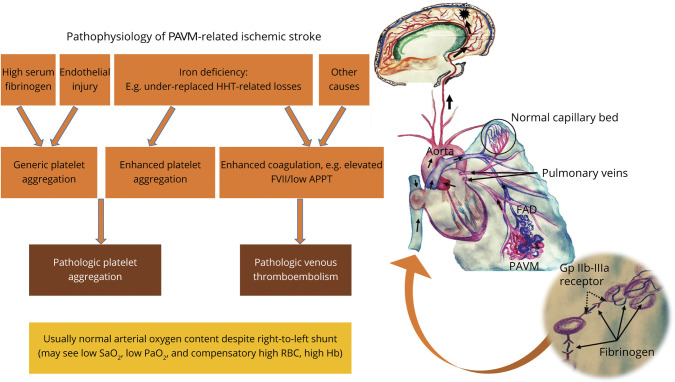
Figure 3. Schematic Model of the Implicative Pathophysiology of Pulmonary Arteriovenous Malformation–Related Ischemic Stroke.
Iron deficiency, which in patients with hereditary hemorrhagic telangiectasia (HHT) is most likely to be a result of underreplacement of hemorrhagic losses, has been shown to mediate a complex serotonergic proaggregatory platelet response facilitated by a dysfunctional platelet monoamine oxidase.3 Patients with ischemic stroke with pulmonary arteriovenous malformations (PAVMs) have also been found to have elevated plasma fibrinogen levels, a protein that normally serves to crosslink the glycoprotein (Gp) IIb-IIIa platelet receptors (inset), further accentuating the role of platelet aggregation.3 While iron deficiency has also been associated with elevated serum factor VIII levels and venous thromboembolism (VTE), clinical3 and epidemiologic19 studies have not found conventional VTE to be commonly associated with PAVM-related ischemic stroke. Recurrent endothelial injury (e.g., from dysplastic vasculature) or other molecular mechanisms of hypercoagulability implicated in different right-to-left shunts (e.g., elevated homocysteine levels in patent foramen ovale)38 may further constitute additional thromboembolic or clot-independent mechanisms of cerebrovascular injury. APPT = activated partial thromboplastin time; FAD = feeding artery diameter; FVII = factor VIII; Hb = hemoglobin; RBC = red blood cell.