Abstract
The association and potential role of the protein hormone adiponectin in autoimmune diseases causing musculoskeletal disorders, including rheumatoid arthritis (RA), are controversial. Conflicting results may arise from the influences of confounding factors linked to genetic backgrounds, disease stage, disease-modifying anti-rheumatic drugs and patients’ metabolic characteristics. Here, we examined serum level of adiponectin and its relationship with disease activity score 28 with erythrocytes sedimentation rate (DAS28[ESR]) and Sharp score in a treatment-naïve Han Chinese RA population. This cross-sectional study enrolled 125 RA patients. Serum level of total adiponectin was assessed by enzyme-linked immunosorbent assay (ELISA). Other important clinical and laboratory parameters were collected from the hospital database. DAS28(ESR) was calculated according to the equation previously published. Sharp score was evaluated based on hands radiographs by an independent radiologist. The correlation between serum adiponectin level and DAS28(ESR) or the Sharp score was investigated by univariate and multivariable linear regression analyses, respectively. Multiple imputation by chained equations was used to account for missing data. Univariate analyses showed a significant positive correlation between DAS28(ESR) and age or C-reactive protein (CRP) (both p = 0.003), while serum adiponectin level was negatively correlated with DAS28(ESR) (p = 0.015). The negative correlation between adiponectin level and DAS28(ESR) remained true in multivariable analyses adjusted for confounders. In addition, the univariate analyses revealed positive correlations of Sharp score to disease duration (p < 0.001), CRP (p = 0.023) and ESR (p < 0.001). In the multivariable model adjusted for confounders, adiponectin was negatively correlated with Sharp score (p = 0.013). In this single-institution cross-sectional study, serum adiponectin level in treatment-naive RA patients is negatively correlated with DAS28(ESR) and the Sharp score after adjustment for prominent identified confounders. Serum adiponectin may be potentially useful for assessing disease activity and radiographic progression of RA.
Subject terms: Immunology, Biomarkers, Rheumatology
Introduction
Rheumatoid arthritis (RA) is a chronic inflammatory-related autoimmune disease that primarily affects joints and causes musculoskeletal disorders1. The characteristics of RA include synovium hyperplasia, lymphocyte infiltration and abnormal proliferation of fibroblast-like synoviocytes, all of which leading eventually to erosive joint destruction2.
Adiponectin is a hormone protein mainly secreted by adipose tissue but also by various other cells, including skeletal myocytes and cardiomyocytes3. It is abundantly present in the circulation, accounting for 0.01% of total plasma proteins4. The potential role of adiponectin in RA has been actively investigated. Conflicting data on its role in RA have been reported. Adiponectin could act as proinflammatory mediator as its serum level positively correlates with disease activity5–9 or radiographic progression10. However, this association has not been unanimously agreed upon, with opposite results being reported from other studies11–14. Given these heterogenous findings coupled with the fact that most of these studies were conducted in Caucasian patients or with relatively higher body mass index (BMI)15–17 and some studies did not adjust for confounders10, we conducted a cross-sectional study to evaluate the relationship between adiponectin and disease activity as well as radiographic outcomes in a cohort of treatment-naïve Chinese RA patients using both univariate and multivariable linear regression methods.
Materials and methods
Study population
Between 2012 and 2020, one hundred twenty-five patients with rheumatoid arthritis diagnosed according to the American College of Rheumatology 1987 criteria were included in this study. Patients with co-morbidities such as hypertension, diabetes, hyperlipidaemia, chronic inflammatory disease, autoimmune disease, or cancer were excluded. At the time of the study, none of the patients had received treatment against RA. All patients belonged to the Han ethnic group. In addition to the RA patients, 34 healthy participants were studied to evaluate the baseline level of total serum adiponectin in the general population. The study was conducted in accordance with the Declaration of Helsinki and was approved by the Ethical Committee of Sichuan Provincial People’s Hospital. All subjects signed informed consent.
Clinical and laboratory measurements
Clinical information and laboratory data were obtained through detailed interviews, self-reported questionnaires, physical examination, and blood tests. The BMI was calculated as [body weight/height2] (kg/m2). Disease activity was measured using modified disease activity score 28 with erythrocyte sedimentation rate (DAS28[ESR])18.
All bloods were collected in the morning, after overnight fasting. Levels of CRP, ESR, rheumatoid factor (RF) and anti-cyclic citrullinated peptide (CCP) antibodies were measured by the Clinical Laboratory of Sichuan Provincial People’s Hospital. Serum concentrations of total adiponectin were measured by enzyme-linked immunosorbent assay (ELISA), using the kit purchased from BioVision, USA. Samples were prepared at appropriate dilutions and assayed according to the manufacturer’s protocol.
Radiographic outcomes
Single-view anterior–posterior X-rays of both hands were scored using the van der Heijde modification of the Sharp method (SHS) (referred to as Sharp score) by a single experienced reader blinded to patient characteristics19.
Statistical analysis
Continuous variables were expressed as means ± standard deviations, and categorical data were expressed as a number (percentage). Statistical significance was calculated using t test (normal distribution) or Kruskal–Wallis test (skewed distribution) unless stated otherwise. A univariate linear regression model was used to analyse each variable’s relationship with DAS28(ESR) or Sharp score where results were presented as regression coefficient β and 95% confidence interval (95% CI). Next, multivariable linear regression models were performed against DAS28(ESR) or Sharp score to examine the β and 95% CI of serum adiponectin level after adjusting for confounders. Gender was selected to adjust for possible differential relationship between genders. Other variables were selected as confounders if they were either significantly associated with the outcome, or if when added to the basic model or removed from the full model, a change in effect estimate of adiponectin level by more than 10% was observed. ‘Basic model’ refers to the unadjusted univariate linear regression model between adiponectin and the outcome, whereas ‘full model’ refers to multivariable linear regression model adjusting for all covariates. Swollen joint count (SJC), tender joint count (TJC) and ESR were excluded from the full model of DAS28(ESR) because they were already captured by the score.
Missing data were noticed in age, disease duration, height, weight, ESR, CRP, RF, CCP, SJC, TJC, Sharp score and adiponectin. To maximise statistical power and minimise potential bias, we used multiple imputation by chained equations to create five imputed datasets to account for missing data20. Analyses were repeated on each of the imputed datasets and final results were obtained by combining the results from each individual analysis according to Rubin’s rules21. In addition, sensitivity analysis was performed to identify whether the created complete data had a significant difference from pre-imputation data. Moreover, smooth curve fitting was used to further characterize the nature of the associations between adiponectin and DAS28(ESR) or Sharp score. All the analyses were performed with R software Version 3.4.3 (http://www.R-project.org) and EmpowerStats (http://www.empowerstats.com, X&Y Solutions, Inc., Boston, MA). A P value less than 0.05 was considered statistically significant.
Ethics approval and consent to participate
This study was approved by the Ethical Committee of Sichuan Provincial People’s Hospital. All subjects signed informed consent.
Consent for publication
All patients signed a consent for publication form.
Results
Clinical and laboratory characteristics of the patients
The characteristics of the healthy controls are summarised in Supplementary Table S1 (Additional file 1). The clinical and laboratory profiles of the RA patients are summarised in Table 1. A total of 125 RA patients with a mean age of 55.7 ± 12.4 years were included, of which 94 (75.2%) were female. Compared to male patients, female patients were significantly younger (54.4 ± 12.7 vs 59.9 ± 10.1, p = 0.033). The average height and weight also showed significant differences between genders (both with p < 0.001), although the two groups had a compatible average BMI, which was overall of 22.6 ± 3.7. The mean DAS28(ESR) of the whole cohort was 5.4 ± 3.3 and the mean value of serum total adiponectin was 25.0 ± 19.1 μg/mL. Serum total adiponectin in an age- and sex-matched healthy population sample was significantly lower (13.6 ± 5.5 μg/mL; p = 0.015) compared with the RA group.
Table 1.
Clinical and laboratory characteristics of the enrolled patients.
| Characteristic | N (%) | Male | Female | Total | p value |
|---|---|---|---|---|---|
| Gender (N, %) | 125 (100) | 31 (24.8) | 94 (75.2) | ||
| Age (year) | 124 (99.2) | 60.9 ± 11.4 | 54.0 ± 12.3 | 55.7 ± 12.4 | 0.007 |
| Height (cm) | 122 (97.6) | 167.2 ± 8.2 | 155.5 ± 5.0 | 158.4 ± 7.8 | < 0.001 |
| Weight (kg) | 122 (97.6) | 64.3 ± 11.0 | 54.4 ± 10.3 | 56.8 ± 11.3 | < 0.001 |
| BMI (kg/m2) | 122 (97.6) | 23.0 ± 3.2 | 22.4 ± 3.8 | 22.6 ± 3.7 | 0.504 |
| Disease duration (month) | 118 (94.4) | 105.2 ± 116.4 | 109.0 ± 107.3 | 108.0 ± 109.1 | 0.871 |
| SJC | 123 (98.4) | 4.5 ± 6.0 | 3.8 ± 6.3 | 4.0 ± 6.2 | 0.637 |
| TJC | 123 (98.4) | 6.8 ± 8.7 | 6.9 ± 9.0 | 6.9 ± 8.9 | 0.844 |
| CRP (mg/dL) | 91 (72.8) | 33.9 ± 38.3 | 40.1 ± 57.3 | 38.5 ± 52.8 | 0.627 |
| ESR (mm/H) | 124 (99.2) | 52.8 ± 30.9 | 56.2 ± 33.0 | 55.4 ± 32.4 | 0.618 |
| DAS28(ESR) | 123 (98.4) | 5.7 ± 3.4 | 5.3 ± 3.3 | 5.4 ± 3.3 | 0.594 |
| RF (IU/mL) | 122 (97.6) | 444.2 ± 685.0 | 321.3 ± 469.3 | 352.5 ± 532.0 | 0.269 |
| Anti-CCP (Ru/mL) | 119 (95.2) | 242.5 ± 173.1 | 230.8 ± 170.4 | 233.7 ± 170.4 | 0.746 |
| Adiponectin (μg/mL) | 118 (94.4) | 21.4 ± 20.3 | 26.3 ± 18.6 | 25.0 ± 19.1 | 0.223 |
| Sharp score | 115 (92.0) | 43.1 ± 63.5 | 44.3 ± 51.7 | 44.0 ± 54.7 | 0.917 |
BMI body mass index, CCP cyclic citrullinated peptides, CRP C-reactive protein, DAS28 disease activity score of 28 joints, ESR erythrocyte sedimentation rate, RF rheumatoid factor, SJC swollen joint count, TJC tender joint count. Continuous variables are expressed as means ± standard deviations and categorical data using number (percentage). p < 0.05 is considered statistically significant and these values are in bold.
Identification of factors correlated with DAS28(ESR) and Sharp score by univariate linear regression analysis
To ensure the correct measurement of adiponectin, we first investigated whether it was correlated with BMI. We found that adiponectin was negatively correlated with BMI (r = − 0.2571, p = 0.0055). The relationships between different clinical parameters and disease activity measured by DAS28(ESR) were then assessed by univariate linear regression analysis. Analyses using the univariate model against DAS28(ESR) indicated significant positive correlations with age and CRP (p = 0.0026 and p = 0.003, respectively; Table 2). In contrast, serum adiponectin was negatively correlated with DAS28(ESR) (p = 0.015). This model did not detect any further significant association between DAS28(ESR) and other variables (p > 0.05) (Table 2).
Table 2.
Univariate linear regression results against DAS28(ESR) or Sharp score.
| Variables | DAS28 (ESR) | Sharp score | ||
|---|---|---|---|---|
| β (95% CI) | p | β (95% CI) | p | |
| Gender | ||||
| Male | 0 | 0 | ||
| Female | − 0.27 (− 1.62, 1.09) | 0.698 | 1.22 (− 21.65, 24.09) | 0.917 |
| Age (year) | 0.07 (0.03, 0.12) | 0.003 | 0.62 (− 0.17, 1.41) | 0.128 |
| Disease duration (month) | 0.00 (− 0.00, 0.01) | 0.177 | 0.30 (0.22, 0.38) | < 0.001 |
| Height (cm) | − 0.03 (− 0.10, 0.05) | 0.497 | − 0.26 (− 1.54, 1.01) | 0.688 |
| Weight (kg) | − 0.03 (− 0.08, 0.03) | 0.331 | − 0.68 (− 1.58, 0.22) | 0.141 |
| BMI (kg/m2) | − 0.06 (− 0.23, 0.10) | 0.444 | − 2.23 (− 5.03, 0.56) | 0.121 |
| RF (IU/mL) | − 0.00 (− 0.00, 0.00) | 0.886 | 0.01 (− 0.01, 0.03) | 0.311 |
| CCP (Ru/mL) | − 0.00 (− 0.01, 0.00) | 0.204 | − 0.01 (− 0.07, 0.03) | 0.853 |
| CRP (mg/dL) | 0.02 (0.01, 0.03) | 0.003 | 0.26 (0.04, 0.049) | 0.023 |
| ESR (mm/H) | N/A | N/A | 0.65 (0.36, 0.94) | < 0.001 |
| SJC | N/A | N/A | 2.05 (0.51, 3.59) | 0.010 |
| TJC | N/A | N/A | 2.07 (1.02, 3.13) | < 0.001 |
| Adiponectin (μg/mL) | − 0.04 (− 0.07, − 0.01) | 0.015 | − 0.39 (− 0.99, 0.20) | 0.198 |
BMI body mass index, CCP cyclic citrullinated peptides, CRP C-reactive protein, RF rheumatoid factor, SJC swollen joint count, TJC tender joint count. p < 0.05 is considered statistically significant and these values are in bold.
Analyses using the univariate model against the Sharp score revealed positive correlations with disease duration, CRP, ESR, SJC and TJC (p < 0.001, p = 0.023, p < 0.001, p = 0.010 and p < 0.001, respectively), whereas no correlation was observed with serum adiponectin level (Table 2).
Analyses by multivariable linear regression showed an independent relationship between adiponectin level and DAS28(ESR) or Sharp score
We further explored the relationship between adiponectin level and the outcomes using multivariable linear regression analyses adjusted for confounders. Age, BMI, gender and CRP were finally selected as confounders for DAS28(ESR). Age, BMI, gender, disease duration, CRP, ESR, SJC and TJC were finally selected as confounders for Sharp score.
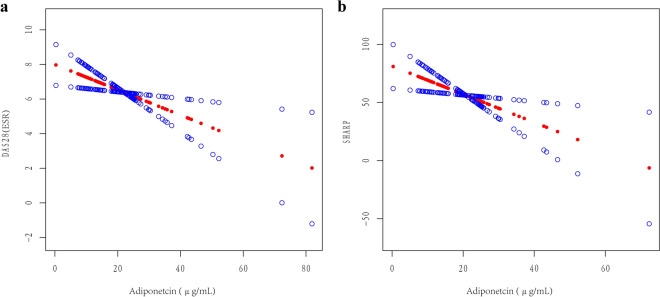
In the crude model, there was no adjustment for confounders, while in model I, three confounders were adjusted when using DAS28(ESR) and four confounders were adjusted when using the Sharp score. Model II was adjusted for all confounders. DAS28(ESR) and adiponectin were significantly and negatively correlated in all three models (Table 3). In the models against the Sharp score, after adjusting for confounders, there was also a negative association between adiponectin level and Sharp score (Table 3). Further analysis using smooth curve fitting confirmed that the associations of adiponectin with DAS28(ESR) or Sharp score was linear after adjusting for all confounders (Fig. 1).
Table 3.
Multivariable linear regression results against DAS28(ESR) or Sharp score, β (95% CI) of adiponectin (μg/mL).
| Outcomes | Crude model | p | Model I | p | Model II | p |
|---|---|---|---|---|---|---|
| DAS28(ESR) | − 0.04 (− 0.07, − 0.01) | 0.015 | − 0.05 (− 0.08, − 0.01)a | 0.007 | − 0.07 (− 0.13, − 0.02)b | 0.008 |
| Sharp score | − 0.39 (− 0.99, 0.20) | 0.198 | − 0.72 (− 1.23, − 0.20)c | 0.008 | − 1.21 (− 2.14, − 0.28)d | 0.013 |
Crude model: univariate model.
aAdjusted for age, BMI and gender.
bAdjusted for age, BMI, gender and CRP.
cAdjusted for age, BMI, gender and disease duration.
dAdjusted for age, BMI, gender, disease duration, CRP, ESR, SJC and TJC.
p < 0.05 is considered statistically significant.
Figure 1.
Association between serum adiponectin level and DAS28(ESR) (a) or Sharp score (b). The smooth curve fitting presented linear association between serum adiponectin and the two outcome measures. The red dotted line and blue dotted line represent the estimated values and their corresponding 95% confidence intervals (CI). Adjusted variables include age, BMI, gender and CRP for DAS28(ESR) and age, BMI, gender, disease duration, CRP, ESR, SJC and TJC for Sharp score.
Sensitivity analysis
The amount of missing data for the different variables ranged from 0 to 27% (Table 1). Eighty-four out of the 125 (67%) patients had complete data for all variables for the main analyses. The distributions of the original and imputed variables are depicted in Supplementary Table S2 (Additional file 1). Regression analyses using multiple imputed datasets gave similar results to those undertaken on the original datasets, as displayed in Supplementary Table S3 (Additional file 1).
Discussion
To evaluate the relationships between serum adiponectin and RA disease activity, we performed a cross-sectional investigation on a Chinese RA population. Adiponectin was negatively correlated with BMI in our study, which is in agreement with other studies22,23, validating the accuracy of our data. After accounting for confounders, multivariable linear regression analyses showed a negative correlation between serum adiponectin level and DAS28(ESR) or Sharp score. The fully-adjusted smooth curve fitting also confirmed a linear association between adiponectin with the outcomes. Since adiponectin was not normally distributed in our cohort, cube root transformation was performed on it and the conclusions remained the same (data not shown). Analyses were repeated after removing potential outlier noted in Fig. 1 (data not shown) and again all conclusions remained the same. To further avoid bias from missing data, we carried out a sensitivity analysis using full imputed data. With this sensitivity analysis, the associations of DAS28(ESR) and Sharp score with serum adiponectin remained statistically significant. These results are in keeping with previous studies showing that serum adiponectin level negatively correlated with disease activity in RA15,24.
In vitro experiments with RA synovial fibroblasts indicated that adiponectin significantly inhibits IL-1-induced RA synovial fibroblasts proliferation25. In a DBA/1 mouse model of collagen-induced arthritis, adiponectin treatment significantly mitigated the severity of arthritis along with a decrease in the expression of TNF-α, IL-1 and MMP-3 in joint tissues26. Anti-TNF treatment in female patients with RA was associated with increased adiponectin levels, which may dampen the systemic inflammatory response associated with RA27. These findings support an anti-inflammatory role for adiponectin in RA. However, several studies, including a metanalysis16, led to the conclusion that there was either no or a positive correlation between adiponectin level and inflammatory markers such as chemokines, CRP or DAS285,6,28,29. This inconsistency may stem from the study designs and sampled populations, but also indicates that adiponectin may play different roles in inflammation, depending on disease characteristics, comorbidities, treatment, genetic backgrounds and physiological characteristics of the patients.
In addition to its role in inflammation, adiponectin has been shown to be protective against insulin resistance (IR) and diabetes30. High total adiponectin concentrations were significantly associated with lower risk for incident type 2 diabetes30. However, conflicting results on the association and prognostic value of blood adiponectin level in patients with coronary artery disease (CAD) have been reported. Some studies found that plasma adiponectin levels were significantly lower in patients with CAD than control subjects31,32, while a recent meta-analysis indicates that elevated adiponectin level is an independent predictor of cardiovascular and all-cause mortality in CAD patients33. It is known that RA patients have a 1.5–2.0 fold increased risk of developing CAD compared with the general population34,35, and the prevalence of IR is also higher in RA patients than in the general population (51–58% in RA vs 19% in controls)36–38. Therefore, combined analysis of inflammation markers and adiponectin could provide us a better understanding of both disease activity and metabolic status in RA patients. However, since patients with co-morbidities such as CAD, hypertension, diabetes, hyperlipidaemia were excluded from our study to avoid interfering with the research purposes, the role of adiponectin in metabolic disorder in our cohort is unclear and further analysis in real-world RA patients is warranted so as to explore the function of adiponectin in the setting of inflammation and metabolic disorder.
Another specificity of our study is that the enrolled patients had relatively long disease duration, high DAS28(ESR) and had not received any treatment for RA. The data from Chinese Registry of Rheumatoid Arthritis showed a mean 2.5-year delay in the diagnosis of RA after symptoms onset, 34.82% Chinese RA patients were found to have moderate disease activity and 47.18% to have high disease activity39. In our study, RA patients had an average disease duration of 108.0 ± 109.1 months without receiving adequate treatment and average DAS28(ESR) was 5.4 ± 3.3, which is classified as high disease activity. There are several challenges in the management of RA in our province which locates in southwest of China: (1) Public awareness is low and even some medical care professionals have limited knowledge of RA. (2) RA patients are unaware of the importance of standard treatment for better prognosis. Some believe in “folk medicine” and “radical therapy”. Until the disease progressed to late stage with severe deformity would they come to seek standard treatment. (3) Many primary care hospitals do not have department of rheumatology and therefore RA is often misdiagnosed. Disease duration has been demonstrated by previous studies as a significant independent predictor of joint damage, that is, bony erosions and joint space narrowing may not occur in the early stage of the disease40. In our study, 115/125 (92%) patients had an evaluable sharp score. Despite the fact we adjusted disease duration in the analysis of the association between adiponectin and Sharp score, whether our conclusion remains valid in patients with a shorter course still needs further verification, especially for patients with early arthritis, when there might be no radiographic change. Other methods, such as ultrasound or MRI, might be adopted to assess the relationship between adiponectin and the imaging outcome in patients within this period. On the other hand, different treatments may affect the level of adiponectin differentially3,27,28,41,42. Therefore, the absence of potential interferences between disease-modifying drugs and adiponectin level may have facilitated the demonstration of negative correlations between adiponectin level and DAS28(ESR) or Sharp score. Similarly, the relative homogeneity of our cohort regarding its ethnicity may have diminished the influence of potential confounding factors such as genetic background, BMI43,44 and adiponectin variants45 on the association between the adiponectin level and disease activity7.
Finally, some limitations and specificities should be taken into consideration when interpreting the data from our study. First, this is a cross-sectional clinical study without longitudinal data, and our results do not imply causation. Second, the participants were restricted to Chinese ethnicity from one district and predominantly females. It is known that Chinese populations have lower average BMI compared to other populations. Finally, as discussed above, there might be unconsidered covariates affecting RA severity or serum adiponectin such as genetic variants (i.e., RA risk HLA alleles and small nucleotide polymorphisms [SNPs] in the adiponectin gene) or adiponectin isoforms7. Further molecular investigation involving in vitro experiments and genetic comparisons in different populations are needed to untangle the pathophysiological role of adiponectin in RA progression.
In conclusion, multivariable linear regression analysis showed a negative and independent correlation between serum adiponectin and DAS28(ESR) or Sharp score after adjusting for important clinical confounders. Therefore, measurement of serum adiponectin may be potentially useful for assessing disease activity and radiographic damage of RA in Chinese patients regardless of ongoing medications.
Supplementary Information
Acknowledgements
The authors thank Dr. Xinglin Chen of the Department of Epidemiology and Biostatistics, X&Y Solutions, Boston, Massachusetts, for her assistance with statistical analysis.
Abbreviations
- BMI
Body mass index
- CCP
Cyclic citrullinated peptide
- CRP
C-reactive protein
- DAS28(ESR)
Disease activity score 28 with erythrocyte sedimentation rate
- ELISA
Enzyme-linked immunosorbent assay
- ESR
Erythrocyte sedimentation rate
- RA
Rheumatoid arthritis
- RF
Rheumatoid factor
- SJC
Swollen joint count
- SNPs
Small nucleotide polymorphisms
- TJC
Tender joint count
Author contributions
X.C., B.Z., L.L. and Q.Z. contributed to the conception and design of the work. X.C., T.L., J.W., T.Z. and J.T. contributed to data collection. K.W. and Q.Z. analysed and interpreted the data. X.C. and K.W. drafted the manuscript. L.L. and Q.Z. substantively revised the manuscript. All authors read and approved the final manuscript.
Funding
This research was supported by the Foundation for Outstanding Young Talents, Science and Technology Department of Sichuan Province (2020JDJQ0067).
Data availability
The datasets used and/or analysed in the current study are available from the corresponding author upon reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Xixi Chen and Kaiwen Wang.
Contributor Information
Li Long, Email: Longlisyy@sina.com.
Qiao Zhou, Email: qiaozhouqz@outlook.com.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-022-06115-9.
References
- 1.McInnes IB, Schett G. The pathogenesis of rheumatoid arthritis. N. Engl. J. Med. 2011;365(23):2205–2219. doi: 10.1056/NEJMra1004965. [DOI] [PubMed] [Google Scholar]
- 2.Cooles FA, Isaacs JD. Pathophysiology of rheumatoid arthritis. Curr. Opin. Rheumatol. 2011;23(3):233–240. doi: 10.1097/BOR.0b013e32834518a3. [DOI] [PubMed] [Google Scholar]
- 3.Brezovec N, et al. Adiponectin deregulation in systemic autoimmune rheumatic diseases. Int. J. Mol. Sci. 2021;22(8):4095. doi: 10.3390/ijms22084095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li P, et al. Low-molecular-weight adiponectin is more closely associated with disease activity of rheumatoid arthritis than other adiponectin multimeric forms. Clin. Rheumatol. 2015;34(6):1025–1030. doi: 10.1007/s10067-015-2899-9. [DOI] [PubMed] [Google Scholar]
- 5.Ebina K, et al. Serum adiponectin concentrations correlate with severity of rheumatoid arthritis evaluated by extent of joint destruction. Clin. Rheumatol. 2009;28(4):445–451. doi: 10.1007/s10067-008-1074-y. [DOI] [PubMed] [Google Scholar]
- 6.Minamino H, et al. Increased circulating adiponectin is an independent disease activity marker in patients with rheumatoid arthritis: A cross-sectional study using the KURAMA database. PLoS One. 2020;15(3):e0229998. doi: 10.1371/journal.pone.0229998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lei Y, et al. Association between adiponectin and clinical manifestations in rheumatoid arthritis. J. Interferon Cytokine Res. 2020;40(10):501–508. doi: 10.1089/jir.2020.0080. [DOI] [PubMed] [Google Scholar]
- 8.Khajoei S, et al. Serum levels of adiponectin and vitamin D correlate with activity of Rheumatoid Arthritis. Mol. Biol. Rep. 2019;46(2):2505–2512. doi: 10.1007/s11033-019-04682-1. [DOI] [PubMed] [Google Scholar]
- 9.Otero M, et al. Changes in plasma levels of fat-derived hormones adiponectin, leptin, resistin and visfatin in patients with rheumatoid arthritis. Ann. Rheum. Dis. 2006;65(9):1198–1201. doi: 10.1136/ard.2005.046540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Giles JT, van der Heijde DM, Bathon JM. Association of circulating adiponectin levels with progression of radiographic joint destruction in rheumatoid arthritis. Ann. Rheum. Dis. 2011;70(9):1562–1568. doi: 10.1136/ard.2011.150813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yoshino T, et al. Elevated serum levels of resistin, leptin, and adiponectin are associated with C-reactive protein and also other clinical conditions in rheumatoid arthritis. Intern. Med. 2011;50(4):269–275. doi: 10.2169/internalmedicine.50.4306. [DOI] [PubMed] [Google Scholar]
- 12.Rho YH, et al. Adipocytokines are associated with radiographic joint damage in rheumatoid arthritis. Arthritis Rheum. 2009;60(7):1906–1914. doi: 10.1002/art.24626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Šenolt L, et al. Increased adiponectin is negatively linked to the local inflammatory process in patients with rheumatoid arthritis. Cytokine. 2006;35(5):247–252. doi: 10.1016/j.cyto.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 14.Baker JF, et al. Insulin-like growth factor 1 and adiponectin and associations with muscle deficits, disease characteristics, and treatments in rheumatoid arthritis. J. Rheumatol. 2015;42:2038–2045. doi: 10.3899/jrheum.150280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Laurberg TB, et al. Plasma adiponectin in patients with active, early, and chronic rheumatoid arthritis who are steroid- and disease-modifying antirheumatic drug-naive compared with patients with osteoarthritis and controls. J. Rheumatol. 2009;36(9):1885–1891. doi: 10.3899/jrheum.080907. [DOI] [PubMed] [Google Scholar]
- 16.Lee YH, Bae SC. Circulating adiponectin and visfatin levels in rheumatoid arthritis and their correlation with disease activity: A meta-analysis. Int. J. Rheum. Dis. 2018;21(3):664–672. doi: 10.1111/1756-185X.13038. [DOI] [PubMed] [Google Scholar]
- 17.Bustos Rivera-Bahena C, et al. Peripheral blood leptin and resistin levels as clinical activity biomarkers in Mexican Rheumatoid Arthritis patients. Reumatol. Clin. 2016;12(6):323–326. doi: 10.1016/j.reuma.2015.11.011. [DOI] [PubMed] [Google Scholar]
- 18.Prevoo ML, et al. Modified disease activity scores that include twenty-eight-joint counts. Development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum. 1995;38(1):44–48. doi: 10.1002/art.1780380107. [DOI] [PubMed] [Google Scholar]
- 19.van der Heijde D. How to read radiographs according to the Sharp/van der Heijde method. J. Rheumatol. 2000;27(1):261–263. [PubMed] [Google Scholar]
- 20.Clark TG, Altman DG. Developing a prognostic model in the presence of missing data: An ovarian cancer case study. J. Clin. Epidemiol. 2003;56(1):28–37. doi: 10.1016/s0895-4356(02)00539-5. [DOI] [PubMed] [Google Scholar]
- 21.Donders AR, et al. Review: A gentle introduction to imputation of missing values. J. Clin. Epidemiol. 2006;59(10):1087–1091. doi: 10.1016/j.jclinepi.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 22.Gerdes S, et al. Adiponectin levels in a large pooled plaque psoriasis study population. J. Dermatol. Treat. 2020;31(5):531–534. doi: 10.1080/09546634.2019.1621979. [DOI] [PubMed] [Google Scholar]
- 23.Staiger H, et al. Relationship of serum adiponectin and leptin concentrations with body fat distribution in humans. Obes. Res. 2003;11(3):368–372. doi: 10.1038/oby.2003.48. [DOI] [PubMed] [Google Scholar]
- 24.Komai N, et al. Anti-tumor necrosis factor therapy increases serum adiponectin levels with the improvement of endothelial dysfunction in patients with rheumatoid arthritis. Mod. Rheumatol. 2007;17(5):385–390. doi: 10.1007/s10165-007-0605-8. [DOI] [PubMed] [Google Scholar]
- 25.Liu D, Luo S, Li Z. Multifaceted roles of adiponectin in rheumatoid arthritis. Int. Immunopharmacol. 2015;28(2):1084–1090. doi: 10.1016/j.intimp.2015.08.013. [DOI] [PubMed] [Google Scholar]
- 26.Lee SW, et al. Adiponectin mitigates the severity of arthritis in mice with collagen-induced arthritis. Scand. J. Rheumatol. 2008;37(4):260–268. doi: 10.1080/03009740801910346. [DOI] [PubMed] [Google Scholar]
- 27.Serelis J, et al. Effect of anti-TNF treatment on body composition and serum adiponectin levels of women with rheumatoid arthritis. Clin. Rheumatol. 2008;27(6):795–797. doi: 10.1007/s10067-008-0855-7. [DOI] [PubMed] [Google Scholar]
- 28.Härle P, et al. No change of serum levels of leptin and adiponectin during anti-tumour necrosis factor antibody treatment with adalimumab in patients with rheumatoid arthritis. Ann. Rheum. Dis. 2006;65(7):970–971. doi: 10.1136/ard.2005.040857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vasileiadis GK, et al. Adipocytokines in untreated newly diagnosed rheumatoid arthritis: Association with circulating chemokines and markers of inflammation. Biomolecules. 2021;11(2):325. doi: 10.3390/biom11020325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhu N, et al. High-molecular-weight adiponectin and the risk of type 2 diabetes in the ARIC study. J. Clin. Endocrinol. Metab. 2010;95(11):5097–5104. doi: 10.1210/jc.2010-0716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ouchi N, et al. Novel modulator for endothelial adhesion molecules: Adipocyte-derived plasma protein adiponectin. Circulation. 1999;100(25):2473–2476. doi: 10.1161/01.cir.100.25.2473. [DOI] [PubMed] [Google Scholar]
- 32.Hotta K, et al. Plasma concentrations of a novel, adipose-specific protein, adiponectin, in type 2 diabetic patients. Arterioscler. Thromb. Vasc. Biol. 2000;20(6):1595–1599. doi: 10.1161/01.atv.20.6.1595. [DOI] [PubMed] [Google Scholar]
- 33.Yang L, et al. Prognostic value of adiponectin level in patients with coronary artery disease: A systematic review and meta-analysis. Lipids Health Dis. 2019;18(1):227. doi: 10.1186/s12944-019-1168-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Solomon DH, et al. Patterns of cardiovascular risk in rheumatoid arthritis. Ann. Rheum. Dis. 2006;65(12):1608–1612. doi: 10.1136/ard.2005.050377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maradit-Kremers H, et al. Increased unrecognized coronary heart disease and sudden deaths in rheumatoid arthritis: A population-based cohort study. Arthritis Rheum. 2005;52(2):402–411. doi: 10.1002/art.20853. [DOI] [PubMed] [Google Scholar]
- 36.Chung CP, et al. Prevalence of the metabolic syndrome is increased in rheumatoid arthritis and is associated with coronary atherosclerosis. Atherosclerosis. 2008;196(2):756–763. doi: 10.1016/j.atherosclerosis.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 37.Mirjafari H, et al. Seropositivity is associated with insulin resistance in patients with early inflammatory polyarthritis: Results from the Norfolk Arthritis Register (NOAR): an observational study. Arthritis Res. Ther. 2011;13(5):R159. doi: 10.1186/ar3476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Giles JT, et al. Insulin resistance in rheumatoid arthritis: Disease-related indicators and associations with the presence and progression of subclinical atherosclerosis. Arthritis Rheumatol. 2015;67(3):626–636. doi: 10.1002/art.38986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tian X, Li M, Zeng X. The current status and challenges in the diagnosis and treatment of rheumatoid arthritis in China: An annual report of 2019. Rheumatol. Immunol. Res. 2021;2(1):49–56. doi: 10.2478/rir-2021-0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Eksioglu E, et al. Articular damage in late rheumatoid arthritis. Clin. Rheumatol. 2007;26(3):314–318. doi: 10.1007/s10067-006-0293-3. [DOI] [PubMed] [Google Scholar]
- 41.Toussirot EBD, Gueugnon C, Dumoulin G. Adiponectin in autoimmune diseases. Curr. Med. Chem. 2012;19(32):7. doi: 10.2174/092986712803833119. [DOI] [PubMed] [Google Scholar]
- 42.Toussirot E, et al. Increased high molecular weight adiponectin and lean mass during tocilizumab treatment in patients with rheumatoid arthritis: A 12-month multicentre study. Arthritis Res. Ther. 2020;22(1):224. doi: 10.1186/s13075-020-02297-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang Y, et al. Elevated adiponectin predicts the development of rheumatoid arthritis in subjects with obesity. Scand. J. Rheumatol. 2020;49(6):452–460. doi: 10.1080/03009742.2020.1753808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chihara K, et al. Re-evaluation of serum leptin and adiponectin concentrations normalized by body fat mass in patients with rheumatoid arthritis. Sci. Rep. 2020;10(1):15932. doi: 10.1038/s41598-020-73068-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhao YL, et al. Association of adiponectin and adiponectin receptor gene polymorphisms with rheumatoid arthritis in a Chinese population. Postgrad. Med. J. 2020;96(1133):149–155. doi: 10.1136/postgradmedj-2018-136372. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analysed in the current study are available from the corresponding author upon reasonable request.