Abstract
The purpose of this study was to determine if Neisseria gonorrhoeae; Chlamydia trachomatis; herpes simplex virus; cytomegalovirus; Epstein-Barr virus; human herpesviruses 6, 7, and 8; or adeno-associated virus influenced the production of cervical intraepithelial neoplasia. Two hundred thirty-one cervical smear samples were tested for the presence of the organisms by PCR. In addition, human papillomavirus types in the samples were determined by PCR and classified into cancer risk types of high, moderate, and low. There was no link with cervical intraepithelial neoplasia status and detection of herpes simplex virus, cytomegalovirus, Epstein-Barr virus, human herpesviruses 6 and 8, gonorrhea, or chlamydia. However, high-grade cervical intraepithelial neoplasia was found more frequently with mixed infection by moderate-risk human papillomavirus types and human herpesvirus 7 than with these papillomavirus types alone. The presence of human herpesvirus 7 may increase the oncogenic potential of moderate-risk human papillomavirus types.
Human papillomaviruses (HPVs) play an essential part in the development of cervical cancer (29), although the risk varies according to the infecting type, e.g., high risk (types 16 and 18), moderate risk (types 33 and 35), and low risk (types 6 and 11). However, the role of other infectious agents is not clear. A history of multiple genital infections is more common in women with cervical cancer (4), suggesting that these agents might increase the risk of developing cancer. However, genital infections may simply be indicators of sexual activity and hence surrogate markers of oncogenic HPV infection.
Both Neisseria gonorrhoeae and Chlamydia trachomatis cause the production of inflammatory cytokines, which stimulates proliferation of cervical cells previously immortalized by high-risk HPVs, providing a selective growth advantage to these cells (31). Therefore, these infections may be a risk factor for progression to high-grade cervical intraepithelial neoplasia (CIN 3) and eventually cervical cancer. Many of the herpesviruses can induce cell cycle transformation either through the production of proteins, as with cytomegalovirus (CMV) (19), or through viral replication, as may occur with Epstein-Barr virus (EBV) (25). Adeno-associated virus (AAV) may also be sexually acquired. This virus has many characteristics of a parvovirus but relies on coinfections with other viruses to replicate (1). AAV has not been associated with any disease in humans despite its ability to integrate into the cell genome. In fact, there is evidence that infection with AAV may reduce the risk of developing cervical cancer (11).
The purpose of this study was to determine if N. gonorrhoeae, C. trachomatis, herpes simplex virus (HSV), CMV, EBV, human herpesviruses 6, 7, and 8 (HHV-6, HHV-7, and HHV-8, respectively); or AAV influenced the development of CIN by determining the prevalence of these infections in colposcopy patients.
MATERIALS AND METHODS
Patients.
The Joint Local Research Ethics Committee Southampton and South West Hampshire unanimously approved this study. Cervical samples were collected from women attending a colposcopy clinic in the Wessex region of southern England. Women were asked for written consent to participate in the study.
Samples.
Specimens from 231 patients were taken for PCR analysis during colposcopic examination by using a sterile plastic spatula (Cellpath, Newtown, United Kingdom) to sample the ectocervix and transformation zone and a sterile cytobrush (Cellpath) to sample the endocervix. Both sampling devices were placed into 2.5 ml of RPMI medium plus 10% fetal calf serum containing 0.05% sodium azide as a preservative. Samples were processed in the laboratory within 6 h of collection. Cells and microbes were detached from the sampling devices by vortex mixing for 10 s. An 0.5-ml aliquot was centrifuged at 60,000 × g for 30 min at 20°C in a Beckman Avanti 30 centrifuge. The pellet was stored frozen at −20°C.
The CIN grade of the patient was determined from a biopsy sample taken at the colposcopy clinic at the same time as the sample for PCR analysis. The grade assigned was the highest grade detected in the biopsy sample. CIN 0 was defined as women found to have no abnormalities.
DNA extraction.
DNA was extracted by alkali lysis of the frozen cell pellet; 200 μl of 2 M ammonium hydroxide (Applied Biosystems, Warrington, United Kingdom) was added to the pellet, which was resuspended by vortex mixing. The sealed tubes were placed in a water bath at 90°C (±1°C) for 10 min to render specimens noninfectious, and then the screw caps were removed and the tubes were reheated for a further 70 min to evaporate all the ammonia.
The tubes were centrifuged at 2,000 × g for 5 min at room temperature to pellet debris, and aliquots from each specimen containing the extracted DNA were stored at −80°C until used for PCR.
PCRs.
HPVs were detected using three consensus primers, GP5+/GP6+ (3), MY09/MY11 (18), and CPI/CPIIG (27), as well as specific primers for HPV type 16 (HPV-16) (forward primer, 5′-GTCAAAAGCCACTGTGTCCT-3′; reverse primer, 5′-CCATCCATTACATCCCGAC-3′, producing a 499-bp product) and HPV-18 (forward primer, 5′-ACAATCCTCCATTTTGCTGTG-3′; reverse primer, 5′-ATAAACTATGTCTGCACAGCTTA-3′, producing a 384-bp product). For the HPV-16 PCR, the reaction mixture consisted of 2 mM MgCl2, PCR buffer (Promega, Southampton, United Kingdom), deoxynucleotide triphosphates (Promega) each at 300 μM, primers at 1 mM, and 1.5 U of Taq DNA polymerase (Promega). Five microliters of extracted DNA (approximately 10 to 100 ng) was used in a total PCR volume of 25 μl. The PCR was performed on an MJ Research PTC-225 machine (Waltham, Mass.) using 40 cycles of denaturation at 94°C for 30 s and annealing at 60°C for 50 s, followed by extension at 72°C for 20 s. The HPV-18-specific PCR used 2.5 mM MgCl2 and an annealing temperature of 62°C; otherwise, the reaction mixture and PCR conditions were the same as those for the HPV-16 PCR. The HPV types detected using consensus primers were identified by sequencing of the PCR product using a fluorescently labeled dideoxy terminator kit (Amersham Pharmacia Biotech, Little Chalfont, United Kingdom), and the sequences generated were compared to HPV sequences at GenBank using the FastA program (program manual for the Wisconsin package; Genetics Computer Group, Madison, Wis.).
The primers used to detect the other infections under study are shown in Table 1. For all pathogens, the PCR mix was the same as that for the HPV-16 PCR; the appropriate MgCl2 concentration and annealing temperature used are also shown in Table 1.
TABLE 1.
Primer sequences used for pathogen detection
| Pathogen | Primer | Sequence 5′→3′a | PCR product size (bp) | Referenceb | MgCl2 (mM) | Annealing temp (°C) |
|---|---|---|---|---|---|---|
| Neisseria gonorrhoeae | HO1 | GCTACGCATACCCGCGTTGC | 392 | 17 | 2 | 55 (multiplex PCR) |
| HO3 | CGAAGACCTTCGAGCAGACA | |||||
| Chlamydia trachomatis | KL1 | TCCGGAGCGAGTTACGAAGA | 236 | 17 | 2 | |
| KL2 | AATCAATGCCCGGGATTGGT | |||||
| HSV | HSVBR | CGGAGCCGCCGACGCCACC | 334 (HSV-1), 310 (HSV-2) | 2 | 65 to 50 (reduced by 0.4°C per cycle) (multiplex PCR) | |
| HSVBA | CCSGACTGCAGCCGCCCGACCTCCGAAG | |||||
| CMV | CMV2 | TCCAGAGGTGGTGGGTTCYTCA | 118 | 2 | ||
| CMV1 | GGGTGCTCAGGAGGAGCRGG | |||||
| EBV | EBV1 | CTCTGGTAGTGATTTGGACCCG | 240 | 12 (modified) | 2 | |
| EBV2 | GTGAAGTCACAAACAAGCCCACT | |||||
| HHV-6 | HHV61 | CTTTGTGTAGGTGGTCGAATGCGAC | 494 | 22 (modified) | 2.5 | 60 |
| HHV62 | ACAGCGCAGCAACATGTTTCAGAGC | |||||
| HS6AE | CGGCCATTTAACGGAACCCTAG | 751 | 5 | 2.5 | 61 | |
| HS6AF | TCCAGAGAAAGGGTGTTGCG | |||||
| HHV-7 | HHV71 | ATCCAGAAATGATAGACAGATGTTGG | 133 | 22 (modified) | 3 | 61 |
| HHV72 | GGTAGCACTAGATTTTTTGAAAAAGATTTAATAAC | |||||
| HHV-8 | KS3-5 | CCCTTCTAGCGTTGGCTAGTC | 608 | 21 | 2 | 60 |
| KS1-3 | TCCGTGTTGTCTACGTCCA | |||||
| AAV | AAV1 | GCGGAGGCCATAGCCC | 218 | 7 | 1.5 | 64 |
| AAV2 | ACGGGAGTCGGGTCTATCTG |
Y is T or C, R is G or A, and S is G or C.
Where no reference is given, the primers were designed with the Gene Runner program (Hastings Software Inc., Hastings, N.Y.).
After the initial PCR with the HS6AE/HS6AF primers, the product was digested with HindIII to distinguish between HHV-6 variants A and B. Variant B produced products of 235 and 516 bp in size when digested; variant A is not cut with this enzyme.
The PCR products were run on a 2.6 to 3% SeaKem agarose gel (Flowgen, Ashby de la Zouch, United Kingdom) depending on the size of the product and then stained for 1 h with Sybr Gold (Cambridge Bioscience, Cambridge, United Kingdom) diluted 1/10,000 in Tris-borate-EDTA buffer. The gel was viewed on a Storm 860 system (Amersham Pharmacia Biotech) using the blue fluorescence filter.
RESULTS
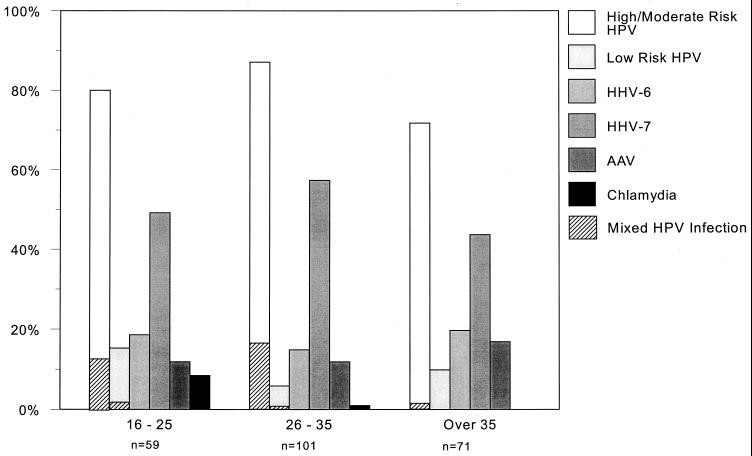
The incidence of the infections was analyzed against patient age and CIN grade. The age-related incidence of the various cervical infections is shown in Fig. 1. The risk type is that defined by Shah and Howley (23) based on the incidence of HPV types in cervical cancers. The high-risk HPV types detected were 16, 18, 31, and 45; moderate-risk types were 33, 35, 39, 51, 52, 56, 58, 59, 67, 68, han831, and ME180; and low-risk HPV types were 6, 11, 30, 40, 42, 43, 53, 54, and 66. The incidence of high- and moderate-risk HPV types was higher in women below 36 years of age (134 of 160 compared to 51 of 71; P value, 0.04), as was the incidence of mixed HPV infections containing high- and moderate-risk HPV types (24 of 160 under 36 years of age compared to 1 of 71 aged 36 years or over; P value, 0.002). No age-related differences were found in the incidence of low-risk HPV types (15 of 160 under 36 years of age compared to 7 of 71 over 36 years; P value, 0.91). High- and moderate-risk HPV types were also more prevalent (106 of 113) in high-grade (CIN 2 and 3) samples compared to 79 of 118 low-grade (CIN 0 and 1) samples (P value, <0.001), whereas low-risk HPV types occurred more often in low-grade CIN samples (17 of 118 compared to 5 of 113 in high-grade CIN samples [P value, 0.01]).
FIG. 1.
Incidence of cervical infections by age. Cervical specimens were tested for the pathogens shown, and the results are presented by patient age group. Hatched markings indicate mixed HPV infections.
Chlamydial infection was detected in five women below 26 years of age and one woman in the 26- to 35-year age range and not detected in women above 36 years of age (P value, 0.02). However, no correlation could be found between current chlamydia infection and grade of CIN. No cases of gonorrhea were detected.
The frequency of AAV remained at 15 to 20% in all age ranges. In the study presented here, 31 samples contained AAV DNA; of these, 9 (29.0%) were CIN 3. In comparison, 200 samples did not contain detectable AAV DNA, of which 59 (29.5%) were CIN 3 (P value, 0.96). Hence, AAV showed no protective effect on the colposcopy patients studied.
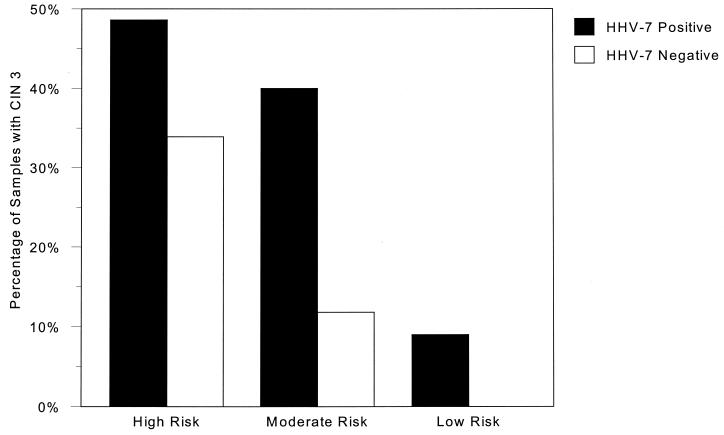
EBV was found in only 5 of 160 women (3%) in the 16- to 35-year age range. Through the age ranges, CMV was found in 2 to 4% of women; the frequency of HHV-6 remained at 15 to 20%, and the incidence of HHV-7 fluctuated from 44 to 57%. One case each of HSV type 1 and HHV-8 was detected in the 26- to 35-year age range. However, DNA from any herpesvirus was detected in 144 patients and absent in 87 patients. Fifty-one (35.4%) of the herpesvirus-positive samples were classified as CIN 3, whereas only 17 (19.5%) herpesvirus-negative samples were CIN 3 (P value, 0.01). In addition, 44 of 118 (37.3%) samples containing HHV-7 were CIN 3, compared to only 24 of 113 (21.2%) not containing HHV-7 (P value, 0.007), regardless of other herpesvirus infections. Further, 36 of 95 (37.9%) samples with HHV-7 as the only herpesvirus present were CIN 3 (P value, 0.006), compared to the 17 of 87 (19.5%) samples containing no herpesvirus DNA. Thus, HHV-7 appeared to occur more often in severe cervical lesions. The correlation between HHV-7 positivity and HPV type was also investigated. Figure 2 shows that, for high-risk HPV types containing HHV-7, 48.6% (35 of 72) were CIN 3, compared to 33.9% (20 of 59) HHV-7-negative samples with high-risk HPV types (P value, 0.09); however, this effect of HHV-7 was more pronounced with moderate-risk HPV types with HHV-7, where 40% (8 of 20) were CIN 3, whereas only 11.8% (4 of 34) HHV-7-negative samples were CIN 3 (P value, 0.016).
FIG. 2.
The percentages of samples containing high-, moderate-, or low-risk HPV types with CIN 3 are shown. Within each HPV risk group, a distinction was made between the proportion of samples containing HHV-7 and those in which HHV-7 was not detected. For high-risk HPV types, the P value is 0.09; for moderate-risk HPV types, the P value is 0.016; and for low-risk HPV types, the P value is 0.3.
DISCUSSION
This study attempted to find out whether genital infections other than those caused by HPV could be implicated in the development of cervical cancer (using CIN 3 as an indicator of a premalignant lesion).
Although chlamydia infection was detected, occurring more often in patients less than 26 years old than in older women attending the colposcopy clinic, there was no correlation with the grade of CIN.
The herpesviruses and AAV cause latent infections (1, 19); hence, detection of a DNA genome from these viruses in cervical cells demonstrates persistent infection at this site or a carrier state. The most common infections detected in this study were those with HHV-7, HHV-6, and AAV. The remaining herpesviruses were each detected at a rate of 3% or less.
In humans, the replication cycle of CMV results in lysis of infected cells; hence, for cellular transformation to occur, nonproductive infection is required. This could happen with a spontaneously produced mutant virus, or more likely, the wild-type virus may express only certain genes in a particular cell type, e.g., cervical epithelial cells. The induction of cervical neoplasia by human CMV in mice (8) may be due to an abortive infection in which few viral proteins are produced. The immediate-early proteins of human CMV, IE1 and IE2, are mutagenic in primary rat cells (24) but are only transiently required, and viral DNA is frequently absent in the transformed cells. Therefore, the immediate-early proteins of human CMV could induce transformation of infected cells in human cervical epithelial cells. If these cells were not permissive for the full replication cycle of the virus, the cells could become malignant. However, the continued presence of the viral proteins could become detrimental to the cell, and thus, cellular growth would favor cells which have lost the viral genome. This may suggest a “hit-and-run” role for CMV in cervical neoplasia, which is difficult to establish in the patients studied here, as only eight patients had evidence of cervical CMV infection.
EBV was found in specimens with CIN 1 (3%) and CIN 3 (5%). These results are comparable with those published by Landers et al. (15), who found that 8% of CIN 2 and CIN 3 samples tested positive for EBV DNA. Again, the presence of EBV DNA within the cervix raises the possibility that this virus may be sexually transmitted. Interestingly, Landers et al. found that, of 18 cases of cervical cancer studied, samples from eight patients contained EBV DNA (44%). In situ hybridization showed the EBV DNA to be located in the nucleus of malignant cells, suggesting that the EBV genome had integrated into the host cell chromosome. Whether this integration event was a cause of malignancy or a consequence is unknown, but not all cases showed integration of EBV DNA, and so it may have been a chance event.
Evidence for HSV having a causal role in CIN is uncertain (2), possibly due to some studies screening for HSV antibodies and others detecting HSV DNA. However, Koffa et al. (14) found that detection of HSV DNA correlated with cervical cancer in specimens that did not contain HPV DNA, suggesting that HSV may have a role in HPV-negative cervical cancer. However, samples from the single patient with HPV-negative CIN 2 found in the study presented here did not contain detectable HSV DNA.
From in vitro studies, AAV has been shown to prevent the replication of HPVs and therefore may protect infected patients from CIN (9). Indeed, Han and coworkers (7) found AAV to be present in 50% of cervical brushing from undiseased patients, and Tobiasch et al. (28) detected AAV in 63% of normal cervical mucosal samples tested. However, more recent studies argue against a protective effect of AAV; Walz et al. (30) detected AAV in 63% of samples from high-grade CIN biopsies, and in a larger study by Strickler et al. (26), no evidence was found of AAV infection in either CIN samples or normal cervical samples. In the study presented here, AAV was found in similar proportions of high- and low-grade CIN samples. In addition, Hermonat et al. (10) analyzed the effect of AAV on bovine papillomavirus (BPV) infection. They discovered that, if the cells were latently infected with AAV, they were more susceptible to BPV transformation than were non-AAV-infected cells. If HPVs infecting the endocervix behave like BPV with regard to AAV infection and if women acquired AAV infection before HPV infection, this could explain the finding of similar levels of AAV in patients with CIN. It is known that primary infection with AAV usually occurs in childhood, but an unstable AAV antibody response may allow lifelong reinfection or reactivation of persisting virus (6). In the study presented here, AAV occurred in similar proportions of all age groups studied, indicating that reactivation of latent infection rather than reinfection would be the most likely source of AAV. This suggests that many women will have latent AAV infection from an early age and hence possible susceptibility to HPV transformation if cells in the endocervix are infected.
Two groups have studied the prevalence of HHV-6 and HHV-7 in the female genital tract. Leach et al. (16) found HHV-6 to be present in 10% of vaginal swabs of women attending a sexually transmitted disease clinic, whereas Okuno et al. (20) found HHV-6 (variant B only) in 19.4% of cervical swabs from 72 pregnant women and 6% of samples from 34 nonpregnant controls and HHV-7 in 2.7% of pregnant women and none in the nonpregnant controls. For the Wessex patients, HHV-6 was detected overall in 18% of specimens, and of 14 samples typed, 12 were HHV-6 variant B and 2 were HHV-6 variant A. The high incidence of HHV-6 and HHV-7 in cervical samples may indicate that these viruses are sexually transmitted.
Unlike HHV-6, HHV-7 can be reactivated from latently infected peripheral blood mononuclear cells by T-cell activation (13), and the ongoing or active HHV-7 replication can, in turn, reactivate latent HHV-6 infections. It may be possible for a latent HHV-7 infection in the cervix to be reactivated by T-cell activation due to the immune response to HPV infection, which could in turn reactivate HHV-6, and therefore, both viruses could be shed and sexually transmitted.
However, HHV-6 DNA has been detected in 88% and HHV-7 DNA has been detected in 100% of samples from the submandibular salivary gland of healthy individuals (22) suggesting that saliva is the important route for transmission of the virus, especially as most people are seropositive for both viruses by the age of 2 years. The presence of HHV-6 and HHV-7 in the cervical samples could be due to macrophages or lymphocytes from the tissue or blood being collected with the cervical cells or latently infected epithelial cells. Detection in the cervix may, therefore, represent dissemination of the patient's systemic infection of the immune system rather than sexually acquired infection. Furthermore, detection of DNA from HHV-6 and HHV-7 in this study does not indicate whether the viruses are latent or reactivated.
No reported studies of HHV-7 have looked at the correlation among HHV-7 infection, CIN grade, and HPV types. We found that samples containing a moderate-risk HPV type and HHV-7 were more likely to be CIN 3 than were samples without any herpesvirus infection. If HPV testing is to be introduced to aid cervical cancer screening, HHV-7 positivity, particularly if detected with a moderate-risk HPV type, may identify a group of patients at additional risk.
ACKNOWLEDGMENTS
We thank Thierry Dupressoir for the gift of the AAV-containing cell line.
This work was funded by a grant from the Medical Research Council.
REFERENCES
- 1.Berns K I. Parvoviridae: the viruses and their replication. In: Fields B N, Knipe D M, Howley P M, editors. Fields virology. 3rd ed. Philadelphia, Pa: Lippincott-Raven Publishers; 1996. pp. 2173–2197. [Google Scholar]
- 2.Boyle D C M, Smith J R. Infection and cervical intraepithelial neoplasia. Int J Gynecol Cancer. 1999;9:177–186. doi: 10.1046/j.1525-1438.1999.99007.x. [DOI] [PubMed] [Google Scholar]
- 3.de Roda Husman A M, Walboomers J M M, van den Brule A J C, Meijer C J L M, Snijders P J F. The use of general primers GP5 and GP6 elongated at their 3′ ends with adjacent highly conserved sequences improves human papillomavirus detection by PCR. J Gen Virol. 1995;76:1057–1062. doi: 10.1099/0022-1317-76-4-1057. [DOI] [PubMed] [Google Scholar]
- 4.de Sanjosé S, Muñoz N, Bosch F X, Reimann K, Pedersen N S, Orfila J, Ascunce N, González L C, Tafur L, Gili M, Lette I, Viladiu P, Tormo M J, Moreo P, Shah K, Wahren B. Sexually transmitted agents and cervical neoplasia in Colombia and Spain. Int J Cancer. 1994;56:358–363. doi: 10.1002/ijc.2910560311. [DOI] [PubMed] [Google Scholar]
- 5.Dewhurst S, MeIntyre K, Schnabel K, Hall C B. Human herpesvirus 6 (HHV-6) variant B accounts for the majority of symptomatic primary HHV-6 infections in a population of U.S. infants. J Clin Microbiol. 1993;31:416–418. doi: 10.1128/jcm.31.2.416-418.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Erles K, Sebökovà P, Schlehofer J R. Update on the prevalence of serum antibodies (IgG and IgM) to adeno-associated virus (AAV) J Med Virol. 1999;59:406–411. doi: 10.1002/(sici)1096-9071(199911)59:3<406::aid-jmv22>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 7.Han L, Parmley T H, Keith S, Kozlowski K J, Smith L J, Hermonat P L. High prevalence of adeno-associated virus (AAV) type 2 rep DNA in cervical materials: AAV may be sexually transmitted. Virus Genes. 1996;12:47–52. doi: 10.1007/BF00370000. [DOI] [PubMed] [Google Scholar]
- 8.Heggie A D, Wentz W B, Reagan J W, Anthony D D. Roles of cytomegalovirus and Chlamydia trachomatis in the induction of cervical neoplasia in the mouse. Cancer Res. 1986;46:5211–5214. [PubMed] [Google Scholar]
- 9.Hermonat P L. Adeno-associated virus inhibits human papillomavirus type 16: a viral interaction implicated in cervical cancer. Cancer Res. 1994;54:2278–2281. [PubMed] [Google Scholar]
- 10.Hermonat P L, Meyers C, Parham G P, Santin A D. Inhibition/stimulation of bovine papillomavirus by adeno-associated virus is time as well as multiplicity dependent. Virology. 1998;247:240–250. doi: 10.1006/viro.1998.9256. [DOI] [PubMed] [Google Scholar]
- 11.Hermonat P L, Santin A D, Batchu R B. The adeno-associated virus Rep78 major regulatory/transformation suppressor protein binds cellular SP1 in vitro and evidence of a biological effect. Cancer Res. 1996;56:5299–5304. [PubMed] [Google Scholar]
- 12.Jiwa N M, Kanavaros P, van der Valk P, Walboomers J M M, Horstman A, Vos W, Mullink H, Meijer C J L M. Expression of c-myc and bcl-2 oncogene products in Reed-Sternberg cells independent of presence of Epstein-Barr virus. J Clin Pathol. 1993;46:211–217. doi: 10.1136/jcp.46.3.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Katsafanas G C, Schirmer E C, Wyatt L S, Frenkel N. In vitro activation of human herpesviruses 6 and 7 from latency. Proc Natl Acad Sci USA. 1996;93:9788–9792. doi: 10.1073/pnas.93.18.9788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koffa M, Koumantakis E, Ergazaki M, Tsatsanis C, Spandidos D A. Association of herpesvirus infection with the development of genital cancer. Int J Cancer. 1995;63:58–62. doi: 10.1002/ijc.2910630112. [DOI] [PubMed] [Google Scholar]
- 15.Landers R J, O'Leary J J, Crowley M, Healy I, Annis P, Burke L, O'Brien D, Hogan J, Kealy W F, Lewis F A, Doyle C T. Epstein-Barr virus in normal, pre-malignant, and malignant lesions of the uterine cervix. J Clin Pathol. 1993;46:931–935. doi: 10.1136/jcp.46.10.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leach C T, Newton E R, McParlin S, Jenson H B. Human herpesvirus 6 infection of the female genital tract. J Infect Dis. 1994;169:1281–1283. doi: 10.1093/infdis/169.6.1281. [DOI] [PubMed] [Google Scholar]
- 17.Mahony J B, Luinstra K E, Tyndall M, Sellors J W, Krepel J. Multiplex PCR for detection of Chlamydia trachomatis and Neisseria gonorrhoeae in genitourinary specimens. J Clin Microbiol. 1995;33:3049–3053. doi: 10.1128/jcm.33.11.3049-3053.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Manos M M, Ting Y, Wright D K, Lewis A J, Broker T R, Wolinsky S M. The use of polymerase chain reaction amplification for the detection of genital human papillomavirus. Cancer Cells. 1989;7:209–214. [Google Scholar]
- 19.Mocarski E S. Cytomegaloviruses and their replication. In: Fields B N, Knipe D M, Howley P M, editors. Fields virology. 3rd ed. Philadelphia, Pa: Lippincott-Raven Publishers; 1996. pp. 2447–2492. [Google Scholar]
- 20.Okuno T, Oishi H, Hayashi K, Nonogaki M, Tanaka K, Yamanishi K. Human herpesviruses 6 and 7 in cervixes of pregnant women. J Clin Microbiol. 1995;33:1968–1970. doi: 10.1128/jcm.33.7.1968-1970.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O'Neill E, Henson T H, Ghorbani A J, Land M A, Webber B L, Garcia J V. Herpes virus-like sequences are specifically found in Kaposi sarcoma lesions. J Clin Pathol. 1996;49:306–308. doi: 10.1136/jcp.49.4.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sada E, Yasukawa M, Ito C, Takeda A, Shiosaka T, Tanioka H, Fujita S. Detection of human herpesvirus 6 and human herpesvirus 7 in the submandibular gland, parotid gland, and lip salivary gland by PCR. J Clin Microbiol. 1996;34:2320–2321. doi: 10.1128/jcm.34.9.2320-2321.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shah K V, Howley P M. Papillomaviruses. In: Fields B N, Knipe D M, Howley P M, editors. Fields virology. 3rd ed. Philadelphia, Pa: Lippincott-Raven Publishers; 1996. pp. 2077–2109. [Google Scholar]
- 24.Shen Y, Zhu H, Shenk T. Human cytomegalovirus IE1 and IE2 proteins are mutagenic and mediate ‘hit-and-run’ oncogenic transformation in cooperation with the adenovirus E1A proteins. Proc Natl Acad Sci USA. 1997;94:3341–3345. doi: 10.1073/pnas.94.7.3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sixbey J W, Lemon S M, Pagano J S. A second site for Epstein-Barr virus shedding: the uterine cervix. Lancet. 1986;ii:1122–1124. doi: 10.1016/s0140-6736(86)90531-3. [DOI] [PubMed] [Google Scholar]
- 26.Strickler H D, Viscidi R, Escoffery C, Rattray C, Kotloff K L, Goldberg J, Manns A, Rabkin C, Daniel R, Hanchard B, Brown C, Hutchinson M, Zanizer D, Palefsky J, Burk R D, Cranston B, Clayman B, Shah K V. Adeno-associated virus and development of cervical neoplasia. J Med Virol. 1999;59:60–65. doi: 10.1002/(sici)1096-9071(199909)59:1<60::aid-jmv10>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 27.Tieben L M, ter Schegget J, Minaar R P, Bavinck B J N, Berkhout R J M, Vermeer B J, Jebbink M F, Smits H L. Detection of cutaneous and genital HPV types in clinical samples by PCR using consensus primers. J Virol Methods. 1993;42:265–280. doi: 10.1016/0166-0934(93)90038-s. [DOI] [PubMed] [Google Scholar]
- 28.Tobiasch E, Rabreau M, Geletneky K, Larue-Charlus S, Severin F, Becker N, Schlehofer J R. Detection of adeno-associated virus DNA in human genital tissue and in material from spontaneous abortion. J Med Virol. 1994;44:215–222. doi: 10.1002/jmv.1890440218. [DOI] [PubMed] [Google Scholar]
- 29.Walboomers J M M, Jacobs M V, Manos M M, Bosch F X, Kummer J A, Shah K V, Snijders P J F, Peto J, Meijer C J L M, Muñoz N. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999;189:12–19. doi: 10.1002/(SICI)1096-9896(199909)189:1<12::AID-PATH431>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 30.Walz C M, Anisi T R, Schlehofer J R, Gissman L, Schneider A, Müller M. Detection of infectious adeno-associated virus particles in human cervical biopsies. Virology. 1998;247:97–105. doi: 10.1006/viro.1998.9226. [DOI] [PubMed] [Google Scholar]
- 31.Woodworth C D, McMullin E, Iglesias M, Plowman G D. Interleukin 1α and tumor necrosis factor α stimulate autocrine amphiregulin expression and proliferation of human papillomavirus-immortalized and carcinoma-derived cervical epithelial cells. Proc Natl Acad Sci USA. 1995;92:2840–2844. doi: 10.1073/pnas.92.7.2840. [DOI] [PMC free article] [PubMed] [Google Scholar]