Abstract
Background and Objectives
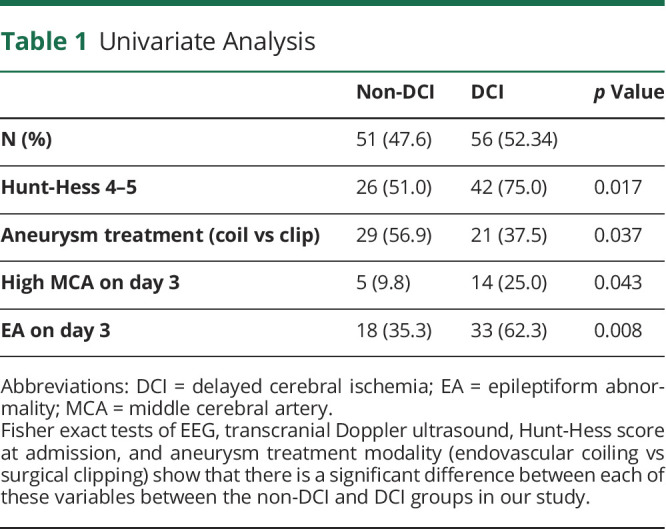
Delayed cerebral ischemia (DCI) is the leading complication of subarachnoid hemorrhage (SAH). Because DCI was traditionally thought to be caused by large vessel vasospasm, transcranial Doppler ultrasounds (TCDs) have been the standard of care. Continuous EEG has emerged as a promising complementary monitoring modality and predicts increased DCI risk. Our objective was to determine whether combining EEG and TCD data improves prediction of DCI after SAH. We hypothesize that integrating these diagnostic modalities improves DCI prediction.
Methods
We retrospectively assessed patients with moderate to severe SAH (2011–2015; Fisher 3–4 or Hunt-Hess 4–5) who had both prospective TCD and EEG acquisition during hospitalization. Middle cerebral artery (MCA) peak systolic velocities (PSVs) and the presence or absence of epileptiform abnormalities (EAs), defined as seizures, epileptiform discharges, and rhythmic/periodic activity, were recorded daily. Logistic regressions were used to identify significant covariates of EAs and TCD to predict DCI. Group-based trajectory modeling (GBTM) was used to account for changes over time by identifying distinct group trajectories of MCA PSV and EAs associated with DCI risk.
Results
We assessed 107 patients; DCI developed in 56 (51.9%). Univariate predictors of DCI are presence of high-MCA velocity (PSV ≥200 cm/s, sensitivity 27%, specificity 89%) and EAs (sensitivity 66%, specificity 62%) on or before day 3. Two univariate GBTM trajectories of EAs predicted DCI (sensitivity 64%, specificity 62.75%). Logistic regression and GBTM models using both TCD and EEG monitoring performed better. The best logistic regression and GBTM models used both TCD and EEG data, Hunt-Hess score at admission, and aneurysm treatment as predictors of DCI (logistic regression: sensitivity 90%, specificity 70%; GBTM: sensitivity 89%, specificity 67%).
Discussion
EEG and TCD biomarkers combined provide the best prediction of DCI. The conjunction of clinical variables with the timing of EAs and high MCA velocities improved model performance. These results suggest that TCD and cEEG are promising complementary monitoring modalities for DCI prediction. Our model has potential to serve as a decision support tool in SAH management.
Classification of Evidence
This study provides Class II evidence that combined TCD and EEG monitoring can identify delayed cerebral ischemia after SAH.
Delayed cerebral ischemia (DCI) is the leading complication of subarachnoid hemorrhage (SAH). Previously, it was believed that DCI is caused solely by large vessel vasospasm, and thus, transcranial Doppler ultrasound (TCD) currently serves as the standard of care for DCI monitoring. Although TCD serves as a noninvasive, portable, bedside monitoring exam, it is done infrequently (at best 1–2 times per day), is operator dependent, can be limited by patient anatomy (poor temporal bone window), and can be affected by other physiologic measures (such as heart rate and blood pressure). In addition, we now know that vasospasm alone does not fully explain DCI.1-3
Continuous EEG (cEEG) has emerged as a promising supplementary diagnostic tool for DCI prediction and addresses some limitations of TCD monitoring. cEEG is noninvasive and portable and, most importantly, can provide several days of continuous data. Studies have demonstrated quantitative cEEG measures such as relative alpha variability and poststimulation alpha/delta ratio4-6 and epileptiform abnormalities (EAs)7,8 to be associated with DCI. There is also evidence that patients usually first exhibit cEEG changes prior to developing DCI and that EEG is more strongly associated with DCI than elevated TCD velocities.8,9 TCD and cEEG offer potentially synergistic information about DCI risk. Yet the combined utility of TCD and cEEG data for DCI prediction has not been assessed.
We sought to address whether integrating TCD and cEEG measures can identify DCI after SAH. We hypothesized that combining TCD and cEEG parameters in a single model will improve DCI prediction compared to either modality alone.
This study provides Class II evidence that combined TCD and EEG monitoring can identify delayed cerebral ischemia after SAH.
Methods
Study Population
We retrospectively evaluated cEEG, TCD, and electronic medical records from 107 patients with moderate to high-grade SAH from the Massachusetts General Hospital (MGH) between September 2011 and January 2015. The inclusion criteria were age ≥18 years; moderate to high-grade SAH (Hunt-Hess grade 4–5 or Fisher group 3–4); nontraumatic SAH; TCD data available; and cEEG lasting at least 24 hours and not discontinued more than 24 hours before diagnosed DCI events. We excluded patients who developed nonconvulsive or convulsive status epilepticus due to confounding of EEG interpretation. We perform daily TCD monitoring as part of standard clinical care, and record peak systolic velocities (PSVs) at the middle (MCA), anterior (ACA), and posterior (PCA) cerebral arteries. We performed cEEG monitoring as part of standard clinical care in all high-grade SAH cases. Monitoring typically began within 48 hours of admission and continued for 10 days.
Standard Protocol Approvals, Registrations, and Patient Consents
For this retrospective analysis, we sought approval from the MGH institutional review board (IRB) to conduct this study (IRB 2013P001024). The IRB approved waiver of participant consent.
Transcranial Doppler
We only looked at MCA PSV, as the sensitivity and specificity of ACA and PCA TCD values for predicting DCI are limited10 and were not consistently available in many patients. We defined high-MCA velocity as MCA PSV measurement ≥200 cm/s. While this is a classic threshold for vasospasm,11-13 we chose to use the term high-MCA velocity in this article to denote arterial narrowing and avoid confusion, given that “vasospasm” and “DCI” have been used interchangeably in the literature.14-16
EEG Recordings
cEEGs were recorded using conventional 10–20 scalp electrode placement. We defined EAs as seizures, epileptiform discharges, lateralized or generalized periodic discharges, and lateralized rhythmic delta activity. The presence or absence of these abnormalities on each day, based on daily cEEG reports generated by fellowship-trained clinical neurophysiologists, was tallied for each patient with “day of bleed” marked as day 0.
DCI Alarms
We defined DCI alarms as the presence of either an EA or high MCA velocity.
DCI Classification
We defined DCI according to an international consensus definition as either (1) new focal neurologic deficits or decrease in the Glasgow Coma Scale of at least 2 points, persisting for a minimum of 1 hour, not explained by other causes (e.g., complications of a procedure, sedation, spike in intracranial pressure, rerupture, hydrocephalus, systemic or metabolic abnormalities) by means of clinical assessment, imaging, or laboratory data; or (2) the presence of cerebral infarction on CT or MRI of the brain, acquired at the discretion of the clinical team, that was not present on any neuroimaging done within the first 48 hours following early aneurysm occlusion, and not attributable to other causes such as surgical clipping or endovascular treatment.14 Although “delayed neurologic deterioration” is a more general term in the absence of angiographic or radiologic evidence, we consider the 2 definitions overlapping. The consensus definition more specifically refers to this as “clinical deterioration caused by DCI,” which we abbreviate to DCI for conciseness.
As previously published,7 we adjudicated the presence or absence of DCI using a multistep process of (1) prospective daily structured research coordinator interviews with the clinical team, (2) independent medical record review by 3 of the authors (E.S.R., M.B.W., S.F.Z.) blinded to cEEG and TCD findings, and (3) consensus adjudication in any case of uncertainty or disagreement.
Data Analysis
We compared baseline characteristics between DCI and non-DCI groups with 2-tailed t tests and Fisher exact tests. We censored longitudinal data once patients developed DCI. We imputed missing data for the MCA PSV via mean (linear) imputation.
We used swimmer plots to visualize the temporal relationship between EAs, high-MCA velocity, and DCI for individual patients. We calculated cumulative distribution functions for the first instance of EA and high-MCA velocity. We used a nonparametric bootstrap with 1,000 replications to estimate 95% confidence intervals (CIs). Then, we compared differences in the incidence of these events across DCI and non-DCI groups with Kolmogorov-Smirnov tests.
We used logistic regression and forward stepwise selection to select TCD and cEEG predictors of DCI. We treated TCD data in 2 different ways: (1) as a binary, max carried forward predictor, whether someone had high-MCA velocity (defined as PSV ≥200 cm/s) on or before each day; and (2) as a continuous, max carried forward predictor using the highest PSV values on or before each day. We also used cEEG data as a binary max carried forward predictor by dichotomizing based on whether a patient had any form of EA present on or before each day. We fit a series of logistic regressions using these TCD and cEEG predictors of DCI and selected the earliest day that was significantly associated with DCI. Then we explored the utility of combining TCD and cEEG in a multivariate regression model. Finally, we calculated model accuracy, sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), updated post-test probabilities, and c statistics. The updated post-test probabilities (denoted as “ΔPPV” and “ΔNPV”) are calculated by subtracting PPV from the study's DCI prevalence and subtracting NPV from the study's non-DCI prevalence, respectively. The c statistic is equal to the area underneath the receiver operator characteristic curve. The closer the c statistic is to 1, the better the model performance. These metrics were calculated through leave-one-out cross-validation (LOO-CV). LOO-CV fits a model on all but 1 patient at a time, and the model predicts the outcome of the observation left out. This process is done iteratively, then pooled to compare the actual outcomes to calculate model performance metrics. We also reported these metrics based on the threshold defined by the Youden index, 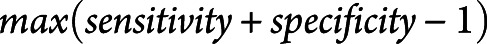 , for DCI prediction. For sensitivity analysis, we compared model prediction for early DCI and late DCI. We defined early DCI as any DCI event occurring on or prior to the median DCI date of our cohort.
, for DCI prediction. For sensitivity analysis, we compared model prediction for early DCI and late DCI. We defined early DCI as any DCI event occurring on or prior to the median DCI date of our cohort.
We used group-based trajectory modeling (GBTM) to describe the evolution of TCD and cEEG over time and test the association of trajectory group membership with DCI. GBTM is a finite mixture model that assumes a population is composed of a specified number of subgroups that follow distinct trajectories of 1 or more repeated measures over time; in this case, MCA PSV and EA. Rather than assuming individuals' group membership a priori, GBTM probabilistically gathers individuals into statistically meaningful subgroups. After each sequential observation, individuals' posterior probability of membership in each trajectory group is updated based on full available data. These posterior probabilities can then enter into predictive models such as logistic regression to test the association of trajectory group membership with outcome.17-20
We included data from the first 10 days after SAH for each patient in GBTMs. We jointly modeled MCA PSV using a beta distribution and EA using a binomial distribution. To select the optimal number of trajectory groups, we compared the Bayesian Information Criteria for each of the models that we fitted. We identified an inflection point that was the best balance between model fit and parsimony. We used LOO-CV to calculate the posterior probability of group membership for each patient on each day. We then entered these posteriors into adjusted outcome models to predict DCI. We assessed model performance by calculating model accuracy, sensitivity, specificity, PPV, NPV, updated post-test probabilities, and c statistics, again using the threshold defined by the Youden index. For the best model, we performed sensitivity analysis by reporting model performance for early and late DCI events.
We also modeled time to DCI using a survival regression model with binary TCD, EEG, and demographic variables as features. To assess performance for each of these survival regression models, we used LOO-CV and reported the cumulative sensitivity, dynamic specificity, and c statistics at each time point.
Statistical analysis was done using R (The R Foundation) and GBTM analysis was done using the Traj package in STATA (StataCorp). Significance was determined based on α = 0.05.
We attempted to address sources of bias via prospective identification of patients and adjudication of DCI classification. There is a risk of selection bias in the inclusion criteria, but it is clinically justified because rates of DCI are higher in high-grade SAH.
Data Availability
The data from this study are available from the corresponding author on request.
Results
Cohort Composition
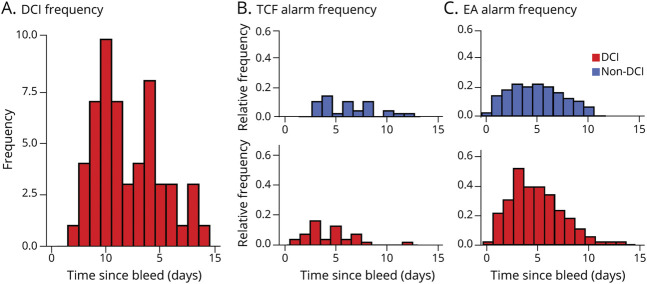
A total of 227 patients were screened and 107 were confirmed eligible and included in the study. No patient was lost to follow-up given the short timeline of DCI development. Of the 107, 56 (52.3%) experienced DCI. The median day of DCI was 6 (interquartile range 5, 9). DCI most commonly occurred on days 5 (10/56 [17.86%]) and 9 (8/56 [14.29%]) (Figure 1A). The mean age was 56.5 years (SD ±14.17) and 75 (70.1%) patients were female. EA and high-MCA velocity incidences peaked on day 3 for the DCI group (Figure 1, B and C). Table 1 presents the variables considered in our analysis.
Figure 1. High Middle Cerebral Artery Velocity and Epileptiform Abnormality Incidences Across Delayed Cerebral Ischemia and Non–Delayed Cerebral Ischemia Groups.
Patients were monitored with transcranial Doppler ultrasound (TCD) for an average (±SD) of 8.98 (±4.30) days with a mean start day 1.75 (±1.18) after subarachnoid hemorrhage (SAH). The mean duration of continuous EEG (cEEG) recordings was 6.32 (±3.22) days with a mean start date of 1.94 (±1.30) days post-SAH. (A) Histogram of delayed cerebral ischemia (DCI) incidence over the first 15 days post-SAH shows that peak DCI incidence occurs on day 5 (10/56 patients with DCI [17.86%]) and day 9 (8/56 patients with DCI [14.28%]). (B) Histogram of TCD alarms over the first 15 days post-SAH shows that, for patients with DCI, peak incidence of TCD alarms occurs on day 3, and peak incidence of TCD alarms for patients without DCI occurs on day 4. The first instance of any TCD alarm occurrence within the non-DCI group occurred on day 3. (C) Histogram of EEG alarms shows that a higher proportion of patients with DCI get EEG alarms. eTable 1 (links.lww.com/WNL/B676) shows counts of DCI and patients without DCI tabulated against DCI alarms occurring at any time during monitoring (prior to DCI).
Table 1.
Univariate Analysis
High-MCA Velocity and Presence of EAs Precede DCI
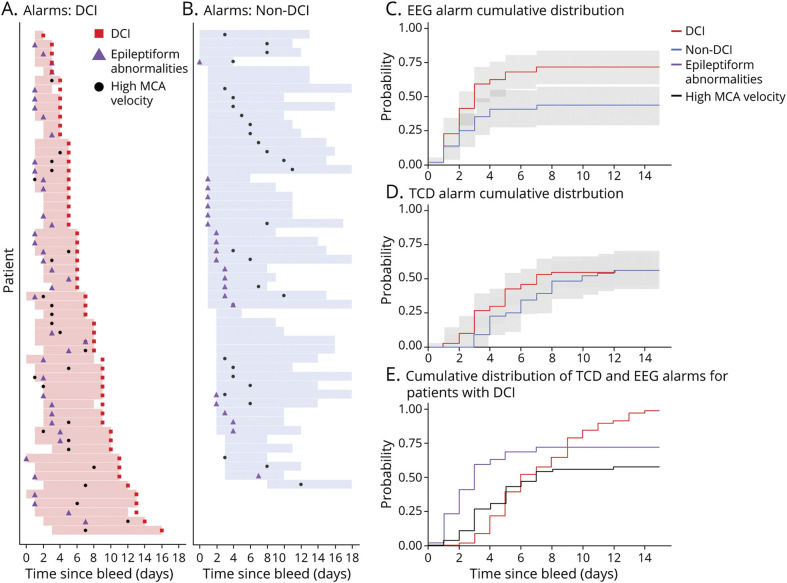
We visualized the time relationship between the first occurrence of high-MCA velocity, EA, and DCI using swimmer plots (Figure 2, A and B). The plot shows that 53/56 (94.64%) patients with DCI experienced at least 1 DCI alarm before their DCI event, compared with 42/51 (82.35%) of patients without DCI (p = 0.08).
Figure 2. Delayed Cerebral Ischemia Alarms and Time of First Occurrence in Relation to Delayed Cerebral Ischemia.
For patients with delayed cerebral ischemia (DCI) (A), most receive at least 1 kind of DCI alarm prior to DCI occurrence. However, many of the patients without DCI (B) also receive DCI alarms. Cumulative probability plots of the first EEG alarm (C) show that patients with DCI receive their first EEG alarm earlier than patients without DCI, though the difference was not significant until day 5. The cumulative probability plots of the first TCD alarm was not different between non-DCI and DCI groups (D). Finally, (E) shows that in general, EEG alarms precede TCD alarms, and both precede DCI occurrence. MCA = middle cerebral artery.
We created cumulative plots to visualize and compare the first instances of EA and high-MCA velocity in the DCI and non-DCI groups. The timing at which the first instance of EA occurs differed in the DCI vs non-DCI groups (Kolmogorov-Smirnov test, p < 0.01), with the separation in the 95% CI occurring on day 5. There was no significant difference in the first instance of high-MCA velocities between the DCI and non-DCI groups (Figure 2, C and D).
For patients with DCI who had both DCI alarms (19/56 [33.93%]), EA often preceded high-MCA velocity (14/19, by a mean of 2.5 days), and both preceded DCI (Figure 2E). When we tested each DCI alarm independently and in combination during a patient's monitoring period to predict DCI, we found that using the occurrence of either EA or high-MCA velocity resulted in much higher sensitivity (94.64%), but lower specificity (17.65%), and using both EA and high-MCA velocity resulted in a much higher specificity (82.35%) but lower sensitivity (33.93%) compared to using a single alarm type to predict DCI. Still, these analyses had limited performance.
Logistic Regressions With Single-Day EEG and TCD Measures
To evaluate the time dependence of the DCI alarms, we fit max carried forward logistic regressions using continuous TCD velocities. Then, we fit logistic regressions using binary max carried forward predictors of cEEG and TCD. These models thus account for data on or before that day. Continuous MCA PSV values were not significantly associated with DCI on any day, but high-MCA velocity occurrence on or before day 3 (p = 0.042) was a significant predictor of DCI. For cEEG, EA occurrence on or before day 3 (p < 0.01), day 5 (p = 0.028), and day 6 (p = 0.028) were significant predictors of DCI. Day 4 was not significant (p = 0.059).
We combined EA (p = 0.007) and high-MCA velocity (p = 0.024) occurrence on or before day 3 as independent predictors of DCI in a multivariate regression model. The model using both high-MCA velocity and EA presence on or before day 3 (sensitivity 76.09%, specificity 56.82%) outperformed the MCA-only (sensitivity 27.45%, specificity 89.36%) and EA-only (sensitivity 66.00%, specificity 61.70%) models in terms of sensitivity, but c statistics remain limited (c = 0.5405, 95% CI 0.4141–0.6670).
GBTM Outcome Modeling Based on Final Group Trajectory Membership
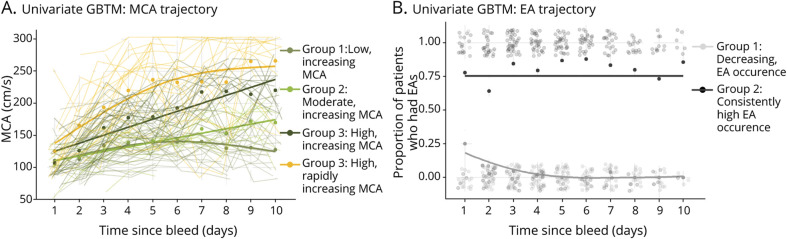
To capture trajectory information over time, we implemented GBTM modeling as detailed in the Methods. We first modeled continuous MCA velocities over time and found that a 4-subgroup GBTM model best fits the data (Figure 3A). Although all 4 subgroups have distinctive trajectories from one another (p < 0.05), only 1 group (yellow, high and rapidly increasing PSV, 17/26 [65.38% DCI]) was significantly associated with DCI (odds ratio [OR] 4.84, p = 0.02, Figure 3A).
Figure 3. Univariate Group-Based Trajectory Modeling.
In univariate group-based trajectory modeling (GBTM) of middle cerebral artery (MCA) (A), only the group experiencing high, rapidly increasing peak systolic velocities (yellow, group 4) had a significant increase of delayed cerebral ischemia (DCI) risk (eTable 2, links.lww.com/WNL/B676; odds ratio [OR] 4.84, p = 0.02). Dots represent average MCA value of individuals across the trajectory group, and the solid lines represent the best fit line of each MCA subgroup. Thin lines represent individual MCA trajectories over time. In univariate GBTM of epileptiform abnormality (EA) (B), group 2 (dark gray) was associated with a significant increase of DCI risk (eTable 3; OR 5.09, p < 0.01) when compared to group 1 (light gray). Opaque dots represent the EA prevalence in the subgroup on each day, and the solid lines represent the best fit line of each EA subgroup. The semitransparent dots centered around 0 and 1 represent individuals who did (1) and did not (0) have an EA on that specific day.
We then modeled EA incidences using GBTM and found that 2 distinct subgroups best fit the data (Figure 3B). Patients in group 2 (dark gray, consistently high EA, 37/53 [69.81% DCI]) had a higher risk of DCI (OR 5.09, p < 0.01) compared to those in group 1 (light gray, decreasing EA, 18/53 [33.96% DCI]). Patients assigned to group 1 experienced either no occurrences of EA or only early during monitoring.
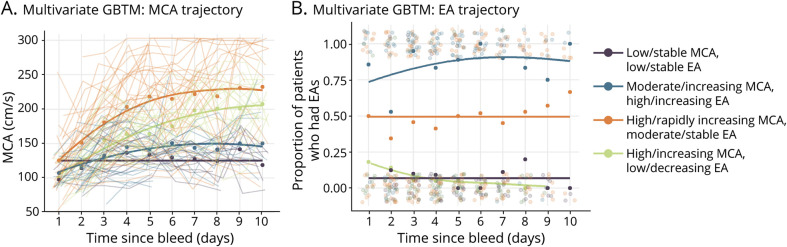
We modeled trajectories of EA and MCA PSV jointly through a multivariate-trajectory model. This type of GBTM model simultaneously accounts for MCA PSV and EA trajectories when determining subgroups, and is thus a distinct model from the ones described previously. The best fit multivariate-trajectory GBTM identified 4 distinct groups when MCA PSV and EA were modeled jointly (Figure 4, A and B). Using group 1 (purple, low/stable PSV and EA, 1/13 [7.69% DCI]) as a reference group, group 4 (navy blue, moderate/increasing PSV and high/increasing EA, 23/32 [71.87% DCI]) had an increased risk of DCI (OR 23.30, p < 0.01). Group 3 (orange, high/rapidly increasing PSV, moderate/stable EA, 22/33 [66.67% DCI]) also had an increased risk of DCI (OR 18.04, p < 0.01) compared to group 1. Group 2 (light green, high/increasing PSV and moderate/stable EA, 6/23 [26.09% DCI]) did not have a significantly different risk of DCI compared to group 1, but the incidence of DCI remained low in both groups.
Figure 4. Multivariate Group-Based Trajectory Modeling.
Multivariate group-based trajectory modeling (GBTM) identified 4 distinct subgroups of middle cerebral artery (MCA) (A) and epileptiform abnormality (EA) (B) when trajectories from both modalities are modeled jointly. Patients in “high risk” groups experience high and rapidly increasing MCA peak systolic velocities (PSVs) along with moderate EA (orange; odds ratio [OR] 18.04, p < 0.01) and moderate and increasing MCA PSVs and increasing EA (navy blue; OR 23.30, p < 0.01) (eTable 4, links.lww.com/WNL/B676). Using trajectory group membership on days 3 and 5 has fair model performance (eTable 5).
GBTM Outcome Modeling Based on Single-Day Trajectory Membership
Regressing the final group trajectory membership probabilities with DCI identified the group trajectory memberships that were significantly associated with DCI. While this was useful to describe a patient's overall risk for DCI, to predict DCI using these group trajectory memberships, we used daily group membership probabilities predicted with LOO-CV and regressed them with DCI outcome.
Daily univariate MCA PSV group trajectory memberships were not significantly associated with DCI. Univariate EA trajectory group membership served as a significant predictor of DCI as early as day 3 (sensitivity 64%, specificity 62.75%) and peaked on day 5 (sensitivity 73.53%, specificity 52.94%).
In the multivariate GBTM, group membership served as a significant predictor on days 3–7 (p < 0.05). The day 3 multitrajectory group membership model (sensitivity 85.11%, specificity 48.98%) had better sensitivity than the day 3 EA-only trajectory membership model. Model performance using group membership on day 5 performed comparably (sensitivity 81.82%, specificity 42.86%), but may be more limited in its clinical utility given 12/56 (21.42%) patients with DCI experienced their DCI event before day 5.
Inclusion of Clinical Predictors in Logistic Regressions and GBTMs
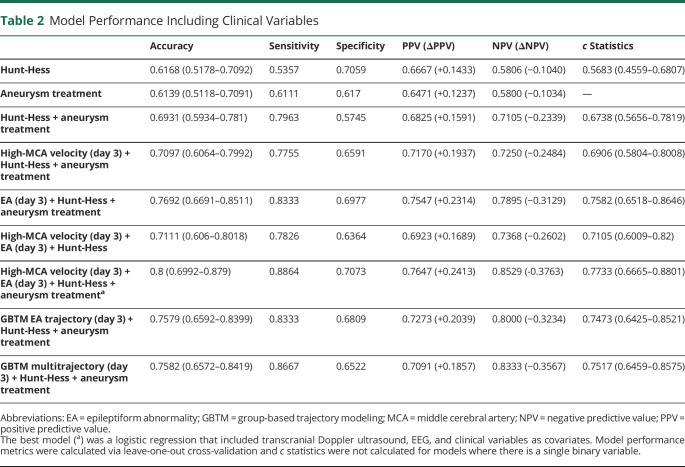
We performed logistic regressions with clinical variables as predictors of DCI and found that higher Hunt-Hess score at admission (p = 0.004; sensitivity 53.57%, specificity 70.59%) and clipping of aneurysm (p = 0.024; sensitivity 61.11%, specificity 61.7%) were significantly associated with increased risk of DCI. A model with only these 2 clinical variables performed with sensitivity of 79.63% and specificity of 57.45%.
We included these clinical variables in the best performing logistic regression model. A final adjusted logistic regression model with the addition of Hunt-Hess score at admission (p = 0.016) and aneurysm treatment (p = 0.013) as independent clinical covariates of DCI along with EA (p = 0.006) and high-MCA velocity (p = 0.072) on or before day 3 resulted in a model with 88.64% sensitivity and 70.73% specificity.
For GBTM models, addition of the Hunt-Hess score improved the univariate EA day 3 model (sensitivity 72.92%, specificity 72.34%). The addition of data on aneurysm treatment also improved the EA day 3 trajectory model in terms of specificity (sensitivity 60.42%, specificity 78.72%), but most notably improved model sensitivity on day 6 (sensitivity 92%, specificity 44.68%). The model with all 3 variables (EA day 3 trajectory membership, Hunt-Hess, and aneurysm treatment) had the best performance (sensitivity 83.33%, specificity 68.09%). Models using trajectory group information on subsequent days had similar performances (sensitivity 80.95%–84.38%, specificity 65.95 from days 4–6).
Inclusion of aneurysm treatment and Hunt-Hess score as clinical variables also improve the day 3 multivariate-trajectory group membership model (sensitivity 86.67%, specificity 65.22%). This adjusted multivariate trajectory model has the best sensitivity on day 6 (sensitivity 87.5%, specificity 60.87%).
The best logistic regression and GBTM models include both significant clinical variables and both monitoring modalities. A summary of these model performances can be found in Table 2, and an overview of models in our study can be found in Figure 5.
Table 2.
Model Performance Including Clinical Variables
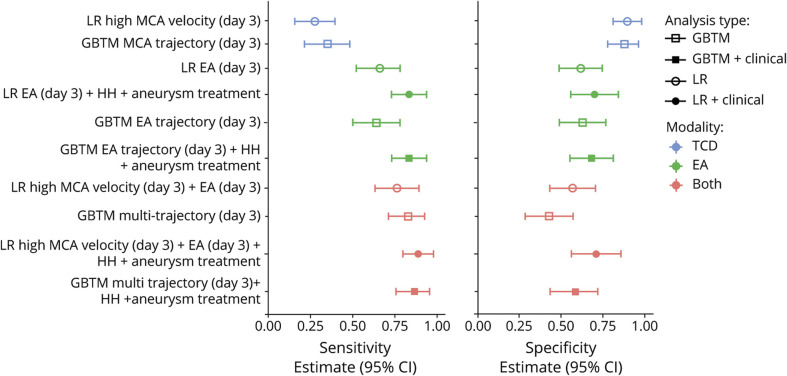
Figure 5. Model Comparison.
A comparison of TCD only (blue), EEG only (green) and combined (red) models show that the best models used both modalities and clinical variables. GBTM models performed comparably to logistic regressions.
Survival Regression Models
The best survival model used only EA, Hunt-Hess, and aneurysm treatment modality. However, model performance prior to day 5 was limited. The best performance (c statistic) did not occur until after 8 days post-SAH (eFigure 1, links.lww.com/WNL/B676).
Sensitivity Analysis
When we assess our best logistic regression (day 3 with TCD, cEEG, and clinical variables) in early vs late DCI events, our model is better at predicting DCI events occurring ≤ day 6 (sensitivity 91.30%, specificity 73.17%, c = 0.8155 [0.7126–0.9183]) compared to > day 6 (sensitivity 85.71%, specificity 70.73%, c = 0.7271 [0.5905–0.8636]). A similar pattern can be found with our best GBTM model (day 3 multivariate trajectory membership and clinical variables) (≤ day 6 [sensitivity 95.24%, specificity 67.39%, c = 0.7857 (0.6787–0.8928)] vs > day 6 [sensitivity 91.67%, specificity 56.52%, c = 0.7219 (0.5979–0.8459)]).
Discussion
We show that combining EEG and TCD data improves prediction of DCI over either modality alone. Most patients with DCI (94.64%) have at least 1 DCI alarm prior to the DCI event. For patients with DCI who had both alarms, EA often preceded high-MCA velocity, and both preceded DCI. The addition of 2 clinical variables (Hunt-Hess score at admission and aneurysm treatment modality [i.e., surgical clipping or endovascular coiling]) further improved model performance.
High MCA velocity alone at any time during monitoring (up to the day of DCI or discontinuation) weakly predicts DCI. Although we tried to analyze TCD velocities as a continuous variable, binary max carried forward variable, or with GBTMs, none of these approaches improved the univariate model performance. Our best TCD-only model (sensitivity 27.45%, specificity 89.36%) had worse sensitivity than what was described in a recent meta-analysis, where TCD vasospasm (defined by mean flow velocity ≥120 cm/s) had an 89% (76–95%) sensitivity and 71% (56–81%) specificity for DCI.21 This is possibly due to variable definitions of DCI, as most studies included in the meta-analysis were published prior to the consensus guideline14 or our use of peak rather than mean flow velocities secondary to data availability. It is also increasingly recognized that DCI can occur without angiographic or radiologic vasospasm, and vice versa.3,22 This may be another potential cause for the TCD models' limited performance in our study.
Based on our previously published finding that EA occurrence is higher in patients with DCI,7 we more closely investigated the timing of EA as a predictive marker of DCI in this study. In EA-only logistic regression models, EA was a significant predictor of DCI as early as day 3 (sensitivity 66%, specificity 61.70%), and model performance peaked at day 6 (sensitivity 67.65%, specificity 57.14%). EA-only logistic regression models could detect impending DCI with a higher sensitivity than TCD-only models, a finding also reported in a previous study by our group.8 For many patients with DCI, EA alarms also preceded TCD velocities crossing the 200 cm/s threshold (Figure 2E). Other studies have shown similar findings where cEEG changes such as decreasing relative alpha variability6 and decreasing alpha and theta power23 preceded detection of vasospasm on TCD.
The univariate GBTM model for EA identified 2 trajectory membership groups associated with DCI risk. The group with consistent EA occurrence over time is associated with a 5-fold increase in odds of DCI (69.81% DCI) compared to the group where EAs occur in the beginning but disappear over time (28.30% DCI). This result suggests that individuals who have persistent EAs tend to be at an increased risk of DCI compared to individuals with transient early EAs.
The multivariate GBTM model identified 4 distinct groups. Groups 1 (purple; low/stable MCA PSVs and EA) and 2 (green; high/increasing MCA PSVs and low/decreasing EAs) can be considered benign trajectories, where most individuals belonging to these groups did not experience DCI. This contrasts with group 4 (navy blue; moderate/increasing PSV and high/increasing EA), where most individuals assigned to this final group trajectory did experience DCI. It seems that EA, rather than MCA PSV, drives the trajectory groups' association with DCI risk. If patients consistently have EAs, as is the case in group 4 (navy blue), DCI risk increases. If EA is decreasing over time, DCI risk does not increase, even in the presence of increasing MCA PSV (groups 2, 3, 4).
GBTMs performed comparably to logistic regressions for univariate EA and the multivariate models. GBTM was recently shown to improve upon the accuracy of logistic regressions in models predicting outcomes of patients after cardiac arrest.20 Thus, we expected the trajectory information to enhance prediction compared to logistic regressions, but this was not the case. This may be because our logistic regression variables are max carried forward and not a time-invariant variable, like those used in the 2019 study to compare GBTM and logistic regressions. Of note, both GBTM and logistic regression models performed better than single-day logistic regressions (that is, time-invariant, not max carried forward). It seems that incorporating longitudinal information improves model performance, without a clear benefit of one model over another. Practically, there is some benefit of this, as implementing a logistic regression model may be easier for clinicians to adapt on a large scale. However, the incorporation of trends through GBTM may be important in capturing the changes that occur across the full window of DCI occurrence. There are dynamic processes that occur after SAH, including early injury factors (e.g., blood–brain barrier disruption, seizures, hydrocephalus, inflammation, and edema) that can happen in the first 72 hours postictus24-26 as well as late injury factors (e.g., delayed cerebral ischemia, delayed hydrocephalus). Looking at trends of both cEEG and TCD may better capture these changes. It is possible that future evaluations of specific EA features, beyond presence or absence of EA, will prove more robust. Incorporating hourly trends may also make the addition of trends information more valuable.
After evaluating both TCD-only and EA-only models, we found that DCI prediction improves when both modalities are considered together. This is because cEEG and TCD help assess different aspects of DCI physiology, namely the metabolic supply–demand mismatch.7,24,27 TCD allows us to directly evaluate reductions in the supply related to large vessel vasospasm. EEG, on the other hand, will enable us to look at markers of excess demand, like EA. By combining these 2 modalities, we attempt to capture data reflecting 2 sides of this delicate balance and end up with a better prediction algorithm.
In our study, higher Hunt-Hess score at admission and clipping of aneurysms were significantly associated with increased DCI risk and improve logistic regression and multivariate GBTM performances. Although the radiographic severity of SAH is also significantly associated with DCI risk based on the literature,28-33 our data were limited to cEEG monitoring mainly in patients with high Fisher scores (3–4). Thus, we were unable to test it as an independent clinical predictor.
These results highlight the importance of clinical variables on the overall prediction of complications like DCI after SAH. Existing literature that uses a combination of clinical and radiographic grading scales to predict DCI has fair discrimination, with c statistics ranging from 0.63 to 0.79.34-36
Our best model, a multivariate logistic regression with binarized, max carried forward EA and TCD values on day 3, Hunt-Hess, and aneurysm treatment, achieved a c statistic of 0.77 (95% CI 0.67–0.88). We note that the other models, which only include radiographic and clinical scales, may be easier to implement when cEEG monitoring is not available. While our model needs to be externally validated, our results show TCD and cEEG as promising complementary monitoring modalities for DCI prediction and can serve as a decision support tool in SAH management.
Our study has a few limitations. We did not have enough TCD measurements available of other arteries to calculate measures such as the Lindergaard ratio or to independently assess the utility of ACA and PCA velocities in DCI prediction. The institutional TCD data velocities were preferentially recorded as PSVs rather than mean flow velocities, and while these values were internally validated to correlate with other modalities for assessing vasospasm, it remains possible that these measurements contributed to the lower sensitivity in our logistic regression models using only TCD information. Mean flow velocity values are better defined in the literature, with at least 17 TCD studies using mean flow to evaluate DCI from 1992 to 2014.21 A comparison of PSV to mean flow velocity performance in other datasets could help elucidate if this was the case. While our dataset is relatively large for a DCI study, we did not have an independent validation cohort. In the future, larger cohorts across multiple institutions would help externally validate our findings. Our study is limited to EEG text reports and the EEG reports extracted did not comment on the trends of spectral patterns. Thus, we limited our analysis to a daily, binary assessment of cEEG as the presence or absence of EA. There is rich information to be gained from cEEG that can be used to enhance the models. Combining spectral cEEG measures associated with DCI, such as alpha-delta ratio,4,5,37 relative alpha variability,5,6 and total power,37 could improve DCI prediction when used with TCD and should be explored further in the future.
This study provides new evidence that cEEG and TCD together provide an improved prediction of DCI. TCD and cEEG provide synergistic information and models using both TCD and cEEG outperformed models using either modality alone. Models that consider the timing of DCI alarms, using different approaches, performed better than models that did not. Simple clinical variables (Hunt-Hess score and aneurysm treatment modality) further improve multimodal performance with the best model using these clinical variables in addition to the presence of either EA or high-MCA velocity up to day 3 for DCI prediction.
Acknowledgment
J. Elmer is supported by the National Institute of Neurological Disorders and Stroke (NINDS) (5K23NS097629, R01NS119825). S.F. Zafer is supported by the NIH (K23NS114201). E.S. Rosenthal was supported by the NIH (1K23NS105950). L.J. Hirsch received research support from The Daniel Raymond Wong Neurology Research Fund at Yale; consultation fees for advising from Aquestive, Ceribell, Marinus, Medtronic, Neuropace, and UCB; royalties for authoring chapters for UpToDate Neurology and from Wiley for coauthoring Atlas of EEG in Critical Care; and honoraria for speaking from Neuropace and Natus. H.P. Zaveri is supported by the NIH (NS109062). K.N. Sheth is supported by the NIH (U24NS107136, U24NS107215, R01NR018335, R01NS107215, U01NS106513, R03NS112859) and the American Heart Association (18TPA34170180, 17CSA33550004, 20SRG35540018). Yale University receives grants to support Dr. Sheth's research from Biogen, Novartis, Hyperfine, and Bard. Dr. Sheth receives equity from Alva and fees from Zoll for his role as a DSMB Chair. N.H. Petersen received funding from the NIH (K23NS110980). M.B. Westover received funding from Glenn Foundation for Medical Research, American Federation for Aging Research (Breakthroughs in Gerontology), the American Academy of Sleep Medicine Strategic Research Award, DoD Moberg ICU Solutions, Inc., subcontract, and the NIH (1R01NS102190, 1R01NS102574, 1R01NS107291, 1RF1AG064312). J.A. Kim received funding from NINDS (R25N065743, K23NS112596-01A1), American Academy of Neurology Clinical Research Training Scholarship, American Heart Association, and Bee Foundation.
Glossary
- ACA
anterior cerebral artery
- cEEG
continuous EEG
- CI
confidence interval
- DCI
delayed cerebral ischemia
- EA
epileptiform abnormality
- GBTM
group-based trajectory modeling
- IRB
institutional review board
- LOO-CV
leave-one-out cross-validation
- MCA
middle cerebral artery
- MGH
Massachusetts General Hospital
- NPV
negative predictive value
- OR
odds ratio
- PCA
posterior cerebral artery
- PPV
positive predictive value
- PSV
peak systolic velocity
- SAH
subarachnoid hemorrhage
- TCD
transcranial Doppler ultrasound
Appendix. Authors
Footnotes
Class of Evidence: NPub.org/coe
Study Funding
The authors report no targeted funding.
Disclosure
The authors report no disclosures relevant to the manuscript. Go to Neurology.org/N for full disclosures.
References
- 1.Vora YY, Suarez-Almazor M, Steinke M, Martin ML, Findlay M. Role of transcranial Doppler monitoring in the diagnosis of cerebral vasospasm after subarachnoid hemorrhage. Neurosurgery. 1999;44(6):1243-1247. [PubMed] [Google Scholar]
- 2.Roos YB, De Haan RJ, Beenen LF, Groen RJ, Albrecht KW, Vermeulen M. Complications and outcome in patients with aneurysmal subarachnoid haemorrhage: a prospective hospital based cohort study in The Netherlands. J Neurol Neurosurg Psychiatry. 2000;68(3):337-341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dankbaar JW, Rijsdijk M, Van Der Schaaf IC, Velthuis BK, Wermer MJH, Rinkel GJE. Relationship between vasospasm, cerebral perfusion, and delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage. Neuroradiology. 2009;51(12):813-819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Claassen J, Hirsch LJ, Kreiter KT, et al. Quantitative continuous EEG for detecting delayed cerebral ischemia in patients with poor-grade subarachnoid hemorrhage. Clin Neurophysiol. 2004;115(12):2699-2710. [DOI] [PubMed] [Google Scholar]
- 5.Rots ML, van Putten MJAM, Hoedemaekers CWE, Horn J. Continuous EEG monitoring for early detection of delayed cerebral ischemia in subarachnoid hemorrhage: a pilot study. Neurocrit Care. 2016;24(2):207-216. [DOI] [PubMed] [Google Scholar]
- 6.Vespa PM, Nuwer MR, Juhász C, et al. Early detection of vasospasm after acute subarachnoid hemorrhage using continuous EEG ICU monitoring. Electroencephalogr Clin Neurophysiol. 1997;103(6):607-615. [DOI] [PubMed] [Google Scholar]
- 7.Kim JA, Rosenthal ES, Biswal S, et al. Epileptiform abnormalities predict delayed cerebral ischemia in subarachnoid hemorrhage. Clin Neurophysiol. 2017;128(6):1091-1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rosenthal ES, Biswal S, Zafar SF, et al. Continuous electroencephalography predicts delayed cerebral ischemia after subarachnoid hemorrhage: a prospective study of diagnostic accuracy. Ann Neurol. 2018;83(5):958-969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rathakrishnan R, Gotman J, Dubeau F, Angle M. Using continuous electroencephalography in the management of delayed cerebral ischemia following subarachnoid hemorrhage. Neurocrit Care. 2011;14(2):152-161. [DOI] [PubMed] [Google Scholar]
- 10.Washington CW, Zipfel GJ. Detection and monitoring of vasospasm and delayed cerebral ischemia: a review and assessment of the literature. Neurocrit Care. 2011;15(2):312-317. [DOI] [PubMed] [Google Scholar]
- 11.Marshall SA, Nyquist P, Ziai WC. The role of transcranial Doppler ultrasonography in the diagnosis and management of vasospasm after aneurysmal subarachnoid hemorrhage. Neurosurgery. 2010;21(2):291-303. [DOI] [PubMed] [Google Scholar]
- 12.Aaslid R, Markwalder TM, Nornes H. Noninvasive transcranial Doppler ultrasound recording of flow velocity in basal cerebral arteries. J Neurosurg. 1982;57(6):769-774. [DOI] [PubMed] [Google Scholar]
- 13.Kirsch JD, Mathur M, Johnson MH, Gunabushanam G, Scoutt LM. Advances in transcranial Doppler US: imaging ahead. Radiographics. 2013;33(1):1-15. [DOI] [PubMed] [Google Scholar]
- 14.Vergouwen MDI, Vermeulen M, van Gijn J, et al. Definition of delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage as an outcome event in clinical trials and observational studies: proposal of a multidisciplinary research group. Stroke. 2010;41(10):2391-2395. [DOI] [PubMed] [Google Scholar]
- 15.Weir BKA, Kongable GL, Kassell NF, et al. Cigarette smoking as a cause of aneurysmal subarachnoid hemorrhage and risk for vasospasm: a report of the Cooperative Aneurysm Study. J Neurosurg. 1998;89(3):405-411. [DOI] [PubMed] [Google Scholar]
- 16.Lynch JR, Wang H, McGirt MJ, et al. Simvastatin reduces vasospasm after aneurysmal subarachnoid hemorrhage: results of a pilot randomized clinical trial. Stroke. 2005;36(9):2024-2026. [DOI] [PubMed] [Google Scholar]
- 17.Nagin DS. Analyzing developmental trajectories: a semiparametric, group-based approach. Psychol Methods. 1999;4(2):139-157. [DOI] [PubMed] [Google Scholar]
- 18.Nagin DS. Group-based modeling of development. Choice Rev. 2005. [Google Scholar]
- 19.Nagin DS, Odgers CL. Group-based trajectory modeling in clinical research. Annu Rev Clin Psychol. 2010;6(1):109-138. [DOI] [PubMed] [Google Scholar]
- 20.Elmer J, Jones BL, Zadorozhny VI, et al. A novel methodological framework for multimodality, trajectory model-based prognostication. Resuscitation. 2019;137:197-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kumar G, Shahripour RB, Harrigan MR. Vasospasm on transcranial Doppler is predictive of delayed cerebral ischemia in aneurysmal subarachnoid hemorrhage: a systematic review and meta-analysis. J Neurosurg. 2016;124(5):1257-1264. [DOI] [PubMed] [Google Scholar]
- 22.Dankbaar JW, De Rooij NK, Velthuis BK, Frijns CJM, Rinkel GJE, Van Der Schaaf IC. Diagnosing delayed cerebral ischemia with different CT modalities in patients with subarachnoid hemorrhage with clinical deterioration. Stroke. 2009;40(11):3493-3498. [DOI] [PubMed] [Google Scholar]
- 23.Gollwitzer S, Groemer T, Rampp S, et al. Early prediction of delayed cerebral ischemia in subarachnoid hemorrhage based on quantitative EEG: a prospective study in adults. Clin Neurophysiol. 2015;126(8):1514-1523. [DOI] [PubMed] [Google Scholar]
- 24.Foreman BP. The pathophysiology of delayed cerebral ischemia. J Clin Neurophysiol. 2016;33(3):174-182. [DOI] [PubMed] [Google Scholar]
- 25.Fujii M, Yan J, Rolland WB, Soejima Y, Caner B, Zhang JH. Early brain injury, an evolving frontier in subarachnoid hemorrhage research. Transl Stroke Res. 2013;4(4):432-446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cossu G, Messerer M, Oddo M, Daniel RT. To look beyond vasospasm in aneurysmal subarachnoid haemorrhage. Biomed Res Int. 2014;2014:628597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dreier JP, Isele T, Reiffurth C, et al. Is spreading depolarization characterized by an abrupt, massive release of gibbs free energy from the human brain cortex? Neuroscientist. 2013;19(1):25-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van der Steen WE, Leemans EL, van den Berg R, et al. Radiological scales predicting delayed cerebral ischemia in subarachnoid hemorrhage: systematic review and meta-analysis. Neuroradiology. 2019;61(3):247-256. [DOI] [PubMed] [Google Scholar]
- 29.Claassen J, Bernardini GL, Kreiter K, et al. Effect of cisternal and ventricular blood on risk of delayed cerebral ischemia after subarachnoid hemorrhage: the Fisher scale revisited. Stroke. 2001;32(9):2012-2020. [DOI] [PubMed] [Google Scholar]
- 30.De Rooij NK, Rinkel GJE, Dankbaar JW, Frijns CJM. Delayed cerebral ischemia after subarachnoid hemorrhage: a systematic review of clinical, laboratory, and radiological predictors. Stroke. 2013;44(1):43-54. [DOI] [PubMed] [Google Scholar]
- 31.Crobeddu E, Mittal MK, Dupont S, Wijdicks EFM, Lanzino G, Rabinstein AA. Predicting the lack of development of delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage. Stroke. 2012;43(3):697-701. [DOI] [PubMed] [Google Scholar]
- 32.Vergouwen MDI. Vasospasm versus delayed cerebral ischemia as an outcome event in clinical trials and observational studies. Neurocrit Care. 2011;15(2):308-311. [DOI] [PubMed] [Google Scholar]
- 33.Frontera JA, Claassen J, Schmidt JM, et al. Prediction of symptomatic vasospasm after subarachnoid hemorrhage: the modified Fisher scale. Neurosurgery. 2006;59(1):21-27. [DOI] [PubMed] [Google Scholar]
- 34.De Oliveira Manoel AL, Jaja BN, Germans MR, et al. The vasograde: a simple grading scale for prediction of delayed cerebral ischemia after subarachnoid hemorrhage. Stroke. 2015;46(7):1826-1831. [DOI] [PubMed] [Google Scholar]
- 35.De Rooij NK, Greving JP, Rinkel GJE, Frijns CJM. Early prediction of delayed cerebral ischemia after subarachnoid hemorrhage: development and validation of a practical risk chart. Stroke. 2013;44(5):1288-1294. [DOI] [PubMed] [Google Scholar]
- 36.Fang YJ, Mei SH, Lu JN, et al. New risk score of the early period after spontaneous subarachnoid hemorrhage: for the prediction of delayed cerebral ischemia. CNS Neurosci Ther. 2019;25(10):1173-1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Labar DR, Fisch BJ, Pedley TA, Fink ME, Solomon RA. Quantitative EEG monitoring for patients with subarachnoid hemorrhage. Electroencephalogr Clin Neurophysiol. 1991;78(5):325-332. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data from this study are available from the corresponding author on request.