Abstract
Skeletal muscle myofibers have differential protein expression resulting in functionally distinct slow- and fast-twitch types. While certain protein classes are well-characterized, the depth of all proteins involved in this process is unknown. We utilized the Human Protein Atlas (HPA) and the HPASubC tool to classify mosaic expression patterns of staining across 49,600 unique tissue microarray (TMA) images using a visual proteomic approach. We identified 2164 proteins with potential mosaic expression, of which 1605 were categorized as “likely” or “real.” This list included both well-known fiber-type-specific and novel proteins. A comparison of the 1605 mosaic proteins with a mass spectrometry (MS)-derived proteomic dataset of single human muscle fibers led to the assignment of 111 proteins to fiber types. We additionally used a multiplexed immunohistochemistry approach, a multiplexed RNA-ISH approach, and STRING v11 to further assign or suggest fiber types of newly characterized mosaic proteins. This visual proteomic analysis of mature skeletal muscle myofibers greatly expands the known repertoire of twitch-type-specific proteins.
Keywords: Human Protein Atlas, proteomics, skeletal muscle, twitch
Graphical Abstract
INTRODUCTION
Skeletal muscle is a striated muscle type responsible for voluntary movement. Skeletal muscles consist of different fiber types delineated by the isoform of the myosin heavy chain they express, their metabolic function, and other properties.1 In humans, slow-twitch fibers (type 1) and some fast-twitch fibers (type 2A) exhibit oxidative metabolic properties, while fast-type 2X fibers exhibit glycolytic metabolic properties.2 Fiber types are variable across different muscles of the body, reflecting different functional needs.2,3
Multiple proteins and protein classes vary across fiber types.1,4 These include isoforms of the myosin heavy and light chains, calcium ATPase pumps, troponin T, and tropomyosin proteins, as well as metabolic proteins, such as pyruvate kinase, GAP dehydrogenase, and succinate dehydrogenase. Beyond these classes, there has been little effort to catalog the entirety of fast/slow-twitch expression differences by proteomics, despite calls for this approach.2
The deepest effort, to date, has been the single fiber proteomics work of the Mann laboratory.5,6 Overall, separate studies of mouse and human single-fiber skeletal muscles reported 1723 and 5115 proteins, respectively. In the deeper human study, among the 5115 proteins described, roughly 875 measured ≥2-fold different across fast- and slow-twitch fiber types. Further expression differences were noted across young vs old individuals’ fibers. As powerful as mass spectrometry-based proteomics can be, the depth of protein discovery is often limited by the input amount, leading to rarer proteins being frequently missed. An approach that can observe these more lowly expressed proteins is the visual proteomic method of immunohistochemistry (IHC).
Mosaic protein expression between slow- and fast-twitch fibers is well-established and easily visualized using IHC or immunofluorescence. The myosin heavy-chain isotypes are often used to observe these patterns. This clear and variable pattern allowed us to ascertain if a more complete understanding of a mosaic expression pattern could be obtained from a powerful, image-rich proteomic resource.
The Human Protein Atlas (HPA) is a comprehensive repository of IHC-stained tissue microarrays for numerous tissues, including skeletal muscle tissues.7 Currently, >15,300 genes and >26,000 antibodies across >1,000,000 images are available for review in this database.8 We have previously built the tool HPASubC as a spatial proteomic tool to rapidly interrogate images of the HPA and characterize novel subproteomes of organs, such as endothelial and smooth muscle cell-specific expression of the heart and nonhepatocyte expression in the liver.9,10 More recently, we identified a mosaic pattern of expression of cardiac myocytes.11
Here, we used the deep dataset of the HPA along with other tools and resources to provide the deepest understanding and characterization of the variably expressed proteome of skeletal muscles.
MATERIALS AND METHODS
HPA and HPASubC
HPA is a comprehensive repository of IHC-stained tissue microarrays for numerous tissues, including skeletal muscles.7,8 The HPASubC tool can rapidly and agnostically interrogate images of HPA to characterize specific staining patterns in organs.9,10,12 HPASubC v1.2.4 was used to download 49,600 unique skeletal muscle tissue microarray images covering 12,620 unique proteins from the HPA website (v18). The images were individually reviewed using HPASubC by K.M.F. to evaluate the presence of a mosaic pattern of protein expression based on IHC staining. The classification of mosaicism was based on a prestudy training set of 300 images from HPA reviewed collaboratively (K.M.F. and M.K.H.). Mosaicism was defined as a dispersed pattern of differential staining in which a significant number of nonadjacent muscle fibers had a higher staining intensity than the surrounding fibers, preferably persisting across the entire microarray. All positive selections made by the trainee were reviewed and rescored, as needed, by a board-certified pathologist (M.K.H.).
After an initial fast review of the images, a secondary review to score the images was performed. A three-tiered classification system was used, indicating increasing certainty of mosaicism: 0 indicated the absence of mosaic staining; 1 indicated unknown mosaic staining; 2 indicated likely mosaic staining; and 3 indicated real mosaic staining. Scoring evaluation was based on the quality of the mosaic pattern, including stain intensity differential between fibers, the presence of “blush”/incomplete staining within cells, and the consistency/completeness of the fiber staining pattern throughout the sample. HPASubC was used on an Apple MacBook Pro running macOS Sierra v10.12.6 with 8 GB RAM and 3.1 GHz CPU and a Dell Precision Tower 3620 running Windows 10 with 16 GB RMA and a 3.7 GHz CPU.
Mass Spectrometry (MS) Data Set
We utilized the human skeletal muscle fiber MS-based proteomic dataset of Murgia et al.6 This dataset contained information from 3585 proteins, based on unique peptide signatures, across 152 fibers from 8 donors.6 The ratios of expression of proteins between type 1 and type 2A cells were determined using Table S6 of Murgia et al. Five hundred and ninety-six proteins with >2.3-fold differences between cell types were selected. Label-free quantification (LFQ) data, from Table S4 of Murgia et al., for the 154 human single-muscle fiber proteomics was obtained. The log2-transformed LFQ data was converted to raw values, and only proteins expressed across all fiber types (n = 94) were considered for plotting UMAP as described.6 Functions of the R-package Seurat (Version 3.1.1) were executed sequentially to derive a UMAP along with its dependency library “uwot (Version 0.1.4)” in R (Version 3.6.1).13,14 A Seurat object of the data matrix was created using “CreateSeuratObject” with default parameters. This data was normalized using the “NormalizeData” function, and outlier proteins were identified using the “FindVariableFeatures.” Proteins across the fiber types were scaled and centered to create a PCA object using “ScaleData” and “RunPCA”, respectively. Further, k-nearest neighbors and shared nearest neighbor for each fiber-type were generated on the Seurat object using “FindNeighbors” and “FindClusters” to plot UMAP using “RunUMAP.” All of these functions were executed using default parameters. The clustering obtained with UMAP was overlaid with the classification of muscle fiber types based on Murgia et al. using ggplot2 (Version 3.2.1). A two-sample t-test assuming equal variances was performed between the 43 slow-twitch myofibers in cluster 1 and the 89 fast-twitch (2A and/or 2X) myofibers in clusters 2–4.
STRING
STRING v11 (string-db.org) was used to identify protein–protein interactions.15 The single protein search option was used on HPA mosaic proteins of known twitch type (based on the MS data of Murgia et al.). All evaluated clusters had at least two proteins of known twitch type. The full complement of proteins in the protein–protein interaction cluster was searched in Gene Ontology (http://geneontology.org/) to identify the etiology of the interactions.16
Multiplexed Immunohistochemistry
Human psoas FFPE tissues were used from the same IRB source described above. Five micrometer sections were cut and placed on Superfrost Plus glass slides (Avantor), baked in a 60 °C oven for 20 min, and then incubated in fresh xylenes (Avantor) three times for 5 min each. Subsequently, the slides were incubated in a decreasing ethanol (Pharmco) gradient for 1 min each. FFPE tissue slides were transferred to a tub of 1X ImmunoDNA Retriever with citrate (Bio SB) and subjected to high pressure and high temperature for 15 min with a Cuisinart CPC-600 6-qt pressure cooker.
Staining was performed by a Lab Vision Autostainer 360, according to the manufacturer’s recommendations (Thermo Fisher Scientific). Slides were each treated with 300 μL of peroxidase blocker (Bio SB) for 5 min; 300 μL of the primary antibody (MilliporeSigma) in diluent (Bio SB) for 45 min; 300 μL of PolyDetector Link (Bio SB) for 15 min; 300 μL of PolyDetector HRP (Bio SB) for 15 min; and 300 μL of the AEC substrate (Vector Laboratories) for 25 min. Information on all primary antibodies is listed in Table S2. Slides were also rinsed once with distilled water and twice with 1X ImmunoDNA Washer (Bio SB) in between applications of each reagent.
After staining, slides were mounted with aqueous medium (MilliporeSigma) and digitally scanned at 20× magnification using a MoticEasyScan Pro (Motic). Images were analyzed using GNU Image Manipulation Program (Version 2.10.20). To create overlays, the MYL3 (slow) and TNNT3 (fast) scans were first desaturated by average HSI intensity and false-colored by adjusting the color balance. The unknowns were then desaturated, false-colored, added to a new layer at 50% opacity, and aligned by hand to the two known patterns to make an assignment of fiber-type.
The coverslip removal was performed in heated distilled water, and AEC destaining was performed using an alcohol gradient series as follows: 140, 180, 200, 180, and 140 proof.
An elution buffer comprised of 800 μL of β-mercaptoethanol (MilliporeSigma), 2 g of SDS (Avantor) in 12.5 mL of 0.5 M Tris-HCl, pH 6.8, and 87.5 mL of distilled water was used to release primary antibodies from their target proteins. Slides were incubated in 40 mL of elution buffer at 56 °C for 30 min, with gentle agitation by hand after 15 min. Afterward, the slides were incubated in fresh distilled water four times for 15 min each on an orbital shaker. Finally, the slides were incubated in fresh TBS-T twice for 30 min each on an orbital shaker.17 After completion of antibody elution, a new antibody was applied following the same methods.
RNA-ISH
Human skeletal muscles were obtained at rapid autopsy (66-year-old male), the latter under an IRB-approved protocol. Tissues were immediately fixed in formalin, and paraffin-embedded blocks were created, from which 5 μm slides were made. Custom probes for RNA in situ hybridization (RISH) were obtained from RNAscope (ACDBio). These probes were designed to detect the following genes: ENO3 (GenBank accession NM_001976.5), CYB5R1 (NM_016243.3), and SRSF11 (NM_004768.5). Each probe set targeted all validated NCBI refseq transcript variants of the gene.
The Multiplex Fluorescent Reagent Kit v2 (ACDBio) was used following the manufacturer’s instructions. Briefly, FFPE tissue slides were baked for 1 h at 60 °C. The slides were subsequently deparaffinized with xylene, rinsed with 100% ethanol, and air-dried. After the application of hydrogen peroxide and washing, slides were treated with the target retrieval reagent in a steamer (>99 °C) for 20 min. Then, the tissue was permeabilized using a protease. Hybridization of the probes to the targeted mRNAs was performed by incubation in a 40 °C oven for 2 h. After washes, the slides were processed for the standard signal amplification and application of fluorescent dye (Opal dye 520, 570, and 620, AKOYA Biosciences) steps. Finally, the slides were counterstained with DIPA, mounted with Prolong Gold Antifade Mounting solution (Invitrogen), and stored in a 4 °C room. The fluorescent images were obtained in the Johns Hopkins Microscope Core Facility using a Zeiss LSM700 laser scanning confocal microscope.
Code Availability
All analysis scripts are available at GitHub (https://github.com/mhalushka/Skeletal_muscle_mosaicism).
RESULTS AND DISCUSSION
HPA-Based Mosaic Protein Discovery
We utilized the HPASubC suite of tools to download and interrogate 49,600 unique images that cover 12,620 proteins from the HPA (Figure 1). Of this group, 2164 proteins had potential mosaic expression in skeletal muscles. Based on the aggregate image scores for any given protein determined by one or more antibodies, each protein was subsetted into categories of “real” mosaicism (374 proteins), “likely” mosaicism (1231 proteins), and “unknown” probability of mosaicism (559 proteins) (Table S1, Figure S1). For analysis purposes, we focused on the 1605 proteins that were in the “real” or “likely” categories to reduce the incidence of false-positive mosaic staining interpretation.
Figure 1.
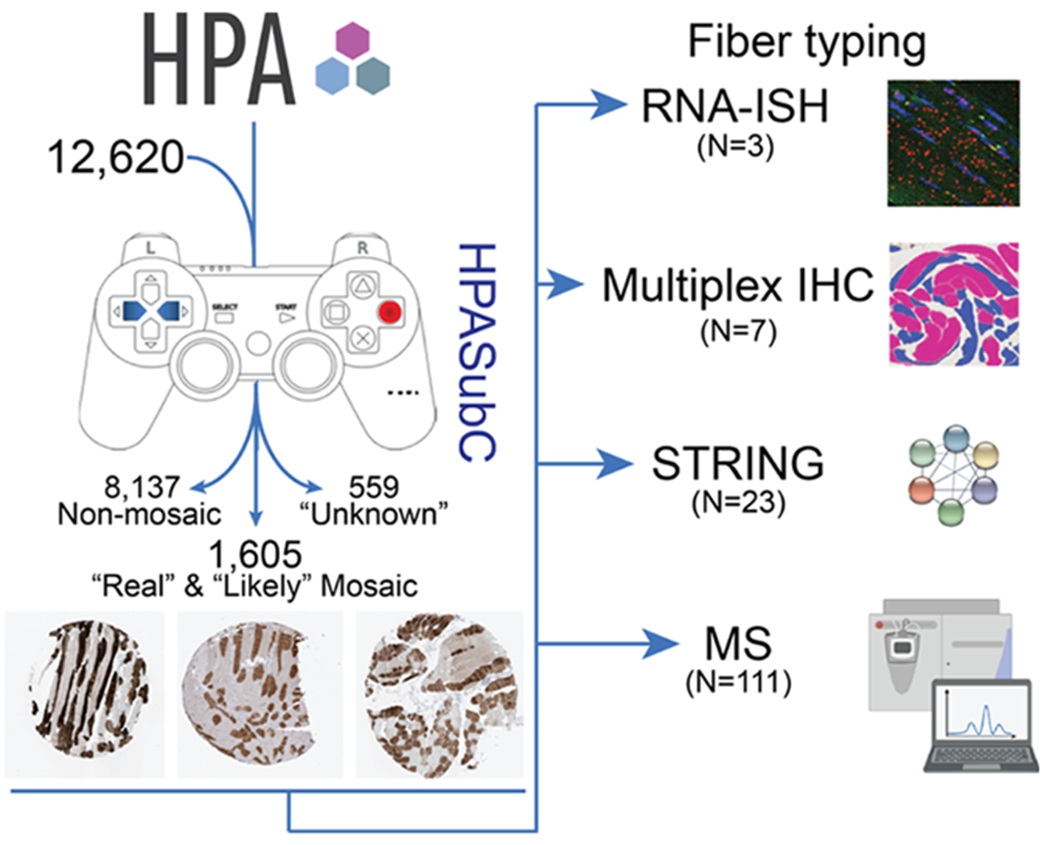
Schematic of the identification of mosaic proteins and fiber typing methods. Images from 12,620 proteins were obtained from the HPA and analyzed through the HPASubC. Of these, 8137 were nonmosaic, 559 were of unknown mosaicism, and 1605 were considered real or likely mosaicism. Of this last group, four methods were employed to assign or suggest a fiber-type of a subset of newly described mosaic expressed proteins. IHC core images from HPA. MS icon obtained from BioRender.com.
This method identified well-known fiber-type-specific proteins such as MYH1, MYH2, MYH4, MHY6, MHY7, and MYH8, which were categorized as both “real” or “likely” based on staining patterns (Table S1). It also identified numerous uncharacterized or poorly characterized proteins, such as 26 zinc finger proteins that included ZNF213, ZNF282, ZNF343, ZNF350, and ZNF408, all of which had “real” patterns of mosaicism (Figure S2).
Cross Comparison of Orthogonal Proteomic Datasets
A limitation of this spatial, IHC-based approach is that each protein image is independent of all other proteins. Thus, one cannot identify co-expression patterns to assign proteins to certain fiber types. To better understand the relationship of these proteins, we attempted several approaches to assign fiber type.
The human skeletal muscle fiber MS data in Murgia et al. consists of over 60,000 peptides mapping to 5115 proteins (4923 uniquely) identified from 152 fibers from eight donors.6 Of this group, 4139 proteins overlapped with the entire HPASubC cohort (Figure 2A). Among mosaic proteins, 558 MS proteins overlapped with the “likely” and “real” mosaic HPASubC data, which could be a potential source for assignment of fiber typing. Murgia et al. had assigned one of five fiber types to all 152 myofibers (slow, mixed slow/2A, fast 2A, fast 2X, and mixed 2A/2X) based on MYH expression patterns.
Figure 2.
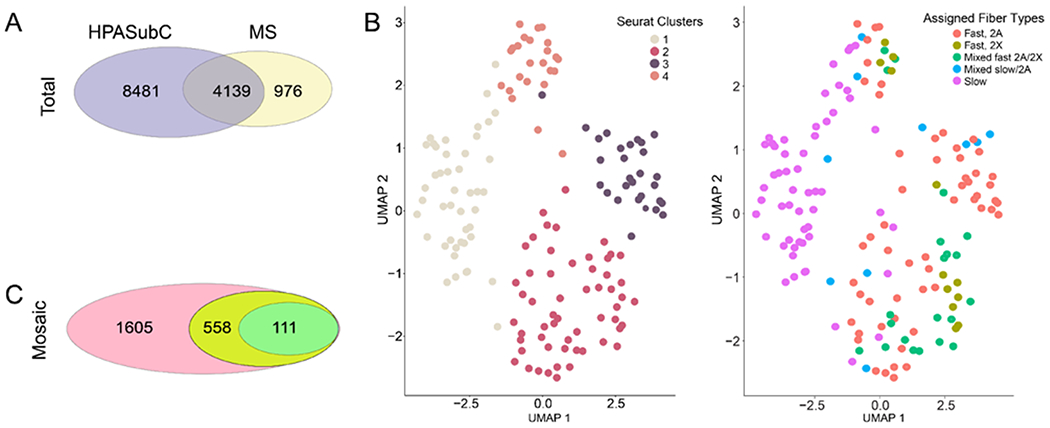
(A) Of the 12,620 HPASubC proteins, 4139 were also present in the MS dataset, while 976 proteins were unique to the MS dataset. (B) UMAPs of MS-based protein data. Seurat identified four cell clusters, which loosely correlated with the assigned fiber types. The slow-type cells are generally Seurat cluster 1. Fast 2A cells are generally in Seurat cluster 3, although they are also detected in clusters 2 and 4. Fast 2X clusters are predominately in Seurat cluster 2. (C) Of the 1605 mosaic proteins, 558 were present in the MS dataset and 111 could be used to assign fiber type.
We analyzed the full label-free quantification (LFQ) dataset of protein expression and constructed a UMAP plot that visualized four clusters (Figure 2B). One cluster was composed primarily of slow-type 1 fibers and was adjacent to a second cluster with a small mixture of slow and other cell types. Two other clusters were primarily a collection of myofibers designated as fast 2A and fast 2X cell types. The difference between cluster and assigned fiber type is the result of a myosin heavy-chain-only approach vs the more holistic expression pattern-based clustering of UMAP.6 We took advantage of both methods to develop a comparison of the assigned slow-twitch fiber samples in cluster 1 against all non slow-twitch fiber samples in clusters 2–4 to discover the proteins with the greatest differential in expression.
In the LFQ dataset, 633 proteins varied across these groups (t-test, p < 0.01). Of these, 111 proteins were also variable in the HPASubC dataset, with 73 being slow-twitch (Table S1). A check of 20 of these proteins showed that in each case the fiber-type assignment was consistent with published reports. This MS proteomics method proved useful in making fiber-type assignments from some proteins, such as Transmembrane 7 Superfamily Member 2 (TM7SF2) and Chromosome 12 Open Reading Frame 4 (C12orf4), which had not previously been described. TM7SF2 is involved in cholesterol biosynthesis and demonstrates increased expression in skeletal muscle cells refed amino acids after starvation.18 C12orf4 has been implicated in autosomal recessive intellectual disability in which two subjects had ataxia/gait disturbance.19
However, the MS dataset was not a complete answer to fiber-type assignment in that it only covered a small percent of all HPASubC-derived mosaic proteins. Additional challenges of this comparison included: (1) some known fiber-type-specific proteins, such as MYLK2, were not significant by t-test in the MS dataset;20 (2) ITPR1 and DIP2B were strongly variable by t-test in the MS dataset but not mosaic in the staining patterns as noted by HPA; and (3) numerous proteins, such as Helicase With Zinc Finger (HELZ), CCR4-NOT Transcription Complex Subunit 4 (CNOT4), and phosphati-dylinositol transfer protein, cytoplasmic 1 (PITPNC1), had clear mosaic patterns in the HPA but were not in the MS dataset (Supporting Information Figure S3).
A likely reason some mosaic proteins were not in the MS dataset was their low expression (HPA gene expression values for HELZ, CNOT4, and PITPNC1 are 2.8, 8, and 1.8 pTPM, respectively). Despite low expression, some of these proteins are compelling additions to the list of variably expressed proteins in skeletal muscles. While no activity in human skeletal muscles is known yet, HELZ was recently found to directly interact with the CCR4-NOT complex.21 Further, CNOT4 was shown to differentially express between skeletal muscles of two different sheep types.22 PITPNC1 was identified as variable between fast and slow avian myotubes in an expression array experiment but not further explored functionally in muscles.23
Assigning Fiber Type by Protein–Protein Associations
We hypothesized that HPA mosaic proteins could be designated to a twitch type if they were in a shared protein pathway with proteins of a known, consistent twitch-type. We used STRING v11 to find interaction clusters based on these shared pathways, assessing protein–protein association clusters. As a proof of concept, we assessed proteins in a myofibril/calcium ion transport cluster, based around CASQ1, a protein identified as fast-twitch in the MS dataset (Supporting Information Figure S4). Of these 21 proteins, an additional 8 were identified as fast-twitch in the MS dataset. Three more, CACNA1S, MYL1, and TFAM, were histologically mosaic but were not identified in the MS dataset. But by clustering with exclusively fast-twitch proteins, these three proteins are suggested to be fast-twitch. The remaining 9 proteins were not mosaic by our HPASubC screen. We then screened a number of additional confirmed twitch-type proteins in STRING to identify similar clusters. We identified clusters based on ACAA1 (fatty acid β-oxidation, slow-twitch), DBT (tricarboxylic acid cycle enzyme complex, slow-twitch), MYOZ2 (myofibril assembly, fast-twitch), and FBP2 (monosaccharide metabolic process, fast-twitch). These four protein clusters were able to suggest 23 additional proteins to twitch type (Table S1). Many of these associations were confirmed by mouse muscle MS data.24
So, while publications of nonhuman datasets and STRING-based associations could be used to suggest human mosaicism, in addition to the MS dataset, we sought to further validate fiber-type using additional visualization methods.
Multiplexed IHC to Define Fiber Type for Newly Characterized Proteins
We utilized a multiplex IHC method to co-register proteins of unknown fiber-type to proteins of established fiber-type. We selected 7 proteins that were a range of known and unknown fiber types that were or were not present in the MS-based data (Table 1). IHC was performed, the image was digitized, the antibodies were removed, and the cycle was repeated 7 times per slide. Using this method, we were able to establish the dominant fiber-type identities of 4 proteins that had not been previously classified by overlaying their expression with the known fiber-type-specific proteins TNNT3 (fast) and MYL3 (slow) (Figure 3). FBXW9 and ZNF408 were discreetly slow-twitch expressed, while CAMSAP2 and TESC appeared to be expressed in both fiber types but more robustly in fast-twitch fibers. A fifth protein, SYNPO, which had an unusual pattern of mosaicism in the HPA dataset, did not co-localize consistently as a fast- or slow-twitch protein (Supporting Information Figure S5).
Table 1.
Seven Proteins Evaluated by Multiplex IHCa
| Protein | multiplex IHC | publication | MS |
|---|---|---|---|
| CAMSAP2 | fast | unknown | not significantly mosaic |
| FBXW9 | slow | unknown | not present |
| MYL3 | slow | slow | slow |
| SYNPO | unknown | unknown | not significantly mosaic |
| TESC | fast | unknown | not present |
| TNNT3 | fast | fast | fast |
| ZNF408 | slow | unknown | not present |
Findings of this method are compared to what was known based on the literature or the MS dataset.
The staining was inconsistently slow or fast in different areas.
Figure 3.
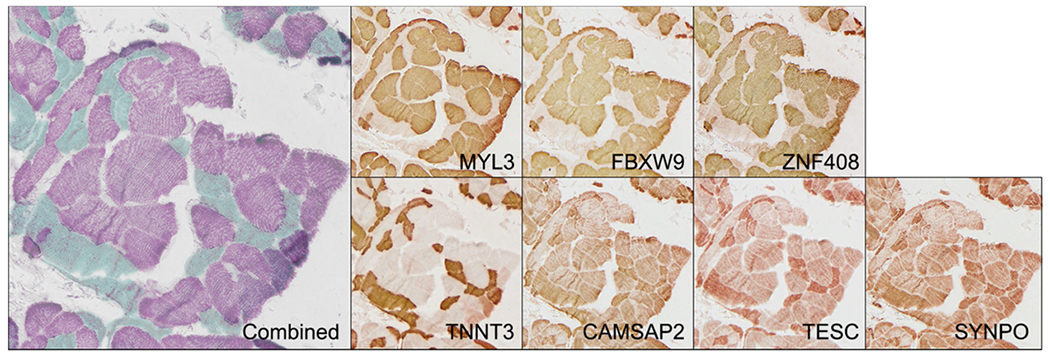
Multiplex IHC on the same tissue section identifies co-registration of proteins by twitch type. Teal fibers are fast and purple fibers are slow based on the known patterns of TNNT3 and MYL3. Individual images of each protein are displayed.
Cross Validation by RNA-ISH
We evaluated a second visualization method, RNA-ISH, to assign fiber-type to two genes, SRSF11 and CYB5R1, whose fiber-type status was poorly characterized or noncharacterized to an established fast-twitch gene (ENO3). These three genes/proteins were chosen for being mosaic by HPASubC and for being identified in the MS dataset. All three genes had expression >19 pTPM by HPA. In the MS dataset, SRSF11 was not variable across the samples by t-test, while CYB5R1 was significantly variable and designated as “slow-twitch.” We observed co-expression of all three genes in human skeletal muscle fast-twitch fibers (Figure 4). Whereas ENO3 and CYB5R1 RNA were diffusely present across human skeletal myofibers, SRSF11 was localized to subcell membrane areas. This visualized CYB5R1 RNA expression ran counter to the expectation based on the MS dataset.
Figure 4.

Representative RNA-ISH of proteins and genes. RNA-ISH demonstrates co-expression of CYB5R1 SRSF11 and ENO3 in fast-twitch myofibers. A presumed slow-twitch fiber is denoted by the star.
CONCLUSIONS
We describe a deep visual proteomic analysis of human skeletal muscles. Utilizing HPA and the HPASubC tool, we identified 1605 proteins that had moderate-to-high confidence as being variably expressed between fast- and slow-twitch fibers. This is a significant expansion over previous characterizations of the human fiber-specific proteome and will be a useful reference for scRNA-seq studies of human myofibers.
The main limitation of this visual proteomic approach employing HPA images is the lack of co-localization of signal such that the myofiber-type cannot be defined for each protein. We demonstrated four approaches, three of which can be utilized going forward. The first was to compare this dataset with a robust MS dataset that was based on known myofiber types. While the depth of that dataset was limiting (only 3585 proteins vs 10,301 proteins reviewed in HPA), it did provide a superficial fast-/slow-twitch comparison that helped us define (or be consistent with known assignments) for 111 proteins. An analysis of STRING protein–protein interactions suggested the twitch type of 23 additional proteins, some of which had been shown to be differentially fast/slow twitched based on mouse muscle data.24 We further used a multiplex IHC approach and a multiplex RNA-ISH approach to demonstrate the assignment of six additional proteins to twitch type. These later two approaches, while robust, require a large investment in antibodies or RNA probes that is beyond the scope of this project. A potential additional method could be based upon a comparison to human single-cell RNA-seq skeletal muscle datasets. As we describe elsewhere, this will be challenging for intact muscle fibers and may have to rely on nuclear RNA-seq approaches.25
A number of limitations should be recognized. As we have stated repeatedly, IHC in the HPA is subject to false-positive staining from shared epitopes, although the requirement of variable, mosaic expression within a single core of tissues reduces this cause of error.9–12 This method also has false negatives for any failed antibodies or antibodies with poor staining as a result of methodologic parameters designed for other tissues. Further, some proteins that are likely mosaic are missing in HPA. Discovery MS is challenged to identify low abundance proteins. Most fibers had between 500 and 700 proteins identified, and only through the overlap across all fibers was a value of 5115 achieved, with most proteins appearing in only a subset of fibers. The STRING analysis could only suggest fiber-type and is not as robust an analysis as the other methods. RNA-ISH is limited by the number of fluorescent channels/probes that can be visualized, which at a commercial level is currently four. Multiplex IHC is limited by the number of reuses of a tissue, but this approach is likely deeper than RNA-ISH.26
In conclusion, we have created a visual proteomic map of fiber-type-specific expression in skeletal muscles identifying hundreds of proteins that appear to demonstrate fiber-type specificity. We utilized complementary methods to demonstrate fiber-type assignment in a subset of these proteins using cross-localization strategies.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank Efrain Ribeiro and Suraj Kannan for their helpful comments on the project. The Mass Spectrometry image in Figure 1 was obtained from BioRender. M.K.H. was supported by grants 1R01HL137811, R01GM130564, and P30CA006973 from the National Institutes of Health and 17GRNT33670405 from the American Heart Association. T.O.N. was supported by grant R01GM130564. M.N.M. was supported by R01HL137811 and the University of Rochester CTSA award number UL1TR002001. A.Z.R. was supported by R01GM130564.
Footnotes
Supporting Information
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.jproteome.0c00673.
Scoring scheme for HPASubC-based skeletal muscle mosaicism (Figure S1); skeletal muscle mosaic staining patterns of 26 ZNF proteins with “real” or “likely” designations (Figure S2); nine representative images of 270 proteins scored as real mosaicism using HPASubC but not identified by MS-based proteomics (Figure S3); STRING v11 protein–protein interactions. Each cluster had at least two proteins of known twitch-type (HPASubC mosaic and identified by the MS dataset). Suggested twitch-type proteins are mosaic in HPASubC but not identified in the MS dataset. For network edges, line thickness is associated with the strength of data support (Figure S4); additional SYNPO staining (Figure S5) (PDF)
All mosaic proteins detected in the HPA Atlas (Table S1) (XLSX)
Primary antibodies used in multiplex immunohistochemistry (Table S2) (XLSX)
Complete contact information is available at: https://pubs.acs.org/10.1021/acs.jproteome.0c00673
The authors declare no competing financial interest.
Contributor Information
Katherine M. Fomchenko, Department of Pathology, Johns Hopkins University School of Medicine, Baltimore, Maryland 21205, United States
Elise M. Walsh, Department of Pathology, Johns Hopkins University School of Medicine, Baltimore, Maryland 21205, United States
Xiaoping Yang, Department of Pathology, Johns Hopkins University School of Medicine, Baltimore, Maryland 21205, United States.
Rohan X. Verma, Department of Pathology, Johns Hopkins University School of Medicine, Baltimore, Maryland 21205, United States
Brian L. Lin, Division of Cardiology, Department of Medicine, Johns Hopkins University School of Medicine, Baltimore, Maryland 21205, United States
Tim O. Nieuwenhuis, Department of Pathology, Johns Hopkins University School of Medicine, Baltimore, Maryland 21205, United States
Arun H. Patil, Department of Pathology, Johns Hopkins University School of Medicine, Baltimore, Maryland 21205, United States
Karen Fox-Talbot, Department of Pathology, Johns Hopkins University School of Medicine, Baltimore, Maryland 21205, United States.
Matthew N. McCall, Department of Biostatistics and Computational Biology, University of Rochester Medical Center, Rochester, New York 14642, United States
David A. Kass, Division of Cardiology, Department of Medicine, Johns Hopkins University School of Medicine, Baltimore, Maryland 21205, United States
Avi Z. Rosenberg, Department of Pathology, Johns Hopkins University School of Medicine, Baltimore, Maryland 21205, United States
Marc K Halushka, Department of Pathology, Johns Hopkins University School of Medicine, Baltimore, Maryland 21205, United States.
REFERENCES
- (1).Okumura N; Hashida-Okumura A; Kita K; Matsubae M; Matsubara T; Takao T; Nagai K Proteomic analysis of slow- and fast-twitch skeletal muscles. Proteomics 2005, 5, 2896–2906. [DOI] [PubMed] [Google Scholar]
- (2).Gonzalez-Freire M; Semba RD; Ubaida-Mohien C; Fabbri E; Scalzo P; Hojlund K; Dufresne C; Lyashkov A; Ferrucci L The Human Skeletal Muscle Proteome Project: a reappraisal of the current literature. J. Cachexia Sarcopenia Muscle 2017, 8, 5–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Schiaffino S; Reggiani C Fiber types in mammalian skeletal muscles. Physiol. Rev 2011, 91, 1447–1531. [DOI] [PubMed] [Google Scholar]
- (4).Drexler HC; Ruhs A; Konzer A; Mendler L; Bruckskotten M; Looso M; Gunther S; Boettger T; Kruger M; Braun T On marathons and Sprints: an integrated quantitative proteomics and transcriptomics analysis of differences between slow and fast muscle fibers. Mol. Cell. Proteomics 2012, 11, No. M111.010801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Murgia M; Nagaraj N; Deshmukh AS; Zeiler M; Cancellara P; Moretti I; Reggiani C; Schiaffino S; Mann M Single muscle fiber proteomics reveals unexpected mitochondrial specialization. EMBO Rep. 2015, 16, 387–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Murgia M; Toniolo L; Nagaraj N; Ciciliot S; Vindigni V; Schiaffino S; Reggiani C; Mann M Single Muscle Fiber Proteomics Reveals Fiber-Type-Specific Features of Human Muscle Aging. Cell Rep. 2017, 19, 2396–2409. [DOI] [PubMed] [Google Scholar]
- (7).Uhlen M; Oksvold P; Fagerberg L; Lundberg E; Jonasson K; Forsberg M; Zwahlen M; Kampf C; Wester K; Hober S; Wernerus H; Bjorling L; Ponten F Towards a knowledge-based Human Protein Atlas. Nat. Biotechnol 2010, 28, 1248–1250. [DOI] [PubMed] [Google Scholar]
- (8).Uhlen M; Fagerberg L; Hallstrom BM; Lindskog C; Oksvold P; Mardinoglu A; Sivertsson A; Kampf C; Sjostedt E; Asplund A; Olsson I; Edlund K; Lundberg E; Navani S; Szigyarto CA; Odeberg J; Djureinovic D; Takanen JO; Hober S; Alm T; Edqvist PH; Berling H; Tegel H; Mulder J; Rockberg J; Nilsson P; Schwenk JM; Hamsten M; von Feilitzen K; Forsberg M; Persson L; Johansson F; Zwahlen M; von Heijne G; Nielsen J; Ponten F Proteomics. Tissue-based map of the human proteome. Science 2015, 347, No. 1260419. [DOI] [PubMed] [Google Scholar]
- (9).Anene DF; Rosenberg AZ; Kleiner DE; Cornish TC; Halushka MK Utilization of HPASubC for the identification of sinusoid-specific proteins in the liver. J. Proteome Res 2016, 15, 1623–1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Cornish TC; Chakravarti A; Kapoor A; Halushka MK HPASubC: A suite of tools for user subclassification of human protein atlas tissue images. J. Pathol. Inf 2015, 6, 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Wang TY; Lee D; Fox-Talbot K; Arking DE; Chakravarti A; Halushka MK Cardiomyocytes have mosaic patterns of protein expression. Cardiovasc. Pathol 2018, 34, 50–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Cheah JX; Nieuwenhuis TO; Halushka MK An expanded proteome of cardiac t-tubules. Cardiovasc. Pathol 2019, 42, 15–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Stuart T; Butler A; Hoffman P; Hafemeister C; Papalexi E; Mauck WM 3rd; Hao Y; Stoeckius M; Smibert P; Satija R Comprehensive Integration of Single-Cell Data. Cell 2019, 177, 1888–1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Becht E; McInnes L; Healy J; Dutertre CA; Kwok IWH; Ng LG; Ginhoux F; Newell EW Dimensionality reduction for visualizing single-cell data using UMAP. Nat. Biotechnol 2019, 37, 38–44. [DOI] [PubMed] [Google Scholar]
- (15).Szklarczyk D; Gable AL; Lyon D; Junge A; Wyder S; Huerta-Cepas J; Simonovic M; Doncheva NT; Morris JH; Bork P; Jensen LJ; Mering CV STRING v11: protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res 2019, 47, D607–D613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Gene Ontology Consortium The Gene Ontology Resource: 20 years and still GOing strong Nucleic Acids Res. 2019, 47D1 D330 D338 DOI: 10.1093/nar/gky1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Gendusa R; Scalia CR; Buscone S; Cattoretti G Elution of High-affinity (>10-9 KD) Antibodies from Tissue Sections: Clues to the Molecular Mechanism and Use in Sequential Immunostaining. J. Histochem. Cytochem 2014, 62, 519–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Iresjö BM; Lundholm K Myosin heavy chain 2A and alpha-actin expression in human and murine skeletal muscles at feeding; particularly amino acids. J. Transl. Med 2012, 10, 238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Philips AK; Pinelli M; de Bie CI; Mustonen A; Maatta T; Arts HH; Wu K; Roepman R; Moilanen JS; Raza S; Varilo T; Scala G; Cocozza S; Gilissen C; van Gassen KL; Jarvela I Identification of C12orf4 as a gene for autosomal recessive intellectual disability. Clin. Genet 2017, 91, 100–105. [DOI] [PubMed] [Google Scholar]
- (20).Stuart CA; Stone WL; Howell ME; Brannon MF; Hall HK; Gibson AL; Stone MH Myosin content of individual human muscle fibers isolated by laser capture microdissection. Am. J. Physiol. Cell Physiol 2016, 310, C381–C389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Hanet A; Rasch F; Weber R; Ruscica V; Fauser M; Raisch T; Kuzuoglu-Ozturk D; Chang CT; Bhandari D; Igreja C; Wohlbold L HELZ directly interacts with CCR4-NOT and causes decay of bound mRNAs. Life Sci. Alliance 2019, 2, No. e201900405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Arora R; S NK; S S; Fairoze MN; Kaur M; Sharma A; Girdhar Y; M SR; Devatkal SK; Ahlawat S; Vijh RK; S MS Transcriptome profiling of longissimus thoracis muscles identifies highly connected differentially expressed genes in meat type sheep of India. PLoS One 2019, 14, No. e0217461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Weimer K; Theobald J; Campbell KS; Esser KA; DiMario JX Genome-wide expression analysis and EMX2 gene expression in embryonic myoblasts committed to diverse skeletal muscle fiber type fates. Dev. Dyn 2013, 242, 1001–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Rakus D; Gizak A; Deshmukh A; Wisniewski JR Absolute quantitative profiling of the key metabolic pathways in slow and fast skeletal muscle. J. Proteome Res 2015, 14, 1400–1411. [DOI] [PubMed] [Google Scholar]
- (25).Fomchenko KM; Verma RX; Kannan S; Lin BL; Yang X; Nieuwenhuis TO; Patil AH; Fox Talbot K; McCall MN; Kwon C; Kass DA; Rosenberg AZ; Halushka MK Proteogenomic single cell analysis of skeletal muscle myocytes bioRxiv 2020. 10.1101/2020.01.23.916791v2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Remark R; Merghoub T; Grabe N; Litjens G; Damotte D; Wolchok JD; Merad M; Gnjatic S In-depth tissue profiling using multiplexed immunohistochemical consecutive staining on single slide. Sci. Immunol 2016, 1, No. aaf6925. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


