influenza-like illness [25]. Additional studies with larger sample sizes are needed to further investigate the epidemiologic patterns, seasonality, clinical presentation, and disease severity between the 2 subgroups, as these might provide clues for diagnosis and clinical distinction between RSV subgroups.
As quantitative differences in the replication of RSV may alter the magnitude of later downstream immunopathological events, multiple studies have investigated the role of RSV viral load in disease severity. The majority of these studies reported a positive correlation of viral load and disease severity, while others reported opposite findings [7–12]. Our study supports the former, demonstrating that a higher severity score at presentation and hospital admission were both associated with lower RSV Ct values, indicating that higher viral loads might have played a role in increased disease severity. In contrast, Garcia-Mauriño et al reported that higher viral loads were associated with decreased disease severity in previously healthy children aged <2 years with RSV-ARI [12]. The fact that our cohort included older children, and those with UMCs, might explain the differences between the 2 studies [12]. The relationship between viral replication and RSV disease severity is important for potential antiviral treatment strategies and should be further explored among different patient populations.
Our study included a severity score with data abstracted at a child’s first presentation for medical care, and we found that a higher severity score was independently associated with hospital admission. On the ROC curve, a severity score at presentation showed promise in accurately identifying the need for ICU admission. Thus, the use of this objective severity score, which collectively incorporates several clinical signs and symptoms, may assist in clinical decision-making. Importantly, since the majority of hospitalized children with RSV in our cohort, like many other RSV studies in hospitalized children, had no known risk factors for severe disease [2], having objective data at presentation to predict illness severity will be helpful. Our findings warrant further validation among different populations and using larger sample sizes and additional RSV seasons.
Our study has several limitations. Ct values were measured using nasal swabs; this location is part of the upper respiratory tract and may not reflect the viral load in the lower respiratory tract. Nonetheless, one study showed that viral loads from nasal vs tracheal aspirations from the same patient correlated well [11]. While we found sufficient evidence that lower Ct values were independently associated with hospitalization and increased severity score, RSV pathophysiology has been attributed to both cytopathic RSV effects and to the host immune response, which potentially overlaps with viral load. We speculate that functional differences in the immune response to RSV among patients in our cohort, as well as genotype differences within the same RSV subgroup, might have affected the disease severity and viral loads [27]. Another limitation is that we included only children from 1 season and 1 geographic location, which affects the generalizability of our conclusions, given the differences in RSV subgroups’ seasonality and severity reported in different seasons. The strengths of our study are that the severity of RSV-ARI at presentation was evaluated and correlated with lower Ct values (higher viral load) using a severity score, along with other markers of severity, including admission status and oxygen use. Additionally, we provided data about the clinical severity of subgroups RSV A and RSV B.
In summary, we demonstrate that RSV continues to be a significant viral pathogen in young children and is associated with increased ARI severity compared with other virus-positive children. We noted that increased viral load, White race, younger age, and higher severity score were associated with hospitalization in RSV-positive children. These findings emphasize the need for effective antiviral therapy and/or preventive measures, such as vaccines, to reduce the burden of disease in young children. Also, future studies should validate the potential benefit of using a severity score to help guide the clinical decision in RSV-ARI in young children.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments . The authors thank William Whitworth who helped with medical chart reviews, clinical trial associates for enrollment, and the families who participated in this study.
Financial support . This work was supported by the Centers for Disease Control and Prevention Emerging Infections Program Cooperative Agreement 1U50CK000491 and Clinical and Translational Science Awards award UL1TR000445.
Potential conflicts of interest . N. B. H. receives grant support from Quidel and Sanofi, served as a consultant for Karius, reports an education grant from Genetech to Spire, and receives vaccine donation and HAI testing for influenza vaccine studies from Sanofi. W. S. reports Data and Safety Monitoring Board fees from Pfizer and consultant fees from VBI Vaccines outside the submitted work. All remaining authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Contributor Information
Zaid Haddadin, Department of Pediatrics, Vanderbilt University Medical Center, Nashville, Tennessee, USA.
Stockton Beveridge, Department of Pediatrics, University of Texas Southwestern Medical Center, Dallas, Texas, USA.
Kailee Fernandez, Department of Pediatrics, Vanderbilt University Medical Center, Nashville, Tennessee, USA.
Danielle A Rankin, Department of Pediatrics, Vanderbilt University Medical Center, Nashville, Tennessee, USA; Vanderbilt Epidemiology PhD Program, Vanderbilt University School of Medicine, Nashville, Tennessee, USA.
Varvara Probst, Department of Pediatrics, Vanderbilt University Medical Center, Nashville, Tennessee, USA.
Andrew J Spieker, Department of Biostatistics, Vanderbilt University Medical Center, Nashville, Tennessee, USA.
Tiffanie M Markus, Department of Health Policy, Vanderbilt University Medical Center, Nashville, Tennessee, USA.
Laura S Stewart, Department of Pediatrics, Vanderbilt University Medical Center, Nashville, Tennessee, USA.
William Schaffner, Department of Health Policy, Vanderbilt University Medical Center, Nashville, Tennessee, USA; Department of Medicine, Vanderbilt University Medical Center, Nashville, Tennessee, USA.
Mary Lou Lindegren, Department of Pediatrics, Vanderbilt University Medical Center, Nashville, Tennessee, USA; Department of Health Policy, Vanderbilt University Medical Center, Nashville, Tennessee, USA.
Natasha Halasa, Department of Pediatrics, Vanderbilt University Medical Center, Nashville, Tennessee, USA.
References
- 1. Halasa NB, Williams JV, Wilson GJ, Walsh WF, Schaffner W, Wright PF. Medical and economic impact of a respiratory syncytial virus outbreak in a neonatal intensive care unit. Pediatr Infect Dis J 2005; 24:1040–4. [DOI] [PubMed] [Google Scholar]
- 2. Hall CB, Weinberg GA, Blumkin AK, et al. Respiratory syncytial virus-associated hospitalizations among children less than 24 months of age. Pediatrics 2013; 132:e341–8. [DOI] [PubMed] [Google Scholar]
- 3. Hall CB, Weinberg GA, Iwane MK, et al. The burden of respiratory syncytial virus infection in young children. N Engl J Med 2009; 360:588–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Breese Hall C. The burgeoning burden of respiratory syncytial virus among children. Infect Disord Drug Targets (Formerly Current Drug Targets-Infectious Disorders) 2012; 12:92–7. [DOI] [PubMed] [Google Scholar]
- 5. Halasa N, Williams J, Faouri S, et al. Natural history and epidemiology of respiratory syncytial virus infection in the Middle East: hospital surveillance for children under age two in Jordan. Vaccine 2015; 33:6479–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. McGuiness CB, Boron ML, Saunders B, Edelman L, Kumar VR, Rabon-Stith KM. Respiratory syncytial virus surveillance in the United States, 2007–2012: results from a national surveillance system. Pediatr Infect Dis J 2014; 33:589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fuller JA, Njenga MK, Bigogo G, et al. Association of the CT values of real-time PCR of viral upper respiratory tract infection with clinical severity, Kenya. J Med Virol 2013; 85:924–32. [DOI] [PubMed] [Google Scholar]
- 8. Fodha I, Vabret A, Ghedira L, et al. Respiratory syncytial virus infections in hospitalized infants: association between viral load, virus subgroup, and disease severity. J Med Virol 2007; 79:1951–8. [DOI] [PubMed] [Google Scholar]
- 9. Yan XL, Li YN, Tang YJ, et al. Clinical characteristics and viral load of respiratory syncytial virus and human metapneumovirus in children hospitaled for acute lower respiratory tract infection. J Med Virol 2017; 89:589–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Thwaites RS, Coates M, Ito K, et al. Reduced nasal viral load and IFN responses in infants with respiratory syncytial virus bronchiolitis and respiratory failure. Am J Respir Crit Care Med 2018; 198:1074–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. El Saleeby CM, Bush AJ, Harrison LM, Aitken JA, Devincenzo JP. Respiratory syncytial virus load, viral dynamics, and disease severity in previously healthy naturally infected children. J Infect Dis 2011; 204:996–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Garcia-Mauriño C, Moore-Clingenpeel M, Thomas J, et al. Viral load dynamics and clinical disease severity in infants with respiratory syncytial virus infection. J Infect Dis 2019; 219:1207–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Anderson LJ, Hierholzer JC, Tsou C, et al. Antigenic characterization of respiratory syncytial virus strains with monoclonal antibodies. J Infect Dis 1985; 151:626–33. [DOI] [PubMed] [Google Scholar]
- 14. Gilca R, De Serres G, Tremblay M, et al. Distribution and clinical impact of human respiratory syncytial virus genotypes in hospitalized children over 2 winter seasons. J Infect Dis 2006; 193:54–8. [DOI] [PubMed] [Google Scholar]
- 15. Laham FR, Mansbach JM, Piedra PA, et al. Clinical profiles of respiratory syncytial virus subtypes A and B among children hospitalized with bronchiolitis. Pediatr Infect Dis J 2017; 36:808–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hall CB, Walsh EE, Schnabel KC, et al. Occurrence of groups A and B of respiratory syncytial virus over 15 years: associated epidemiologic and clinical characteristics in hospitalized and ambulatory children. J Infect Dis 1990; 162:1283–90. [DOI] [PubMed] [Google Scholar]
- 17. Hornsleth A, Klug B, Nir M, et al. Severity of respiratory syncytial virus disease related to type and genotype of virus and to cytokine values in nasopharyngeal secretions. Pediatr Infect Dis J 1998; 17:1114–21. [DOI] [PubMed] [Google Scholar]
- 18. Straliotto SM, Roitman B, Lima JB, Fischer GB, Siqueira MM. Respiratory syncytial virus (RSV) bronchiolitis: comparative study of RSV groups A and B infected children. Rev Soc Bras Med Trop 1994; 27:1–4. [DOI] [PubMed] [Google Scholar]
- 19. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap) --a metadata-driven methodology and workflow process for providing translational research informatics support. Journal of biomedical informatics 2009; 42:377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tal A, Bavilski C, Yohai D, Bearman JE, Gorodischer R, Moses SW. Dexamethasone and salbutamol in the treatment of acute wheezing in infants. Pediatrics 1983; 71:13–8. [PubMed] [Google Scholar]
- 21. García CG, Bhore R, Soriano-Fallas A, et al. Risk factors in children hospitalized with RSV bronchiolitis versus non-RSV bronchiolitis. J Pediatr 2010; 126:e1453–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Simoes EA. Environmental and demographic risk factors for respiratory syncytial virus lower respiratory tract disease. J Pediatr 2003; 143:118–26. [DOI] [PubMed] [Google Scholar]
- 23. Sangaré L, Curtis MP, Ahmad S. Hospitalization for respiratory syncytial virus among California infants: disparities related to race, insurance, and geography. J Pediatr 2006; 149:373–7. [DOI] [PubMed] [Google Scholar]
- 24. Cintra OA, Owa MA, Machado AA, et al. Occurrence and severity of infections caused by subgroup A and B respiratory syncytial virus in children in southeast Brazil. J Med Virol 2001; 65:408–12. [DOI] [PubMed] [Google Scholar]
- 25. Liu W, Chen D, Tan W, et al. Epidemiology and clinical presentations of respiratory syncytial virus subgroups A and B detected with multiplex real-time PCR. PLoS One 2016; 11:e0165108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Haynes AK, Prill MM, Iwane MK. Respiratory syncytial virus activity—United States, July 2011–January 2013. MMWR Morb Mortal Wkly Rep 2013; 62:141–4 [PMC free article] [PubMed] [Google Scholar]
- 27. Mella C, Suarez-Arrabal MC, Lopez S, et al. Innate immune dysfunction is associated with enhanced disease severity in infants with severe respiratory syncytial virus bronchiolitis. J Infect Dis 2013; 207:564–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.