Abstract
γ-Tocopherol (γT) is a major form of vitamin E in the US diet and the second most abundant vitamin E in the blood and tissues, while α-tocopherol (αT) is the predominant vitamin E in tissues. During the last >25 years, research has revealed that γT has unique antioxidant and anti-inflammatory activities relevant to disease prevention compared to αT. While both compounds are potent lipophilic antioxidants, γT but not αT can trap reactive nitrogen species by forming 5-nitro-γT, and appears to show superior protection of mitochondrial function. γT inhibits ionophore-stimulated leukotrienes by blocking 5-lipoxygenase (5-LOX) translocation in leukocytes, decreases cyclooxygenase-2 (COX-2)-catalyzed prostaglandins in macrophages and blocks the growth of cancer cells but not healthy cells. For these activities, γT is stronger than αT. Moreover, γT is more extensively metabolized than αT via cytochrome P-450 (CYP4F2)-initiated side-chain oxidation, which leads to formation of metabolites including 13’-carboxychromanol (13’-COOH) and carboxyethyl-hydroxychroman (γ-CEHC). 13’-COOH and γ-CEHC are shown to be the predominant metabolites found in feces and urine, respectively. Interestingly, γ-CEHC has natriuretic activity and 13’-COOH inhibits both COX-1/-2 and 5-LOX activity. Consistent with these mechanistic findings of γT and metabolites, studies show that supplementation of γT mitigates inflammation and disease symptoms in animal models with induced inflammation, asthma and cancer. In addition, supplementation of γT decreased inflammation markers in patients with kidney diseases and mild asthma. These observations support that γT may be useful against inflammation-associated diseases.
Graphical Abstract
INTRODUCTION
γ-Tocopherol (γT) belongs to the vitamin E family that comprises eight lipophilic molecules, including α-, β-, γ-, δ-tocopherol (αT, βT, γT, δT) (Figure 1) and the corresponding tocotrienols that differ from tocopherols in containing double bonds on the side chain. γT, like other vitamin E forms, is known to be an effective lipophilic antioxidant and capable of scavenging lipid peroxyl radicals. γT is often the most abundant vitamin E in US diets and the second most abundant in the blood and tissues. While most research on vitamin E has historically focused on αT [1], the predominant form of vitamin E in tissues and responsible for preventing vitamin E deficiency [2], during the last >25 years, mechanistic studies combined with preclinical animal models have indicated that compared to αT, γT appears to have different biological properties that may be useful in its own right for prevention and therapy against chronic diseases. Furthermore, γT is more extensively metabolized than αT. Specific metabolites have been shown to have unique bioactivities and exhibit stronger anti-inflammatory effects than the un-metabolized vitamin E. In this review, we summarize current knowledge of γT and metabolites including antioxidant and anti-inflammatory effects in mechanistic and animal models and discuss the impact of dietary or therapeutic interventions with γT on diseases in human studies.
Figure 1. The structures of tocopherols –
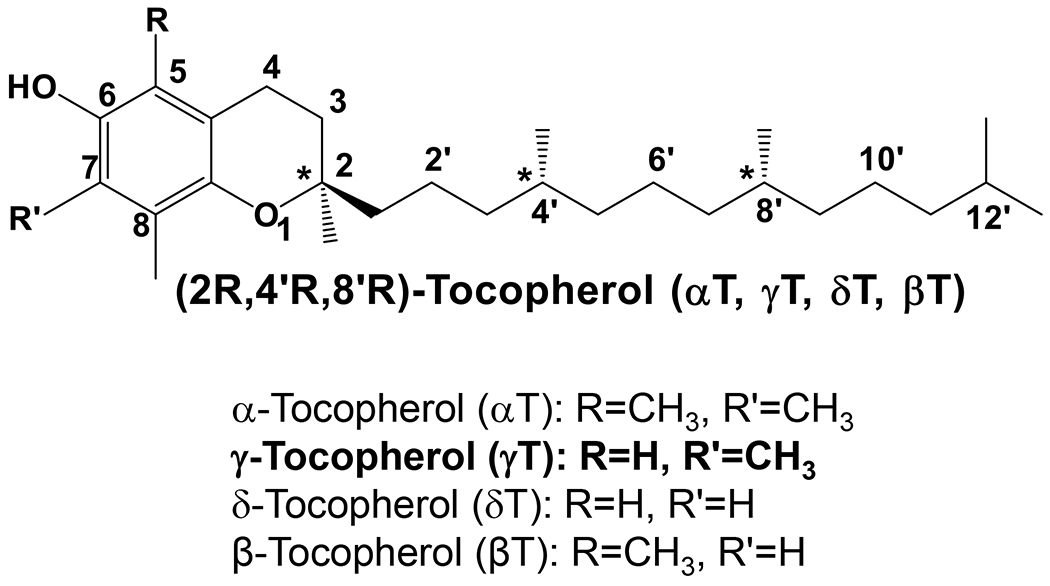
Naturally-occurring tocopherols have three chiral centers with R-configuration at 2-, 4’- and 8’-position.
1. FOOD SOURCES, BIOAVAILABILITY, CELLULAR DISTRIBUTION AND METABOLISM
1.1. Food sources
αT and γT as well as other vitamin E forms are rich in plant seeds including commonly-used nuts, seeds and relevant plant oils. αT is the most abundant vitamin E in peanuts, almonds and sunflower seeds, while γT is the major vitamin E in walnuts, pecans, pistachios and sesame seeds [3, 4]. αT and γT are found in many food oils including corn, soybean and peanut oil [3, 4]. Because of the widespread use of com and soybean oil, γT and aT account for ~60-70 % and 20-25% vitamin E consumed in the typical US diet, respectively [3]. In contrast, αT is the most abundant vitamin E in European diets because of popular consumption of olive and sunflower oil [5]. Interestingly, γT-rich oils appear to have more n-6 fatty acids than αT-rich ones, suggesting that γT intake may correlate with consumption of dietary n-6 fatty acids [6].
1.2. Bioavailability of αT and γT in humans
Despite its higher dietary abundance in the US diet, γT is often several folds lower than αT in the blood [1] and αT is the predominant form of vitamin E in the body. Specifically, plasma concentrations of γT and αT often range from 1-5 μM and 18-25 μM, respectively, in individuals whose sources of vitamin E are from diets [1, 6]. Supplementation of pharmaceutical doses of γT (~1200 mg) temporarily increases this vitamin E in the plasma to up to 30-40 μM [7, 8]. However, γT disappears much more rapidly than αT, indicating relatively short lifetime of γT [9]. In tissues, γT is also the second most abundant vitamin E, although its concentrations appear to be higher in human skin, adipose and muscle than those in the plasma. In a human study, muscle concentrations of γT (107 nmoles/g) were found to be comparable to those of αT (155 nmoles/g), which yields the ratio of αT:γT was ~1.4, whereas the ratio of αT:γT was 5-7 in the plasma [10]. In the same study, concentrations of αT and γT in adipose tissues were 440 and 176 nmol/g, respectively, which gives the ratio of αT/γT aT 2.5 [10]. In another study, the concentrations of αT and γT in adipose was found to be 119 and 15.6 nmol/g (ratio of αT/γT ~7.6), respectively, while αT/γT in the plasma was > 26, which is consistent with higher intake of αT than γT in the European diet [11]. Overall, these data indicate that αT and γT are the most and 2nd most abundant vitamin E forms in the body, and γT is more bioavailable in tissues than the plasma.
1.3. Absorption, transport, intracellular distribution, and metabolism of vitamin E forms
Dietary γT and other vitamin E forms are absorbed along with dietary fats in the intestine and then transported by chylomicron via lymphatic system to the peripheral tissues including muscle, brain, adipose and skin. Subsequently chylomicron remnants are taken up by the liver [1, 2, 12]. Vitamin E forms likely enter the hepatocytes by receptor-mediated endocytosis, although the detailed mechanisms remain to be fully elucidated [13]. αT has been shown to bind to tocopherol transfer protein (TTP) in the late endosomal membranes and this interaction facilitates the transport of αT to the plasma membrane and secretion with lipoproteins into the circulation [14, 15]. Inside cells, αT is not only present in lysosome and cytoplasmic membrane, but also in the endoplasmic reticulum (ER), mitochondria and peroxisome [16, 17]. As to other vitamin E forms, γT is found to mainly correlate with subcellular organelle markers of ER, plasma membrane and lysosome [16].
The preferential tissue retention of αT over γT is largely rooted in their different metabolic fate in the liver. TTP has much stronger affinity toward αT (100%) than γT (10-30%) or other vitamin E forms, and therefore most αT is bound to TTP, which prevents αT from being catabolized. In contrast, γT is largely catabolized via cytochrome P450 (CYP4F2) initiated ω-hydroxylation and oxidation in the ER followed by β-oxidation of the phytyl chain in the peroxisome and mitochondrion to generate 13’-hydroxychromanol (13’-OH), 13’-carboxychromanol (13’-COOH), various intermediate carboxychromanols and terminal metabolite 3’-carboxychromanol (3’-COOH) or (2’-carboxyethyl)-6-hydroxychromans (CEHC) (Figure 2) [6, 18]. Conjugation such as sulfation of the phenolic on the chromanol may take place in parallel with β-oxidation when there is high intake of vitamin E forms [19–21].
Figure 2. Metabolism of γT -.
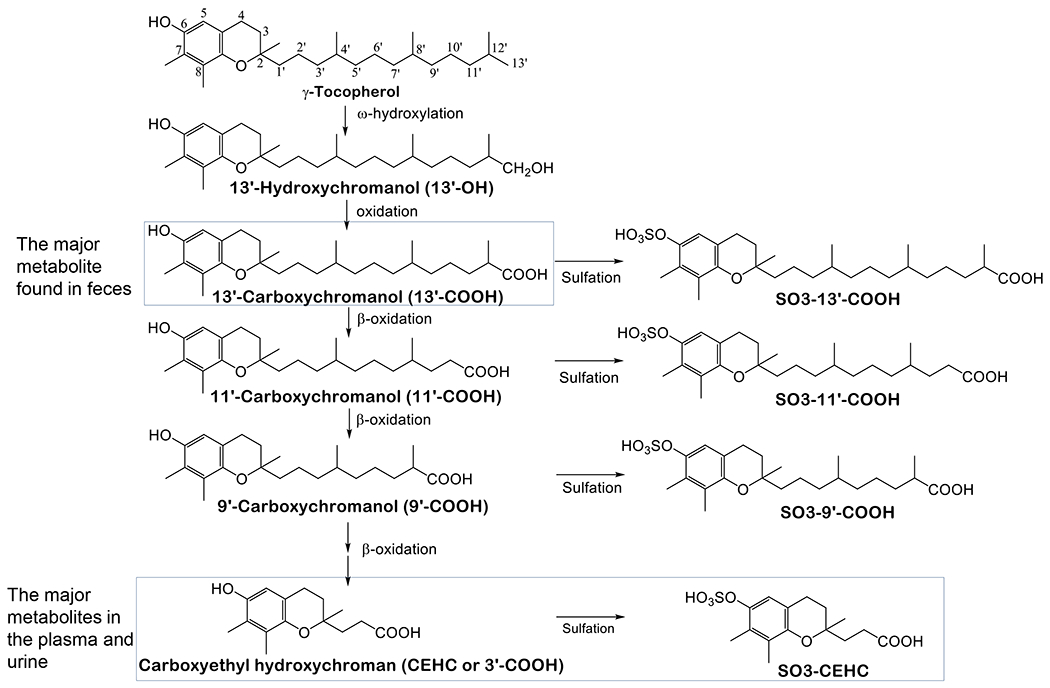
γT is metabolized by CYP4F2-catalyzed ω-hydroxylation and ω-oxidation to form 13’-OH and 13’-COOH. 13’-COOH is further metabolized via β-oxidation to generate shorter-chain carboxychromanols and terminal metabolite, γ-CEHC. Studies in animal and humans show that 13’-COOH appears to be the most abundant metabolite found in feces, and γ-CEHC and conjugated γ-CEHC are the major metabolites in the blood and urine.
Many studies in humans and animals have documented that γT is more extensively metabolized than αT, as indicated by higher γ-CEHC than α-CEHC in the urine and higher 13’-COOH from γT than αT in feces. For instance, Brigelius-Flohe et al. [22] showed that less than 1% of 30 mg deuterium-labeled αT was excreted as urinary α-CEHC, whereas ~7.5% of deuterated γT appeared as γ-CEHC in the urine in a human study. Supplementation of γT aT high doses results in the increase of γ-CEHC to up to several μM in the plasma. Specifically, Burbank et al. [7] reported that after participants consumed γT-enriched geltabs containing γT, αT, βT and δT at 612, 7, 28 and 8 mg, respectively, every 12 hrs for 3 doses, γ-CEHC in the plasma increased from baseline of 0.2 (0.06-0.5) μM to 7.3 (1.8-14.8) μM, and γ-CEHC remained elevated even 12 hours after the last dosing. These investigators also noticed large interpersonal differences in the elevation of γ-CEHC in the plasma. Recently, γ-CEHC and 5’-COOH were found to be elevated in the prostate as a result of supplementation of γT-rich tocopherols (containing αT, γT and δT at 128, 200 and 71 mg) in men diagnosed with localized prostate cancer undergoing radical prostatectomy [23]. As to long-chain metabolites, studies in animals revealed that 13’-COOH as the unconjugated form is the predominant metabolite found in feces in response to supplementation of γT or mixed tocopherols [24–26]. In addition, higher levels of fecal excretion of 13’-COOH from γT than αT were also observed when mice were fed diets supplemented with the same dose of these tocopherols, and consistently, more αT than γT as the un-metabolized form was excreted in feces [27]. These studies demonstrate higher amounts of metabolites formed from γT than αT, and thus validates more extensive metabolism of γT.
2. BIOLOGICAL ACTIVITIES AND MECHANISMS OF γT AND METABOTLIES IN CELL-BASED AND MECHANISTIC STUDIES
This section focuses on bioactivities of γT and metabolites identified in cell-based and mechanistic studies, including antioxidant, anti-inflammatory and anticancer effects (summarized in Table 1 and Figures 3 & 4). These findings provide mechanistic insights into the role of γT in prevention and management of diseases observed in animal and human studies (section 3–6). More comprehensive review of different vitamin E metabolites can be found elsewhere [28].
Table 1.
Bioactivities and mechanisms of γT and metabolites
| Activities in cells or animal models | Mechanisms | Ref | |
|---|---|---|---|
| Activity of γT; Comparing γT and αT | Scavenging NOx ↓nitrotyrosine, ↓nitrite γT but not αT shows these effects |
forming 5-NγT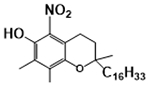
|
[30–32] |
| ↓LTB4, ↓cysteinyl leukotrienes: γT’s IC50 ~5-25μM, αT’s IC50≥50μM |
Blocking ionophore-stimulated Ca2+ influx and 5-LOX translocation | [65] | |
| ↓PGE2, ↓PGD2: γT’s IC50 ~4-7.5μM αT’s IC50 > 50 μM |
Modest inhibition of COX-2 after chronic incubation with macrophages or generating 13’-COOH that inhibits COX-2 | [56] | |
| ↓TxB2 in platelets γT’s IC50 ~8-25 μM αT’s IC50 ≥ 50 μM |
Inhibit human recombinant COX-1 activity | [59] | |
| ↓cancer cell growth: γT’s IC50 ~25-50μM αT’s IC50> 100μM |
Modulating sphingolipids including intracellular accumulation of dihydroceramides and dihydrosphingosine | [71] | |
| 13’-COOH | ↓LTB4 | Inhibition of 5-LOX activity (IC50 0.04-1 μM) | [65–67] |
| ↓ PGE2, ↓TxB2 | Inhibit COX-1 and COX-2 enzyme (IC50 ~4 μM) | [57] [59] | |
| ↓cancer cell growth | Modulating sphingolipids including intracellular accumulation of ceramides and dihydroceramides | [66] | |
| γ-CEHC | Natriuretic | Inhibit 70 pS potassium channel in the kidney thick ascending limb cells | [41] |
| ↓PGE2, ↓PGD2 | Inhibit COX-2 activity (IC50 ≥ 30μM) | [56] | |
| ↓JNK-p, prevent IκBα degradation | Not determined | [140] |
Figure 3. Antioxidant activities of αT and γT.
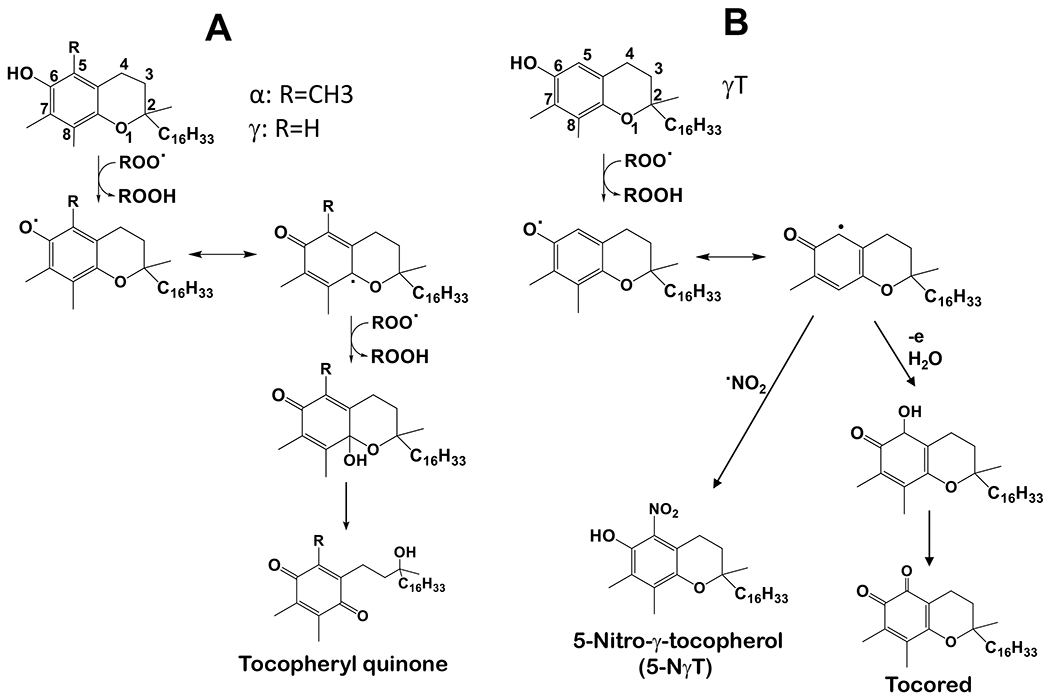
(A) – Both αT and γT can stop lipid peroxidation via donating electrons and forming tocopherylquinone. (B) - γT uniquely scavenges NOx including NO2, ONOO− and SOD/H2O2/NO2− via forming 5-NγT.
Figure 4. Antiinflammatory activities of γT and metabolite 13’-COOH –
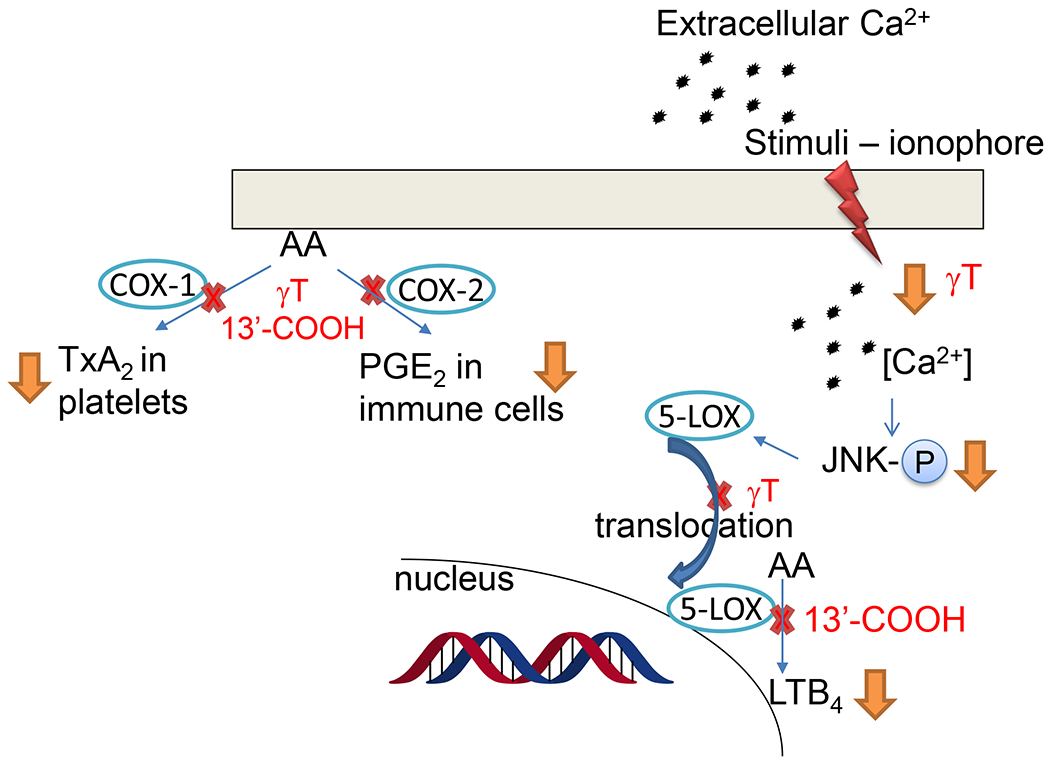
γT blocks ionophore (or sphingosine 1-phosphate)-stimulated calcium influx, JNK phosphorylation (p-JNK), 5-LOX translocation from cytosol to the nucleus and LTB4 formation in neutrophils. 13’-COOH are inhibitors of COX-1, COX-2 and 5-LOX. Details see Table 1.
2.1 –. γT has antioxidant activities including scavenging reactive nitrogen species (NOx) via formation of 5-nitro-γT (5-NγT) and protects mitochondrial functions
Because γT and αT as well as other vitamin E forms possess a phenolic group on the chromanol ring, these compounds have potent antioxidant activities by donating electrons to scavenge lipid peroxyl radicals (Figure 3A) [1]. Interestingly, γT possesses an un-substituted 5-position on the chromanol and is therefore able to trap electrophiles including NOx to form 5-NγT [29–32]. In contrast, αT has a fully substituted aromatic ring on the chromanol and cannot form a nitrated adduct. Consequently, γT is shown to be superior to αT in detoxifying nitrogen dioxide (NO2), peroxynitrite (ONOO−) and copper-zinc superoxide dismutase (SOD)/H2O2/NO2−), via formation of 5-NγT (Figure 3B) [1, 29–32]. Consistently, 5-NγT was elevated in zymosan induced-peritonitis in rats [33] and during FeCl3 patch-induced occlusive thrombus formation in rats [34].
Mitochondria represent both the main source and target of reactive oxygen species including NOx. Oxidative stress-caused neuronal mitochondrial dysfunction is believed to contribute to aging-associated neurological disorders including Alzheimer’s disease (AD) [35, 36]. Interestingly, Williamson et al [37] observed significant elevation of 5-NγT, evidence of NOx, in cortical regions of the Alzheimer’s disease brain compared to normal brain. More importantly, γT, but not αT, attenuated ONOO−-producing compound-caused inactivation of the Kreb’s cycle enzyme α-ketoglutarate dehydrogenase in rat’s brain mitochondria [37]. In another study, both αT and γT were found to protect 7-ketocholoestrol-induced mitochondrial and peroxisomal dysfunction in microglial BV-2 cells [38]. In a recent study, Pahrudin Arrozi et al [39] compared the effect of αT and γT on mitochondrial functions in cellular model of Alzheimer’s disease. Specifically, SH-SY5Y cells were stably transfected with wild-type or mutant amyloid precursor protein (APP) gene that is known to increase the formation of amyloid-beta (Aβ). These investigators observed that while both αT and γT dose-dependently reduced mitochondrial reactive oxygen species, only γT significantly increased ATP levels and complex V enzyme activity [39]. αT and γT treatment also reduced cytochrome c release and the ratio of Bcl-2-associated X (BAX)/Bcl-2, indicating protection against cell death. Furthermore, γT, but not αT, reduced mutant APP-associated increase of cyclophilin D, a regulator of mitochondrial membrane permeability, inhibition of which is known to be protective to mitochondrial functions [39, 40]. These observations indicate potential superior protective effects of γT over αT on mitochondrial function partially via scavenging NOx.
2.2. γ-CEHC has natriuretic activity
Wechter et al. [41] demonstrated that γ-CEHC possesses natriuretic activity via inhibition of the 70 pS potassium channel in the thick ascending limb cells of the kidney, but α-CEHC does not appear to have this activity. Consistent with γ-CEHC’s natriuretic activity, Yoshikawa et al. [42] reported that 4-week supplementation of γT non-significantly increased urinary sodium excretion compared to placebo and significantly enhanced sodium excretion αT one week following cessation of γT supplementation in human participants.
2.3. γT and metabolites block cyclooxygenase-1 (COX-1)- and cyclooxygenase-2 (COX-2)-catalyzed biosynthesis of thromboxane and prostaglandins
Chronic inflammation plays significant roles in the etiology of degenerative diseases including cardiovascular diseases, diabetes and cancer [43–45]. Prostaglandins and thromboxanes are key lipid mediators regulating cellular physiology and immune response including inflammation [46]. During inflammation, COX-2 is often upregulated in macrophages and epithelial cells where prostaglandin E2 (PGE2) is synthesized via COX-2-catalyzed oxidation of arachidonic acid (AA). PGE2 is known to causes pain and fever [47, 48] and stimulate cytokine formation [49]. In cancer tissues, PGE2 and COX-2 are elevated and shown to contribute to cancer-promoting microenvironment [50]. Thromboxane A2 (TxA2) is synthesized by COX-1-mediated reaction in platelets, and known to stimulate platelet aggregation. Overproduction of TxA2 increases the risk of cardiovascular diseases [51] and promotes inflammation and cancer metastasis [52]. Consistently, COX inhibitors, which are non-steroidal anti-inflammatory drugs (NSAIDs) including aspirin, have proven effective in suppression of inflammation and prevention of colorectal cancer and cardiovascular diseases [50, 53–55].
Cell-based and mechanistic studies have demonstrated that γT and metabolite 13’-COOH have anti-inflammatory effects by inhibition of COX-2-mediated prostaglandins. Specifically, Jiang et al. [56, 57] have shown that γT and γ-CEHC inhibited COX-2-mediated PGD2 and PGE2 formation in LPS-stimulated macrophages and IL-1β-activated A549 cells. However, γT does not inhibit COX-2 activity in enzyme assays or have any impact on COX-2 expression. Subsequent studies show that decrease of PGE2 in macrophages stems from modest inhibition of COX-2 in the cellular environment, like other weak inhibitors of COX including salicylate [56, 58]. Interestingly, γT’s inhibition of PGE2 in A549 cells was diminished by co-incubation with sesamin, an inhibitor vitamin E metabolism [57]. This observation suggests that metabolites formed during incubation of γT with A549 cells may contribute to the inhibition of PGE2. Consistent with this hypothesis, conditioned media containing γT- or δT-derived long-chain carboxychromenols were found to inhibit COX-2 activity [57]. Further, purified 13’-COOH derived from δT (δT-13’-COOH) inhibits COX-1 and COX-2 with IC50s of 4-5 μM, and for these effects, is stronger than 9’-COOH (IC50 at 6 μM in cells) and γ-CEHC (3’-COOH) (IC50 of 30-70 μM in cells) [57]. In addition, enzyme kinetic studies indicate that δT-13’-COOH competitively inhibits COX-1 and COX-2 [57].
Recently, we show that αT and γT inhibited human recombinant COX-1 enzyme activity with IC50 of 12 and 2.5 μM, respectively [59]. Consistent with this observation, in rats’ platelets, αT and γT decreased TxB2 (TxA2 metabolite) formation induced by collagen, a physiologically relevant stimulus, with IC50s of 50 and 8-10 μM, respectively. Moreover, γT, but not αT, decreased ionophore-induced TxB2 with an IC50 of ~25 μM [59]. In addition, in agreement with our previous observation that 13’-COOHs are COX-1 inhibitors, δT-13’-COOH and δTE-13’-COOH (metabolite of δ-tocotrienol (δTE)) blocked thromboxane in collagen- or ionophore-stimulated platelets with IC50s of 1.5-2.5 μM [59]. These activities offer a mechanistic explanation of the observation that γT and αT are found to inhibit platelet aggregation in human studies [60, 61].
2.4. γT and metabolite 13’-COOH block 5-lipoxygenase (5-LOX)-mediated leukotrienes by inhibition of 5-LOX activating signaling and the enzyme activity, respectively
Leukotriene B4, C4 and D4 (LTB4, LTC4 and LTD4) are produced by 5-LOX-catalyzed oxidation of arachidonic acid in neutrophils, eosinophils and mast cells. LTB4 is a potent pro-inflammatory and chemotactic agent [62], and LTC4 and LTD4 play key roles in allergic inflammatory diseases and asthma [63]. 5-LOX inhibitor Zileuton has clinically been used to treat asthma [64]. In addition, 5-LOX and leukotrienes have been shown to promote cancer development and are potential targets for cancer prevention [50].
γT and metabolites are shown to inhibit 5-LOX-catalyzed LTB4 via distinct mechanisms. Specifically, γT and δT inhibited ionophore-stimulated LTB4 and LTC4 with IC50 of ~5-20 μM in neutrophil- and eosinophil-like HL60 cells as well as human neutrophils isolated from peripheral blood, while αT was much less effective for these effects [65]. However, γT, δT or αT do not inhibit human recombinant 5-LOX activity at physiologically relevant concentrations [65]. Further investigation shows that γT decreased LTB4 in cells via suppressing ionophore-stimulated Ca2+ influx, phosphorylation of INK and 5-LOX translocation from cytosol to the nucleus, a key event for activation of 5-LOX and for its catalyzed synthesis of leukotrienes [65]. Unlike δT and γT, δT-13’-COOH and γT-13’-COOH (our unpublished data) inhibit human 5-LOX activity with IC50s of ~0.5-1 μM. Consistently, 13’-COOHs decrease ionophore- or other stimuli-induced LTB4 in neutrophils [65]. Subsequently, other 13’-COOHs including δTE-13’-COOH and αT-13’-COOH (αT metabolite) are found to potently inhibit 5-LOX activity with IC50s of 0.04-1 μM and dampen LTB4 formation in neutrophils [59, 66, 67]. Recently, we show that 13’-COOHs competitively inhibit 5-LOX based on enzyme kinetic data, but do not affect 5-LOX translocation [59].
2.5. Effects of γT on inducible nitric oxide synthase (iNOS), cytokine/chemokine and gene expression in immunce cells including macrophages, T cells, lung epithelial cells and mast/basophils
In macrophages, we observed that γT at 10 μM inhibited LPS-stimulated nitrite formation and decreased iNOS expression [56]. In another study, Zingg et al. [68] reported that γT appeared to inhibit activation of T cells, as indicated by the observation that CD3/CD28-stimulated T cells isolated from old mice supplemented with γT at 500 mg/kg produced less amounts of cytokines and chemokines than those from mice fed with 30 mg/kg of γT. In contrast, supplementation αT modestly enhanced T cell activation. In human lung epithelial cells, γT showed stronger inhibition of IL-13-induced eotaxin-3 than αT, but was weaker than γ-tocotrienol for this effect [69]. In addition, Mills et al [70] assessed the effect of γT on IgE-mediated degranulation and mediators using a rat basophil cell line, RBL SX-38, which like mast cells, expresses human FcεRI receptor (high affinity IgE receptor) and secrete inflammatory mediators and cγtokines upon allergen stimulation. Pretreatment with γT at 20 μM significantly inhibited IgE-stimulated β-hexosaminidase release. Furthermore, γT pretreatment of these cells significantly reduced production of the TH2 cytokines IL-4 & IL-13 and cysteinyl leukotrienes (CysLTs) [70].
2.6. Anticancer effects and mechanisms of γT and 13’-COOHs in cell-based studies
γT has been shown to have anticancer effects in various types of cancer cells. For instance, we showed that γT inhibited the growth of human prostate LNCaP and PC-3 cancer cells and induce apoptosis in LNCaP cells, but had no impact on the proliferation of healthy prostate epithelial cells. In contrast, αT does not show anti-proliferation of prostate cancer cells [71]. Combinations of γT or γT-rich mixed tocopherols with δT or γTE enhanced anti-proliferative effects compared to individual agents [71, 72]. We also reported that γT inhibited the growth of human HCT116 colon cancer cells [66]. Moreover, Gopalan et al. [73] observed that γT induced apoptosis and upregulated death receptor-5 expression in human breast cancer cells. Mechanistic investigation revealed that γT treatment induced intracellular accumulation of dihydroceramides and dihydrosphingosine in prostate cancer cells [71], and ceramides and dihydroceramides in breast cancer cells [73]. Inhibition of de novo ceramide biosynthesis by chemical inhibitors diminished the ability of γT to induce apoptosis [71, 73]. These studies demonstrate that γT exert anticancer effects via modulation of de novo synthesis of sphingolipids. As to the metabolites, although there was no direct study with γT-13’-COOH, other 13’-COOHs including δT-13’-COOH and δTE-13’-COOH have been shown to inhibit proliferation of colon cancer cells but showed little influence on healthy cells [66].
3. ANTI-INFLAMMATORY AND ANTIOXIDANT ACTIVITIES IN PRECLINICAL MODELS WITH INFLAMMATION AND INFLAMMATION-ASSOCIATED DISEASES
Since γT and metabolites have anti-inflammatory properties based on mechanistic studies, potential effects of this vitamin E form on inflammation including eicosanoids, cytokines and nitrotyrosine have been examined in many preclinical models of inflammation-associated diseases. For instance, in a model resembling joint disease, γT but not αT significantly inhibited carrageenan-induced elevation of PGE2, LTB4 and 8-isoprostane and attenuated inflammation-associated damage in rats [74]. In the same air-pouch model, combining aspirin with γT, but not aspirin with αT, prolonged aspirin’s anti-inflammation effects and attenuated aspirin-induced stomach lesions [75]. In zymosan-induced peritonitis in rats, supplementation of γT significantly decreased formation of protein bound 3-nitrotyrosine, attenuated ascorbate oxidation in the kidney, and prevented starvation-induced ascorbate decrease [76]. In another study, Hamahata et al. [77] found that γT nebulization improved pulmonary function in sheep suffering from 40% total body surface area burn and smoke-inhalation injury, as indicated by attenuation of pulmonary edema caused by burn and smoke inhalation injury, fall in oxygenation, nitrotyrosine and cytokines including IL-6 and IL-8. In a similar ovine model, Yamamoto et al. [78, 79] showed that γT nebulization mitigated oxidative stress and lung injury after burn and smoke inhalation.
It is well-recognized that inflammation plays a key role in atherosclerosis. γT has been tested and shown to exert anti-atherogenic effects in animal models relevant to atherosclerosis. Specifically, γT but not αT attenuated balloon catheter-induced increase of the ratio of neointima to media (a vascular injury marker) and 3-nitrotyrosine in insulin resistant rats [80]. In another study, Saldeen et al. [81] reported that γT was more potent than αT in attenuating FeCl3-induced platelet aggregation, superoxide production and occlusive thrombus in rats.
The effect of γT on colonic inflammation has been evaluated in experimental colitis model induced by dextran sulfate sodium (DSS). Li et al. [82] observed that γT-rich mixed tocopherols (γTmT) alleviated DSS (1%)-induced oxidative damage, and attenuated elevation of PGE2 and leukocyte infiltration in colon tissues. Further, these protective effects appeared to be independent of the nuclear factor (erythroid-derived 2)-like 2 (NFE2L2 or Nrf2), a key antioxidant transcription factor, as γTmT showed similar protection in Nrf2 knockout mice to that in wild-type animals [82]. In addition, we recently showed that both γT and αT mitigated DSS-induced colitis symptoms and decreased colitis-associated increase of IL-6 [27]. Importantly, both tocopherols inhibited colitis-induced loss of the tight junction protein occludin and blocked elevation of LPS-binding protein in the plasma, a surrogate marker of intestinal barrier dysfunction. These data suggest protection of γT on gut barrier integrity. Consistently, αT and γT mitigated cytokine-induced impairment of trans-epithelial electrical resistance in human intestinal epithelial Caco-2 cell monolayer [27]. Interestingly, using 16S rRNA gene sequencing of fecal DNA, we observed that γTmT but not αT separated gut microbial composition from controls under the diseased condition and attenuated DSS-caused depletion of Roseburia, which contains butyrate producing bacteria and is decreased in IBD patients [27]. This observation indicates that γT shows stronger modulation of gut microbiota than αT, although the modulatory effects were only seen in DSS-treated animals.
4. THE EFFECT OF γT ON ASTHMA AND ALLERGIC AIRWAY INFLAMMATION IN ANIMAL MODELS
Asthma is a chronic airway inflammatory disease. Leukotrienes produced by 5-LOX are key players mediating bronchoconstriction and inflammatory responses in the pathophysiology of asthma [83]. Zileuton, the only clinically used 5-LOX inhibitor to date, has been used for asthma treatment [64, 83]. Since γT and metabolite 13’-COOH inhibited 5-LOX-catalyzed leukotrienes in neutrophils [65, 67], these compounds may be useful anti-asthmatic agents. Accordingly, the impact of γT on airway inflammation has been tested in various animal models with induced asthma and/or allergy.
Consistent with mechanistic findings, beneficial effects of γT have been observed in allergic and non-allergic asthma models, which are often associated with eosinophilic and neutrophilic asthma, respectively. For instance, Wagner et al. [84, 85] reported beneficial effects of γT on allergic asthma in ovalbumin (OVA)-sensitized rat models. Specially, oral administration of γT at 100 mg/kg bw for 4 days inhibited OVA-induced allergic inflammation by reducing airway eosinophilia and mucous cell hyperplasia in both pulmonary and nasal airways of male Brown Norway rats [84]. In this study, γT treatment also decreased inflammatory mediators including PGE2, LTB4 and cysteinyl leukotrienes, in the lung, and nasal expression of Th2 cytokines (IL-4, IL-5, and IL-13) and IFN-γ [84]. Moreover, under the exacerbating condition with exposure to ozone, oral administration of γT (100 mg/kg bw) to OVA-sensitized Brown Norway rats for 4 days also alleviated eosinophilic infiltration and key mediators in the lung and bronchoalveolar lavage (BAL), including cysteinyl leukotrienes, monocyte chemoattractant protein (MCP)-1, IL-6, IL-5 and IL-13 [85]. Similar protection was also seen with ozone-induced exacerbation of allergic rhinitis and sinusitis [86]. Consistent with these observations, in the OVA-sensitized BALB/c mice, Wu et al. [87] reported that i.p. injection of γT reduced eosinophil infiltration, goblet cell hyperplasia and the levels of eotaxin and IL-4 in serum and BAL fluid. Interestingly, the modulatory effect of γT was deemed to be comparable to that of dexamethasone, a glucocorticoid. In addition to the beneficial effects observed in allergic asthma models, γT appears to be beneficial to LPS-induced neutrophilic airway inflammation in rats [88, 89]. In particular, oral administration of γT inhibited LPS-stimulated airway recruitment of neutrophils and inflammatory markers, including PGE2 and secreted mucin. In this study, γT also inhibited LPS-stimulated BAL PGE2, mucin secretion, and cytokines including neutrophil-chemotactic cytokines (MIP-2 and GRO-KC) and mucus-production cytokines (Muc5AC) [88, 89].
Despite above-mentioned anti-asthmatic and anti-inflammatory effects, contradictory effects of γT on asthma were also reported. In the OVA-induced murine asthma model, γT at 0.2 mg/day via s.c. injection increased inflammatory cell infiltration in BAL and this effect was reversed by αT, whereas γT at 2 mg/day reduced leukocyte infiltration and inflammatory cytokines in BAL [90]. However, γT (2mg/day) in ethoxylated castor oil via s.c. injection during OVA-challenge increased immune cell infiltrate in BAL and lung and counteracted anti-inflammatory actions of αT, but surprisingly, γT did not alter expression of inflammatory cytokines, chemokines, and adhesion molecules [91]. Moreover, although γT appears to exacerbate house dust mite (HDM)-induced BAL leukocytes, immune cells in γT-treated mice were two-fold lower than those from αT-treated mice that was deemed to lower eosinophilia compared to the controls [92]. These data suggest somewhat inconsistency in the model. In the same publication, asthma risk was found to be the lowest in people that have relatively high plasma αT with medium levels of γT [92], suggesting that both tocopherols may be needed to attenuate asthma. In addition, when given during pregnancy and lactation in OVA-sensitized allergic female mice, γT increased the numbers of eosinophils in BAL and the lung in their pups [93], as well as the CD11c+CD11b+ inflammatory dendritic cells (DCs) in the lungs of pups, suggesting potential impact of γT on development of allergic responses in neonatal life [93, 94].
Overall, animal studies have revealed inconsistent results concerning the role of γT in asthma and allergic airway inflammation. The reason underlying conflicting observations is not clear, but several factors may be considered. First, critical differences in the methods of drug administration, s.c vs. oral, may produce different outcomes because oral administration of γT results in formation of metabolites including 13’-COOHs, whereas s.c avoids liver metabolism of drugs. It is notable that s.c. dosing with γT in mice caused significant depression of αT levels in both plasma and lung tissue, whereas the natural dietary intake of γT by rats did not have the similar effect on αT levels [86]. Thus, sufficient levels of both γT and αT may be required for optimal regulation of inflammatory responses in allergic lung, which is supported by asthma risk association data in humans [92]. Second, γT was found to promote inflammation at a low dose but showed anti-inflammatory at a high dose in one study [90]. Potentially dose-dependent effects should be further examined. Moreover, in another study, although supplementation of γT was associated with increased immune cells, γT had no impact on cytokine production or cell activation [91], which makes observed increase of immune cell number puzzling. In addition, the observation of perinatal γT’s impact on new-born pups potentially has important implications on developmental origins of disease but should be further investigated. Finally, given that few therapies have been developed or translated to humans based on induced asthma in rodent models, further studies concerning the effect of αT and γT on asthma should be carried out in models more closely relevant to the pharmacodynamics and pathobiology of human diseases [95].
5. CHEMOPREVENTIVE EFFECTS OF γT IN CANCER MODELS
COXs- and 5-LOX-mediated eicosanoids play important roles in the early-, intermediate-stage cancer progression and even late-stage cancer including metastasis [50]. Since γT and metabolites have been shown to block eicosanoids in mechanistic studies [56, 57, 65] and inhibit cancer cell growth [96], potential anticancer effects of γT and γT-rich toopherols have been tested in different types of cancer models. This review will focus on in vivo studies in prostate, colon, esophageal and breast cancer models.
The impact of γT and sometimes along with other tocopherols on the progression of prostate cancer has been examined and compared in various animal models. For instance, γT-rich diet was reported to attenuate N-methyl-N-nitrosourea (MNU)-induced epithelial dysplasia, cell proliferation, COX-2 and MMP-9 activity in the ventral prostate in rats [97]. γT, δT and αT (all at 0.2% diet) as well as γTmT (0.3% diet) inhibited 2-Amino-l-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP)-induced prostate intraepithelial neoplasia (PIN), an early precancerous lesion in the CYP1A-humanized mice, and δT appeared to be stronger than γT or αT for these effects [98]. Moreover, in the transgenic rat for adenocarcinoma of prostate and transgenic murine prostate mice, γT (at 50, 100 and 200 mg/kg diet) or γTmT (at 0.1% diet), but not αT (at 50 mg/kg diet), suppressed cancer progression from PIN to adenocarcinoma and decreased palpable tumor incidence [99–101]. In addition, γT (at eq. 0.054% diet) and δT (0.3% diet), but not αT (0.3% diet), moderately suppressed tumor growth in the LNCaP-implant xenograft model [102, 103]. Combining γT and methaneselenic acid more strongly blocked tumor development than either agent alone in 22Rvl-implanted xenograft model in mice [104]. Despite these positive outcomes, γT or its combination with lycopene failed to have any impact on tumor growth of Dunning R3327H adenocarcinoma in rats [105], probably because of relatively low dose used (0.02% diet). Overall, these observations indicate that γT and γT-rich tocopherols inhibited the development of early-stage PIN formation and progression from PINs to adenocarcinoma, but exhibited modest inhibition of relatively late-stage tumor.
COX inhibitors and inhibition of 5-LOX have been shown to prevent colon cancer development in human and preclinical animal studies [50, 55, 106]. Since γT and metabolites block COX- and 5-LOX-mediated eicosanoids, it is hypothesized that γT may be effective for chemoprevention of colon cancer. Thus, studies have been conducted to investigate the effect of γT on colon cancer in chemically-induced colon cancer models. Specifically, γT or δT at 0.2% diet and γTmT (0.1% diet) inhibited azoxymethane (AOM)-induced aberrant crypt foci (ACF), which are clinically relevant precancerous lesions in F344 rats, whereas αT (at 0.05% or 0.2% diet) did not exhibit any effect on ACF [107, 108]. Ju et al. [109] reported that γTmT (at 0.3% diet) suppressed AOM/dextran sodium sulfate (DSS)-induced colitis-associated colon cancer and eicosanoids (PGE2 and LTB4) in mice. In the murine AOM/DSS-induced colitis-associated colon cancer model, we observed that γT at 0.1% diet attenuated moderate but not severe colitis induced tumorigenesis. In this study, high levels of 13’-COOH derived from γT were detected in the feces of mice supplemented with γT [110]. In addition, in the CYP1A-humanized mice, γT and δT but not αT (all at 0.2% diet) were found to inhibit PhIP/DSS-induced tumor multiplicity when the intervention of these compounds started before PhIP (carcinogen) injection. However, when the intervention started after PhIP/DSS treatment, the anticancer efficacy significantly diminished [111]. These studies demonstrate that γT and γTmT, when administrated at the initiating stage of carcinogenesis, show cancer preventive effects against colon cancer, and are stronger than αT for these activities. Nevertheless, considering that all these studies were conducted in chemically-induced models, anticancer efficacy of γT should be further examined in spontaneously tumor-developing cancer models bearing genetic mutations that resemble human cancers.
In addition to colon cancer, potential effect of tocopherols on esophageal carcinogenesis was investigated in chemically-induced preclinical models in rats and mice. Specifically, dietary supplementation of γTmT, δT, or αT (all at 0.15% diet) significantly suppressed N-nitrosomethylbenzylamine (NMBA)-induced esophageal squamous cell carcinoma in F344 rats, as indicated by decreased tumor incidence, multiplicity and volume in animals supplemented with these tocopherols compared to those fed the control diet. Further biochemical analyses revealed that tocophehrols attenuated NF-κB activation and CXCR3-mediated inflammation [112]. These observations suggest that tocopherols including γTmT may be promising chemopreventive agents against esophageal cancer.
Chemo-preventive effects of γT and γTmT on breast cancer has also been extensively evaluated in various mammary tumor models, many of which were conducted by Dr. Suh and coworkers. In particular, γTmT dose-dependently inhibited tumor growth in the MNU-induced hormone-dependent mammary tumor and increased p21, p27 and PPAR-γ in female Sprague Dawley rats [113]. In the same model, γT and δT, but not αT (all at 0.3% diet), reduced tumor multiplicity and weight and increased apoptotic markers [114]. Furthermore, γTmT at 0.3 and 0.5% in diet suppressed tumor size in estrogen-promoted mammary tumors, stimulated antioxidant regulator Nrf2 and increased expression of PPAR-γ in the August-Copenhagen Irish (ACI) rats, which were subcutaneously implanted with silastic tubing filled with 9 mg of 17β-estradiol (E2) and exhibit 80-100% tumor incidence upon prolonged exposure to estrogen [115]. In the same model, γT or γTmT (0.2% diet) but not αT reduced estrogen-mediated mammary tumor volume by ~60% and γT treatment induced robust gene expression changes including downregulation of CXCR2, IGFBP3 and IGF1, as indicated by RNA-Seq analysis in the ACI rats [116]. In another study, γTmT at 0.05, 0.1, 0.3 and 0.5% diet for 9 wk decreased mammary tumor development in ER+ MCF7 breast cancer cells orthotopically implanted, immunodeficient mice [115]. In the same MCF7-xenograft model, γT or γTmT (0.2% diet) for 5 weeks blocked estrogen-stimulated MCF-7 growth and reduced oxidative stress and DNA damage [117]. Similarly, γT at 0.2% diet inhibited mammary cancer growth and lung metastasis in murine 66c1-4 or MDA-MB231-GFP breast cancer cell-implanted mice [118, 119]. Interestingly, αT was found to counteract γT’s anticancer effects. Despite these positive outcomes, γT or γTmT (0.3% diet) did not decrease tumor growth in HER-2 overexpressed MMTV/ErbB2/neu transgenic mice [114]. These results indicate that γT or γTmT appeared to be effective for preventing estrogen-dependent mammary tumors and less effective in the model resembling relatively advanced breast cancer.
6. HEALTH BENEFITS IN HUMAN CLINICAL STUDIES
6.1. Brief discussion of observational studies on association of serum concentrations of γT and chronic diseases in humans
Some epidemiological studies have examined potential correlation between serum γT with chronic diseases, and reported mixed results. For instance, serum concentrations of γT have been found to be inversely associated with prostate cancer [120, 121], whereas such correlation was not observed in another study [122]. A meta-analysis revealed that total circulating tocopherols but not αT alone were inversely associated with early onset coronary artery diseases [123]. However, Chai et al. [124] observed inverse association between all-cause mortality and αT, but positive association with γT. The reason underlying the positive association between γT and all-cause mortality is not clear. Interestingly, γT is also found to be associated with inflammation markers such as C-reactive protein (CRP) and poor lifestyles including reduced physical activities and poor diets [125]. Therefore, it is possible that high γT intake is associated with certain unhealthy lifestyles. In this regard, γT is found in plant oils enriched with n-6 fatty acids including soybean and corn oil, whereas αT is often found in oils with high portions of monounsaturated fatty acids such as olive oil [6]. Despite the necessity of further investigation, existing evidence suggests that consumption of n-6 fatty acids and their enriched vegetable oils may promote inflammation and even death in humans [126, 127].
6.2. Supplementation of γT in clinical intervention studies
Encouraged by promising anti-inflammatory and antioxidant effects observed in mechanistic and animal studies, potential beneficial effects of γT on inflammation and inflammation-associated diseases have been evaluated in some clinical intervention studies including those in patients with chronic kidney diseases, multiple sclerosis, diabetes and asthma (Table 2).
Table 2.
Beneficial effects of γT or mixed tocopherols in human intervention studies
| Type of study | Subject characteristics | Supplementation details | Effect and major outcomes | Ref. |
|---|---|---|---|---|
| Patients with kidney diseases | ||||
| Randomized, double-blinded, crossover | Patients on chronic hemodialysis therapy; n=15 patients and 15 healthy participants | 300 mg αT or 300 mg γT-rich mixed toocpherols (60% γT, 28% δT and 10% αT) for 14 d | Supplementation of γT-rich mixed tocopherols decreased CRP in the plasma, whereas αT had on effect on CRC but increased IL-6. | [128] |
| Randomized, double-blinded, placebo-controlled | Hemodialysis maintenance patients; | 308 mg γT plus 800 mg DHA (docosahexaenoic acid) for 4 and 8 wk; supplement (n=31) vs. placebo (n=30) | Compared to placeboes, supplementation of γT plus DHA decreased white blood cells, IL-6, neutrophils and erythropoietin index, but did not affect plasma CRP, F2-isoprostane or carbonyls. | [130] |
| Prospective, double-blind, randomized and placebo-controlled trial | Patients with chronic kidney disease undergoing coronary procedures | 350 mg αT, or 300 mg γT, or placebo (n=101-102 per group) for 5 days prior to coronary procedure and 2 days afterwards | Prophylaxis administration with αT or γT decreased the risk of contrast-induced acute kidney injury in chronic kidney disease patients. | [132] |
| Prospective, placebo-controlled, double-blind, randomized | Patients undergoing maintenance hemodialysis therapy | A combination of mixed tocopherols (666 IU/d) plus α-lipoic acid (600mg/d) for 6 months (n=353) | The treatment, while generally safe and well-tolerated, had no significant effect on plasma CRP, IL-6, and F2 isoprostane and did not improve the erythropoietic response. | [133] |
| Patients with multiple sclerosis | ||||
| randomized, blind, placebo-controlled | Patients with relapsing-remitting multiple sclerosis / Total n = 80 | Group A: n-3 (EPA+DHA)/n-6 fatty acids at 1:1 with αT (22mg); group B: A plus γT (760mg); group C: γT (760mg); placebo, for 30 months. n=20 per group | The combination of n-3/n-6 (1:1) fatty acids and γT (group B) reduced relapse rate of multiple sclerosis by 64%, delayed disability progression by 72% and decreased the risk of the sustained progression disability by 85%. | [131] |
| Patients with metabolic syndromes | ||||
| Double-blind, placebo-controlled trial | Type 2 diabetic patients | 500 mg of RRR-αT, or γT-rich mixed tocopherols (75mg αT, 315mg γT and 110mg δT), and placebo (n=18-19 per group), for 6 wk. | αT or γT-rich mixed tocopherols decreased plasma F2-isoprostane, while increased blood pressure, without affecting inflammation markers. γT + αT reduced LTB4 from stimulated neutrophils in vitro. | [138, 139] |
| Randomized, placebo-controlled double-blind trial | Participants with metabolic syndromes / | 800 mg αT, or 800 mg γT, or their combination, or placebo (n=20 per group) for 6 wk | The combination of αT and γT decreased CRP, nitrotyrosine and oxidation markers, while αT and γT alone showed partial benefits regarding these markers. | [137] |
| The effect on asthma-related endpoints in healthy and mild asthma | ||||
| Open-label, Phase I dosing study of two doses | Adults (18-50 y) with/without asthma (moderate to severe); n=16; allergic asthma n=8 | γT-enriched (623 mg γT, 61.1 mg αT, 11.1 mg βT, 231mg δT)×1 or 2/d; 8d→8d-washout-→8d; | γT-rich tocopherols decreased serum concentrations of 5-nitro-γT, suggesting attenuation of oxidative stress and inhibited PBMC response to ex vivo LPS challenge. | [140] |
| Open-label, single-arm | Adults (22-43 y), n=5 healthy participants and n=5 mild asthma | γT-enriched tocopherols (612 mg γT, 7 mg αT, 28 mg βT, 8 mg δT)×2; every 12hr for 3 doses; | γT supplementation decreased ex vivo LPS-induced IL-6 and IL-1β production from PBMCs | [7] |
| Open-label, single-arm | Adults with dust mite allergy, n=20 | γT-enriched supplement (612 mg γT, 7 mg αT, 28 mg βT, 8 mg δT)x2 daily for 7 days | γT treatment reduced IgE-mediated basophil activation with dust mite allergen. | [70] |
| Phase IIa randomized, double-blinded, placebo-controlled crossover | Healthy adults (19-33 y), challenged by intranasal endotoxin (LPS); (n=13) | γT-rich tocopherols (540mg γT, 50mg αT, and 240mg βT+δT)×2/d; 1wk prior to endotoxin inhalation challenge→ washout→1wk | γT-enriched supplement reduced intranasal LPS-induced infiltration of airway (sputum) neutrophils, reduced % eosinophils in sputum and neutralized LPS-induced increase of IL-β. | [88] |
| Randomized, double-blinded, placebo-controlled, phase IIa crossover | Adults (20-47 y) with mild asthma; total n=23; atopic n=17 | γT-enriched supplement (612 mg γT, 7 mg αT, 28 mg βT, 8 mg δT) or placebo for 2wk, then treated with LPS inhalation →3-wk washout→2wk | γT reduced (pre-LPS) sputum eosinophils and mucins including mucin 5AC, and inhibited inhaled LPS challenge-induced airway neutrophil recruitment. γT also prevented post-LPS challenge caused slow-down of mucociliary clearance. | [8] |
| Randomized, double blind, placebo-controlled crossover study | Adults with mild intermittent allergic asthma, n=15 | γT-enriched geltabs (600 mg, 89.5% γT)x2 and placebo (safflower oil 700 mg) every 12 hours for 4 doses; participants are then exposed to 0.25ppm O3 for 3 hours | γT pre-treatment did not affect ozone-induced airway inflammation. | [141] |
| The effect of γT on cardiovascular disease-relevant endpoints | ||||
| Placebo controlled study | platelet aggregation after tocopherol supplementation in healthy volunteers | Mixed tocopherols (100mg γT, 40mg δT and 20mg αT) (n=18) or all-rac-αT acetate at 100mg (n=18), or placebo (n=10) for 8 wk | Mixed tocopherols suppressed ADP-induced platelet aggregation and induced endothelial nitric oxide synthase and nitric oxide release more strongly than αT. Both induced SOD and inhibited PKC activation. | [60] |
| Randomized placebo controlled | Healthy sedentary subjects in strenuous exercise | 300 mg γT, or 400IU αT, every day or every other day for 6 wk (n=36) | γT but not αT ameliorated exercise-induced decrease of APTT (activated partial thromboplastin time) and exercise-increased platelet aggregation induced by collagen. | [134] |
| Randomized, crossover, single-blind design | Healthy men with oral glucose tolerance test following overnight fasting | Mixed tocopherols (500mg γT, 60mg αT, 170mg δT and 9mg βT) daily for 5 days (n = 15) | γT-rich tocopherols attenuated glucose-induced decrease of brachial artery flow-mediated dilation, lipid peroxidation and disruption in NO homeostasis as well as dicarbonyl methylglyoxal. | [135, 136] |
All the major findings (outcomes) are statistically significant.
Kidney disease and multiple sclerosis patients -
Supplementation of γT was reported to decrease inflammation marker C-reactive protein (CRP) in the plasma of hemodialysis patients, while αT did not show such benefits to hemodialysis or end-stage renal disease patients [128, 129]. In another study, supplementation of combined γT and docosahexaenoic acid (DHA) led to reduction of inflammation markers including IL-6 and white blood cell counts without influencing CRP in hemodialysis-maintenance patients [130]. Interestingly, γT combined with DHA-rich n-3/n-6 fatty acids significantly attenuated relapse of multiple sclerosis and decreased the risk of sustained progression of disability in multiple sclerosis patients [131]. Moreover, 5-d supplementation of αT and γT attenuated contrast-induced kidney injury in patients with chronic kidney disease [132]. However, a combination of α-lipoic acid and mixed tocopherols, whose tocopherol contents were not specified, did not significantly affect biomarkers of inflammation and oxidative stress or the erythropoietic response in patients undergoing maintenance hemodialysis therapy [133]. These observations suggest that γT and its combination with DHA may be beneficial to patients with chronic kidney diseases and multiple sclerosis, whereas combining mixed tocopherols with lipoic acid does not offer protective effects.
The impact of γT on type 2 diabetes and cardiovascular relevant parameters -
Potential modulatory effects of γT on cardiovascular relevant parameters and diabetes have been investigated in several human studies. Supplementation with γT or γT-rich mixed tocopherols but not αT is found to attenuate strenuous exercise-increased platelet coagulation [134] or ADP-induced platelet aggregation [60]. γT-rich tocopherols alleviated postprandial hyperglycemia-caused impairment of endothelial function, enhancement of lipid peroxidation and disruption in NO homeostasis [135, 136]. In addition, in diabetic patients, γT or its combination with αT suppressed CRP and attenuated oxidative stress [137, 138]. Despite these positive results, αT or mixed tocopherols increased blood pressure without affecting cytokines or endothelium-dependent and independent vasodilation in type 2 diabetes patients [139]. Therefore, these studies suggest that γT or γT-rich tocopherols may potentially offer beneficial effects on cardiovascular diseases, whereas mixed results were observed concerning the impact of γT and αT in patients with diabetes.
The impact on asthma and related endpoints -
Despite controversial data in animal models regarding the role of γT in asthma, potential effects of γT or γT-rich tocopherols on asthma-related endpoints have been evaluated in several clinical studies in healthy individuals and/or patients with mild asthma. In an open-label, phase I dosing study, γT-enriched tocopherols decreased serum 5-NγT and suppressed LPS-stimulated pro-inflammatory cytokines secreted by peripheral blood mononuclear cells (PBMCs) in ex vivo studies. Further, γT, γ-CEHC, and α-CEHC, but not αT, inhibited LPS-induced degradation of IκBα, the inhibitor of nuclear factor (NF) kappa B in the PBMCs from allergic asthmatic volunteers, suggesting inhibition of NF-κB [140]. Consistently, a short course of supplementation with γT-enriched tocopherols reduced ex vivo LPS-induced IL-6 and IL-1β production in PBMCs from participants with mild asthma. Interestingly, there was a negative correlation between changes in plasma γ-CEHC concentration and changes in LPS-induced IL-8 formation, suggesting potential contribution of this metabolite to γT’s effects [7]. In addition to asthma, one week of daily oral supplementation with γT in dust mite-allergic volunteers reduced ex vivo IgE-mediated upregulation of CD63 & CD203c, indicating reduced basophil activation and beneifical effects against allergic inflammation [70].
These early-phase human studies support the hypothesis that γT has anti-inflammatory and anti-allergic action in vivo. Subsequently, in phase IIa clinical studies, supplementation with γT was found to ameliorate endotoxin inhalation-induced increase of neutrophilic inflammation in healthy volunteers as reflected in assessment of sputum samples recovered after challenge [88]. In a second double-blinded placebo controlled intervention study in volunteers with mild asthma, two week dosing with γT-enriched tocopherols reduced constitutive eosinophilic airway inflammation and mucin levels and endotoxin challenge-induced sputum neutrophilia, and prevented slowing of post-challenge associated mucociliary clearance [8]. These proof-of-concept clinical studies provide further evidence that γT can reduce Th2-airway inflammation in mild asthma and subsequent endotoxin challenge-induced neutrophilia, which is also consistent with observations in animal studies [88, 89]. In contrast, a short course of γT supplementation over 48 hours did not offer protection of ozone-exacerbated airway inflammation in adults with mild asthma [141]. Collectively, these studies suggest that at least one week of treatment with γT supplementation would be needed to observe clinical benefits in airway diseases.
In summary, existing clinical studies suggest that γT and γT-rich tocopherols, in combination with DHA may offer anti-inflammatory and beneficial effects in patients with elevated inflammation including kidney diseases, multiple sclerosis and asthma.
7. SUMMARY AND CONCLUSION REMARKS
During the last >25 years, there has been great advance in our understanding of biological activities of γT including anti-inflammatory effects and new functions of metabolites (Table 1 and Figures 3 and 4). Specifically, γT is capable of scavenging NOx via forming 5-NγT, whereas αT cannot trap NOx in this way. γT inhibits ionophore-stimulated 5-LOX activation and leukotrienes in neutrophils and decreased PGE2 in stimulated macrophages. For these activities, γT is much stronger than αT. Unlike αT that is mostly retained in tissues as a result of its strong binding to TTP, γT is substantially metabolized to generate metabolites including 13’-COOH and γ-CEHC, the predominant metabolites in feces and urine, respectively. γ-CEHC but not α-CEHC has natriuretic activity. 13’-COOHs are potent inhibitors of COX-1/2 and 5-LOX, whereas tocopherols do not inhibit COX-2 or 5-LOX activity. 13’-COOHs also show stronger anti-proliferation and induction of apoptosis in cancer cells than γT. Because of these activities, these metabolites may contribute to the beneficial effects of γT. In agreement with mechanistic findings, γT supplementation has been shown to be beneficial in managing inflammation-associated diseases in animal models and some human studies.
Despite advance in our knowledge of γT and metabolites, several future directions may be pursued to further facilitate translation of the use of γT for disease management and to address fundamental questions relevant to vitamin E biology. First, 13’-COOH shows stronger anti-inflammatory and anticancer effects than γT. Our recent publication indicate that δTE-13’-COOH, a metabolite of δTE, appears to be more effective than δTE in blocking colitis associated tumorigenesis in mice [142]. However, there is no direct evidence that metabolites play significant roles in γT’s or other vitamin E forms’ beneficial effects in vivo. Future studies are needed to address this question, which will contribute to the understanding of vitamin E functions. In addition, the bioavailability of metabolites like 13’-COOH should be further evaluated.
Second, although γT has anti-inflammatory effects, its combination with other bioactive compounds may offer improved or even synergistic benefits. In particular, γT combined with DHA or DHA-rich fatty acids showed promising protective effects in chronic kidney disease patients and multiple sclerosis patients (Table 2). Thus, the combination of γT and DHA may be further examined in relevant preclinical disease models regarding anti-inflammatory and chemopreventive effects. If animal studies support the combination therapies, clinical studies in humans should be performed to validate potential superior beneficial effects of the combinations. Mechanistic investigations should also be conducted to elucidate the mechanisms. Another noteworthy combinatory therapy is combining αT and γT for disease prevention and mitigation. We now know that αT and γT have different activities and likely complement each other for fighting inflammation-associated diseases. It is also known that supplementation of either tocopherol may decrease tissue levels of the other tocopherol. Therefore, therapeutic interventions with combining αT and γT as well as γT-rich tocopherols should be tested in future clinical studies.
Finally, although early stage proof-of-concept studies of γT supplementation shows protective effects in mild asthma patients in phase II studies, adequately powered phase II/III clinical trials focused on clinical endpoints are needed to demonstrate safety and efficacy of this intervention. Dosing studies and use of γT in combination with αT and possibly other agents should be assessed in asthma and other diseases including cancer as well.
γ-Tocopherol (γT), but not α-tocopherol (αT), can trap NOx via forming 5-nitro-γT
γT is stronger than αT in blocking 5-lipoxygenase activation, cyclooxygenase-1-catalyzed thromboxane and cancer cell proliferation
γT’s metabolite 13’-carboxychromanol is an inhibitor of cyclooxygenase-1/-2 and 5-lipoxygenase
γT is stronger than αT in attenuating inflammation-associated damages and cancer prevention in animal models
γT shows protective effects in patients with kidney diseases and asthma
SPECIAL ACKNOWLEDGEMENT –
Qing Jiang (the lead author of this review) is grateful to Professor Bruce Ames for his mentorship and being a role model of a true scientist. Qing’s interest of gamma-tocopherol started from her postdoctoral research with Professor Ames at UC Berkeley and Chori. Qing is now a Professor of Nutritional Biochemistry and has been doing research on different forms vitamin E and novel metabolites. To this day, Qing Jiang is still inspired by Professor Ames’ passion for science and influential thinking.
FUNDING
This work was supported in part by USDA Hatch 1022869 (QJ), Research Awards from the Purdue Center for Cancer Research, NIH grant P30 CA023168 (QJ) and 1021659 (JGW).
Abbreviations:
- AA
arachidonic acid
- AOM
azoxymethane (AOM)
- αT, βT, γT and δT
α-, β-, γ- and δ-tocopherol
- αTE, βTE, γTE and δTE
α-, β-, γ- and δ-tocotrienol
- BAL
bronchoalveolar lavage
- CEHC also called 3’-COOH
carboxyethyl-hydroxychromans
- 13’-COOH
13’-carboxychromanol
- COX-1 and COX-2
cyclooxygenase-1/-2
- CRP
C-reactive protein
- CysLTs
cysteinyl leukotrienes
- 5-LOX
5-lipoxygenase
- LTB4
leukotriene B4
- 5-NγT
5-nitro-γ-tocopherol (5-NO2-γT)
- NOx
reactive nitrogen species
- NSAIDs
non-steroidal anti-inflammatory drugs
- PGE2
prostaglandin E2
- γTmT
γ-tocopherol rich tocopherols
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].Jiang Q, Christen S, Shigenaga MK, Ames BN, gamma-tocopherol, the major form of vitamin E in the US diet, deserves more attention, The American journal of clinical nutrition 74(6) (2001) 714–22. [DOI] [PubMed] [Google Scholar]
- [2].Brigelius-Flohe R, Traber MG, Vitamin E: function and metabolism, Faseb J 13(10) (1999) 1145–55. [PubMed] [Google Scholar]
- [3].McLaughlin PJ, Weihrauch JL, Vitamin E content of foods, Journal of the American Dietetic Association 75(6) (1979) 647–65. [PubMed] [Google Scholar]
- [4].Dreher ML, Pistachio nuts: composition and potential health benefits, Nutrition reviews 70(4) (2012) 234–40. [DOI] [PubMed] [Google Scholar]
- [5].Wagner KH, Kamal-Eldin A, Elmadfa I, Gamma-tocopherol--an underestimated vitamin?, Annals of nutrition & metabolism 48(3) (2004) 169–88. [DOI] [PubMed] [Google Scholar]
- [6].Jiang Q, Natural forms of vitamin E: metabolism, antioxidant, and anti-inflammatory activities and their role in disease prevention and therapy, Free radical biology & medicine 72 (2014) 76–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Burbank AJ, Duran CG, Almond M, Wells H, Jenkins S, Jiang Q, Yang C, Wang T, Zhou HB, Hernandez ML, Peden DB, A short course of gamma tocopherol mitigates LPS-induced inflammatory responses in humans ex vivo, J Allergy Clin Immun 140(4) (2017) 1179-+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Burbank AJ, Duran CG, Pan Y, Burns P, Jones S, Jiang Q, Yang C, Jenkins S, Wells H, Alexis N, Kesimer M, Bennett WD, Zhou H, Peden DB, Hernandez ML, Gamma tocopherol-enriched supplement reduces sputum eosinophilia and endotoxin-induced sputum neutrophilia in volunteers with asthma, The Journal of allergy and clinical immunology 141(4) (2018) 1231–1238 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Traber MG, Burton GW, Hughes L, Ingold KU, Hidaka H, Malloy M, Kane J, Hyams J, Kayden HJ, Discrimination between forms of vitamin E by humans with and without genetic abnormalities of lipoprotein metabolism, Journal of lipid research 33(8) (1992) 1171–82. [PubMed] [Google Scholar]
- [10].Burton GW, Traber MG, Acuff RV, Walters DN, Kayden H, Hughes L, Ingold KU, Human plasma and tissue alpha-tocopherol concentrations in response to supplementation with deuterated natural and synthetic vitamin E [see comments], The American journal of clinical nutrition 67(4) (1998) 669–684. [DOI] [PubMed] [Google Scholar]
- [11].Gleize B, Steib M, Andre M, Reboul E, Simple and fast HPLC method for simultaneous determination of retinol, tocopherols, coenzyme Q(10) and carotenoids in complex samples, Food chemistry 134(4) (2012) 2560–4. [DOI] [PubMed] [Google Scholar]
- [12].Traber MG, Vitamin E regulatory mechanisms, Annual review of nutrition 27 (2007) 347–62. [DOI] [PubMed] [Google Scholar]
- [13].Qian J, Morley S, Wilson K, Nava P, Atkinson J, Manor D, Intracellular trafficking of vitamin E in hepatocytes: the role of tocopherol transfer protein, Journal of lipid research 46(10) (2005) 2072–82. [DOI] [PubMed] [Google Scholar]
- [14].Horiguchi M, Arita M, Kaempf-Rotzoll DE, Tsujimoto M, Inoue K, Arai H, pH-dependent translocation of alpha-tocopherol transfer protein (alpha-TTP) between hepatic cytosol and late endosomes, Genes to cells : devoted to molecular & cellular mechanisms 8(10) (2003) 789–800. [DOI] [PubMed] [Google Scholar]
- [15].Chung S, Ghelfi M, Atkinson J, Parker R, Qian J, Carlin C, Manor D, Vitamin E and Phosphoinositides Regulate the Intracellular Localization of the Hepatic alpha-Tocopherol Transfer Protein, The Journal of biological chemistry 291(33) (2016) 17028–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Irias-Mata A, Sus N, Flory S, Stock D, Woerner D, Podszun M, Frank J, alpha-Tocopherol transfer protein does not regulate the cellular uptake and intracellular distribution of alpha- and gamma-tocopherols and -tocotrienols in cultured liver cells, Redox biology 19 (2018) 28–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Mustacich DJ, Leonard SW, Patel NK, Traber MG, Alpha-tocopherol beta-oxidation localized to rat liver mitochondria, Free radical biology & medicine 48(1) (2010) 73–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Sontag TJ, Parker RS, Cytochrome P450 omega-hydroxylase pathway of tocopherol catabolism. Novel mechanism of regulation of vitamin E status, The Journal of biological chemistry 277(28) (2002) 25290–6. [DOI] [PubMed] [Google Scholar]
- [19].Freiser H, Jiang Q, Gamma-tocotrienol and gamma-tocopherol are primarily metabolized to conjugated 2-(beta-carboxyethyl)-6-hydroxy-2,7,8-trimethylchroman and sulfated long-chain carboxychromanols in rats, The Journal of nutrition 139(5) (2009) 884–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Freiser H, Jiang Q, Optimization of the enzymatic hydrolysis and analysis of plasma conjugated gamma-CEHC and sulfated long-chain carboxychromanols, metabolites of vitamin E, Analytical biochemistry 388(2) (2009) 260–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Jiang Q, Freiser H, Wood KV, Yin X, Identification and quantitation of novel vitamin E metabolites, sulfated long-chain carboxychromanols, in human A549 cells and in rats, Journal of lipid research 48(5) (2007) 1221–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Brigelius-Flohe R, Roob JM, Tiran B, Wuga S, Ribalta J, Rock E, Winklhofer-Roob BM, The effect of age on vitamin E status, metabolism, and function: metabolism as assessed by labeled tocopherols, Annals of the New York Academy of Sciences 1031 (2004) 40–3. [DOI] [PubMed] [Google Scholar]
- [23].Goodin S, Kim I, Lee MJ, Shih WJ, Orlick M, Zheng X, Yang CS, Plasma, Prostate and Urine Levels of Tocopherols and Metabolites in Men after Supplementation with a gamma-Tocopherol-Rich Vitamin E Mixture, Nutrition and cancer (2020) 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Liu KY, Jiang Q, Tocopherols and Tocotrienols Are Bioavailable in Rats and Primarily Excreted in Feces as the Intact Forms and 13′-Carboxychromanol Metabolites, The Journal of nutrition 150(2) (2020)222–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Bardowell SA, Duan F, Manor D, Swanson JE, Parker RS, Disruption of mouse cytochrome p450 4f14 (Cyp4f14 gene) causes severe perturbations in vitamin E metabolism, The Journal of biological chemistry 287(31) (2012) 26077–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Jiang Q, Xu T, Huang J, Jannasch AS, Cooper B, Yang C, Analysis of vitamin E metabolites including carboxychromanols and sulfated derivatives using liquid chromatography tandem mass spectrometry, Journal of lipid research 56(11) (2015) 2217–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Liu KY, Nakatsu CH, Jones-Hall Y, Kozik A, Jiang Q, Vitamin E alpha- and gamma-tocopherol mitigate colitis, protect intestinal barrier function and modulate the gut microbiota in mice, Free radical biology & medicine 163 (2021) 180–189. [DOI] [PubMed] [Google Scholar]
- [28].Jiang Q, Metabolism of natural forms of vitamin E and biological actions of vitamin E metabolites Free radical biology & medicine In press (2021). [DOI] [PMC free article] [PubMed]
- [29].Singh RJ, Goss SP, Joseph J, Kalyanaraman B, Nitration of gamma-tocopherol and oxidation of alpha-tocopherol by copper-zinc superoxide dismutase/H2O2/NO2-: role of nitrogen dioxide free radical, Proceedings of the National Academy of Sciences of the United States of America 95(22) (1998)12912–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Christen S, Woodall AA, Shigenaga MK, Southwell-Keely PT, Duncan MW, Ames BN, gamma-tocopherol traps mutagenic electrophiles such as NO(X) and complements alpha-tocopherol: physiological implications, Proceedings of the National Academy of Sciences of the United States of America 94(7) (1997) 3217–3222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Cooney RV, Franke AA, Harwood PJ, Hatch-Pigott V, Custer LJ, Mordan LJ, γ-Tocopherol detoxification of nitrogen dioxide: Superiority to α-tocopherol, Proc. Natl. Acad. Sci. USA 90(5) (1993) 1771–1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Cooney RV, Harwood PJ, Franke AA, Narala K, Sundström A-K, Berggren P-O, Mordan LJ, Products of γ-tocopherol reaction with NO2 and their formation in rat insulinoma (RINm5F) cells, Free Rad. Biol. Med 19(3) (1995) 259–269. [DOI] [PubMed] [Google Scholar]
- [33].Christen S, Jiang Q, Shigenaga MK, Ames BN, Analysis of plasma tocopherols alpha, gamma, and 5-nitro-gamma in rats with inflammation by HPLC coulometric detection, Journal of lipid research 43(11) (2002) 1978–85. [DOI] [PubMed] [Google Scholar]
- [34].Hensley K, Benaksas EJ, Bolli R, Comp P, Grammas P, Hamdheydari L, Mou S, Pye QN, Stoddard MF, Wallis G, Williamson KS, West M, Wechter WJ, Floyd RA, New perspectives on vitamin E: gamma-tocopherol and carboxyelthylhydroxychroman metabolites in biology and medicine, Free radical biology & medicine 36(1) (2004) 1–15. [DOI] [PubMed] [Google Scholar]
- [35].Bolanos JP, Moro MA, Lizasoain I, Almeida A, Mitochondria and reactive oxygen and nitrogen species in neurological disorders and stroke: Therapeutic implications, Advanced drug delivery reviews 61(14) (2009) 1299–315. [DOI] [PubMed] [Google Scholar]
- [36].Johri A, Beal MF, Mitochondrial dysfunction in neurodegenerative diseases, The Journal of pharmacology and experimental therapeutics 342(3) (2012) 619–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Williamson KS, Prasad Gabbita S, Mou S, West M, Pye QN, Markesbery WR, Cooney RV, Grammas P, Reimann-Philipp U, Floyd RA, Hensley K, The Nitration Product 5-Nitro-gamma-tocopherol Is Increased in the Alzheimer Brain, Nitric Oxide 6(2) (2002) 221–227. [DOI] [PubMed] [Google Scholar]
- [38].Debbabi M, Nury T, Zarrouk A, Mekahli N, Bezine M, Sghaier R, Gregoire S, Martine L, Durand P, Camus E, Vejux A, Jabrane A, Bretillon L, Prost M, Moreau T, Ammou SB, Hammami M, Lizard G, Protective Effects of alpha-Tocopherol, gamma-Tocopherol and Oleic Acid, Three Compounds of Olive Oils, and No Effect of Trolox, on 7-Ketocholesterol-Induced Mitochondrial and Peroxisomal Dysfunction in Microglial BV-2 Cells, International journal of molecular sciences 17(12) (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Pahrudin Arrozi A, Shukri SNS, Wan Ngah WZ, Mohd Yusof YA, Ahmad Damanhuri MH, Jaafar F, Makpol S, Comparative Effects of Alpha- and Gamma-Tocopherol on Mitochondrial Functions in Alzheimer’s Disease In Vitro Model, Scientific reports 10(1) (2020) 8962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Amanakis G, Murphy E, Cyclophilin D: An Integrator of Mitochondrial Function, Frontiers in physiology 11 (2020) 595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Wechter WJ, Kantoci D, Murray ED Jr., D’Amico DC, Jung ME, Wang WH, A new endogenous natriuretic factor: LLU-α, Proceedings of the National Academy of Sciences of the United States of America 93(12) (1996) 6002–6007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Yoshikawa S, Morinobu T, Hamamura K, Hirahara F, Iwamoto T, Tamai H, The effect of gamma-tocopherol administration on alpha-tocopherol levels and metabolism in humans, European journal of clinical nutrition 59(8) (2005) 900–5. [DOI] [PubMed] [Google Scholar]
- [43].McGeer PL, McGeer EG, Inflammation, autotoxicity and Alzheimer disease, Neurobiol Aging 22(6) (2001) 799–809. [DOI] [PubMed] [Google Scholar]
- [44].Libby P, Ridker PM, Maseri A, Inflammation and atherosclerosis, Circulation 105(9) (2002) 1135–43. [DOI] [PubMed] [Google Scholar]
- [45].Balkwill F, Mantovani A, Inflammation and cancer: back to Virchow?, Lancet 357(9255) (2001) 539–45. [DOI] [PubMed] [Google Scholar]
- [46].Funk CD, Prostaglandins and leukotrienes: advances in eicosanoid biology, Science 294(5548) (2001) 1871–5. [DOI] [PubMed] [Google Scholar]
- [47].Samad TA, Moore KA, Sapirstein A, Billet S, Allchorne A, Poole S, Bonventre JV, Woolf CJ, Interleukin-1beta-mediated induction of Cox-2 in the CNS contributes to inflammatory pain hypersensitivity, Nature 410(6827) (2001) 471–5. [DOI] [PubMed] [Google Scholar]
- [48].Vane JR, Prostaglandins as mediators of inflammation, Adv Prostaglandin Thromboxane Res 2 (1976) 791–801. [PubMed] [Google Scholar]
- [49].Williams JA, Shacter E, Regulation of macrophage cytokine production by prostaglandin E2. Distinct roles of cyclooxygenase-1 and -2, The Journal of biological chemistry 272(41) (1997) 25693–25699. [DOI] [PubMed] [Google Scholar]
- [50].Wang D, Dubois RN, Eicosanoids and cancer, Nature reviews. Cancer 10(3) (2010) 181–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Lebas H, Yahiaoui K, Martos R, Boulaftali Y, Platelets Are at the Nexus of Vascular Diseases, Frontiers in cardiovascular medicine 6 (2019) 132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Dovizio M, Alberti S, Guillem-Llobat P, Patrignani P, Role of platelets in inflammation and cancer: novel therapeutic strategies, Basic & clinical pharmacology & toxicology 114(1) (2014) 118–27. [DOI] [PubMed] [Google Scholar]
- [53].Gupta RA, Dubois RN, Colorectal cancer prevention and treatment by inhibition of cyclooxygenase-2, Nat Rev Cancer 1(1) (2001) 11–21. [DOI] [PubMed] [Google Scholar]
- [54].Wynne HA, Campbell M, Pharmacoeconomics of nonsteroidal anti-inflammatory drugs (NSAIDs), Pharmacoeconomics 3(2) (1993) 107–23. [DOI] [PubMed] [Google Scholar]
- [55].Maresso KC, Tsai KY, Brown PH, Szabo E, Lippman S, Hawk ET, Molecular cancer prevention: Current status and future directions, CA: a cancer journal for clinicians 65(5) (2015) 345–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Jiang Q, Elson-Schwab I, Courtemanche C, Ames BN, gamma-tocopherol and its major metabolite, in contrast to alpha- tocopherol, inhibit cyclooxygenase activity in macrophages and epithelial cells, Proceedings of the National Academy of Sciences of the United States of America 97(21) (2000) 11494–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Jiang Q, Yin X, Lill MA, Danielson ML, Freiser H, Huang J, Long-chain carboxychromanols, metabolites of vitamin E, are potent inhibitors of cyclooxygenases, Proceedings of the National Academy of Sciences of the United States of America 105(51) (2008) 20464–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Mitchell JA, Saunders M, Barnes PJ, Newton R, Belvisi MG, Sodium salicylate inhibits cyclo-oxygenase-2 activity independently of transcription factor (nuclear factor kappaB) activation: role of arachidonic acid, Molecular pharmacology 51(6) (1997) 907–12. [DOI] [PubMed] [Google Scholar]
- [59].Park N, Im S, Jiang Q, Different forms of vitamin E and metabolite 13′-carboxychromanols inhibit cyclooxygenase-1-catalyzed thromboxane in platelets, and tocotrienols and 13′-carboxychromanols are competitive inhibitors of 5-lipoxygenase Journal of Nutritional Biochemistry 108884 (2021) 10.1016/j.jnutbio.2021.108884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Liu M, Wallmon A, Olsson-Mortlock C, Wallin R, Saldeen T, Mixed tocopherols inhibit platelet aggregation in humans: potential mechanisms, The American journal of clinical nutrition 77(3) (2003) 700–6. [DOI] [PubMed] [Google Scholar]
- [61].Freedman JE, Farhat JH, Loscalzo J, Keaney JF Jr., alpha-tocopherol inhibits aggregation of human platelets by a protein kinase C-dependent mechanism, Circulation 94(10) (1996) 2434–40. [DOI] [PubMed] [Google Scholar]
- [62].Yokomizo T, Izumi T, Shimizu T, Leukotriene B4: metabolism and signal transduction, Archives of biochemistry and biophysics 385(2) (2001) 231–41. [DOI] [PubMed] [Google Scholar]
- [63].Samuelsson B, Dahlen SE, Lindgren JA, Rouzer CA, Serhan CN, Leukotrienes and lipoxins: structures, biosynthesis, and biological effects, Science 237(4819) (1987) 1171–6. [DOI] [PubMed] [Google Scholar]
- [64].Fanning LB, Boyce JA, Lipid mediators and allergic diseases, Annals of allergy, asthma & immunology : official publication of the American College of Allergy, Asthma, & Immunology 111(3) (2013)155–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Jiang Z, Yin X, Jiang Q, Natural forms of vitamin E and 13′-carboxychromanol, a long-chain vitamin E metabolite, inhibit leukotriene generation from stimulated neutrophils by blocking calcium influx and suppressing 5-lipoxygenase activity, respectively., J Immunology 186(2) (2011) 1173–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Jang Y, Park NY, Rostgaard-Hansen AL, Huang J, Jiang Q, Vitamin E metabolite 13′-carboxychromanols inhibit pro-inflammatory enzymes, induce apoptosis and autophagy in human cancer cells by modulating sphingolipids and suppress colon tumor development in mice, Free radical biology & medicine 95 (2016) 190–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Pein H, Ville A, Pace S, Temml V, Garscha U, Raasch M, Alsabil K, Viault G, Dinh CP, Guilet D, Troisi F, Neukirch K, Konig S, Bilancia R, Waltenberger B, Stuppner H, Wallert M, Lorkowski S, Weinigel C, Rummler S, Birringer M, Roviezzo F, Sautebin L, Helesbeux JJ, Seraphin D, Mosig AS, Schuster D, Rossi A, Richomme P, Werz O, Koeberle A, Endogenous metabolites of vitamin E limit inflammation by targeting 5-lipoxygenase, Nature communications 9(1) (2018) 3834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Zingg JM, Han SN, Pang E, Meydani M, Meydani SN, Azzi A, In vivo regulation of gene transcription by alpha- and gamma-tocopherol in murine T lymphocytes, Archives of biochemistry and biophysics 538(2) (2013) 111–9. [DOI] [PubMed] [Google Scholar]
- [69].Wang Y, Moreland M, Wagner JG, Ames BN, Illek B, Peden DB, Jiang Q, Vitamin E forms inhibit IL-13/STAT6-induced eotaxin-3 secretion by up-regulation of PAR4, an endogenous inhibitor of atypical PKC in human lung epithelial cells, The Journal of nutritional biochemistry 23(6) (2012) 602–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Mills K, Lay J, Wu W, Robinette C, Kesic MJ, Dreskin SC, Peden DB, Hernandez M, Vitamin E, gamma-tocopherol, diminishes ex vivo basophil response to dust mite allergen, Allergy 69(4) (2014) 541–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Jiang Q, Wong J, Fyrst H, Saba JD, Ames BN, {gamma}-Tocopherol or combinations of vitamin E forms induce cell death in human prostate cancer cells by interrupting sphingolipid synthesis, Proc Natl Acad Sci U S A 101(51) (2004) 17825–17830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Sato C, Kaneko S, Sato A, Virgona N, Namiki K, Yano T, Combination Effect of delta-Tocotrienol and gamma-Tocopherol on Prostate Cancer Cell Growth, Journal of nutritional science and vitaminology 63(5) (2017) 349–354. [DOI] [PubMed] [Google Scholar]
- [73].Gopalan A YW, Jiang Q, Jang Y, Sanders BG, Kline K, Involvement of de novo ceramide synthesis in gamma-tocopherol and gamma-tocotrienol-induced apoptosis in human breast cancer cells., Mol Nutr Food Res 56(12) (2012) 1803–11. [DOI] [PubMed] [Google Scholar]
- [74].Jiang Q, Ames BN, Gamma-tocopherol, but not alpha-tocopherol, decreases proinflammatory eicosanoids and inflammation damage in rats, The FASEB journal : official publication of the Federation of American Societies for Experimental Biology 17(8) (2003) 816–22. [DOI] [PubMed] [Google Scholar]
- [75].Jiang Q, Moreland M, Ames BN, Yin X, A combination of aspirin and gamma-tocopherol is superior to that of aspirin and alpha-tocopherol in anti-inflammatory action and attenuation of aspirin-induced adverse effects., The Journal of nutritional biochemistry 20(11) (2009) 894–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Jiang Q, Lykkesfeldt J, Shigenaga MK, Shigeno ET, Christen S, Ames BN, gamma-tocopherol supplementation inhibits protein nitration and ascorbate oxidation in rats with inflammation, Free radical biology & medicine 33(11) (2002) 1534–42. [DOI] [PubMed] [Google Scholar]
- [77].Hamahata A, Enkhbaatar P, Kraft ER, Lange M, Leonard SW, Traber MG, Cox RA, Schmalstieg FC, Hawkins HK, Whorton EB, Horvath EM, Szabo C, Traber LD, Herndon DN, Traber DL, gamma-Tocopherol nebulization by a lipid aerosolization device improves pulmonary function in sheep with burn and smoke inhalation injury, Free radical biology & medicine 45(4) (2008) 425–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Yamamoto Y, Sousse LE, Enkhbaatar P, Kraft ER, Deyo DJ, Wright CL, Taylor A, Traber MG, Cox RA, Hawkins HK, Rehberg SW, Traber LD, Herndon DN, Traber DL, gamma- tocopherol nebulization decreases oxidative stress, arginase activity, and collagen deposition after burn and smoke inhalation in the ovine model, Shock 38(6) (2012) 671–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Yamamoto Y, Enkhbaatar P, Sousse LE, Sakurai H, Rehberg SW, Asmussen S, Kraft ER, Wright CL, Bartha E, Cox RA, Hawkins HK, Traber LD, Traber MG, Szabo C, Herndon DN, Traber DL, Nebulization with gamma-tocopherol ameliorates acute lung injury after burn and smoke inhalation in the ovine model, Shock 37(4) (2012) 408–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Takahashi K, Komaru T, Takeda S, Takeda M, Koshida R, Nakayama M, Kokusho Y, Kawakami Y, Yamaguchi N, Miyazawa T, Shimokawa H, Shirato K, gamma-tocopherol, but not alpha-tocopherol, potently inhibits neointimal formation induced by vascular injury in insulin resistant rats, Journal of molecular and cellular cardiology 41(3) (2006) 544–54. [DOI] [PubMed] [Google Scholar]
- [81].Saldeen T, Li D, Mehta JL, Differential effects of alpha- and gamma-tocopherol on low-density lipoprotein oxidation, superoxide activity, platelet aggregation and arterial thrombogenesis [see comments] [published erratum appears in J Am Coll Cardiol 2000 Jan;35(l):263], J Am Coll Cardiol 34(4)(1999)1208–1215. [DOI] [PubMed] [Google Scholar]
- [82].Li G, Lee MJ, Liu AB, Yang Z, Lin Y, Shih WJ, Yang CS, The antioxidant and anti-inflammatory activities of tocopherols are independent of Nrf2 in mice, Free radical biology & medicine 52(7) (2012) 1151–8. [DOI] [PubMed] [Google Scholar]
- [83].Bruno F, Spaziano G, Liparulo A, Roviezzo F, Nabavi SM, Sureda A, Filosa R, D’Agostino B, Recent advances in the search for novel 5-lipoxygenase inhibitors for the treatment of asthma, European journal of medicinal chemistry 153 (2018) 65–72. [DOI] [PubMed] [Google Scholar]
- [84].Wagner JG, Jiang Q, Harkema JR, Ames BN, Illek B, Roubey RA, Peden DB, Gamma-tocopherol prevents airway eosinophilia and mucous cell hyperplasia in experimentally induced allergic rhinitis and asthma, Clinical and experimental allergy : journal of the British Society for Allergy and Clinical Immunology 38(3) (2008) 501–11. [DOI] [PubMed] [Google Scholar]
- [85].Wagner JG, Jiang Q, Harkema JR, Illek B, Patel DD, Ames BN, Peden DB, Ozone enhancement of lower airway allergic inflammation is prevented by gamma-tocopherol, Free radical biology & medicine 43(8) (2007) 1176–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Wagner JG, Harkema JR, Jiang Q, Illek B, Ames BN, Peden DB, Gamma-tocopherol attenuates ozone-induced exacerbation of allergic rhinosinusitis in rats, Toxicologic pathology 37(4) (2009) 481–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Wu Y-M, Xue Z-W, Zhang L-L, Gao N-M, Du X-M, Zhang X-Y, Zhang Z-H, Zhang Z-G, Comparable Function of γ-Tocopherols in Asthma Remission by Affecting Eotaxin and IL-4, Advances in clinical and experimental medicine: official organ Wroclaw Medical University 25(4) (2016) 643–648. [DOI] [PubMed] [Google Scholar]
- [88].Hernandez ML, Wagner JG, Kala A, Mills K, Wells HB, Alexis NE, Lay JC, Jiang Q, Zhang H, Zhou H, Peden DB, Vitamin E, γ-tocopherol, reduces airway neutrophil recruitment after inhaled endotoxin challenge in rats and in healthy volunteers, Free Radic Biol Med 60 (2013) 56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Wagner JG, Birmingham NP, Jackson-Humbles D, Jiang Q, Harkema JR, Peden DB, Supplementation with γ-tocopherol attenuates endotoxin-induced airway neutrophil and mucous cell responses in rats, Free Radical Biology and Medicine 68 (2014) 101–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].McCary CA, Abdala-Valencia H, Berdnikovs S, Cook-Mills JM, Supplemental and highly elevated tocopherol doses differentially regulate allergic inflammation: reversibility of α-tocopherol and γ-tocopherol’s effects, J Immunol 186(6) (2011) 3674–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Berdnikovs S, Abdala-Valencia H, McCary C, Somand M, Cole R, Garcia A, Bryce P, Cook-Mills JM, Isoforms of vitamin E have opposing immunoregulatory functions during inflammation by regulating leukocyte recruitment, J Immunol 182(7) (2009) 4395–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Cook-Mills J, Gebretsadik T, Abdala-Valencia H, Green J, Larkin EK, Dupont WD, Shu XO, Gross M, Bai C, Gao Y-T, Interaction of vitamin E isoforms on asthma and allergic airway disease, Thorax 71(10) (2016) 954–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Abdala-Valencia H, Soveg F, Cook-Mills JM, γ-Tocopherol supplementation of allergic female mice augments development of CD11c+CD11b+ dendritic cells in utero and allergic inflammation in neonates, Am J Physiol Lung Cell Mol Physiol 310(8) (2016) L759–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Abdala-Valencia H, Berdnikovs S, Soveg FW, Cook-Mills JM, α-Tocopherol supplementation of allergic female mice inhibits development of CD11c+CD11b+ dendritic cells in utero and allergic inflammation in neonates, Am J Physiol Lung Cell Mol Physiol 307(6) (2014) L482–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Kol A, Arzi B, Athanasiou KA, Farmer DL, Nolta JA, Rebhun RB, Chen X, Griffiths LG, Verstraete FJ, Murphy CJ, Borjesson DL, Companion animals: Translational scientist’s new best friends, Science translational medicine 7(308) (2015) 308ps21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Jiang Q, Natural forms of vitamin E and metabolites-regulation of cancer cell death and underlying mechanisms, IUBMB life 71(4) (2019) 495–506. [DOI] [PubMed] [Google Scholar]
- [97].Sanches LD, Santos SA, Carvalho JR, Jeronimo GD, Favaro WJ, Reis MD, Felisbino SL, Justulin LA Jr., Protective effect of gamma-tocopherol-enriched diet on N-methyl-N-nitrosourea-induced epithelial dysplasia in rat ventral prostate, International journal of experimental pathology 94(6) (2013) 362–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Chen JX, Li G, Wang H, Liu A, Lee MJ, Reuhl K, Suh N, Bosland MC, Yang CS, Dietary tocopherols inhibit PhIP-induced prostate carcinogenesis in CYP1A-humanized mice, Cancer letters 371(1) (2016) 71–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Takahashi S, Takeshita K, Seeni A, Sugiura S, Tang M, Sato SY, Kuriyama H, Nakadate M, Abe K, Maeno Y, Nagao M, Shirai T, Suppression of prostate cancer in a transgenic rat model via gamma-tocopherol activation of caspase signaling, The Prostate 69(6) (2009) 644–51. [DOI] [PubMed] [Google Scholar]
- [100].Barve A, Khor TO, Nair S, Reuhl K, Suh N, Reddy B, Newmark H, Kong AN, Gamma-tocopherol-enriched mixed tocopherol diet inhibits prostate carcinogenesis in TRAMP mice, International journal of cancer. Journal international du cancer 124(7) (2009) 1693–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Huang Y, Khor TO, Shu L, Saw CL, Wu TY, Suh N, Yang CS, Kong AN, A gamma-tocopherol-rich mixture of tocopherols maintains Nrf2 expression in prostate tumors of TRAMP mice via epigenetic inhibition of CpG methylation, The Journal of nutrition 142(5) (2012) 818–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Jiang Q, Rao X, Young Kim C, Freiser H, Zhang Q, Jiang Z, Li G, Gamma-tocotrienol induces apoptosis and autophagy in prostate cancer cells by increasing intracellular dihydrosphingosine and dihydroceramide, Int J Cancer (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Huang H, He Y, Cui XX, Goodin S, Wang H, Du ZY, Li D, Zhang K, Tony Kong AN, DiPaola RS, Yang CS, Conney AH, Zheng X, Potent inhibitory effect of delta-tocopherol on prostate cancer cells cultured in vitro and grown as xenograft tumors in vivo, Journal of agricultural and food chemistry 62(44) (2014) 10752–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Singh CK, Ndiaye MA, Siddiqui IA, Nihal M, Havighurst T, Kim K, Zhong W, Mukhtar H, Ahmad N, Methaneseleninic acid and gamma-Tocopherol combination inhibits prostate tumor growth in Vivo in a xenograft mouse model, Oncotarget 5(11) (2014) 3651–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Lindshield BL, Ford NA, Canene-Adams K, Diamond AM, Wallig MA, Erdman JW Jr., Selenium, but not lycopene or vitamin E, decreases growth of transplantable dunning R3327-H rat prostate tumors, PloS one 5(4) (2010) e10423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Cheon EC, Khazaie K, Khan MW, Strouch MJ, Krantz SB, Phillips J, Blatner NR, Hix LM, Zhang M, Dennis KL, Salabat MR, Heiferman M, Grippo PJ, Munshi HG, Gounaris E, Bentrem DJ, Mast cell 5-lipoxygenase activity promotes intestinal polyposis in APCDelta468 mice, Cancer research 71(5) (2011) 1627–36. [DOI] [PubMed] [Google Scholar]
- [107].Guan F, Li G, Liu AB, Lee MJ, Yang Z, Chen YK, Lin Y, Shih W, Yang CS, delta- and gamma-tocopherols, but not alpha-tocopherol, inhibit colon carcinogenesis in azoxymethane-treated F344 rats, Cancer Prev Res (Phila) 5(4) (2012) 644–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Chung H, Wu D, Han SN, Gay R, Goldin B, Bronson RE, Mason JB, Smith DE, Meydani SN, Vitamin E supplementation does not alter azoxymethane-induced colonic aberrant crypt foci formation in young or old mice, The Journal of nutrition 133(2) (2003) 528–32. [DOI] [PubMed] [Google Scholar]
- [109].Ju J, Hao X, Lee MJ, Lambert JD, Lu G, Xiao H, Newmark HL, Yang CS, A gamma-tocopherol-rich mixture of tocopherols inhibits colon inflammation and carcinogenesis in azoxymethane and dextran sulfate sodium-treated mice, Cancer Prev Res (Phila) 2(2) (2009) 143–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Jiang Q, Jiang Z, Hall YJ, Jang Y, Snyder PW, Bain C, Huang J, Jannasch A, Cooper B, Wang Y, Moreland M, Gamma-tocopherol attenuates moderate but not severe colitis and suppresses moderate colitis-promoted colon tumorigenesis in mice, Free radical biology & medicine 65 (2013) 1069–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Chen JX, Liu A, Lee MJ, Wang H, Yu S, Chi E, Reuhl K, Suh N, Yang CS, delta- and gamma-tocopherols inhibit phIP/DSS-induced colon carcinogenesis by protection against early cellular and DNA damages, Molecular carcinogenesis 56(1) (2017) 172–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Yang H, Xu M, Lu F, Zhang Q, Feng Y, Yang CS, Li N, Jia X, Tocopherols inhibit esophageal carcinogenesis through attenuating NF-kappaB activation and CXCR3-mediated inflammation, Oncogene 37(29) (2018) 3909–3923. [DOI] [PubMed] [Google Scholar]
- [113].Lee HJ, Ju J, Paul S, So JY, DeCastro A, Smolarek A, Lee MJ, Yang CS, Newmark HL, Suh N, Mixed tocopherols prevent mammary tumorigenesis by inhibiting estrogen action and activating PPAR-gamma, Clinical cancer research : an official journal of the American Association for Cancer Research 15(12) (2009) 4242–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Smolarek AK, So JY, Burgess B, Kong AN, Reuhl K, Lin Y, Shih WJ, Li G, Lee MJ, Chen YK, Yang CS, Suh N, Dietary Administration of delta- and gamma-Tocopherol Inhibits Tumorigenesis in the Animal Model of Estrogen Receptor-Positive, but not HER-2 Breast Cancer, Cancer Prev Res (Phila) 5(11) (2012) 1310–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Das Gupta S, Sae-tan S, Wahler J, So JY, Bak MJ, Cheng LC, Lee MJ, Lin Y, Shih WJ, Shull JD, Safe S, Yang CS, Suh N, Dietary gamma-Tocopherol-Rich Mixture Inhibits Estrogen-Induced Mammary Tumorigenesis by Modulating Estrogen Metabolism, Antioxidant Response, and PPARgamma, Cancer Prev Res (Phila) 8(9) (2015) 807–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Das Gupta S, Patel M, Wahler J, Bak MJ, Wall B, Lee MJ, Lin Y, Shih WJ, Cai L, Yang CS, Suh N, Differential Gene Regulation and Tumor-Inhibitory Activities of Alpha-, Delta-, and Gamma-Tocopherols in Estrogen-Mediated Mammary Carcinogenesis, Cancer Prev Res (Phila) 10(12) (2017) 694–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Bak MJ, Das Gupta S, Wahler J, Lee HJ, Li X, Lee MJ, Yang CS, Suh N, Inhibitory Effects of gamma- and delta-Tocopherols on Estrogen-Stimulated Breast Cancer In Vitro and In Vivo, Cancer Prev Res (Phila) 10(3) (2017) 188–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Yu W, Jia L, Park SK, Li J, Gopalan A, Simmons-Menchaca M, Sanders BG, Kline K, Anticancer actions of natural and synthetic vitamin E forms: RRR-alpha-tocopherol blocks the anticancer actions of gamma-tocopherol, Mol Nutr Food Res 53(12) (2009) 1573–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Yu W, Jia L, Wang P, Lawson KA, Simmons-Menchaca M, Park SK, Sun L, Sanders BG, Kline K, In vitro and in vivo evaluation of anticancer actions of natural and synthetic vitamin E forms, Mol Nutr Food Res 52(4) (2008) 447–56. [DOI] [PubMed] [Google Scholar]
- [120].Helzlsouer KJ, Huang HY, Alberg AJ, Hoffman S, Burke A, Norkus EP, Morris JS, Comstock GW, Association Between alpha-Tocopherol, gamma-Tocopherol, Selenium, and Subsequent Prostate Cancer, J Natl Cancer Inst 92(24) (2000) 2018–2023. [DOI] [PubMed] [Google Scholar]
- [121].Weinstein SJ, Wright ME, Pietinen P, King I, Tan C, Taylor PR, Virtamo J, Albanes D, Serum alpha-tocopherol and gamma-tocopherol in relation to prostate cancer risk in a prospective study, Journal of the National Cancer Institute 97(5) (2005) 396–9. [DOI] [PubMed] [Google Scholar]
- [122].Cui R, Liu ZQ, Xu Q, Blood alpha-tocopherol, gamma-tocopherol levels and risk of prostate cancer: a meta-analysis of prospective studies, PloS one 9(3) (2014) e93044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Li G, Li Y, Chen X, Sun H, Hou X, Shi J, Circulating tocopherols and risk of coronary artery disease: A systematic review and meta-analysis, European journal of preventive cardiology 23(7) (2016) 748–57. [DOI] [PubMed] [Google Scholar]
- [124].Chai W, Maskarinec G, Franke AA, Monroe KR, Park SY, Kolonel LN, Wilkens LR, Le Marchand L, Cooney RV, Association of serum gamma-tocopherol levels with mortality: the Multiethnic Cohort Study, European journal of clinical nutrition 74(1) (2020) 87–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Morimoto Y, Beckford F, Cooney RV, Franke AA, Maskarinec G, Adherence to cancer prevention recommendations and antioxidant and inflammatory status in premenopausal women, The British journal of nutrition 114(1) (2015) 134–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Ramsden CE, Zamora D, Majchrzak-Hong S, Faurot KR, Broste SK, Frantz RP, Davis JM, Ringel A, Suchindran CM, Hibbeln JR, Re-evaluation of the traditional diet-heart hypothesis: analysis of recovered data from Minnesota Coronary Experiment (1968-73), BMJ 353 (2016) i1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Saunders EF, Ramsden CE, Sherazy MS, Gelenberg AJ, Davis JM, Rapoport SI, Reconsidering Dietary Polyunsaturated Fatty Acids in Bipolar Disorder: A Translational Picture, The Journal of clinical psychiatry 77(10) (2016) e1342–e1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Himmelfarb J, Kane J, McMonagle E, Zaltas E, Bobzin S, Boddupalli S, Phinney S, Miller G, Alpha and gamma tocopherol metabolism in healthy subjects and patients with end-stage renal disease, Kidney international 64(3) (2003) 978–91. [DOI] [PubMed] [Google Scholar]
- [129].Smith KS, Lee CL, Ridlington JW, Leonard SW, Devaraj S, Traber MG, Vitamin E supplementation increases circulating vitamin E metabolites tenfold in end-stage renal disease patients, Lipids 38(8) (2003) 813–9. [DOI] [PubMed] [Google Scholar]
- [130].Himmelfarb J, Phinney S, Ikizler TA, Kane J, McMonagle E, Miller G, Gamma-tocopherol and docosahexaenoic acid decrease inflammation in dialysis patients, Journal of renal nutrition : the official journal of the Council on Renal Nutrition of the National Kidney Foundation 17(5) (2007) 296–304. [DOI] [PubMed] [Google Scholar]
- [131].Pantzaris MC, Loukaides GN, Ntzani EE, Patrikios IS, A novel oral nutraceutical formula of omega-3 and omega-6 fatty acids with vitamins (PLP10) in relapsing remitting multiple sclerosis: a randomised, double-blind, placebo-controlled proof-of-concept clinical trial, BMJ open 3(4) (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Tasanarong A, Vohakiat A, Hutayanon P, Piyayotai D, New strategy of alpha- and gamma-tocopherol to prevent contrast-induced acute kidney injury in chronic kidney disease patients undergoing elective coronary procedures, Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association 28(2) (2013) 337–44. [DOI] [PubMed] [Google Scholar]
- [133].Himmelfarb J, Ikizler TA, Ellis C, Wu P, Shintani A, Dalal S, Kaplan M, Chonchol M, Hakim RM, Provision of antioxidant therapy in hemodialysis (PATH): a randomized clinical trial, Journal of the American Society of Nephrology : JASN 25(3) (2014) 623–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Vucinic L, Singh I, Spargo FJ, Hawley JA, Linden MD, Gamma tocopherol supplementation prevents exercise induced coagulation and platelet aggregation, Thrombosis research 125(2) (2010) 196–9. [DOI] [PubMed] [Google Scholar]
- [135].Mah E, Noh SK, Ballard KD, Park HJ, Volek JS, Bruno RS, Supplementation of a gamma-tocopherol-rich mixture of tocopherols in healthy men protects against vascular endothelial dysfunction induced by postprandial hyperglycemia, The Journal of nutritional biochemistry 24(1) (2013) 196–203. [DOI] [PubMed] [Google Scholar]
- [136].Masterjohn C, Mah E, Guo Y, Koo SI, Bruno RS, gamma-Tocopherol abolishes postprandial increases in plasma methylglyoxal following an oral dose of glucose in healthy, college-aged men, The Journal of nutritional biochemistry 23(3) (2012) 292–8. [DOI] [PubMed] [Google Scholar]
- [137].Devaraj S, Leonard S, Traber MG, Jialal I, Gamma-tocopherol supplementation alone and in combination with alpha-tocopherol alters biomarkers of oxidative stress and inflammation in subjects with metabolic syndrome, Free radical biology & medicine 44(6) (2008) 1203–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Wu JH, Ward NC, Indrawan AP, Almeida CA, Hodgson JM, Proudfoot JM, Puddey IB, Croft KD, Effects of alpha-tocopherol and mixed tocopherol supplementation on markers of oxidative stress and inflammation in type 2 diabetes, Clinical chemistry 53(3) (2007) 511–9. [DOI] [PubMed] [Google Scholar]
- [139].Ward NC, Wu JH, Clarke MW, Puddey IB, Burke V, Croft KD, Hodgson JM, The effect of vitamin E on blood pressure in individuals with type 2 diabetes: a randomized, double-blind, placebo-controlled trial, Journal of hypertension 25(1) (2007) 227–34. [DOI] [PubMed] [Google Scholar]
- [140].Wiser J, Alexis NE, Jiang Q, Wu W, Robinette C, Roubey R, Peden DB, In vivo γ-tocopherol supplementation decreases systemic oxidative stress and cytokine responses of human monocytes in normal and asthmatic subjects, Free Radical Biology and Medicine 45(1) (2008) 40–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Burbank AJ, Hernandez ML, Robinette C, Wang T, Zhou H, Alexis N, Bennett WD, Peden DB, Short course gamma tocopherol did not mitigate effects of ozone on airway inflammation in asthmatics, Inhalation Toxicology 32(7) (2020) 279–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Yang C, Zhao Y, Im S, Nakatsu C, Jones-Hall Y, Jiang Q, Vitamin E delta-tocotrienol and metabolite 13′-carboxychromanol inhibit colitis-associated colon tumorigenesis and modulate gut microbiota in mice, The Journal of nutritional biochemistry 89 (2021) 108567. [DOI] [PubMed] [Google Scholar]


