Abstract
Chemotherapy-induced peripheral neuropathy (CIPN) is one of the most frequently reported adverse effects of cancer treatment. CIPN often persists long after treatment completion and has detrimental effects on patient’s quality of life. There are no efficacious FDA-approved drugs for CIPN. We recently demonstrated that nasal administration of mesenchymal stem cells (MSC) reverses the cognitive deficits induced by cisplatin in mice.
Here we show that nasal administration of MSC after cisplatin- or paclitaxel treatment-completely reverses signs of established CIPN, including mechanical allodynia, spontaneous pain, and loss of intraepidermal nerve fibers (IENF) in the paw. The resolution of CIPN is associated with normalization of the cisplatin-induced decrease in mitochondrial bioenergetics in DRG neurons. Nasally administered MSC enter rapidly the meninges of the brain, spinal cord and peripheral lymph nodes to promote IL-10 production by macrophages.
MSC-mediated resolution of mechanical allodynia, recovery of IENFs and restoration of DRG mitochondrial function critically depends on IL-10 production. MSC from IL-10 knockout animals are not capable of reversing the symptoms of CIPN. Moreover, WT MSC do not reverse CIPN in mice lacking IL-10 receptors on peripheral sensory neurons. In conclusion, only two nasal administrations of MSC fully reverse CIPN and the associated mitochondrial abnormalities via an IL-10 dependent pathway. Since MSC are already applied clinically, we propose that nasal MSC treatment could become a powerful treatment for the large group of patients suffering from neurotoxicities of cancer treatment.
Keywords: Mesenchymal Stem Cells (MSC), cisplatin, peripheral neuropathy, Interleukin-10 (IL-10), meninges, sensory neurons, mitochondria
1. Introduction
Chemotherapy-induced peripheral neuropathy (CIPN) is a frequent side effect of many chemotherapy regimens (1–4). In a recent systematic review the authors showed that CIPN prevalence was 68.1% at the first month of chemotherapy to 30% six-months after chemotherapy, with a wide variance in prevalence from 12.1–96.2% (3). Up to 30 % of the affected patients still suffer from CIPN months to years after treatment completion representing a long-lasting negative impact on quality of life (2, 3, 5–7). CIPN symptoms include pain, mechanical, heat, and cold allodynia, as well as numbness and tingling (8–10). CIPN develops in a symmetric distal “stocking and glove” type distribution (11). To date, there are no effective FDA-approved treatments for CIPN. In view of the increasing number of cancer survivors, an efficacious therapeutic strategy to treat CIPN is urgently needed.
The mechanisms associated with the pathogenesis of CIPN are not completely understood. It is well-accepted that mitochondrial impairment of peripheral sensory neurons is one of the underlying causes of CIPN (9, 12–19). Preclinical studies using rodent models demonstrated that prevention of mitochondrial damage in DRG and peripheral nerve, protects against CIPN (12, 13, 20–25). For example, we recently showed that preventing the cisplatin-induced early p53 accumulation at the mitochondria of DRG neurons using co-administration of the small molecule pifithrin-μ (PFT- μ), completely prevented CIPN (13, 20).
Advances in regenerative medicine have shown that mesenchymal stem cells (MSC) stimulate tissue repair and ameliorate the outcome in preclinical models of cerebral trauma and neurodegenerative disorders (26, 27). We have shown that the nasal route is an effective and safe route of administration of MSC to reverse brain damage induced by hypoxic-ischemic events or in models of subarachnoid hemorrhage in rodents (28–34).
Notably, nasal MSC administration also reversed cognitive deficiencies as a result of cisplatin treatment and this beneficial effect was associated with restoration of brain synaptosomal mitochondrial function (35). There is evidence that intrathecal or intravenous administration of MSC can alleviate pain in the spared nerve injury model of neuropathic pain (36) or in a model of diabetic neuropathy (36–39).
However, intravenous administration has the disadvantage that many MSCs will be sequestered to the lung and liver and only few of the donated MSCs will arrive at the site of action (40). A disadvantage of intrathecal administration of MSC is that it is highly invasive.
Several mechanisms have been suggested to mediate the beneficial effects of MSC, e.g. transfer of healthy mitochondria from MSCs to damaged neurons, secretion of neurotrophic and angiogenic factors, and increased production of immunosuppressive factors including IL-10 (41–43). We and others have shown that IL-10 signaling is a crucial pathway in the spontaneous resolution of pain in models of CIPN, nerve injury, and in exercise-induced analgesia (44–46). In this study we investigated the effect of nasal administration of MSC on signs of cisplatin-induced peripheral neuropathy, including mechanical allodynia, spontaneous pain and loss of intraepidermal nerve fibers. In addition, we examined whether the resolution of CIPN after nasal MSC administration was associated with a repair of mitochondrial dysfunction in dorsal root ganglia. As a possible mechanism of the beneficial effects of nasal MSC, we explored the contribution of IL-10 production by MSC and the role of IL-10 receptor signaling on peripheral sensory neurons for the resolution of CIPN.
2. Results
2.1. Nasal administration of MSC reverses cisplatin-induced mechanical allodynia in both male and female mice
To induce CIPN male and female mice were treated with two cycles of cisplatin for 5 days (2.3 mg/kg/day, i.p.) with 5 days of rest in between as we have published before (14, 20, 35). MSC (1 × 106 per dose) were administered nasally at 48h and 96h after the last dose of cisplatin. Mechanical allodynia was measured over time using Von Frey hairs. The results in Figures 1A and B demonstrate that cisplatin induces mechanical allodynia in both female and male mice. Nasal MSC administration completely reversed the already existing mechanical allodynia in both sexes. The reversal of cisplatin-induced mechanical allodynia in response to nasal MSC administration developed slowly over time; the first beneficial effects were detected on day 8 after MSC administration and MSC-treated mice had completely recovered from cisplatin-induced mechanical allodynia 21 days after the last dose of MSC (Fig. 1A and B). Mechanical allodynia persisted in mice that had received cisplatin only since no evidence of spontaneous recovery was observed for at least 70 days after the first cisplatin injection (14). Nasal administration of bone marrow-derived MSC of human origin (hMSC) reduced mechanical allodynia in cisplatin-treated mice as well (Fig.1C). To investigate whether MSC also reverse paclitaxel-induced mechanical allodynia, mice were treated with PBS or paclitaxel (2mg/kg/day, i.p) every other day for 2 weeks. Mice displayed a prolonged allodynia after paclitaxel treatment which was completely reversed by nasal administration of MSC at 48 and 96 hours after completion of paclitaxel treatment (Fig.1D). The kinetics of resolution of paclitaxel-induced allodynia were faster than after cisplatin treatment since a full resolution was observed after 12 days (Fig. 1D).
Figure 1. Nasal MSC application reverses cisplatin- and paclitaxel-induced allodynia.
Female (A) or male (B, C) mice were treated i.p. with 2 cycles of 5 days of cisplatin (2.3 mg/kg/day i.p.) or PBS with 5 days of rest in between; 48 h and 96 h after the last cisplatin dose, 1×106 murine MSC (mMSC or PBS; A, n=4/group and B, n=7–12/group) or hMSC or PBS (C, n=8/group) were administered via the nasal route. Mechanical allodynia was measured using von Frey hairs. Data were analyzed using two-way repeated measure ANOVA followed by Bonferroni’s post-hoc test. Cisplatin versus cisplatin + MSC * P<0.05, *** P<0.001. (D) Male mice (n= 4/group) were treated i.p. with paclitaxel (2 mg/kg/day) or vehicle every other day for 2 weeks followed by nasal MSC administration. Mechanical allodynia data were analyzed using two-way repeated measure ANOVA followed by Bonferroni’s post-hoc test. ** P<0.01, *** P<0.001 (E) Male mice were treated with PBS (n=7–10/group) or cisplatin (n=12/group) followed by mMSC or PBS as in (A). Spontaneous pain was measured by the conditioned place preference test starting 3 weeks after mMSC treatment. Data represent the change in time spent in the light, analgesic-paired chamber from baseline to testing. Data are shown as mean ± SEM and were analyzed using two-way ANOVA followed by Sidak’s post-hoc test. * P<0.05.
2.2. Nasal administration of MSC reverses cisplatin-induced spontaneous pain in both male and female mice
Spontaneous pain is an important complaint of patients with CIPN (17). To assess the effect of MSC on spontaneous pain induced by cisplatin, we used a conditioned place preference (CPP) paradigm with the nerve blocker retigabine as the conditioning stimulus (14, 47). The CPP paradigm assessed 3 weeks after the last administration of MSC. Cisplatin-treated mice showed an increase in time spent in the retigabine-paired bright chamber indicating ongoing pain. In contrast, mice treated with cisplatin followed by MSC did not show an increase in time in the retigabine paired chamber, suggesting that nasal MSC administration had reversed spontaneous pain in these mice (Fig. 1E).
2.3. Nasal administration of MSC reverses cisplatin-induced decrease in intraepidermal nerve density
Next, we determined whether nasal MSC administration affects the reduction in IENF density in the plantar surface of the hind paw after cisplatin treatment. As shown before (14, 48), the density of IENF was reduced in cisplatin-treated animals. Importantly, nasal administration of two doses of MSC was sufficient to completely restore IENF density (Fig.2A and B).
Figure 2. Nasal administration of mMSC reverses the loss of intra-epidermal nerve fibers in the hind paw of cisplatin-treated mice.
Mice were treated with cisplatin and MSC as in Figure 1. Skin biopsies were collected from the plantar surface of the hind paws 24 days after the last mMSC administration and IENF density was quantified using the pan-neuronal marker protein gene product PGP9.5 (white). Sections were stained for collagen (green) for orientation purposes. (A) Representative confocal images from saline, cisplatin and cisplatin + mMSC-treated mice. The basement membrane is indicated by the dashed lines, nerve fibers crossing the basement membrane are indicated by arrows. (B) Quantification of intra-epidermal nerve fiber (IENF) density is expressed as the number of nerve fibers crossing the basement membrane per mm length of the basement membrane. 5–8 sections of each paw were quantified. n = 4 male mice/group. Scale bars represent 25 μm. Data are expressed as mean ± SEM and were analyzed using One-way ANOVA followed by Bonferroni’s post-hoc analysis. * P<0.05, *** P<0.001.
2.4. Effect of MSC on mitochondrial function in DRG neurons from cisplatin-treated mice
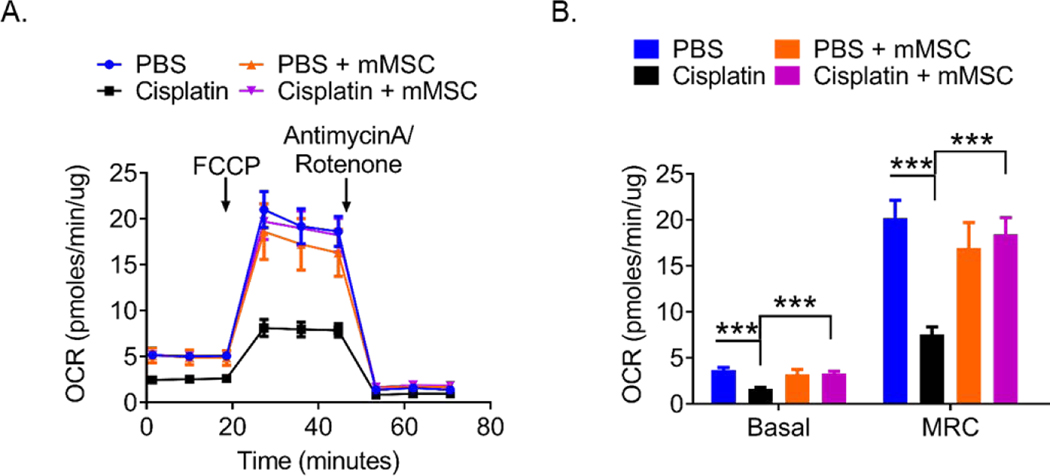
Cisplatin-induced peripheral neuropathy is associated with impaired mitochondrial bioenergetics in dorsal root ganglion neurons (9, 12, 14–16, 20, 48). We assessed the effect of nasal MSC administration on mitochondrial bioenergetics in DRG neurons 3 weeks after completion of MSC treatment. At this time point, cisplatin-treated mice displayed persistent mechanical allodynia, while mice treated with nasal MSC had fully recovered. Treatment of mice with cisplatin decreased both the basal and maximal oxygen consumption rate of DRG neurons (Fig. 3). Notably, nasal MSC treatment normalized basal and maximal mitochondrial respiration of DRG neurons (Fig. 3B). MSC treatment by itself did not have any effect on mitochondrial bioenergetics in control mice.
Figure 3. Nasally administered mMSC restore mitochondrial bioenergetics in DRG neurons of cisplatin-treated mice.
Mitochondrial bioenergetics in DRG neurons from mice that had received 2 rounds of cisplatin treatment followed by 2 doses of mMSC. Tissues were collected 24 days after the last mMSC administration. (A) Oxygen consumption rates (OCR) were measured over time using a Seahorse XFe 24 Analyzer and normalized to protein content. (B) Mean basal and maximum respiratory capacity (MRC) normalized to protein content were calculated. Results are expressed as means ± SEM and data were analyzed using Two-way ANOVA followed by Bonferroni’s post-hoc test. *** P<0.001. PBS n= 6 mice/group, PBS + MSC n= 4 mice/group, Cisplatin and Cisplatin + MSC n= 11 mice/group.
2.5. Nasally delivered MSC migrate to the meninges of the brain and lumbar spinal cord as well as to peripheral lymph nodes
To explore the fate of the nasally administered MSC, we administered MSC from GFP-transgenic mice (GFP+-MSC) 48h after the last dose of cisplatin. We have shown before that a low number of MSC can be found in the parenchyma of the brain, including the hippocampus, thalamus and cortex where they arrive 12 hours after administration and completely disappear within 72 hours after nasal administration (35). The data in Figure 4 show that MSC can already be detected in the meninges of the brain in high numbers at 2 hours after nasal administration. We detected GFP+-MSC within the superior sagittal sinus (SSS) (Fig. 4A and B) of the meninges of the brain at all time points tested (Fig. 4C–G). The highest number of MSC in the meninges of the brain was detected at 2 and 24 hours after nasal administration. At 7 days after nasal administration, we still detected up to 50% of the number of MSC that were present at 2–24 hours in the meninges of the brain. This is in sharp contrast to what we previously showed for the brain parenchyma, where the GFP signal had completely disappeared within 72 hours (35).
Figure 4. Nasal MSC migrate to the meninges of the brain.
Mice were treated with 2 cycles of cisplatin and 48 hours after the last cisplatin dose, 2×106 GFP+-mMSC were administered nasally. Meninges were collected 2 h, 24 h, 72 h and 7 days after MSC administration and analyzed by confocal microscopy. (A) Overview of meninges of the brain stained with DAPI to label all nuclei. The box contains the superior sagittal sinus (SSS) (Á). Representative images of nasally administered GFP-MSC in SSS at 2h (B and D), 24h (E), 72h (F) and 7 days (G) after nasal administration. (C) Quantification of the number of GFP+-MSC in the SSS. Data are represented as means ± SEM and were analyzed using one-way ANOVA followed by Bonferroni’s post-hoc analysis. n= 4 mice/group. Regions denoted by a white line represent magnifications of the regions in the dotted lines.
The meninges of the brain extend towards the spinal cord forming the spinal cord meninges. The data in Figure 5 demonstrate that nasally administered GFP+-MSC also reach the spinal cord meninges, albeit in relatively low numbers. We detected GFP+-MSC in the spinal cord meninges at 12 and 24 hours after nasal administration (Fig. 5A). No GFP+-MSC were yet detected in the spinal cord meninges at 2 hours after administration or after 7 days of administration. Moreover, we did not detect MSC in the spinal cord parenchyma.
Figure 5. Detection of GFP+-MSC in the lumbar spinal cord meninges and lymph nodes.
Mice were treated with cisplatin followed by GFP+-mMSC as in Figure 4. (A) Spinal cord (SC) meninges, (B) deep cervical (dcLN), (C) inguinal and (D) lumbar lymph nodes were dissected and GFP+-MSC were traced at different time points after nasal administration. GFP+ cells were only detected at 12h and 24h after MSC administration in the meninges of the spinal cord, whereas GFP+ cells were detected at 12, 72 hours and 7 days in DCLN (B) as well as 24, 72 hours and 7 days in inguinal and lumbar lymph nodes (C and D). Regions denoted by a white line indicate magnified images (right panel). Scale bars represent 25 μm. Representative images from 4 independent mice per group.
We also traced GFP+-MSC in the deep cervical, inguinal, and lumbar lymph nodes for up to 7 days after nasal administration (Fig. 5B–D). These results indicate that MSC are also transported via the lymphatic route.
2.6. MSC-derived IL-10 is necessary for resolution of cisplatin-induced peripheral neuropathy
We recently showed that IL-10 signaling is required for the spontaneous resolution of CIPN and therefore we evaluated a possible contribution of MSC-derived IL-10 in the mechanism of resolution of CIPN. We compared the effect of nasal administration of MSC from WT and Il10−/− mice (Il10−/− MSC) to cisplatin-treated mice on mechanical allodynia. The results in Fig. 6A show that Il10−/− MSC did not have any positive effect on cisplatin-induced mechanical allodynia, while mechanical allodynia resolved in response to the control WT MSC.
Figure 6. Nasal administration of Il10−/− mMSC does not reverse cisplatin-induced mechanical allodynia and loss of mitochondrial bioenergetics in DRG neurons.
Mice received two rounds of cisplatin followed by nasal administration of WT or Il10−/− MSC and mechanical allodynia was measured as in figure 1. (A) Data were analyzed using two-way repeated measure ANOVA followed by Bonferroni’s post-hoc test. *** P<0.001 Cisplatin + WT mMSC versus cisplatin + Il10−/− mMSC. n= 12 mice/group. Mitochondrial bioenergetics in DRG neurons (B, C) were measured 24 days after the last MSC dose. Oxygen consumption rates (OCR) normalized to protein content are represented in. Data were analyzed using two-way ANOVA followed by Bonferroni’s post-hoc test. * P<0.05, ** P<0.01. PBS n= 4 mice/group, Cisplatin n= 12 mice/group, Cisplatin + WT MSC n= 12 mice/group, Cisplatin + IL10−/− MSC n= 12 mice/group.
To test whether IL-10 production by MSC is also needed for resolution of the cisplatin-induced mitochondrial dysfunction in the peripheral nervous system, we assessed mitochondrial bioenergetics in DRG neurons isolated from mice treated with either WT or Il10−/− MSC. In line with what we observed for mechanical allodynia, nasal administration of Il10−/− MSC failed to normalize the deficits in mitochondrial bioenergetics in DRG that were induced by cisplatin. In contrast, nasal administration of WT MSC normalized mitochondrial bioenergetics in the DRG (Fig. 6B and C).
2.7. Presence of IL-10R1 on sensory neurons is essential for resolution of cisplatin-induced mechanical allodynia
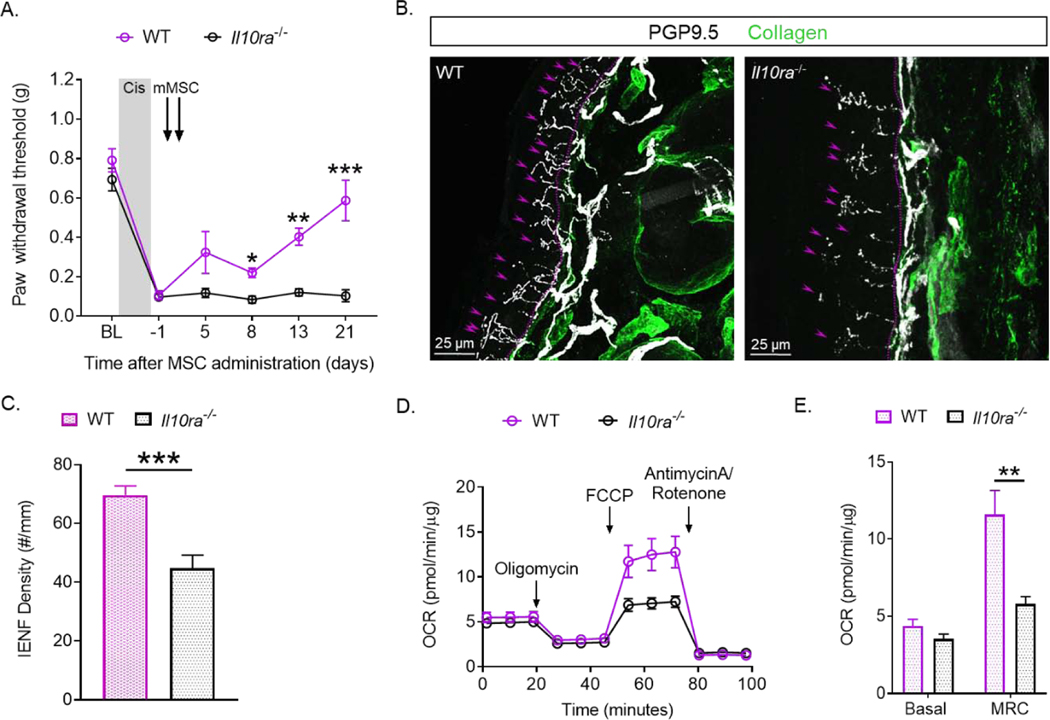
In a recent study, we showed that expression of IL-10R1 on Advillin-positive sensory neurons (Avil-Il10ra−/− mice) is required for the spontaneous resolution of CIPN after a short course (3 days) of cisplatin treatment (49). Here we used Il10ra−/− mice to determine the contribution of IL-10 receptors on sensory neurons to the resolution of CIPN after the full dosing regimen of cisplatin (10 gifts of 2.3 mg/kg) and treatment with nasal administration of MSC. Il10ra−/− mice showed a similar onset and severity of allodynia as WT mice. However, nasal MSC treatment did not reverse cisplatin-induced mechanical allodynia in mice lacking IL-10R1 on sensory neurons, whereas mechanical allodynia completely resolved in WT mice (Fig. 7A). Expression of IL-10R1 on sensory neurons was also required for reversal of the cisplatin-induced loss of IENF in response to nasal MSC administration. (Fig. 7B and C).
Figure 7. IL-10R1 expression in Advillin-positive sensory neurons is necessary for resolution of cisplatin-induced mechanical allodynia in response to nasal MSC treatment.
Wild type (WT) and Avil-Il10ra−/− mice were treated with cisplatin and MSC as in Figure 1. (A) Mechanical allodynia was followed over time. Data were analyzed using Two-way ANOVA followed by Bonferroni’s post-hoc test. * P<0.05, ** P<0.01, *** P<0.001. n= 6 mice/group. IENFs density in the hind paw plantar surface was quantified as in figure 2. (B) Representative confocal images from WT and Il10ra−/− mice. The basement membrane is indicated by the dashed lines, the nerve fibers crossing the basement membrane are indicated by arrows. (C) Quantification of intra-epidermal nerve fiber (IENF) density expressed as the number of nerve fibers crossing the basement membrane/length of the basement membrane (mm). 5–8 sections of each paw were quantified. Scale bars represent 25 μm. Data were analyzed using unpaired t-test with Welch’s correction. *** P<0.001. WT n = 9 mice/group, Il10ra−/− n = 10 mice/group. Mitochondrial bioenergetics were measured in DRG neurons (D, E) 24 days after the second mMSC dose. Oxygen consumption rates (OCR) normalized to protein content are represented in (D). (E) Mean basal and maximum respiratory capacity (MRC) normalized to protein are represented in. Data were analyzed using unpaired t-test with Welch’s correction. **P<0.01. n= 6 mice/group.
Next, we probed whether the presence of IL-10R on sensory neurons is required for MSC to resolve cisplatin-induced mitochondrial dysfunction of DRG. While MSC induced full recovery of mitochondrial bioenergetics in the DRG of WT mice, we did not detect any improvement in mitochondrial function of DRG of Il10ra−/− mice after nasal administration of MSC (Fig. 7D and E). These results clearly show that resolution of CIPN in response to nasal MSC requires the expression of IL-10 receptors on sensory neurons.
2.8. Macrophages increase their IL-10 production after nasal MSC administration.
There is increasing evidence that at least part of the beneficial effects of MSC is mediated via a crosstalk between MSC and macrophages (50–53). To explore the mechanism of action via which nasally administered MSC reverse CIPN, we hypothesized that part of the positive effect of MSC is due to ‘education of macrophages in the meningeal compartment to produce IL-10. Figure 8 shows that nasal administration of MSC to cisplatin-treated mice significantly increased the percentage of IL-10 producing macrophages of the meningeal compartment of the brain (Fig. 8C and D). Cisplatin treatment alone did not change IL-10 production by meningeal macrophages (Fig. 8B and D) as compared to PBS-treated mice (Fig. 8A).
Figure 8. Nasal MSC treatment increases IL-10 production by macrophages.
Mice received either PBS or cisplatin followed by MSC as in Figure 1. Meninges were collected and single cell suspensions were analyzed for intracellular IL-10 and the macrophage cell surface marker F4/80 detection. Cells were then analyzed by flow cytometry. (A and C) Representative dot plots of macrophages (F4/80+) in the meninges (A) and inguinal lymph nodes (C) of mice treated with either PBS, cisplatin or cisplatin and MSC. (B and D) Quantification of the percentage of F4/80+ IL-10+ macrophages normalized to data of PBS-treated mice. Data are expressed as means ± SEM and were analyzed using One-way ANOVA followed by Bonferroni’s post-hoc test. Meninges, PBS n= 9 mice/group, Cisplatin n= 6 mice/group and Cisplatin + MSC n= 7 mice/group. ** P<0.01, *** P<0.001. Inguinal lymph nodes, PBS n= 5 mice/group, Cisplatin n= 5 mice/group and Cisplatin + MSC n= 6 mice/group. ** P<0.01, *** P<0.001.
We also evaluated IL-10 production by macrophages in the inguinal lymph nodes (iLNs). Figure 8 shows that MSC treatment is associated with an increase of the percentage of Il-10 producing macrophages in the iLNs as compared to PBS- treated mice, while cisplatin treatment decreases the percentage of IL-10 producing macrophages (Fig. 8C and D).
3. Discussion
We report here that two nasal administrations of only 1×106 of MSC completely reverses multiple signs of CIPN including mechanical allodynia, spontaneous pain, and the loss of IENF. Nasal MSC treatment did not only reverse CIPN caused by cisplatin treatment but also promoted resolution of CIPN as a result of paclitaxel treatment.
Mitochondrial dysfunction in peripheral neurons is one of the important underlying mechanisms of CIPN (12, 14, 15). Notably, nasal administration of MSC fully reverses the impaired mitochondrial bioenergetics in DRG neurons of cisplatin-treated mice. Finally, we identify a key role of IL-10 produced by MSC in the resolution of CIPN. Moreover, we show that IL-10 signaling to IL-10 receptors on Advillin-positive primary sensory neurons is required for resolution of CIPN as well as for normalization of DRG mitochondrial function in response to MSC treatment.
The unexpected finding that MSC administered via the nose restore CIPN and mitochondrial health in DRG indicates a long ranging, disease modifying effect of MSC. This is important from a clinical as well as fundamental perspective. Nasally administered MSC rapidly migrated to the meninges of the brain within 2 hours and could be detected in low numbers in the meninges of the lumbar spinal cord of cisplatin-treated mice after 12 hours. Galeano et al. have reported that nasally administered MSC cross the olfactory epithelium, and as they pass through the cribiform plate, they can enter the subarachnoidal space via its extension adjacent to the fila olfactoria (40). From this cerebral meningeal compartment, MSC can reach the meningeal compartment of the spinal cord by migrating along the meninges, the meningeal vasculature and/or via the cerebrospinal fluid. We did not observe GFP+-cells in the parenchyma of the spinal cord tissue or DRG (data not shown). We showed previously that GFP+-MSC, when nasally administered to cisplatin-treated mice, penetrate the brain parenchyma as well after 12–24 hours albeit in low numbers (35). MSC do not survive for a long time in the brain; GFP+-MSC were no longer detectable in the brain parenchyma at 72 hours after administration (35). However, in the meninges of the brain we detected the GFP+-MSC as early as 2 hours and up to 7 days after nasal administration. Moreover, GFP+-MSC could also be traced in deep cervical, inguinal and lumbar lymph nodes where they were still detectable 7 days after administration, which was the last time point tested. These findings show that MSC traffic to multiple distal sites where they directly or indirectly (via activation of other cell types) could promote reversal of CIPN and restoration of neuronal function.
We show here that MSC have to express IL-10 in order to be capable of resolving CIPN. Moreover, selective deletion of IL-10 receptors from peripheral sensory neurons, prevented the beneficial effects of MSC on CIPN. We have previously shown that endogenous IL-10 signaling plays a crucial role in the spontaneous resolution of inflammatory pain and of CIPN induced by a short 3 day- course of cisplatin or paclitaxel (49, 54, 55). Specifically, we showed that genetic deletion of IL-10 or intrathecal administration of anti-IL-10 delayed the resolution of inflammatory pain and CIPN (54). Seminal studies by Watkins and co-workers have shown that intrathecal administration of an IL-10 encoding vector suppresses inflammatory and neuropathic pain in multiple models (56) (46, 57). However, the mechanism via which IL-10 promotes resolution of pain has only been partially elucidated. We demonstrated recently that in vitro, IL-10 transiently suppresses the abnormal spontaneous firing of peripheral sensory neurons from paclitaxel-treated rats (54) or cisplatin-treated mice (49). Similarly, we showed that intrathecal administration of a stable IL-10 fusion protein reduced chemotherapy-induced allodynia, but was not sufficient for complete reversal (54). These findings indicate that long-lasting increased production of IL-10 is required for maintaining resolution of CIPN. This would imply that MSC may, apart from producing IL-10, activate other resident cell types, including macrophages, to produce IL-10 in the long term.
We showed previously that depletion of mice from macrophages delays resolution of inflammatory pain (55). In addition, accumulating evidence suggests that MSC promote the conversion of pro-inflammatory macrophage populations into anti-inflammatory wound healing cells producing IL-10 (58, 59). Our current data indicate that nasal administration of MSC promotes the production of IL-10 by meningeal macrophages which could travel to the periphery to directly regulate neuronal function. Moreover, nasally administered MSC also travel to the lymph nodes where they also promote the switch to IL-10 producing macrophages as we show here for the iLNs. These macrophages could subsequently migrate to the sites of action, the peripheral sensory nerve. We therefore propose that MSC can exert their effect at multiple sites where they can interact with macrophages.
One of the ways MSC could ‘educate’ macrophages to reach an M2 state is via transfer of MSC-derived mitochondria from MSC to macrophages. In a model of murine asthma, it has been shown that MSC applied to the lungs transfer their mitochondria to the alveolar macrophages to promote a reparative, M2 state with bioenergetics changes and increased IL-10 production (60). Jackson et al. reported similar effects of MSC on alveolar macrophages in a model of acute respiratory distress syndrome (50).
We show here that IL-10 signaling to sensory neurons is also required for the normalization of mitochondrial bioenergetics. Interestingly, IL-10 can change the metabolic state of cells by decreasing the glycolytic state and rescuing the oxidative state in macrophages (61). Therefore, we hypothesize that IL-10 signaling contributes to the metabolic reprogramming of the sensory nerve ultimately leading to the resolution of CIPN. Once reprogramming is stable, MSC would no longer be necessary to tune or educate the macrophages.
IENF loss has been reported both in patients treated with chemotherapy and in rodent models of CIPN (12, 14). The reduction in mitochondrial function in peripheral nerves is thought to lead to degeneration and/or retraction of peripheral nerve endings and thus in a reduction in IENF density (12). Consistent with our previous reports, we demonstrate here that cisplatin treatment decreased IENF density in the hind paws (14). Importantly, we demonstrate that two doses of nasal MSC treatment fully reverses cisplatin-induced IENF loss. This restoration of IENF density was associated with normalization of mitochondrial function, and was dependent on IL-10 signaling. These findings indicate that MSC promote metabolic reprogramming of sensory nerves and the regrowth of IENF via an IL-10 dependent pathway.
We have shown earlier that nasal MSC treatment is safe and has lifelong efficacy in mice after hypoxic ischemic damage (33). At 14 months after the hypoxic-ischemic event, we did not detect any neoplasia in the nasal turbinates, brain, or any other organ of hypoxic-ischemic mice treated with MSCs. Moreover, Chiu et al. demonstrated that nasal administration of MSC does not interfere with the anti-tumor effects of cisplatin or with tumor growth in a heterotopic syngeneic murine model of human papilloma virus (HPV)-related head and neck cancer (35, 62, 63). As has been shown before, allogeneic MSCs can be used in humans and we show here that xenogeneic MSCs can be used since human MSCs were effective in resolving CIPN in mice (64).
The fact that MSC also reverse paclitaxel-induced CIPN underlines the importance of MSC treatment for reversal of CIPN since treatment of cancer patients often involves combination of chemotherapy. Taken together, our results identify nasal MSC administration as a potential powerful easy accessible therapeutic strategy for reversal of chemotherapy-induced neurotoxicities including peripheral neuropathy and chemotherapy-induced cognitive deficits as we have shown before (35). This is an important finding as to date, there are no effective FDA-approved therapeutics for the treatment of CIPN or chemobrain in cancer patients and survivors.
4. Materials and Methods
4.1. Animals
Male and female C57BL/6J, female GFP+ mice, and female Il10−/− mice in a C57BL/6J of 8 – 10 weeks were obtained from Jackson Laboratories. To obtain Avil-Il10ra−/− mice, we bred Avil-cre+/− mice with Il10rafloxflox mice (65). Animals were housed at The University of Texas MD Anderson Cancer Center animal facility in Houston, TX on a reversed 12-hour dark/light cycle and had free access to water and food. Animals were randomly assigned to treatment groups and experiments were performed by investigators blinded to group. Il10ra−/− mice were compared to their WT flox flox littermates as controls. All procedures were in accordance with the National Institute of Health Guidelines for the Care and Use of Laboratory Animals and the Ethical Issues of the International Association for the Study of Pain and were approved by the local Institutional Animal Care and Use Committee.
4.2. Chemotherapy and MSC Treatment
Cisplatin (2.3 mg/kg/day; TEVA, Petah Tiva, Israel) or phosphate-buffered saline (PBS) was injected intraperitoneally (i.p.) for 2 cycles of 5 daily injections followed by 5 days of rest (14, 48). Paclitaxel (2mg/kg/day; PREMIER ProRx, Charlotte, NC, USA) or PBS was administered i.p. every other day for 2 weeks. C57BL/6 murine bone marrow MSC were either purchased from Invitrogen (Carlsbad, CA, USA) or isolated from tibia and femur of 8 weeks old female WT, GFP-transgenic mice or Il10−/− mice. Mouse MSC were cultured in 5% CO2 at 37 °C in DMEM/F12 medium with GlutaMax-I, containing 10% MSC-qualified fetal bovine serum and 5 μg/mL gentamycin (all from GIBCO, Carlsbad, CA, USA). Cells were harvested using TrypLE-express (GIBCO). MSCs were positive for MSC-associated antigens CD29, CD44, CD73, CD105, CD106, Sca-1, and negative for hematopoietic markers CD11b and CD45. Human MSC (hMSC, Lonza, Basel, Switzerland) from female donors were grown in 5% CO2 at 37 °C in MEM-alpha medium (Corning, Corning, NY, USA) supplemented with 5% platelet lysate (PLTMax, Mill Creek Life Sciences, Rochester, MN, USA), 2 mM GlutaMax (GIBCO, Carlsbad, CA, USA), 100 units/ml of Penicillin/Streptomycin (Corning) and 2 units/ml of Heparin (Hospira, Lake Forest, IL, USA). Cells were harvested using 0.05% Trypsin (GE Healthcare Bio-Sciences, Pittsburgh, PA, USA). 1 × 106 MSCs were administered 48 and 96 h after the last dose of cisplatin (35, 66) or 72 h and 120 h after the last dose of paclitaxel. Before MSC administration, mice received 3 μl of hyaluronidase in PBS in each nostril (100 U per mouse, Sigma-Aldrich) to temporarily increase the permeability of the mucosa lining the nasal cavity (31, 35, 66, 67). MSC or PBS were given to mice 30 min later, as two doses of 3 μl in each nostril, for a total of 12 μl and 1 × 106 MSC per mouse per day.
4.3. Mechanical allodynia
Mechanical allodynia was measured using von Frey hairs and the up and down method (68) starting with the 0.16 g filament and a range of 0.02–1.4 g filaments (14, 20, 47, 48, 69). Data represent group means of the average of both hind paws for each mouse.
4.4. Spontaneous pain
Spontaneous pain was tested using a conditioned place preference (CPP) paradigm with retigabine as the conditioned stimulus (14). During the first day, mice were first allowed to freely explore the CPP apparatus, consisting of 2 chambers (18 × 20 cm, 1 dark, 1 bright light) connected by a 15 cm hallway (Stoelting, Wood Dale, IL), for 15 minutes. During the four days of the conditioning phase, intraperitoneal PBS injections were paired with exposure to the dark chamber and retigabine injections (10 mg/kg in PBS; #R-100; Alomone Labs, Jerusalem, Israel) paired with exposure to the bright chamber. On day 5, mice were allowed to freely explore the two chambers for 15 minutes without any injections. An increase in the time spent in the bright, retigabine-paired chamber as compared to the pre-conditioning session was interpreted as evidence for sponatenous pain. Preconditioning and postconditioning test results were recorded and analyzed using EthoVision XT video tracking software (Noldus Information Technology Inc, Leesburg, VA).
4.5. IENF density
Biopsies from the plantar surface of the hind paws were collected and processed as described (14, 47, 48, 69). Briefly, 25 μm frozen sections were incubated with antibodies for the pan-neuronal marker PGP9.5 (rabbit; Bio-Rad AbD Serotec, Oxford, United Kingdom) and collagen IV (goat; Southern Biotech, Birmingham, AL) followed by Alexa-594 donkey anti-rabbit and Alexa-488 donkey anti-goat secondary antibodies (Life Technologies, Carlsbad, CA). Images of IENF density were captured using an SPE Leica Confocal Microscope (Leica Microsystems, Buffalo Grove, IL, USA) with a 40 X objective, and analyzed using LAS X software. IENF density was expressed as the total number of nerve fibers crossing the basement membrane/length of the basement membrane (mm). 5–8 random pictures/mouse were quantified by researchers blinded to treatment.
4.6. Assessment of mitochondrial bioenergetics
Mitochondrial function in DRG neurons was assessed as described (14). Lumbar DRGs were digested with 1.25% collagenase (Thermo Fisher Scientific, Waltham, MA) and 2% trypsin (Sigma-Aldrich). DRGs were dissociated by triturating in Ham’s F10 medium (Corning Inc., Corning, NY) and dissociated neurons were layered on a 10 ml gradient of sterile 26% Percoll (GE Healthcare Life Sciences, Little Chalfont, United Kingdom) in PBS, and centrifuged at 800 × g for 20 minutes at room temperature. Cell pellet was resuspended in Ham’s F10 medium supplemented with N2 supplement (Thermo Fisher Scientific). Cells were cultured in an XF24 microplate overnight. 1 hour before starting the assay, the cell maintenance medium was changed to XF base media (Seahorse Biosciences) containing 11 mM glucose, 2 mM glutamine, and 1 mM pyruvate. Oligomycin (2 μM), FCCP (4 μM) and rotenone/antimycin A (2 μM each) were used with a 3-time repeat of a 2-minute mix, 3-minute wait, and 2-minute measure assay cycle. OCR values were normalized to the total protein content of each well.
4.7. Isolation and immunofluorescence analysis of brain, spinal cord meninges and lymph nodes
Mice were euthanized by CO2 exposure and transcardially perfused with 10 ml ice-cold PBS containing 5 units/ml of heparin. Meninges were collected from skullcaps fixed in PBS with 4% PFA for 24h at 4°C as described by Louveau et al. (70, 71). Spinal columns were fixed in PBS with 4% PFA for 2 days at 4°C and meninges were peeled off the spinal cord. Lymph nodes were fixed in 4% PFA for 24h at 4°C, sucrose protected, embedded in OCT and sliced into 10 μm thick sections. Lymph node sections, brain and spinal cord meninges were blocked in PBS containing 1% BSA, 2% of normal serum (either donkey or goat), 0.1% Triton-X-100 and 0.05% of Tween 20 for 1 h at room temperature, followed by incubation with anti-GFP (Invitrogen, A-21311) overnight at 4°C in PBS with 1% BSA and 0.5% Triton-X-100. Images were acquired using an SPE Leica Confocal Microscope (Leica Microsystems, Buffalo Grove, IL, USA) with either a 40 X or 60 X objective, and analyzed using the LAS X software.
4.8. Flow cytometry
Meninges were collected in PBS with 2% BSA and 1 mg/ml collagenase IV and digested for 40 minutes at 37 °C. Meninges and inguinal lymph nodes were minced through a 70 μm cell strainer and homogenized in FACS buffer containing 2% FBS, 0.5 mM EDTA and 0.1 % sodium azide in PBS. Homogenized samples were centrifuged at 300 × g for 5 minutes to pellet cells and stored on ice. For intracellular IL-10 staining, cells were resuspended in MSC media and stimulated with protein transport inhibitor containing Brefeldin A (BD GolgiPlug, 555029, Franklin Lakes, NJ) for 3 hours. Cells were collected and washed with FACS buffer. Cells were stained with the following antibodies: Brilliant Violet 570 anti-CD45 (Biolegend, clone 30-F11, 103136, San Diego, CA), Pacific Blue anti F4/80 (Biolegend, clone BM8, 123124) for 30 minutes at 4 °C. Cells were washed with FACS buffer, fixed for 30 minutes at 4°C, and then permeabilized for 30 minutes at room temperature using mouse Foxp3 buffer set (BD Pharmingen, 560409) containing mouse Foxp3 fixation and permeabilization buffers. Cells were washed with FACS buffer and stained intracellularly with Alexa Fluor 647 anti-IL10 antibody (clone JES5–16E3, 505014) for 30 minutes at 4°C. Cells were resuspended in FACS buffer and acquired on a Gallios flow cytometer (Beckman Coulter, Brea, CA) at The University of Texas MD Anderson Cancer Center Flow Cytometry & Cellular Imaging Core Facility. Data for all flow cytometric evaluations was analyzed using Kaluza 2.1.1 software (Beckman Coulter).
4.9. Statistical analysis
Data are shown as mean ± standard error of the mean (SEM) of at least 3 independent experiments. Data were analyzed by one-way or two-way analysis of variance (ANOVA) with or without repeated measure followed by either Bonferroni’s or Sidak’s correction for multiple comparisons, or using t-test, as indicated in the legends.
Supplementary Material
Highlights.
Mesenchymal stem cells resolve chemotherapy-induced peripheral neuropathy.
Mesenchymal stem cells reverse mitochondrial dysfunction in sensory neurons.
Mesenchymal stem cells need to produce IL-10 to reverse all symptoms of neuropathy.
Mesenchymal stem cells boost IL-10 release by meningeal and lymph node macrophages.
IL-10 receptors on sensory neurons are required for resolution of neuropathy.
Acknowledgments
This work was supported by National Institute of Health Grants R01CA208371, RO1 NS073939 and RO1CA227064.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Zajaczkowska R, et al. (2019) Mechanisms of Chemotherapy-Induced Peripheral Neuropathy. Int J Mol Sci 20(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Seretny M, et al. (2014) Incidence, prevalence, and predictors of chemotherapy-induced peripheral neuropathy: A systematic review and meta-analysis. Pain 155(12):2461–2470. [DOI] [PubMed] [Google Scholar]
- 3.Molassiotis A, et al. (2019) Are we mis-estimating chemotherapy-induced peripheral neuropathy? Analysis of assessment methodologies from a prospective, multinational, longitudinal cohort study of patients receiving neurotoxic chemotherapy. BMC Cancer 19(1):132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cavaletti G, Alberti P, Frigeni B, Piatti M, & Susani E (2011) Chemotherapy-induced neuropathy. Curr Treat Options Neurol 13(2):180–190. [DOI] [PubMed] [Google Scholar]
- 5.Pike CT, Birnbaum HG, Muehlenbein CE, Pohl GM, & Natale RB (2012) Healthcare costs and workloss burden of patients with chemotherapy-associated peripheral neuropathy in breast, ovarian, head and neck, and nonsmall cell lung cancer. Chemother Res Pract 2012:913848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Banach M, Juranek JK, & Zygulska AL (2017) Chemotherapy-induced neuropathies-a growing problem for patients and health care providers. Brain Behav 7(1):e00558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miltenburg NC & Boogerd W (2014) Chemotherapy-induced neuropathy: A comprehensive survey. Cancer Treat Rev 40(7):872–882. [DOI] [PubMed] [Google Scholar]
- 8.Alberti P, Cortinovis D, Frigeni B, Bidoli P, & Cavaletti G (2013) Neuropathic pain and chemotherapy-induced peripheral neurotoxicity: the issue. Pain Manag 3(6):417–419. [DOI] [PubMed] [Google Scholar]
- 9.Ma J, Kavelaars A, Dougherty PM, & Heijnen CJ (2018) Beyond symptomatic relief for chemotherapy-induced peripheral neuropathy: Targeting the source. Cancer 124(11):2289–2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marmiroli P, Scuteri A, Cornblath DR, & Cavaletti G (2017) Pain in chemotherapy-induced peripheral neurotoxicity. J Peripher Nerv Syst 22(3):156–161. [DOI] [PubMed] [Google Scholar]
- 11.Kim JH, Dougherty PM, & Abdi S (2015) Basic science and clinical management of painful and non-painful chemotherapy-related neuropathy. Gynecol Oncol 136(3):453–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bennett GJ, Doyle T, & Salvemini D (2014) Mitotoxicity in distal symmetrical sensory peripheral neuropathies. Nat Rev Neurol 10(6):326–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krukowski K, Nijboer CH, Huo X, Kavelaars A, & Heijnen CJ (2015) Prevention of chemotherapy-induced peripheral neuropathy by the small-molecule inhibitor pifithrinmu. Pain 156(11):2184–2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krukowski K, et al. (2017) HDAC6 inhibition effectively reverses chemotherapy-induced peripheral neuropathy. Pain 158(6):1126–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Flatters SJ & Bennett GJ (2006) Studies of peripheral sensory nerves in paclitaxel-induced painful peripheral neuropathy: evidence for mitochondrial dysfunction. Pain 122(3):245–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Flatters SJ (2015) The contribution of mitochondria to sensory processing and pain. Prog Mol Biol Transl Sci 131:119–146. [DOI] [PubMed] [Google Scholar]
- 17.Flatters SJL, Dougherty PM, & Colvin LA (2017) Clinical and preclinical perspectives on Chemotherapy-Induced Peripheral Neuropathy (CIPN): a narrative review. Br J Anaesth 119(4):737–749. [DOI] [PubMed] [Google Scholar]
- 18.Jin HW, Flatters SJ, Xiao WH, Mulhern HL, & Bennett GJ (2008) Prevention of paclitaxel-evoked painful peripheral neuropathy by acetyl-L-carnitine: effects on axonal mitochondria, sensory nerve fiber terminal arbors, and cutaneous Langerhans cells. Exp Neurol 210(1):229–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barriere DA, et al. (2012) Paclitaxel therapy potentiates cold hyperalgesia in streptozotocin-induced diabetic rats through enhanced mitochondrial reactive oxygen species production and TRPA1 sensitization. Pain 153(3):553–561. [DOI] [PubMed] [Google Scholar]
- 20.Maj MA, Ma J, Krukowski KN, Kavelaars A, & Heijnen CJ (2017) Inhibition of Mitochondrial p53 Accumulation by PFT-mu Prevents Cisplatin-Induced Peripheral Neuropathy. Front Mol Neurosci 10:108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xiao WH & Bennett GJ (2012) Effects of mitochondrial poisons on the neuropathic pain produced by the chemotherapeutic agents, paclitaxel and oxaliplatin. Pain 153(3):704–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Janes K, et al. (2013) Bioenergetic deficits in peripheral nerve sensory axons during chemotherapy-induced neuropathic pain resulting from peroxynitrite-mediated post-translational nitration of mitochondrial superoxide dismutase. Pain 154(11):2432–2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xiao WH, Zheng H, & Bennett GJ (2012) Characterization of oxaliplatin-induced chronic painful peripheral neuropathy in the rat and comparison with the neuropathy induced by paclitaxel. Neuroscience 203:194–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zheng H, Xiao WH, & Bennett GJ (2012) Mitotoxicity and bortezomib-induced chronic painful peripheral neuropathy. Exp Neurol 238(2):225–234. [DOI] [PubMed] [Google Scholar]
- 25.Trecarichi A & Flatters SJL (2019) Mitochondrial dysfunction in the pathogenesis of chemotherapy-induced peripheral neuropathy. Int Rev Neurobiol 145:83–126. [DOI] [PubMed] [Google Scholar]
- 26.Volkman R & Offen D (2017) Concise Review: Mesenchymal Stem Cells in Neurodegenerative Diseases. Stem Cells 35(8):1867–1880. [DOI] [PubMed] [Google Scholar]
- 27.Guo S, Zhen Y, & Wang A (2017) Transplantation of bone mesenchymal stem cells promotes angiogenesis and improves neurological function after traumatic brain injury in mouse. Neuropsychiatr Dis Treat 13:2757–2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van Velthoven CT, Kavelaars A, van Bel F, & Heijnen CJ (2010) Repeated mesenchymal stem cell treatment after neonatal hypoxia-ischemia has distinct effects on formation and maturation of new neurons and oligodendrocytes leading to restoration of damage, corticospinal motor tract activity, and sensorimotor function. J Neurosci 30(28):9603–9611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van Velthoven CT, Kavelaars A, van Bel F, & Heijnen CJ (2010) Nasal administration of stem cells: a promising novel route to treat neonatal ischemic brain damage. Pediatr Res 68(5):419–422. [DOI] [PubMed] [Google Scholar]
- 30.van Velthoven CT, Kavelaars A, van Bel F, & Heijnen CJ (2010) Mesenchymal stem cell treatment after neonatal hypoxic-ischemic brain injury improves behavioral outcome and induces neuronal and oligodendrocyte regeneration. Brain Behav Immun 24(3):387–393. [DOI] [PubMed] [Google Scholar]
- 31.Donega V, et al. (2013) Intranasal mesenchymal stem cell treatment for neonatal brain damage: long-term cognitive and sensorimotor improvement. PLoS One 8(1):e51253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Donega V, et al. (2014) Intranasal administration of human MSC for ischemic brain injury in the mouse: in vitro and in vivo neuroregenerative functions. PLoS One 9(11):e112339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Donega V, et al. (2015) Assessment of long-term safety and efficacy of intranasal mesenchymal stem cell treatment for neonatal brain injury in the mouse. Pediatr Res 78(5):520–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Donega V, et al. (2014) Intranasally administered mesenchymal stem cells promote a regenerative niche for repair of neonatal ischemic brain injury. Exp Neurol 261:53–64. [DOI] [PubMed] [Google Scholar]
- 35.Chiu GS, et al. (2018) Nasal administration of mesenchymal stem cells restores cisplatin-induced cognitive impairment and brain damage in mice. Oncotarget 9(85):35581–35597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sacerdote P, et al. (2013) Systemic administration of human adipose-derived stem cells reverts nociceptive hypersensitivity in an experimental model of neuropathy. Stem Cells Dev 22(8):1252–1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Evangelista AF, et al. (2018) Bone marrow-derived mesenchymal stem/stromal cells reverse the sensorial diabetic neuropathy via modulation of spinal neuroinflammatory cascades. J Neuroinflammation 15(1):189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hosseini M, Yousefifard M, Aziznejad H, & Nasirinezhad F (2015) The Effect of Bone Marrow-Derived Mesenchymal Stem Cell Transplantation on Allodynia and Hyperalgesia in Neuropathic Animals: A Systematic Review with Meta-Analysis. Biol Blood Marrow Transplant 21(9):1537–1544. [DOI] [PubMed] [Google Scholar]
- 39.Brini AT, et al. (2017) Therapeutic effect of human adipose-derived stem cells and their secretome in experimental diabetic pain. Sci Rep 7(1):9904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Galeano C, et al. (2018) The Route by Which Intranasally Delivered Stem Cells Enter the Central Nervous System. Cell Transplant 27(3):501–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kyurkchiev D, et al. (2014) Secretion of immunoregulatory cytokines by mesenchymal stem cells. World J Stem Cells 6(5):552–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Polydoro M, et al. (2014) Soluble pathological tau in the entorhinal cortex leads to presynaptic deficits in an early Alzheimer’s disease model. Acta Neuropathol 127(2):257–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gebler A, Zabel O, & Seliger B (2012) The immunomodulatory capacity of mesenchymal stem cells. Trends Mol Med 18(2):128–134. [DOI] [PubMed] [Google Scholar]
- 44.Leung A, Gregory NS, Allen LA, & Sluka KA (2016) Regular physical activity prevents chronic pain by altering resident muscle macrophage phenotype and increasing interleukin-10 in mice. Pain 157(1):70–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ji RR, Chamessian A, & Zhang YQ (2016) Pain regulation by non-neuronal cells and inflammation. Science 354(6312):572–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ledeboer A, et al. (2007) Intrathecal interleukin-10 gene therapy attenuates paclitaxel-induced mechanical allodynia and proinflammatory cytokine expression in dorsal root ganglia in rats. Brain Behav Immun 21(5):686–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Laumet G, Edralin JD, Dantzer R, Heijnen CJ, & Kavelaars A (2019) Cisplatin educates CD8+ T cells to prevent and resolve chemotherapy-induced peripheral neuropathy in mice. Pain 160(6):1459–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ma J, et al. (2019) Cell-specific role of HDAC6 in chemotherapy-induced mechanical allodynia and loss of intraepidermal nerve fibers. Pain. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Laumet G, et al. (2020) Interleukin-10 resolves pain hypersensitivity induced by cisplatin by reversing sensory neuron hyperexcitability. Pain. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jackson MV, et al. (2016) Mitochondrial Transfer via Tunneling Nanotubes is an Important Mechanism by Which Mesenchymal Stem Cells Enhance Macrophage Phagocytosis in the In Vitro and In Vivo Models of ARDS. Stem Cells 34(8):2210–2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kim J & Hematti P (2009) Mesenchymal stem cell-educated macrophages: a novel type of alternatively activated macrophages. Exp Hematol 37(12):1445–1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Eggenhofer E & Hoogduijn MJ (2012) Mesenchymal stem cell-educated macrophages. Transplant Res 1(1):12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Takeda K, et al. (2018) Mesenchymal Stem Cells Recruit CCR2(+) Monocytes To Suppress Allergic Airway Inflammation. J Immunol 200(4):1261–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Krukowski K, et al. (2016) CD8+ T Cells and Endogenous IL-10 Are Required for Resolution of Chemotherapy-Induced Neuropathic Pain. J Neurosci 36(43):11074–11083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Willemen HL, et al. (2014) Monocytes/Macrophages control resolution of transient inflammatory pain. J Pain 15(5):496–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Milligan ED, et al. (2005) Controlling pathological pain by adenovirally driven spinal production of the anti-inflammatory cytokine, interleukin-10. Eur J Neurosci 21(8):2136–2148. [DOI] [PubMed] [Google Scholar]
- 57.Milligan ED, et al. (2006) Intrathecal polymer-based interleukin-10 gene delivery for neuropathic pain. Neuron Glia Biol 2(4):293–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nemeth K, et al. (2009) Bone marrow stromal cells attenuate sepsis via prostaglandin E(2)-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat Med 15(1):42–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chiossone L, et al. (2016) Mesenchymal Stromal Cells Induce Peculiar Alternatively Activated Macrophages Capable of Dampening Both Innate and Adaptive Immune Responses. Stem Cells 34(7):1909–1921. [DOI] [PubMed] [Google Scholar]
- 60.Braza F, et al. (2016) Mesenchymal Stem Cells Induce Suppressive Macrophages Through Phagocytosis in a Mouse Model of Asthma. Stem Cells 34(7):1836–1845. [DOI] [PubMed] [Google Scholar]
- 61.Ip WKE, Hoshi N, Shouval DS, Snapper S, & Medzhitov R (2017) Anti-inflammatory effect of IL-10 mediated by metabolic reprogramming of macrophages. Science 356(6337):513–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Spanos WC, et al. (2009) Immune response during therapy with cisplatin or radiation for human papillomavirus-related head and neck cancer. Arch Otolaryngol Head Neck Surg 135(11):1137–1146. [DOI] [PubMed] [Google Scholar]
- 63.Vichaya EG, et al. (2016) Sickness behavior induced by cisplatin chemotherapy and radiotherapy in a murine head and neck cancer model is associated with altered mitochondrial gene expression. Behav Brain Res 297:241–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Squillaro T, Peluso G, & Galderisi U (2016) Clinical Trials With Mesenchymal Stem Cells: An Update. Cell Transplant 25(5):829–848. [DOI] [PubMed] [Google Scholar]
- 65.Laumet G, et al. (2015) G9a is essential for epigenetic silencing of K(+) channel genes in acute-to-chronic pain transition. Nat Neurosci 18(12):1746–1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Boukelmoune N, Chiu GS, Kavelaars A, & Heijnen CJ (2018) Mitochondrial transfer from mesenchymal stem cells to neural stem cells protects against the neurotoxic effects of cisplatin. Acta Neuropathol Commun 6(1):139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Danielyan L, et al. (2009) Intranasal delivery of cells to the brain. Eur J Cell Biol 88(6):315–324. [DOI] [PubMed] [Google Scholar]
- 68.Chapman CR, et al. (1985) Pain measurement: an overview. Pain 22(1):1–31. [DOI] [PubMed] [Google Scholar]
- 69.Singhmar P, et al. (2018) Orally active Epac inhibitor reverses mechanical allodynia and loss of intraepidermal nerve fibers in a mouse model of chemotherapy-induced peripheral neuropathy. Pain 159(5):884–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Louveau A, et al. (2018) CNS lymphatic drainage and neuroinflammation are regulated by meningeal lymphatic vasculature. Nat Neurosci 21(10):1380–1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Laumet G, et al. (2018) Resolution of inflammation-induced depression requires T lymphocytes and endogenous brain interleukin-10 signaling. Neuropsychopharmacology 43(13):2597–2605. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.