Abstract
Introduction:
Numerous studies have shown that the oral rotavirus vaccines are less effective in infants born in low income countries compared to those born in developed countries. Identifying the specific factors in developing countries that decrease and/or compromise the protection that rotavirus vaccines offer, could lead to a path for designing new strategies for the vaccines’ improvement.
Areas covered:
We accessed PubMed to identify rotavirus vaccine performance studies (i.e., efficacy, effectiveness and immunogenicity) and correlated performance with several risk factors. Here, we review the factors that might contribute to the low vaccine efficacy, including passive transfer of maternal rotavirus antibodies, rotavirus seasonality, oral polio vaccine (OPV) administered concurrently, microbiome composition and concomitant enteric pathogens, malnutrition, environmental enteropathy, HIV, and histo blood group antigens.
Expert commentary:
We highlight two major factors that compromise rotavirus vaccines’ efficacy: the passive transfer of rotavirus IgG antibodies to infants and the co-administration of rotavirus vaccines with OPV. We also identify other potential risk factors that require further research because the data about their interference with the efficacy of rotavirus vaccines are inconclusive and at times conflicting.
Keywords: Rotavirus, vaccines, efficacy, immunogenicity, review
1. Introduction
Rotavirus is the most important cause of severe gastroenteritis in children worldwide [1]. The main symptoms of rotavirus gastroenteritis are low-grade fever, vomiting, and acute watery diarrhea. Vaccines represent the optimal practice for preventing the severe consequences of rotavirus infection, especially in impoverished regions where resources and access to medical care are usually limited. Two live attenuated oral rotavirus vaccines were licensed in 2006. Rotarix (RV1, GSK Biologics) is a two-dose monovalent (G1P[8]) human rotavirus vaccine. RotaTeq (RV5, Merck & Co.) is a three-dose pentavalent vaccine consisting of a mixture of bovine-human mono-reassortants carrying the genes encoding the human G1, G2, G3, G4, and P[8] in the genetic background of a bovine rotavirus WC3 (G6P[5]) [2]. In 2009, the WHO recommended implementation of rotavirus vaccines worldwide. Rotavirus vaccine is recommended to be administered in infancy concurrently with polio, diphtheria-tetanus-pertussis, and pneumococcal (PCV) vaccines as early as 6 weeks of age [3,4]. Currently, rotavirus vaccines are introduced into national immunization programs of 85 countries and in a phase introduction of 7, including 41 GAVI-eligible countries with financial support for vaccine procurement [5]. Implementation of rotavirus vaccines into national vaccination programs has led to substantial declines in the burden of severe gastroenteritis in several countries [5-7].
RV1 and RV5 were preceded by RotaShield® (RRV-TV, Wyeth, U.S.A.), the first live attenuated oral rotavirus vaccine based on a Rhesus monkey rotavirus strain (RRV) that was reassorted with human rotavirus VP7 proteins representing the G-types G1, G2, and G4 [8]. With RRV as G-type 3, this was called ‘tetravalent’ or RRV-TV vaccine. However, this vaccine was withdrawn from the market after it was found to be associated with intussusception, a rare form of intestinal invagination [9]. Four other oral rotavirus vaccines are currently licensed in national markets: Lanzhou lamb rotavirus vaccine (LLR, Lanzhou Institute of Biological Products, China) containing a live attenuated lamb rotavirus strain, G10P[10], Rotavin-M1 (POLYVAC, Vietnam) containing a live attenuated human rotavirus strain, G1P[8], ROTAVAC (Bharat Biotech, India) containing a live attenuated neonatal rotavirus strain, G9P[11] (aka 116E), and ROTASIIL (Serum Institute, India) containing five bovine-human reassortant rotavirus strains (G1, G2, G3, G4, G9) [10]. In April 2016, ROTAVAC was launched in the routine immunization programs in four states in India and has been expanded to an additional five states in 2017. LLR and Rotavin-M1 are only available on the private market in China and Vietnam, respectively [10]. In two recent phase 3 trials in Niger and India, ROTASIIL showed efficacies of 67% and 39.5%, respectively [11,12]. Results for other two rotavirus vaccines were favorable for a neonatal dose in stage II clinical trials. In New Zealand, one neonatal dose with two additional infant doses of RV3-BB, a monovalent human rotavirus vaccine, performed comparably to a three-dose infant schedule [13]. In Ghana, one neonatal dose followed by one infant dose of RRV-TV had a vaccine efficacy of 63% [14]. Estimates suggest that rotavirus vaccines have the potential to prevent 2.46 million childhood deaths and 83 million disability-adjusted life-years between 2011 through 2030 [15].
Additionally, inactivated rotavirus particles and subunit rotavirus proteins have been proposed as an alternative to the current live oral vaccines [16,17]. First, the P2-VP8* candidate (PATH), a truncated recombinant VP8* protein of human rotavirus genotypes P[8] expressed in Escherichia coli, was tested and found to be safe and immunogenic in phase I and II clinical trials [18-21]. A trivalent P2-VP8-P[8]/P[6]/P[4] vaccine is being tested to determine its safety and immunogenicity in South African children. Second, the inactivated rotavirus vaccine (IRV), CDC-9 strain (G1P[8]) is being developed for intramuscular and intradermal vaccination by US Centers for Disease Control and Prevention (CDC, U.S.A.). Studies showed that this monovalent IRV was effective in inducing homotypic (against the vaccine-type strain) and heterotypic (against non-vaccine-type strain) neutralizing antibody to different human strains, and protection against an oral challenge with a virulent human virus in animals [22,23]. The CDC researchers have prepared a pilot vaccine and are planning the first-in-human studies. Third, other subunit approaches include virus-like particles (VLPs) [24] in various formats—usually the inner capsid VP6 antigen, with or without the outer capsid proteins VP7 and/or VP4, and in some platforms combined with norovirus VLPs [25]. Both of these strategies are in early preclinical R&D.
High vaccine efficacy (85–98%) against severe rotavirus disease has been reported for both RV1 and RV5 in high- and middle-income settings with sustained protection until 2 years of age [26]. High levels of both homotypic and heterotypic protection are induced by both vaccines in such settings [27]. However, the majority (>90%) of childhood deaths due to rotavirus gastroenteritis occur in low-income countries in Africa and Asia [1], and clinical trials have shown lower efficacy (50–64%) in these settings. These differences in efficacy are not explained by strain variation in these environments [27-37]. Moreover, striking reductions in efficacy were reported in the second year of life compared with the first year [33], particularly in sub-Saharan Africa where rotavirus is still a significant pathogen at that age [38]. Despite lower efficacy in developing countries, the mortality rate from rotavirus-associated disease was lowered in 27 countries that introduced rotavirus vaccine into their national routine [39]. Similar reductions were seen across mortality strata.
Many oral vaccines, primarily live ones, have shown reduced immunogenicity and efficacy when used in low-income compared with high-income countries. Reduced performance of oral polio vaccine (OPV) in developing countries is well recognized as a significant obstacle for the eradication of polio by vaccination [40-45]. Also, CVD 103-HgR live cholera vaccine 4144, B subunit-inactivated Vibrio cholera whole cell combination vaccine [46], and SC602 live Shigella flexneri 2a vaccine [47] were less effective in low-income settings. This gradient immunogenicity or protection has been seen in all age groups, from young infants to adults. The causes for reduced efficacy are likely multifactorial and their identification could allow the design of strategies for vaccine improvement. Because of the high burden of rotavirus disease, even a modest improvement in vaccine effectiveness in the individual could nonetheless have significant overall public health impact. In this review, we aim to systematically describe biological and environmental factors associated with low performance of rotavirus vaccines by reviewing the current literature.
2. Passive transfer of maternal rotavirus antibodies
2.1. Breastmilk rotavirus antibodies
We assessed the effect of breastfeeding on the response to two- or three-dose oral rotavirus vaccines (Table 1). Information of history of infants’ feeding practices was obtained from parents or guardians in all the studies. Seven studies analyzed the effect of breastfeeding as a factor protecting against rotavirus gastroenteritis. In Germany, the researchers observed a statistically significant association between breastfeeding and rotavirus vaccines’ (RV1/RV5) failure [48]. Two pooled analyses from Africa (Ghana, Kenya, and Mali) and Asia (Bangladesh and Vietnam) showed a slightly decreased efficacy of RV5 in children with exclusive breastfeeding, compared with children with nonexclusive breastfeeding who were immunized, but this difference was not statistically significant [49]. Also, in Europe, marginally decreased efficacy was observed in infants who were breastfed after 2 years of RV1, which was not statistically significant, either [50]. On the other hand, the efficacies of RRV-S1, RRV-TV, or WC3 in the U.S.A., and RV1 in Botswana were similar in infants who were breastfed and non-breastfed [35,51,52]. Four studies analyzed the effect of breastfeeding on the immunogenicity of rotavirus vaccines. In Mexico (RV1), breastfeeding was significantly associated with reduction of both IgA seroresponse and vaccine shedding [53]. In Israel, an analysis with RRV-TV showed a decreased IgA seroconversion in children who were breastfed compared with non-breastfed were immunized, but this difference was not statistically significant [54]. However, in the U.S.A. (RRV-S1, RRV-TV) and Europe (RV1) the immunogenicity was similar in infants who were breastfed and non-breastfed [50,52]. Thus, in the majority of studies, breastfeeding did not interfere significantly with rotavirus vaccine performance.
Table 1.
Rotavirus vaccine response in infants with and without history of breastfeedinga.
| Reference | Location | Income groupc | Vaccine | No of Doses | Measured outcomes | Type of feeding |
p-Value | |
|---|---|---|---|---|---|---|---|---|
| BF | Non-BF | |||||||
| Protection against RVGE | ||||||||
| Gastanaduy et al. 2016 [35] | Botswana | UM | RV1 | 2 | Effectivenessb after 1 or 2 years (95% CI) | 50 (−12, 78) | 51 (−18, 80) | NS |
| Gruber et al. 2017 [49] | Kenya and Mali | L, LM | RV5 | 3 | Efficacy after 1 year (95% CI) | 49 (28–63) | 52 (−13, 80) | NS |
| Efficacy after 2 years (95% CI) | 29 (14–41) | 37 (−4, 62) | NS | |||||
| Gruber et al. 2017 [49] | Bangladesh and Vietnam | LM | RV5 | 3 | Efficacy after 1 year (95% CI) | 53 (25–71) | 61 (−23, 88) | NS |
| Efficacy after 2 years (95% CI) | 38 (13–56) | 60 (12, 82) | NS | |||||
| Rennels et al. 1995 [52] | USA | H | RRV-S1 | 3 | Efficacy after 2 years (95% CI) | 28 (−43, 63) | 39 (−19, 69) | NS |
| RRV-TV | 3 | Efficacy after 2 years (95% CI) | 51 (−6–77) | 50 (0–75) | NS | |||
| Goveia et al. 2008 [51] | USA | H | WC3 | Efficacy after 1 year (95% CI) | 68 (54–78) | 68 (46–82) | NS | |
| Vesikari et al. 2012 [50] | Europe | H | RV1 | 2 | Efficacy after 1 year (95% CI) | 86 (77–92) | 91 (73–98) | NS |
| Efficacy after 2 years (95% CI) | 69 (56–78) | 89 (64–97) | NS | |||||
| Immunogenicity | ||||||||
| Bautista-Marquez et al. 2016 [53] | Mexico | UM | RV1 | 2 | RV-IgA seroresponse GMT (95% CI) | 236 (147–378) | 578 (367–910) | 0.007 |
| Stool RV-vaccine shedding rate | 22% | 43% | 0.016 | |||||
| Friedman et al. 1993 [54] | Israel | H | RRV-TV | 2 | RV-IgA seroconversion rate | 50% | 60% | NS |
| Rennels et al. 1995 [52] | USA | H | RRV-S1 | 3 | RV-IgA seroconversion rate | 68% | 71% | NS |
| RRV-TV | 3 | RV-IgA seroconversion rate | 71% | 72% | NS | |||
| Vesikari et al. 2012 [50] | Europe | H | RV1 | 2 | RV-IgA seroconversion rate | 86% | 89% | NS |
| RV-IgA seroresponse GMT (95% CI) | 185 (161–214) | 232 (186–288) | NS | |||||
Full-dose vaccination studies assessing the effect breast-feeding vs bottle feeding. History of infants’ feeding practices was collected by parents or guardians.
Against severe RVGE.
World Bank list of economies (December 2016) low-income (L) economies are defined as those with a GNI per capita, of $1,025 or less in 2015; lower middle-income (LM) economies are those with a GNI per capita between $1,026 and $4,035; upper middle-income (UM) economies are those with a GNI per capita between $4,036 and $12,475; high-income (H) economies are those with a GNI per capita of $12,476 or more.
RV: rotavirus; BF: rreastfeeding; RVGE: rotavirus gastroenteritis; NS: non-statistically significant (p > 0.05); OR: odds ratio, GMT: geometric mean titer; RRV-S1: rhesus-human reassortant monovalent serotype 1; RRV-TV: rhesus-human reassortant tetravalent; RV1: rotarix; RV5: rotateq.
We analyzed the levels of breastmilk or colostrum’ RV IgA and corresponding infants’ IgA seroconversion post dose 1 or 2 of rotavirus vaccines in mother–infant pairs (Table 2) [55-59]. In India and Zambia, higher breastmilk IgA titers were significantly associated with non-IgA seroconversion to RV1. The same tendency was found in two studies in Nicaragua (RV5) and New Zealand (RV3-BB), but the differences were not statistically significant.
Table 2.
Pre dose 1 breast milk rotavirus-IgA by infants’ rotavirus vaccines IgA seroconversiona.
| Reference | Location | Income groupd | Vaccine | No of Doses | Measured outcomes | Rotavirus vaccines IgA seroconversion |
p-Value | |
|---|---|---|---|---|---|---|---|---|
| R | NR | |||||||
| Rongsen-Chandola et al. 2014 [55] | India | LM | RV1 | 2 | Pre dose 1 breastmilk RV-IgA, OR (95% CI) | 0.76 (0.59–0.97) | Ref | 0.029 |
| Pre dose 2 breastmilk RV-IgA, OR (95% CI) | 0.70 (0.54–0.91) | Ref | 0.007 | |||||
| Becker-Dreps 2015 [58] | Nicaragua | LM | RV5 | 1 | Pre dose 1 breastmilk RV-IgA, median (IQR) | 160 [79, 320] | 320 [159, 320] | NS |
| Chilengi et al. 2016 [56] | Zambia | LM | RV1 | 2 | Pre dose 1 breastmilk RV-IgA, median (IQR) | 80 (40–160) | 160 (80–320) | 0.001 |
| Moon et al. 2016 [57] | South Africa | UM | RV1 | 2 | Pre dose 1 breastmilk RV-IgA, median (range) | 20 (10–80) | 40 (10–80) | NS |
| Pre dose 2 breastmilk RV-IgA, median (range) | 40 (5–2,560) | 40 (1–10,240) | NS | |||||
| Chen et al. 2017 [59] | New Zealand | H | RV3-BB | 3 | Neonatal scheduleb: colostrum RV-IgA median (IQR) | 80 (20–160) | 640 (160–1,280) | NS |
| Infant schedulec: pre dose 1 breastmik RV-IgA, median (IQR) | 80 (40–160) | 80 (65–140) | NS | |||||
RV: rotavirus; R: responders; NR: non responders; NS: non-statistically significant (p > 0.05); OR: odds ratio; IQR: interquartile range; RV1: rotarix; RV5: rotateq; RV3-BB: live attenuated neonatal rotavirus strain; G3P[6].
Studies that analyzed mother–infant pairs.
Doses administered at 0, 8, 15 weeks of age,
Doses administered at 8, 15, 24 weeks of age.
World Bank list of economies (December 2016) low-income (L); lower middle-income (LM); upper middle-income (UM); high-income (H).
To investigate whether a transient abstention from breastfeeding at the time of vaccination would improve the immunogenicity of RV1, three randomized control trials were performed in South Africa, Pakistan, and India (Table 3) [55,60,61]. Lactating women and their infants were recruited and randomly allocated to groups that withheld breastfeeding for 1 h (South Africa and Pakistan) or 30 min (India) before and after RV1 vaccination. Control groups breastfed normally. Despite high compliance of the mothers, none of the three studies reported significantly higher IgA seroconversion post dose 2 in infants who had breastmilk withheld around vaccination compared to those who did not.
Table 3.
Rotarix IgA seroconversion with and without abstention from breastfeeding at the time of vaccinationa.
| Reference | Location | Income groupd | Vaccine | RV1 IgA seroconversion post dose 2 |
p-Value | |
|---|---|---|---|---|---|---|
| Withholding breastfeedingb | Non-withholding breastfeedingc | |||||
| Rongsen-Chandola et al. 2014 [55] | India | LM | RV1 | 26% | 27% | NS |
| Ali et al. 2015 [61] | Pakistan | LM | RV1 | 28% | 38% | NS |
| Groome et al. 2014 [60] | South Africa | UM | RV1 | 63% | 58% | NS |
SC: seroconversion, RV1: Rotarix, NS: Non-statistically significant (p > 0.05).
Post licensure randomized control trials.
Abstention from breastfeeding for at least 30 or 60 min before and after each dose or Rotarix.
Unrestricted breastfeeding was encouraged.
World Bank list of economies (December 2016) low-income (L); lower middle-income (LM); upper middle-income (UM); high-income (H).
We hypothesize that rotavirus IgA present in the breastmilk may diminish the rotavirus vaccine response when infant breastfeeding is a common practice. We concluded that breastmilk anti-rotavirus IgA levels negatively impact the immunogenicity of rotavirus vaccines in some studies. In human colostrum and mature milk, IgA is the predominant immunoglobulin, accounting for 88–90% of its immunoglobulins [62]. The antibodies found in breast milk occur as a result of antigenic stimulation of maternal mucosa-associated lymphoid tissue and bronchial tree (broncho mammary pathway) [63]. These antibodies target the infectious agents encountered by the mother during the perinatal period, meaning that they also target the infectious agents most likely to be encountered by the infant. On the other hand, transient withholding of breastfeeding does not improve the immunogenicity of rotavirus vaccines. Antibodies or other immune factors may persist in infants’ gastrointestinal tract for longer periods than the interval during which breastfeeding was withheld in these studies. An infant’s gastric half-emptying time is between 47 and 56 minutes [64-66]. Therefore, despite withholding of breastfeeding before immunization, the vaccine still may have come into contact with breastmilk in the stomach or the intestines.
2.2. Transplacentally acquired rotavirus IgG
Three studies in Nicaragua (RV5), India (RV1), and South Africa (RV1) found that higher levels of pre dose 1 mothers’ RV-IgG were significantly associated with non-IgA seroconversion in vaccinated infants (Table 4(a)) [55,57,58]. Five studies analyzed the interference of transplacentally acquired RV-IgG with IgA seroconversion to rotavirus vaccines in infants (Table 4(b)). High titers of preexisting RV-IgG were significantly associated with non-IgA seroconversion to the 116E in India and to RV1 in South Africa [57,67]. The same trend was observed in Nicaragua (RV5), Zambia (RV1), and New Zealand (RV3-BB), although in these studies, trends lacked statistical significance [56,58,59].
Table 4.
Mothers’ (a) and infants’ (b) pre dose 1 serum rotavirus-IgG by infants’ rotavirus vaccines IgA seroconversiona.
| Reference | Location | Income groupd |
Vaccine | No of Doses |
Measured factors | Rotavirus vaccines IgA seroconversion |
p-Value | |
|---|---|---|---|---|---|---|---|---|
| R | NR | |||||||
| Becker-Dreps 2015 [58] | Nicaragua | LM | RV5 | 1 | Mothers’ pre dose 1 sera RV-IgG, median (IQR) | 2560 [4360, 5119] | 5120 [3119, 12,240] | 0.020 |
| Rongsen-Chandola et al. 2014 [55] | India | LM | RV1 | 2 | Mothers’ pre dose 1 sera RV-IgG, OR (95% CI) | 0.75 (0.60–0.93) | Ref | 0.011 |
| Moon et al. 2016 [57] | South Africa | UM | RV1 | 1 | Mothers’ pre dose 1 sera RV-IgG, median (IQR) | 5120 (80–81 920) | 10,240 (640–163,840) | 0.031 |
| 2 | 10,240 (80–163,840) | 10,240 (640–163,840) | NS | |||||
| Appaiahgari et al. 2014 [67] | India | LM | 116E | 2 | Infants’ pre dose 1 sera RV-IgG, Median AU/ml [range] | 7278 [1121–48,532] | 15,243 [3742–84,360] | <0.001 |
| Becker-Dreps 2015 [58] | Nicaragua | LM | RV5 | 1 | Infants’ pre dose 1 sera RV-IgG, median (IQR) | 1280 [640–4560] | 560 [1280–3119] | NS |
| Chilengi et al. 2016 [56] | Zambia | LM | RV1 | 2 | Infants’ pre dose 1 sera RV-IgG, GMT (95% CI) | 3988 (3340–4762) | 4833 (3998–5842) | NS |
| Moon et al. 2016 [56] | South Africa | UM | RV1 | 1 | Infants’ pre dose 1 sera RV-IgG, median (IQR) | 1280 (20–10,240) | 1280 (80–20,480) | 0.010 |
| 2 | 1280 (20–10,240) | 1280 (80–20,480) | 0.072 | |||||
| Chen et al. 2017 [59] | New Zealand | H | RV3-BB | 3 | Neonatal scheduleb: Infants’ pre dose 1 sera RV-IgG, Units median (range) | 18,599 (7169–29,223) | 28,437 (26,397–34,566) | NS |
| Infant schedulec: Infants’ pre dose 1 sera RV-IgG, Units median (range) | 15,879 (8485–29,157) | 31,100 (24,303–41,746) | NS | |||||
RV: rotavirus; R: responders; NR: non responders; NS: non-statistically significant (p > 0.10); IQR: interquartile range; RV1: rotarix; RV5: rotateq; RV3-BB: live attenuated neonatal rotavirus strain, G3P[7].
Studies that analyzed mother–infant pairs
Doses administered at 0, 8, 15 weeks of age
Doses administered at 8, 15, 24 weeks of age;
World Bank list of economies (December 2016) low-income (L); lower middle-income (LM); upper middle-income (UM); high-income (H).
The negative effect of both mothers’ and infants’ pre dose 1 anti-rotavirus-IgG on the immunogenicity of rotavirus vaccines was seen in several geographic locations. Even with the neonatal rotavirus strain RV3-BB, the nonresponders had higher titers of pre dose 1 RV-IgG, although the difference was not significant. In South Africa and Nicaragua, a significant correlation was found between levels of RV-IgG in sera of mother–infant pairs before their first rotavirus immunization, suggesting direct transplacental transmission of this antibody from mothers to infants [57,58]. During pregnancy, maternal IgG is transported over the placenta (transplacental transport) by an active, FcRn receptor mediated process and protects infants against different infections during the first months of life [68]. This transplacentally acquired RV-IgG is also one of the proposed factors for reduced infant vaccine efficacy in other pediatric vaccines [69], such as measles [70,71], tetanus [72], and pneumococcal vaccines [72,73].
3. Rotavirus seasonality
In Zambia, IgA seroconversion post RV1 was lower in children receiving their first vaccine dose during a rotavirus season, although with a marginal level of significance (Figure 1(a)) [56]. The same trend was found in Bangladesh and South Africa, but none of the studies detected significantly smaller IgA sercoconversion values in children receiving their first vaccine dose during a rotavirus season [57,74]. Additionally, in five studies from the Americas, the effectiveness of RV1 and RV5 was lower for children born during rotavirus season, but the effect was not significant in any single country (Figure 1(b)) [75-78]. The definition of a rotavirus season for Zambia, Bangladesh, and South Africa was based on data previously published [79-83]. The definition of a rotavirus season for each Latin American countries, rotavirus seasons was based on data from the WHO’s global surveillance network for rotavirus [84]. For the U.S.A., the rotavirus season was defined based on data from the National Respiratory and Enteric Virus Surveillance System [85]. We observed a pattern of lower rotavirus vaccine performance in children either born or receiving their first dose during rotavirus season. However, there are a few factors that vary seasonally and could support the observed patterns. First, maternal rotavirus-antibody levels in mothers are likely to be much higher during the rotavirus season [60]. These passively acquired antibodies, which are transferred from mother to child either transplacentally or through breastfeeding, could potentially influence rotavirus vaccine immune response by neutralizing the vaccine and decreasing the response of rotavirus vaccine in children receiving their first dose or born during months with higher rotavirus activity [86]. A second possibility relates to an active ongoing rotavirus infection during the rotavirus season that could interfere with rotavirus vaccine performance due to the damage to the intestinal epithelium and the ongoing immune response. Third, infections with multiple enteric pathogens are very common in many developing settings [87,88]. Infection with other enteric pathogens at the time of immunization could interfere and impair response to vaccine [89]. If enteric pathogens do interfere with vaccine performance, then, norovirus, which tend to co-circulate during the rotavirus season, could be more likely to interfere with vaccine performance than bacterial enteric pathogens, which are more prevalent during the non-rotavirus season.
Figure 1. Rotavirus vaccines IgA seroconversion by season of their first dose (a) and rotavirus vaccine effectiveness in the first year of life by season of birth (b).
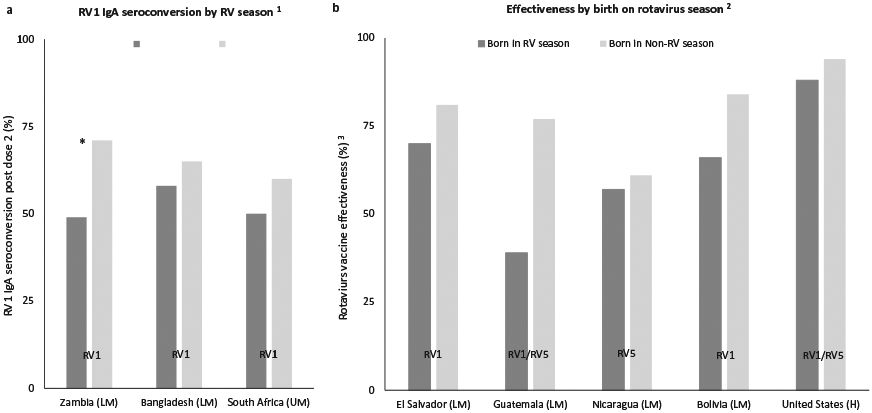
Abbreviations: SC: seroconversion, RV1: Rotarix, RV5: Rotateq * statistically significant at p < 0.1; 1[56,57,74]; 2[75-78]; 3against RVGE; € World Bank list of economies (December 2016) low-income (L); lower middle-income (LM); upper middle-income (UM); high-income (H).
4. Changes in rotavirus vaccination schedules
In Ghana, Kenya, and Mali, significantly higher pooled vaccine efficacy was observed in infants receiving their first dose at ages of 8 weeks or older compared with those receiving first dose before 8 weeks of age (Figure 2) [49]. The same trend was observed in pooled data from Bangladesh and Vietnam, but without statistical significance [49].
Figure 2. Rotateq efficacy by age at first dose.
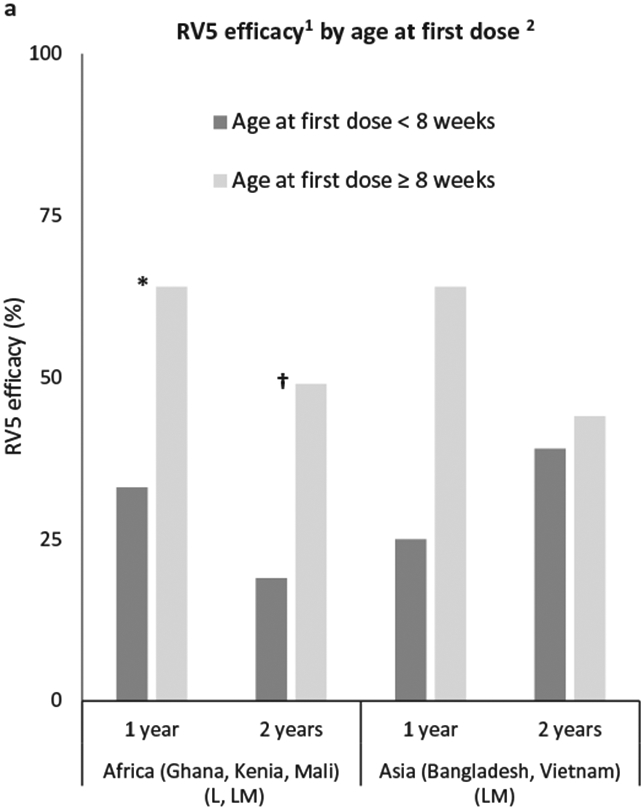
Abbreviations: SC: seroconversion, RV5: Rotateq; †Statistically significant (p < 0.05); *Statistically significant at p < 0.1; 1against RVGE; 2[49].
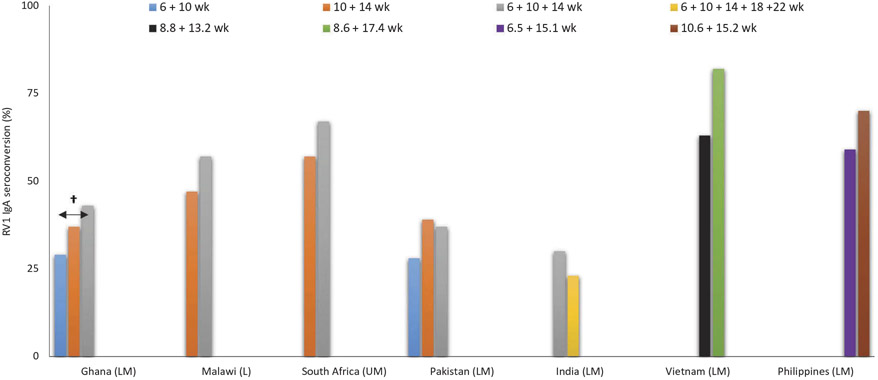
Immunogenicity studies specifically with Rotarix in Africa and Asia suggest a slight benefit in modulating dose schedule (Figure 3) [31,32,90-93]. In a post-licensure study from Ghana [91], the authors tested the immunogenicity after an additional, third dose of RV1 given at 14 weeks of age versus the standard two-dose schedule at 6 and 10 weeks of age. IgA seroconversion was significantly higher in the three-dose arm, but still low in absolute terms. A lesser benefit in IgA seroconversion was seen using a delayed two-dose schedule at 10 and 14 weeks of age, which did not reach statistical significance. These data are in line with findings from a clinical trial of RV1 from South Africa and Malawi performed prior to vaccine introduction. That study compared three doses given at 6, 10, and 14 weeks of age with two doses at ages 10 and 14 weeks to placebo. The IgA seroconversion and efficacy increased with the three-dose schedule over that provided by two doses, though the study was underpowered to analyze each separate schedule [31,32]. On the other hand, trials from Pakistan and India did not show higher IgA seroconversion with a three-dose or five-dose RV1 schedule [90,92]. In Vietnam, a later second dose schedule of RV1 showed a higher IgA seroconversion (although not statistically significant). Additionally, in Philippines, a later first dose showed a higher IgA seroconversion (also not statistically significant) [93].
Figure 3. Rotarix IgA seroconversion by number of doses and schedules1.
Abbreviations: SC: seroconversion, RV1: Rotarix; †Statistically significant (p < 0.05); 1[31,32,90-93]; € World Bank list of economies (December 2016) low-income (L); lower middle-income (LM); upper middle-income (UM); high-income (H).
An even later delay of dosing was tested in Bangladesh, in a controlled efficacy trial of RV1 given at 10 and 17 weeks after birth. In this study, effectiveness against severe rotavirus gastroenteritis was 74% (95% CI, 46–87%) [94], higher than the RV1 effectiveness of 41.4% (95% CI, 23–55%), reported in that country when vaccine doses were given at 6 and 10 weeks of age [95].
Both, delaying the rotavirus vaccination schedule and giving additional doses slightly improved vaccine immunogenicity. These schedules might decrease the impact of maternal antibody in reducing immune responses when vaccine is administered in early infancy as described before. There are high levels of circulating rotavirus antibodies in mothers living in developing countries. Another reason could be that the infant immune system is more immature at earlier time points and capable of more robust memory responses with age. Many studies have shown that the primary t-cell-dependent antibody responses induced in the neonatal period differ from adult responses [96]. Neonatal antibody responses are delayed in beginning, reach lower peak levels, are of shorter duration, differ in the distribution of IgG isotypes (with neonates showing lower IgG2 than adults), and are of lower average affinity and reduced heterogeneity. Reduced antibody responses might be partially caused by the presence of maternal antibodies.
In order to address the problem of reduced duration of protection, in a trial in Bangladesh, a third dose of RV1 was given at 9 months of age [97]. The third dose of RV1 enhanced its immunogenicity, mostly among those infants who were either seronegative or had low antibody titers prior to the third dose. In the previously mentioned two trials of alternate schedules (Malawi and South Africa), no adverse effects were attributed to RV1. Both trials were too small to detect intussusception [98]. Since the relative risk of intussusception was considered to be higher between those receiving the first dose after 3 months of age [99], age restrictions were placed on the timing of immunization with RV1 and RV5. Initially, WHO recommended that the first dose should be given by 15 weeks of age and the last dose by 32 weeks of age [100]. Post-introduction studies have shown that RV1 and RV5 were associated with an increased risk of intussusception primarily right after the first dose, but at a much lower level than that associated with RRV-TV [101]. Infants in low-income countries often do not receive prompt vaccination, and because any increase in deaths from intussusception is expected to be far outweighed by rotavirus deaths prevented through vaccination, the age restriction recommendation was consequently abandoned by the WHO to maximize the opportunity for infants to be immunized [98].
The vaccination later in infancy is expected to decrease the effect of maternal antibody in reducing immunogenicity as opposed to when the vaccine is administered in early infancy as described before. However, a timely vaccination in low-income countries is recommended because of the early natural exposure to rotavirus [102]. Early rotavirus vaccination could decrease the burden of rotavirus gastroenteritis in the first year of life, when infants are the most vulnerable to the symptomatic disease [4]. Immunization with the neonatal RV3-BB strain during the first 7 days of life generated immunogenicity comparable to the conventional vaccination schedule [13]. In developing countries, reinfection is common and is, in general, associated with milder disease [102-104]. However, an Indian cohort study has shown that infants can be symptomatically infected multiple times, even with a strain closely related to that of previous infections [102].
5. OPV administered concurrently
We reviewed several studies from various regions of the world that evaluated the influence of OPV on the immunogenicity of rotavirus vaccines (Figure 4) [74,105-112]. Five studies [South Africa (RV1), Bangladesh (RV1), Chile (RV1), and Latin America (RV1 or RV5)] found that co-administration of an OPV with rotavirus vaccines significantly decreased RV-IgA seroconversion. One of those, in South Africa [105], suggests that this effect may be more relevant at the time of the first dose of RV1. Also, in Bangladesh and Chile, both monovalent and bivalent OPV have shown significant reduction of IgA seroconversion to RV1 [74,109]. Three studies with RV1 in South Africa, Bangladesh, and China also found that infants that had co-administration of OPV with RV1 had lower RV-IgA seroconversion, but the difference was not statistically significant. Emperador et al, reported an inhibitory effect of OPV on RV1 in early stages of virus replication, although the mechanism of interference still needs to be defined [74]. Despite the lower immunogenicity, one efficacy study in middle-income Latin American countries showed no decrease in efficacy of RV1 in infants receiving concurrent OPV [110,111].
Figure 4. Rotavirus vaccine IgA seroconversion with and without OPV concurrently.
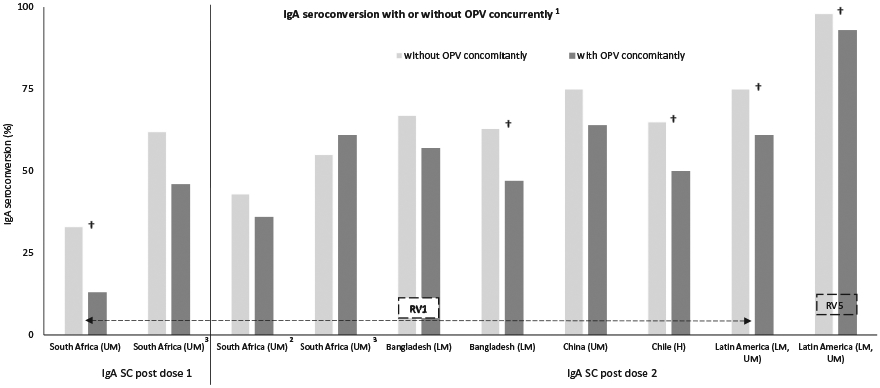
Abbreviations: SC: seroconversion, RV1: Rotarix, RV5: Rotateq † Statistically significant (p < 0.05); 1[75,106-113]; 22 doses at 6 and 10 weeks of age; 32 doses at 10 and 14 weeks of age; € World Bank list of economies (December 2016) low-income (L); lower middle-income (LM); upper middle-income (UM); high-income (H).
6. Microbiome composition and concomitant intestinal infections
We reviewed studies evaluating the interactions of the gut microbiome with rotavirus vaccines immunogenicity (Table 5) [113-115]. In Ghana, researchers explored differences in pre-vaccination fecal microbiota composition between infants with and those without IgA seroconversion following RV1 vaccination and healthy, rotavirus-unvaccinated, Dutch infants of the same age, who were assumed to be rotavirus vaccine responders [113]. The authors found that RV1 response correlated with an increased abundance of Streptococcus bovis and a decreased abundance of the Bacteroidetes phylum in comparisons between both Ghanaian RV1 responders and nonresponders, and Dutch infants and Ghanaian nonresponders. In Pakistan, the authors found that RV1 immunogenicity correlated with a higher abundance of bacteria belonging to Clostridium cluster XI and Proteobacteria, including bacteria related to Serratia and Escherichia coli. Surprisingly, abundance of these Proteobacteria was also significantly higher in Dutch infants when compared to Pakistanian RV1-nonresponders [116]. Additionally, concurrent enterovirus infections correlated significantly with poor IgA seroconversion to RV1 in Bangladesh [114]. A study in Ecuador showed higher plasma IgA responses to rotavirus vaccine and OPV in children of helminth-infected mothers, compared to that of children of helminth-uninfected mothers [115]. However, the pathogenic mechanism for the observed difference is unclear, but may involve the transfer of helminth-induced cytokines (e.g. IL-10) across the placenta or in breastmilk. Additionally, the helminth infections were not associated with reduced immune responses to other infant vaccines.
Table 5.
Gut microbiome and concomitant intestinal infections by Rotarix IgA seroconversion.
| Reference | Location | Income groupa |
Vaccine | No of Doses |
Measured factors | RV vaccines IgA seroconversion |
p-Value | |
|---|---|---|---|---|---|---|---|---|
| R | NR | |||||||
| Harris et al. 2016 [114] | Ghana | LM | RV1 | 2 | Pre dose 1 fecal microbiome composition, correlation | Streptococcus bovis (FDR = 0.008) | Bacteroidetes phylum (FDR = 0.003) | - |
| Harris et al. 2017 [117] | Pakistan | LM | RV1 | 2/3 | Pre dose 1 fecal microbiome composition, correlation | Clostridium, (p = 0.02) Proteobacteria (p = 0.04) | ||
| Taniuchi et al. 2016 [115] | Bangladesh | LM | RV1 | 2 | Pre dose 1 fecal enterovirus quantity, OR (95% CI) | 0.78 (0.64–0.96) | 0.022 | |
| Clark et al. 2016 [116] | Ecuador | UM | RV1 | 2 | Maternal antenatal helminth infections, OR (95% CI) | 1.31 (1.06–1.59) | 0.011 | |
R: responders; NR: non responders; IQR: interquartile range; OR: odds ratio; FDR: false discovery rate; RV1: rotarix.
World Bank list of economies (December 2016) low-income (L); lower middle-income (LM); upper middle-income (UM); high-income (H).
Regarding the microbiome studies in Ghana and Pakistan, the authors speculate that they may complement one another—Proteobacteria and E. coli-derived lipopolysaccharide (LPS) might boost RV1 responses in some populations whereas Bacteroidetes-derived LPS might inhibit RV1 responses in others. Also, because the intestinal microbiome differs significantly in different geographic populations, hypothesis are arising that differences in the intestinal microbiome may help explain this gradient in RV vaccine immunogenicity. In humans, the intestinal microbiota does not become stable and mature until about 2 years of age (post-weaning), and several studies have shown the microbial composition prior to this time period is highly variable and sensitive to environmental exposures [117,118]. Given the central role that microbiota have on immune system development, it is a natural extension that the microbiota will impact upon live vaccine efficacy [119]. Additionally, recent work in animal models has demonstrated the significance of the microbiota and associated products (e.g. bacterial LPS) for the replication of enteric viruses [120]. Acute and persistent infections with diverse pathogens in the intestine and their interactions with microbiome can affect immune homeostasis and gut health, which could have a direct effect on vaccine performance [121,122]. Enteric live attenuated vaccines replicate and may interact with the gut microbiota in the intestinal tract. Therefore, the microbiota is also likely to directly and/or indirectly affect efficient vaccine strain replication, which is necessary to elicit a protective local immune response. The microbiota of children living in low-income countries has been shown to be more diverse in its composition, and more variable over time, compared with the microbiota of children living in high-income countries [123-125].
7. Probiotics
We found two studies that examined the impact of supplementation with Lactobacillus rhamnosus GG (LGG) on the immunogenicity to rotavirus vaccines (Table 6). In India, daily supplementation with a LGG in conjunction with zinc resulted in a significant rise in IgA seroconversion compared with infants receiving placebo in a cohort of infants immunized with RV1 [126]. In a small study in Finland, LGG supplementation resulted in a significant increase in IgA seroconversion post RRV-S1 [127]. The two intervention studies with probiotics that increased the immunogenicity of rotavirus vaccines had small sample size and were different in study design (e.g. administration schedule, dose, and probiotic strain used), population-specific microbiota, and gut health. Combined supplementation of probiotic and zinc deserves further investigation. Additionally, studies in gnotobiotic pigs showed that LGG, B. lactis Bb12 (Bb12), and L. acidophilus (LA) probiotics had beneficial effects on AttHRV vaccine protective efficacy and immunogenicity and they moderated the severity of diarrhea, but only when given at least 21 days prior to human rotavirus challenge [128-130]. Severe rotavirus diarrhea in children has also been successfully treated using antibodies derived from hyperimmune bovine colostrum of immunized cows [131-133].
Table 6.
Probiotic supplementation by rotavirus vaccine IgA seroconversion.
| Reference | Location | Income groupb | Vaccine | No of Doses | Measured outcomes | Supplementation with LGG® |
p-Value | |
|---|---|---|---|---|---|---|---|---|
| Yes | No | |||||||
| Lazarus et al. 2017 [127] | India | LM | RV1 | 2 | IgA seroconversion rate | 39%a | 27% | 0.04 |
| Isolauri et al. 1995 [128] | Finland | H | RRV-S1 | 1 | IgA seroconversion rate | 93% | 74% | 0.05 |
RV1: rotarix; RRV-S1: rhesus-human reassortant monovalent serotype 1; LGG®: Lactobacillus rhamnosus.
With additional zinc supplementation.
World Bank list of economies (December 2016) low-income (L); lower middle-income (LM); upper middle-income (UM); high-income (H).
8. Undernutrition
Undernutrition, which is prevalent in the world’s most impoverished regions, has been associated with failure of resisting infections and recovering from disease, especially in children under the age of 5 [134,135]. Studies on undernutrition and rotavirus vaccines response showed heterogeneous and non-consistent effects. Gastanaduy et al, showed a significant correlation between undernutrition (weight for length z score < −2, based on the WHO growth standards) and low effectiveness of RV1 in Botswana after 1ne or 2 years of follow-up [35] (Table 7). Also, underweight infants (weight for age z score < −2) from three African countries (RV5) had a nonsignificant trend toward lower combined efficacy after 1 and 2years [49]. In pooled data from Bangladesh and Vietnam (RV5), undernourished infants (weight for age z score < −2) had slightly higher efficacy in the first year of follow-up, however, after the second year they showed lower efficacy [49]. In Latin America (RV1), however, undernutrition status (weight for age z score < −22) did not affect vaccine efficacy [136]. Additionally, in Bangladesh, IgA seroconversion post RV1 was not affected by the undernutrition status (weight for length z score < −2) of the children [74].
Table 7.
Rotavirus vaccine response in infants with and without undernutrition.
| Reference | Location | Income groupc | Vaccine | No of Doses | Measured outcomes | Undernurished |
p-Value | |
|---|---|---|---|---|---|---|---|---|
| Yes | No | |||||||
| Protection against severe RVGE | ||||||||
| Gastanaduy et al. 2016 [35] | Botswanaa | UM | RV1 | 2 | Effectiveness after 1 or 2 years (95% CI) | −28 (−309, 60) | 75 (41, 89) | 0.02 |
| Gruber et al. 2017 [49] | Ghana, Kenya and Malib | L, LM | RV5 | 3 | Efficacy after 1 year (95% CI) | 33 (−64, 73) | 72 (48, 85) | NS |
| Efficacy after 2 years (95% CI) | 7 (−83, 53) | 44 (24, 59) | NS | |||||
| Gruber et al. 2017 [49] | Bangladesh and Vietnamb | LM | RV5 | 3 | Efficacy after 1 year (95% CI) | 66 (−229, 96) | 50 (11, 72) | NS |
| Efficacy after 2 years (95% CI) | 23 (−245, 83) | 50 (24,66) | NS | |||||
| Perez-Schael et al. 2007 [137] | Brazil, Mexico, Venezuelab | UM | RV1 | 2 | Efficacy after 1 year (95% CI) | 73 (11, 92) | 74 (52, 86) | NS |
| Immunogenicity | ||||||||
| Emperador et al. 2016 [75] | Bangladesha | LM | RV1 | 2 | IgA seroconversion rate | 55% | 57% | NS |
R: responders; NR: non responders; NS: non-statistically significant (p > 0.05); RV1: rotarix; RV5: rotateq.
Undernutrition was defined as a weight-for-length z score <−2 based on World Health Organization growth standards.
Undernutrition was defined as a weight-for-age z score <−2 based on World Health Organization growth standards.
World Bank list of economies (December 2016) low-income (L); lower middle-income (LM); upper middle-income (UM); high-income (H).
We discovered that there is conflicting evidence on the impact of nutritional status on the performance of rotavirus vaccines in developing countries. Nutrition can impact the function of the mammalian adaptive immune system, and therefore, the responses to vaccines in children [137]. Several micronutrients are relevant for immune role and vaccine efficacy, including vitamins A and D, and zinc [138,139]. For example, vitamin A deficiency in mice has been shown to modulate trafficking of vaccine-specific CD8+T cells to the gastrointestinal tract in an ovalbumin/simian immunodeficiency virus vaccine model by interfering with retinoic acid-dependent upregulation of mucosal homing integrins in vaccine-specific CD8+T cells [140]. Several in vivo studies comprising adult mice vaccinated subcutaneously or intramuscularly with inactivated vaccines co-administered with 1,25-(OH)2D3 (the most active form of vitamin D that is transported to target tissues) showed the production of antigen-specific mucosal immunity and enhanced systemic immune responses [141-143]. The studies included IPV [141], Haemophilus influenzae type b oligosaccharide conjugated to diphtheria toxoid vaccine [142], and hepatitis B surface antigen [143]. The observation of induction of mucosal immunity is significant, as the traditional paradigm suggests this requires direct antigen presentation at the mucosal surface [144]. However, whether vitamin D will prove to be an adjuvant for rotavirus vaccination will require further study. Considering the effect of diet to the composition of the intestinal microbiome, it is possible that malnutrition modifies the microbiome significantly [145]. However, it is still unknown how these diet-driven microbiota changes affect rotavirus vaccine efficacy. Additionally, zinc plays a key role in the adaptive immune system, and deficiency is associated with depressed T cell function [146]. Studies have tested the effect of supplementation with zinc on the response to vaccination, including OPV and inactivated oral cholera vaccine [137,147-150]. In a study in rural Pakistan, supplementation with 10 mg zinc daily from birth to 18 weeks of age had no impact on seroconversion after four doses of trivalent OPV [147]. Zinc supplementation did increase serum vibriocidal antibody titers in children and adults following administration of inactivated oral cholera vaccine, although this effect was not apparent in infants 6–9 months old [148-150].
9. Environmental enteropathy markers
Environmental enteropathy (EE)—also referred as ‘environmental enteric dysfunction’—is a subclinical condition characterized by histological and functional abnormalities in the small intestine, which seem to be almost ubiquitous in children living in resource-poor settings [151]. A prospective longitudinal study of infants in an urban slum from Bangladesh showed that fecal alpha-1-antitrypsin and IL-10 (biomarkers of enteric and systemic inflammation, respectively) were significantly correlated with non-IgA seroconversion after RV1 vaccination (Table 8) [152]. In Nicaragua, the researchers found that two fecal biomarkers of EE: myeloperoxidase (MPO) and calprotectin (CAL) were statistically associated with diminished IgA seroconversion post RV5 [153]. The two studies have shown that EE biomarkers were associated with lower rotavirus vaccine immunogenicity.
Table 8.
Environmental enteropathy biomarkers by rotavirus vaccine IgA seroconversion.
| Reference | Location | Income groupa |
Vaccine | No of Doses |
Measured factors | Rotavirus vaccines IgA seroconversion |
p-Value | |
|---|---|---|---|---|---|---|---|---|
| R | NR | |||||||
| Naylor et al. 2015 (153) | Bangladesh | LM | RV1 | 2 | Biomarker of EE (fecal alpha-1-antitrypsin), R2 | 0.24 | * | |
| Becker-Dreps et al. 2017 (154) | Nicaragua | LM | RV5 | 1 | 4 Biomarkers of EE, median combined score (IQR) | 4.5 (2.8–5.8) | 6.5 (4.5–9.5) | 0.017 |
R: responders; NR: non responders; NS: non-statistically significant (p > 0.05); EE: environmental enteropathy; RV1: rotarix; RV5: rotateq.
No reported but significant.
World Bank list of economies (December 2016) low-income (L); lower middle-income (LM); upper middle-income (UM); high-income (H).
10. HIV
Diarrheal disease is a major cause of sickness and death in HIV-infected (HIV+) children; some studies reported more severe rotavirus infection in HIV+ children [154-158]. Two studies in Botswana and South Africa measured the RV1 effectiveness in HIV-exposed-uninfected compared to HIV-unexposed-uninfected children and found no statistical difference between the groups (Table 9) [35,159]. In Zambia, the researchers measured the IgA seroconversion post RV1 in HIV-exposed-uninfected compared to HIV-unexposed-uninfected children and found no statistical difference between the groups either [56]. The evidence available to date showed no impact of HIV-exposure on the performance of RV1 in African countries. Additionally, one placebo-controlled trial of the safety and immunogenicity of RV5 administered to HIV+ and HIV-exposed-uninfected infants was performed in four African countries [160]. RV5 showed an IgA seroconversion of 85% in both HIV+ and HIV-exposed-uninfected infants, regardless of significant differences in inflammation and immune activation at the start of the immunization series in both groups [160,161].
Table 9.
Rotarix response in HIV-exposed and HIV-unexposed-uninfected infants.
| Reference | Location | Income groupd |
Vaccine | No of doses |
Measured factors | HIV-uninfected infantsa |
p-Value | |
|---|---|---|---|---|---|---|---|---|
| HIV-exposed | HIV-unexposed | |||||||
| Gastanaduy et al. 2016 [35] | Botswana | UM | RV1 | 2 | Effectivenessb after 1 or 2 years (95% CI) | 32 (−121, 79) | 44 (−34, 76) | NS |
| Groome et al. 2014 [155] | South Africa | UM | RV1 | 1 | Effectivenessc after 1 or 2 years (95% CI) | 61 (22,81) | 24 (−17, 51) | NS |
| 2 | 64 (34, 80) | 54 (31, 69) | ||||||
| Chilengi et al. 2016 [56] | Zambia | LM | RV1 | 2 | IgA seroconversion rate | 54% | 62% | NS |
HIV: human immunodeficiency virus; NS: non-statistically significant (p > 0.05).
An HIV-uninfected child was judged to be HIV-exposed if the mother gave history of testing HIV positive during pregnancy, or HIV-unexposed if the mother had a history of negative HIV test during pregnancy.
Against severe RVGE;
Against any RVGE;
World Bank list of economies (December 2016) low-income (L); lower middle-income (LM); upper middle-income (UM); high-income (H).
Unfortunately, the small sample size of this study and its absence of an HIV-unexposed control group limit their ability to make conclusive statements about RV5 in HIV+ infants. Nevertheless, in perinatally infected infants, they demonstrated no effect of HIV-associated inflammation and immune activation on the immunogenicity to RV5. Although many HIV+ infants have received live rotavirus vaccines since the WHO recommended them, information on the safety and immunogenicity of rotavirus vaccines in HIV+ infants is limited to approximately 100 infants who received RV1 [159,162], and less than 50 infants who received RV5 [29,163]. Despite HIV+ infants may benefit from rotavirus vaccines, these vaccines have been implicated in prolonged gastroenteritis with persistent shedding of vaccine-strain virus in infants with severe combined immunodeficiency, and other live viral vaccines have caused disease in infants with advanced HIV infection [164-167].
11. Histo-blood group antigens
Certain histo-blood group antigens (HBGAs) expressed on enterocytes have been proposed as receptors for the VP8* of rotaviruses (VP8* is the globular head fragment of the spike protein, VP4) [168,169]. Additionally, several studies demonstrated an HBGA correlation with rotavirus disease [170-174]. HBGAs are synthesized by glycosyltransferases encoded by ABO, Lewis, and secretor gene families. Recent investigations have suggested that the sialic acid-independent human rotaviruses recognize certain HBGAs in a P genotype-dependent manner. VP8* sequencing identified segregation of animal and human rotaviruses into five P genogroups, and researchers have hypothesized that strains within a genogroup may interact with a specific HBGA epitope [169]. HBGA phenotype correlates significantly with rotavirus vaccine IgA seroconversion. In Pakistani infants, the IgA seroconversion after three doses of RV1 differed significantly by salivary HBGA phenotype, with the lowest rate (19%) among infants who were nonsecretors (i.e. who did not express the carbohydrate synthesized by FUT2), an intermediate rate (30%) among secretors with non-blood group O, and the highest rate (51%) among secretors with O blood group [175]. How this lower seroresponse impacts on clinical protection is not yet clear and may require additional studies. For instance, while nonsecretors may be less prone to respond to vaccination, they will also be less susceptible to natural infection with certain genotypes. The study in Pakistan showed that secretor and salivary ABO blood group antigen status predicted rotavirus vaccine IgA seroconversion. This finding is consistent with an in vitro data that showed recombinant VP8* and cell-culture-adapted P[8] strains interacted with Lewis b and H type 1 antigens [168] and that RV1 VP8*-GST fusion protein bound to saliva samples from secretors but not those from nonsecretors [176]. Epidemiological studies with some population diversity have found that children with rotavirus disease from P[8] strains are significantly more likely to be secretors, compared with the general population [177]. Epidemiological data from one location have also suggested that children with rotavirus disease from P[6] strains are more likely to be Lewis negative [170].
12. Conclusions
There was a nonsignificant trend of lower rotavirus vaccines performance in breastfed infants. The rotavirus IgG transplacentally transferred was negatively associated with vaccine response. We show a nonsignificant trend of lower rotavirus performance in children either born or receiving their first dose during a rotavirus season. Both delaying the rotavirus vaccination schedule and giving additional doses slightly improved vaccine immunogenicity. This might be due to the fact that the baseline antibodies have waned in those infants, and also that the infant immune system is capable of more robust memory responses with age. Co-administration with the OPV also decreases vaccine immunogenicity. In addition, intestinal microbiome differs significantly in rotavirus vaccines’ responders and nonresponders and in different geographic populations. On the other hand, the role of undernutrition still remains controversial and further studies are needed. Two clinical trials have shown that L. rhamnosus GG increased immunogenicity of rotavirus vaccines. Furthermore, EE biomarkers were associated with lower rotavirus vaccine immunogenicity, but HIV status appeared to have no impact on the performance of RV1 in Africa. Recent data suggest potential roles of host genetic factors (e.g. HBGA) in rotavirus vaccine response. Understanding the risk factors for low rotavirus vaccines’ performance is critical for maximizing the public health impact of the current oral vaccines and developing the next generation of rotavirus vaccines.
13. Expert commentary
Since rotavirus vaccines were introduced into routine national immunization programs in 2006, it had a tremendous public health impact, as evidenced by reductions in diarrhea-associated mortality in low and middle-income settings, and reductions of hospitalizations in high-income settings. However, in low-income settings, the lower vaccine efficacy and initial indications that rotavirus vaccine protection might decline beyond the first year of life pose ongoing challenges to sustainability of early vaccine success. Consequently, mechanisms for lower vaccine efficacy in low-income countries and practical strategies to modify contributing factors are being explored. IgG rotavirus antibodies passively transferred to the infants and co-administration with OPV were generally associated with the reduced rotavirus vaccine’s immunogenicity.
Despite a nonsignificant interaction between breastfeeding practices and rotavirus vaccination in some studies, breastfeeding should be strongly recommended during immunization counseling. The still maturing immunologic systems of infants benefit greatly from breastfeeding’s modulating effect on responses to pathogen challenges. Ideally, accurate descriptions of breastfeeding practices should be included in research databases of vaccine efficacy and safety trials. Randomized trials could evaluate the impact of vaccine administration schedule on efficacy in Africa and Asia with a high disease burden.
There is a need for an understanding of the relationship between the composition of microbiota to responses to rotavirus vaccines. For instance, studies correlating the use of antibiotics (that cause a major effect on microbial diversity) before vaccination, followed by analysis of vaccine-responses, would provide major insights into changes in the microbiota and rotavirus vaccine responses. Furthermore, additional research and analysis is needed on the role of particular species within communities and their correlation with rotavirus vaccine responses. And, the tools are now readily available (community sequencing, metagenomics, metabolomics, bioinformatics, etc.). Also, it is essential to further explore the importance of the whole microbiome in the gut as a potential modulator of responses to rotavirus vaccines and evaluating the impact of nutritional status of the infants on the performance of rotavirus vaccines in developing countries. More in vivo studies are needed to comprehend the role of probiotics’ impact and its applications as a vaccine adjuvant.
Additional information about rotavirus vaccines in HIV-positives and immunocompromised infants is desirable because protective antibody responses can be impaired in infants with untreated HIV infection, and robust responses may not be achieved even when vaccine is administered after initiating antiretroviral therapy early in life. In the future, accurate assessment of the safety of rotavirus vaccines in HIV-exposed-uninfected and HIV+ infants will require larger-scale effectiveness studies because performing placebo-controlled efficacy trials are not deemed to be ethical. Additionally, it is important to understand if and how the expression of HBGAs in different populations influences the performance of rotavirus vaccines. More data is required to answer the question of whether the expression of particular HBGAs in infants will determine their susceptibility to RV infections and interfere with the uptake of RV vaccines. While we analyzed the data of the individual risk factors affecting the vaccines’ efficacy, the impact of the combination of the specific risk factors has yet to be explored.
Parenterally administered, nonreplicating rotavirus vaccines could provide a valuable addition to the current range of available rotavirus vaccines and may elude some of the barriers discussed here. In addition to improved efficacy in developing countries, these vaccines might be produced at a low cost and can potentially be combined with other childhood vaccines, thus facilitating the vaccine delivery. Further, parenteral vaccines may avoid concerns about replicating vaccines and the associated risk of intussusception and possible vaccine strain transmission.
14. Five-year view
Adjustment of vaccine schedules may be implemented in low-resource areas if considered effective, including earlier and additional booster doses. Hopefully, ongoing large-scale vaccination campaigns that are already in place can be exploited to evaluate the link between microbiome composition and rotavirus vaccine effectiveness. The potential role of infant nutritional status and host genetic factors (as HBGA) on the vaccine performance should continue to be assessed for potential enhancement of vaccine’s performance in resource-poor settings. Parenteral rotavirus vaccines currently under development may be licensed and available to possibly overcome the barriers to orally administered vaccines in developing countries.
Key issues.
Two rotavirus vaccines, Rotarix (RV1) and RotaTeq (RV5), were licensed for global use since 2006.
After clinical trials showed high efficacy (85–98%) of both vaccines in high-income and upper-middle-income countries in the Americas, Asia, and Europe, many countries in these regions implemented national rotavirus vaccination programs.
Subsequent clinical trials conducted in low-income countries of Africa and Asia showed modest efficacy (50–64%). The reasons for this phenomenon have not been fully elucidated.
Infants who were breastfed had a non-significant lower protection compared to non-breastfed infants (ranges: 28–86% vs. 39–91% after 1 year, and 29–69% vs. 37–89% after 2 years). However, 3 trials showed that a transient abstention from breastfeeding did not improve the immunogenicity of rotavirus vaccines.
Titers of RV IgG at pre dose 1 in either the infants or their mothers was about two times higher in non-vaccine seroresponders compared to responders.
There was a non-significant trend of lower protection in children born during a RV season compared with children born in other months (~72 vs. ~84%).
Delaying the rotavirus vaccination schedule and/or giving additional doses of vaccine slightly improved vaccine immunogenicity.
Infants who had co-administration with OPV had generally lower seroresponse to RV vaccination than infants without OPV (~59 vs. ~68%).
Intestinal microbiome differs significantly in RV1’ responders and non-responders.
Undernutrition does not have a significant impact on the vaccine performance.
Biomarkers of environmental enteropathy were associated with lower rotavirus vaccine immunogenicity.
There was no impact of HIV-exposure on the performance of RV1 in African countries.
Funding
This paper was part of work for hire as government employees. No pharmaceutical companies supported this work.
Footnotes
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties. Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
References
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers.
- 1.Tate JE, Burton AH, Boschi-Pinto C, et al. Global, regional, and national estimates of rotavirus mortality in children <5 years of age, 2000-2013. Lancet Infect Dis. 2016;62 Suppl 2:S96–S105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Angel J, Franco MA, Greenberg HB. Rotavirus vaccines: recent developments and future considerations. Nat Rev Microbiol. 2007;5(7):529–539. [DOI] [PubMed] [Google Scholar]
- 3.WHO. Meeting of the Strategic Advisory Group of Experts on immunization, October 2009 - conclusions and recommendations. Biologicals. 2010;38(1):170–177. . [DOI] [PubMed] [Google Scholar]
- 4.WHO. Rotavirus vaccines. WHO position paper - January 2013. Vaccine. 2013;31(52):6170–6171. [DOI] [PubMed] [Google Scholar]
- 5.Leshem E, Moritz RE, Curns AT, et al. Rotavirus vaccines and health care utilization for diarrhea in the United States (2007-2011). Pediatrics. 2014;134(1):15–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rha B, Tate JE, Payne DC, et al. Effectiveness and impact of rotavirus vaccines in the United States - 2006-2012. Expert Rev Vaccines. 2014;13(3):365–376. [DOI] [PubMed] [Google Scholar]
- 7.Jonesteller CL, Burnett E, Yen C, et al. Effectiveness of rotavirus vaccination: a systematic review of the first decade of global post-licensure data, 2006-2016. Lancet Infect Dis. 2017;65(5):840–850. [DOI] [PubMed] [Google Scholar]
- 8.Midthun K, Greenberg HB, Hoshino Y, et al. Reassortant rotaviruses as potential live rotavirus vaccine candidates. J Virol. 1985;53(3):949–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murphy TV, Gargiullo PM, Massoudi MS, et al. Intussusception among infants given an oral rotavirus vaccine. N Engl J Med. 2001;344(8):564–572. [DOI] [PubMed] [Google Scholar]
- 10.Burnett E, Yen C, Tate JE, et al. Rotavirus vaccines: current global impact and future perspectives. Future Virol. 2016;11(10):699–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kulkarni PS, Desai S, Tewari T, et al. A randomized phase III clinical trial to assess the efficacy of a bovine-human reassortant pentavalent rotavirus vaccine in Indian infants. Vaccine. 2017;35(45):6228–6237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Isanaka S, Guindo O, Langendorf C, et al. Efficacy of a low-cost, heat-stable oral rotavirus vaccine in Niger. N Engl J Med. 2017;376(12):1121–1130. [DOI] [PubMed] [Google Scholar]
- 13.Bines JE, Danchin M, Jackson P, et al. Safety and immunogenicity of RV3-BB human neonatal rotavirus vaccine administered at birth or in infancy: a randomised, double-blind, placebo-controlled trial. Lancet Infect Dis. 2015;15(12):1389–1397. [DOI] [PubMed] [Google Scholar]
- 14.Armah GE, Kapikian AZ, Vesikari T, et al. Efficacy, immunogenicity, and safety of two doses of a tetravalent rotavirus vaccine RRV-TV in Ghana with the first dose administered during the neonatal period. J Infect Dis. 2013;208(3):423–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Atherly DE, Lewis KD, Tate J, et al. Projected health and economic impact of rotavirus vaccination in GAVI-eligible countries: 2011-2030. Vaccine. 2012;30 Suppl 1:A7–A14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Azevedo MSP, Gonzalez AM, Yuan L, et al. An oral versus intranasal prime/boost regimen using attenuated human rotavirus or VP2 and VP6 virus-like particles with immunostimulating complexes influences protection and antibody-secreting cell responses to rotavirus in a neonatal gnotobiotic pig model. Clin Vaccine Immunol. 2010;17(3):420–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li T, Lin H, Zhang Y, et al. Improved characteristics and protective efficacy in an animal model of E. coli-derived recombinant double-layered rotavirus virus-like particles. Vaccine. 2014;32(17):1921–1931. [DOI] [PubMed] [Google Scholar]
- 18.Wen X, Cao D, Jones RW, et al. Construction and characterization of human rotavirus recombinant VP8* subunit parenteral vaccine candidates. Vaccine. 2012;30(43):6121–6126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wen X, Cao D, Jones RW, et al. Tandem truncated rotavirus VP8* subunit protein with T cell epitope as non-replicating parenteral vaccine is highly immunogenic. Hum Vaccin Immunother. 2015;11(10):2483–2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fix AD, Harro C, McNeal M, et al. Safety and immunogenicity of a parenterally administered rotavirus VP8 subunit vaccine in healthy adults. Vaccine. 2015;33(31):3766–3772. [DOI] [PubMed] [Google Scholar]
- 21.Groome MJ, Koen A, Fix A, et al. Safety and immunogenicity of a parenteral P2-VP8-P[8] subunit rotavirus vaccine in toddlers and infants in South Africa: a randomised, double-blind, placebo-controlled trial. Lancet Infect Dis. 2017;17(8):843–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Velasquez DE, Wang Y, Jiang B. Inactivated human rotavirus vaccine induces heterotypic antibody response: correction and development of IgG avidity assay. Hum Vaccin Immunother. 2015;11(2):531–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang Y, Vlasova A, Velasquez DE, et al. Skin vaccination against rotavirus using microneedles: proof of concept in gnotobiotic piglets. PLoS One. 2016;11(11):e0166038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bertolotti-Ciarlet A, Ciarlet M, Crawford SE, et al. Immunogenicity and protective efficacy of rotavirus 2/6-virus-like particles produced by a dual baculovirus expression vector and administered intramuscularly, intranasally, or orally to mice. Vaccine. 2003;21(25–26):3885–3900. [DOI] [PubMed] [Google Scholar]
- 25.Lappalainen S, Pastor AR, Malm M, et al. Protection against live rotavirus challenge in mice induced by parenteral and mucosal delivery of VP6 subunit rotavirus vaccine. Arch Virol. 2015;160(8):2075–2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tate JE, Parashar UD. Rotavirus vaccines in routine use. Lancet Infect Dis. 2014;59(9):1291–1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Velasquez DE, Parashar UD, Jiang B. Strain diversity plays no major role in the varying efficacy of rotavirus vaccines: an overview. Infect Genet Evol. 2014;28:561–571.25460825 • Concise overview of overall and strain-specific rotavirus vaccine effectiveness in high and middle income settings.
- 28.Madhi SA, Cunliffe NA, Steele D, et al. Effect of human rotavirus vaccine on severe diarrhea in African infants. N Engl J Med. 2010;362(4):289–298. [DOI] [PubMed] [Google Scholar]
- 29.Armah GE, Sow SO, Breiman RF, et al. Efficacy of pentavalent rotavirus vaccine against severe rotavirus gastroenteritis in infants in developing countries in sub-Saharan Africa: a randomised, double-blind, placebo-controlled trial. Lancet (London, England). 2010;376(9741):606–614. [DOI] [PubMed] [Google Scholar]
- 30.Zaman K, Dang DA, Victor JC, et al. Efficacy of pentavalent rotavirus vaccine against severe rotavirus gastroenteritis in infants in developing countries in Asia: a randomised, double-blind, placebo-controlled trial. Lancet (London, England). 2010;376(9741):615–623. [DOI] [PubMed] [Google Scholar]
- 31.Madhi SA, Kirsten M, Louw C, et al. Efficacy and immunogenicity of two or three dose rotavirus-vaccine regimen in South African children over two consecutive rotavirus-seasons: a randomized, double-blind, placebo-controlled trial. Vaccine. 2012;30(Supplement 1):A44–AA51. [DOI] [PubMed] [Google Scholar]
- 32.Cunliffe NA, Witte D, Ngwira BM, et al. Efficacy of human rotavirus vaccine against severe gastroenteritis in Malawian children in the first two years of life: a randomized, double-blind, placebo controlled trial. Vaccine. 2012;30(Supplement 1):A36–A43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bar-Zeev N, Jere KC, Bennett A, et al. Population impact and effectiveness of monovalent rotavirus vaccination in urban Malawian children 3 years after vaccine introduction: ecological and case-control analyses. Lancet Infect Dis. 2016;62 Suppl 2:S213–S219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Beres LK, Tate JE, Njobvu L, et al. A preliminary assessment of rotavirus vaccine effectiveness in Zambia. Lancet Infect Dis. 2016;62 Suppl 2:S175–S182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gastanaduy PA, Steenhoff AP, Mokomane M, et al. Effectiveness of monovalent rotavirus vaccine after programmatic implementation in Botswana: a multisite prospective case-control study. Lancet Infect Dis. 2016;62 Suppl 2:S161–S167. [DOI] [PubMed] [Google Scholar]
- 36.Sahakyan G, Grigoryan S, Wasley A, et al. Impact and effectiveness of monovalent rotavirus vaccine in Armenian children. Lancet Infect Dis. 2016;62 Suppl 2:S147–S154. [DOI] [PubMed] [Google Scholar]
- 37.Abeid KA, Jani B, Cortese MM, et al. Monovalent rotavirus vaccine effectiveness and impact on rotavirus hospitalizations in Zanzibar, Tanzania: data from the first 3 years after introduction. J Infect Dis. 2017;215(2):183–191. [DOI] [PubMed] [Google Scholar]
- 38.Kotloff KL, Nataro JP, Blackwelder WC, et al. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case-control study. Lancet (London, England). 2013;382(9888):209–222. [DOI] [PubMed] [Google Scholar]
- 39.Burnett E, Jonesteller CL, Tate JE, et al. Global impact of rotavirus vaccination on childhood hospitalizations and mortality from diarrhea. J Infect Dis. 2017;215(11):1666–1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.WHO. Polio vaccines: WHO position paper, January 2014–recommendations. Vaccine. 2014;32(33):4117–4118. [DOI] [PubMed] [Google Scholar]
- 41.Sutter RW, Platt L, Mach O, et al. The new polio eradication end game: rationale and supporting evidence. J Infect Dis. 2014;210 Suppl 1:S434–S438. [DOI] [PubMed] [Google Scholar]
- 42.Serazin AC, Shackelton LA, Wilson C, et al. Improving the performance of enteric vaccines in the developing world. Nat Immunol. 2010;11(9):769–773. [DOI] [PubMed] [Google Scholar]
- 43.Grassly NC, Jafari H, Bahl S, et al. Waning intestinal immunity after vaccination with oral poliovirus vaccines in India. J Infect Dis. 2012;205(10):1554–1561. [DOI] [PubMed] [Google Scholar]
- 44.Grassly NC, Jafari H, Bahl S, et al. Asymptomatic wild-type poliovirus infection in India among children with previous oral poliovirus vaccination. J Infect Dis. 2010;201(10):1535–1543. [DOI] [PubMed] [Google Scholar]
- 45.John J Role of injectable and oral polio vaccines in polio eradication. Expert Rev Vaccines. 2009;8(1):5–8. [DOI] [PubMed] [Google Scholar]
- 46.Hallander HO, Paniagua M, Espinoza F, et al. Calibrated serological techniques demonstrate significant different serum response rates to an oral killed cholera vaccine between Swedish and Nicaraguan children. Vaccine. 2002;21(1–2):138–145. [DOI] [PubMed] [Google Scholar]
- 47.Levine MM, Kotloff KL, Barry EM, et al. Clinical trials of Shigella vaccines: two steps forward and one step back on a long, hard road. Nature reviews. Microbiology. 2007;5(7):540–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Adlhoch C, Hoehne M, Littmann M, et al. Rotavirus vaccine effectiveness and case-control study on risk factors for breakthrough infections in Germany, 2010-2011. Pediatr Infect Dis J. 2013;32(2):e82–e89. [DOI] [PubMed] [Google Scholar]
- 49.Gruber JF, Hille DA, Liu GF, et al. Heterogeneity of rotavirus vaccine efficacy among infants in developing countries. Pediatr Infect Dis J. 2017;36(1):72–78. [DOI] [PubMed] [Google Scholar]
- 50.Vesikari T, Prymula R, Schuster V, et al. Efficacy and immunogenicity of live-attenuated human rotavirus vaccine in breast-fed and formula-fed European infants. Pediatr Infect Dis J. 2012;31(5):509–513. [DOI] [PubMed] [Google Scholar]
- 51.Goveia MG, DiNubile MJ, Dallas MJ, et al. Efficacy of pentavalent human-bovine (WC3) reassortant rotavirus vaccine based on breastfeeding frequency. Pediatr Infect Dis J. 2008;27(7):656–658. [DOI] [PubMed] [Google Scholar]
- 52.Rennels MB, Wasserman SS, Glass RI, et al. Comparison of immunogenicity and efficacy of rhesus rotavirus reassortant vaccines in breastfed and nonbreastfed children. US Rotavirus Vaccine Efficacy Group. Pediatrics. 1995;96(6):1132–1136. [PubMed] [Google Scholar]
- 53.Bautista-Marquez A, Velasquez DE, Esparza-Aguilar M, et al. Breastfeeding linked to the reduction of both rotavirus shedding and IgA levels after Rotarix(R) immunization in Mexican infants. Vaccine. 2016;34(44):5284–5289. [DOI] [PubMed] [Google Scholar]
- 54.Friedman MG, Segal B, Zedaka R, et al. Serum and salivary responses to oral tetravalent reassortant rotavirus vaccine in newborns. Clin Exp Immunol. 1993;92(2):194–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rongsen-Chandola T, Strand TA, Goyal N, et al. Effect of withholding breastfeeding on the immune response to a live oral rotavirus vaccine in North Indian infants. Vaccine. 2014;32 Suppl 1:A134–A139. [DOI] [PubMed] [Google Scholar]
- 56.Chilengi R, Simuyandi M, Beach L, et al. Association of maternal immunity with rotavirus vaccine immunogenicity in Zambian infants. PLoS One. 2016;11(3):e0150100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Moon SS, Groome MJ, Velasquez DE, et al. Prevaccination rotavirus serum IgG and IgA are associated with lower immunogenicity of live, oral human rotavirus vaccine in South African infants. Lancet Infect Dis. 2016;62(2):157–165. •• One of several studies to show an inhibitory effect of rotavirus IgG antibodies passively transferred to the infants on oral rotavirus vaccine immunogenicity.
- 58.Becker-Dreps S, Vilchez S, Velasquez D, et al. Rotavirus-specific IgG antibodies from mothers’ serum may inhibit infant immune responses to the pentavalent rotavirus vaccine. Pediatr Infect Dis J. 2015;34(1):115–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chen MY, Kirkwood CD, Bines J, et al. Rotavirus specific maternal antibodies and immune response to RV3-BB neonatal rotavirus vaccine in New Zealand. Hum Vaccin Immunother. 2017;13(5):1126–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Groome MJ, Moon SS, Velasquez D, et al. Effect of breastfeeding on immunogenicity of oral live-attenuated human rotavirus vaccine: a randomized trial in HIV-uninfected infants in Soweto, South Africa. Bull World Health Organ. 2014;92(4):238–245.24700991 • One of the three randomized trials to show no beneficial effect of temporarily withholding breastfeeding on oral rotavirus vaccine immunogenicity.
- 61.Ali A, Kazi AM, Cortese MM, et al. Impact of withholding breastfeeding at the time of vaccination on the immunogenicity of oral rotavirus vaccine–a randomized trial. PLoS One. 2015;10(6):e0127622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hurley WL, Theil PK. Perspectives on immunoglobulins in colostrum and milk. Nutrients. 2011;3(4):442–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Goldman AS. Modulation of the gastrointestinal tract of infants by human milk. Interfaces and interactions. An evolutionary perspective. J Nutr. 2000;130(2S Suppl):426s–431s. [DOI] [PubMed] [Google Scholar]
- 64.Billeaud C, Guillet J, Sandler B. Gastric emptying in infants with or without gastro-oesophageal reflux according to the type of milk. Eur J Clin Nutr. 1990;44(8):577–583. [PubMed] [Google Scholar]
- 65.Cavkll B Gastric emptying in infants fed human milk or infant formula. Acta Paediatr. 1981;70(5):639–641. [PubMed] [Google Scholar]
- 66.Van Den Driessche M, Peeters K, Marien P, et al. Gastric emptying in formula-fed and breast-fed infants measured with the 13C-octanoic acid breath test. J Pediatr Gastroenterol Nutr. 1999;29(1):46–51. [DOI] [PubMed] [Google Scholar]
- 67.Appaiahgari MB, Glass R, Singh S, et al. Transplacental rotavirus IgG interferes with immune response to live oral rotavirus vaccine ORV-116E in Indian infants. Vaccine. 2014;32(6):651–656. [DOI] [PubMed] [Google Scholar]
- 68.Malek A, Sager R, Kuhn P, et al. Evolution of maternofetal transport of immunoglobulins during human pregnancy. Am J Reprod Immunol. 1996;36(5):248–255. [DOI] [PubMed] [Google Scholar]
- 69. Voysey M, Kelly DF, Fanshawe TR, et al. The influence of maternally derived antibody and infant age at vaccination on infant vaccine responses: an individual participant meta-analysis. JAMA Pediatr. 2017;171(7):637–646.28505244 • Shows the inhibitory effect of maternal antibodies across a wide range of oral and parenteral vaccines.
- 70.Garly ML, Bale C, Martins CL, et al. Measles antibody responses after early two dose trials in Guinea-Bissau with Edmonston-Zagreb and Schwarz standard-titre measles vaccine: better antibody increase from booster dose of the Edmonston-Zagreb vaccine. Vaccine. 2001;19(15-16):1951–1959. [DOI] [PubMed] [Google Scholar]
- 71.BorrÀS E, Urbiztondo L, Costa J, et al. Measles antibodies and response to vaccination in children aged less than 14 months: implications for age of vaccination. Epidemiol Infect. 2011;140(9):1599–1606. [DOI] [PubMed] [Google Scholar]
- 72.Jones C, Pollock L, Barnett SM, et al. The relationship between concentration of specific antibody at birth and subsequent response to primary immunization. Vaccine. 2014;32(8):996–1002. [DOI] [PubMed] [Google Scholar]
- 73.Francis JP, Richmond PC, Pomat WS, et al. Maternal antibodies to pneumolysin but not to pneumococcal surface protein A delay early pneumococcal carriage in high-risk Papua New Guinean infants. Clin Vaccine Immunol. 2009;16(11):1633–1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Emperador DM, Velasquez DE, Estivariz CF, et al. Interference of monovalent, bivalent, and trivalent oral poliovirus vaccines on monovalent rotavirus vaccine immunogenicity in Rural Bangladesh. Lancet Infect Dis. 2016;62(2):150–156. •• One of several studies to show a diminished effect of co-administration with OPV on oral rotavirus vaccine immunogenicity.
- 75.Patel MM, Patzi M, Pastor D, et al. Effectiveness of monovalent rotavirus vaccine in Bolivia: case-control study. BMJ (Clinical Research Ed.). 2013;346:f3726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.De Palma O, Cruz L, Ramos H, et al. Effectiveness of rotavirus vaccination against childhood diarrhoea in El Salvador: case-control study. BMJ (Clinical Research Ed.). 2010;340:c2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Patel M, Pedreira C, De Oliveira LH, et al. Duration of protection of pentavalent rotavirus vaccination in Nicaragua. Pediatrics. 2012;130(2):e365–e372. [DOI] [PubMed] [Google Scholar]
- 78.Payne DC, Boom JA, Staat MA, et al. Effectiveness of pentavalent and monovalent rotavirus vaccines in concurrent use among US children <5 years of age, 2009-2011. Lancet Infect Dis. 2013;57(1):13–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mwenda JM, Ntoto KM, Abebe A, et al. Burden and epidemiology of rotavirus diarrhea in selected African countries: preliminary results from the African Rotavirus Surveillance Network. J Infect Dis. 2010;202 Suppl:S5–S11. [DOI] [PubMed] [Google Scholar]
- 80.Mpabalwani M, Oshitani H, Kasolo F, et al. Rotavirus gastro-enteritis in hospitalized children with acute diarrhoea in Zambia. Ann Trop Paediatr. 1995;15(1):39–43. [DOI] [PubMed] [Google Scholar]
- 81.Cunliffe NA, Kilgore PE, Bresee JS, et al. Epidemiology of rotavirus diarrhoea in Africa: a review to assess the need for rotavirus immunization. Bull World Health Organ. 1998;76(5):525–537. [PMC free article] [PubMed] [Google Scholar]
- 82.Mapaseka SL, Dewar JB, Van Der Merwe L, et al. Prospective hospital-based surveillance to estimate rotavirus disease burden in the Gauteng and North West Province of South Africa during 2003-2005. J Infect Dis. 2010;202 Suppl:S131–S138. [DOI] [PubMed] [Google Scholar]
- 83.Das SK, Begum D, Ahmed S, et al. Geographical diversity in seasonality of major diarrhoeal pathogens in Bangladesh observed between 2010 and 2012. Epidemiol Infect. 2014;142(12):2530–2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.WHO. Generic protocol for monitoring impact of rotavirus vaccination on gastroenteritis disease burden and viral strains. Geneva: WHO. 2008. [Google Scholar]
- 85.CDC. The National Respiratory and Enteric Virus Surveillance System (NREVSS). Available at www.cdc.gov/surveillance/nrevss/rotavirus/index.html. Accessed January 2017.
- 86.Chan J, Nirwati H, Triasih R, et al. Maternal antibodies to rotavirus: could they interfere with live rotavirus vaccines in developing countries? Vaccine. 2011;29(6):1242–1247. [DOI] [PubMed] [Google Scholar]
- 87.Urbina D, Arzuza O, Young G, et al. Rotavirus type A and other enteric pathogens in stool samples from children with acute diarrhea on the Colombian northern coast. Int Microbiol. 2003;6(1):27–32. [DOI] [PubMed] [Google Scholar]
- 88.Bodhidatta L, McDaniel P, Sornsakrin S, et al. Case-control study of diarrheal disease etiology in a remote rural area in Western Thailand. Am J Trop Med Hyg. 2010;83(5):1106–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wang H, Moon S, Wang Y, et al. Multiple virus infection alters rotavirus replication and expression of cytokines and Toll-like receptors in intestinal epithelial cells. Virus Res. 2012;167(1):48–55. [DOI] [PubMed] [Google Scholar]
- 90.Ali SA, Kazi AM, Cortese MM, et al. Impact of different dosing schedules on the immunogenicity of the human rotavirus vaccine in infants in Pakistan: a randomized trial. J Infect Dis. 2014;210(11):1772–1779. [DOI] [PubMed] [Google Scholar]
- 91.Armah G, Lewis KD, Cortese MM, et al. A randomized, controlled trial of the impact of alternative dosing schedules on the immune response to human rotavirus vaccine in Rural Ghanaian infants. J Infect Dis. 2016;213(11):1678–1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kompithra RZ, Paul A, Manoharan D, et al. Immunogenicity of a three dose and five dose oral human rotavirus vaccine (RIX4414) schedule in south Indian infants. Vaccine. 2014;32 Suppl 1:A129–A133. [DOI] [PubMed] [Google Scholar]
- 93.Anh DD, Carlos CC, Thiem DV, et al. Immunogenicity, reactogenicity and safety of the human rotavirus vaccine RIX4414 (Rotarix) oral suspension (liquid formulation) when co-administered with expanded program on immunization (EPI) vaccines in Vietnam and the Philippines in 2006-2007. Vaccine. 2011;29(11):2029–2036. [DOI] [PubMed] [Google Scholar]
- 94.Colgate ER, Haque R, Dickson DM, et al. Delayed dosing of oral rotavirus vaccine demonstrates decreased risk of rotavirus gastroenteritis associated with serum zinc: a randomized controlled trial. Lancet Infect Dis. 2016;63(5):634–641. [DOI] [PubMed] [Google Scholar]
- 95.Zaman K, Sack DA, Neuzil KM, et al. Effectiveness of a live oral human rotavirus vaccine after programmatic introduction in Bangladesh: a cluster-randomized trial. PLoS Med. 2017;14(4):e1002282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Siegrist C-A. Neonatal and early life vaccinology. Vaccine. 2001;19(25):3331–3346. [DOI] [PubMed] [Google Scholar]
- 97.Zaman K, Fleming JA, Victor JC, et al. Noninterference of rotavirus vaccine with measles-rubella vaccine at 9 months of age and improvements in antirotavirus immunity: a randomized trial. J Infect Dis. 2016;213(11):1686–1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Parashar UD, Cortese MM, Payne DC, et al. Value of post-licensure data on benefits and risks of vaccination to inform vaccine policy: the example of rotavirus vaccines. Am J Prev Med. 2015;49(6 Suppl 4):S377–S382. [DOI] [PubMed] [Google Scholar]
- 99.Jiang J, Jiang B, Parashar U, et al. Childhood intussusception: a literature review. PLoS One. 2013;8(7):e68482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Yen C, Healy K, Tate JE, et al. Rotavirus vaccination and intussusception – science, surveillance, and safety: A review of evidence and recommendations for future research priorities in low and middle income countries. Hum Vaccin Immunother. 2016;12(10):2580–2589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Parashar UD, Cortese MM, Payne DC, et al. Value of post-licensure data on benefits and risks of vaccination to inform vaccine policy: the example of rotavirus vaccines. Vaccine. 2015;33 Suppl 4:D55–D59. [DOI] [PubMed] [Google Scholar]
- 102.Gladstone BP, Ramani S, Mukhopadhya I, et al. Protective effect of natural rotavirus infection in an Indian birth cohort. N Engl J Med. 2011;365(4):337–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Fischer TK, Valentiner-Branth P, Steinsland H, et al. Protective immunity after natural rotavirus infection: a community cohort study of newborn children in Guinea-Bissau, West Africa. J Infect Dis. 2002;186(5):593–597. [DOI] [PubMed] [Google Scholar]
- 104.Velázquez FR, Matson DO, Calva JJ, et al. Rotavirus infection in infants as protection against subsequent infections. New England J Med. 1996;335(14):1022–1028. [DOI] [PubMed] [Google Scholar]
- 105.Steele AD, De Vos B, Tumbo J, et al. Co-administration study in South African infants of a live-attenuated oral human rotavirus vaccine (RIX4414) and poliovirus vaccines. Vaccine. 2010;28(39):6542–6548. [DOI] [PubMed] [Google Scholar]
- 106.Steele AD, Reynders J, Scholtz F, et al. Comparison of 2 different regimens for reactogenicity, safety, and immunogenicity of the live attenuated oral rotavirus vaccine RIX4414 coadministered with oral polio vaccine in South African infants. J Infect Dis. 2010;202 Suppl:S93–S100. [DOI] [PubMed] [Google Scholar]
- 107.Zaman K, Sack DA, Yunus M, et al. Successful co-administration of a human rotavirus and oral poliovirus vaccines in Bangladeshi infants in a 2-dose schedule at 12 and 16 weeks of age. Vaccine. 2009;27(9):1333–1339. [DOI] [PubMed] [Google Scholar]
- 108.Li R-C, Huang T, Li Y, et al. Immunogenicity and reactogenicity of the human rotavirus vaccine, RIX4414 oral suspension, when co-administered with routine childhood vaccines in Chinese infants. Hum Vaccin Immunother. 2016;12(3):785–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ramani S, Mamani N, Villena R, et al. Rotavirus serum IgA immune response in children receiving rotarix coadministered with bOPV or IPV. Pediatr Infect Dis J. 2016;35(10):1137–1139. [DOI] [PubMed] [Google Scholar]
- 110.Ruiz-Palacios GM, Pérez-Schael I, Velázquez FR, et al. Safety and efficacy of an attenuated vaccine against severe rotavirus gastroenteritis. N Engl J Med. 2006;354(1):11–22. [DOI] [PubMed] [Google Scholar]
- 111.Tregnaghi MW, Abate HJ, Valencia A, et al. Human rotavirus vaccine is highly efficacious when coadministered with routine expanded program of immunization vaccines including oral poliovirus vaccine in Latin America. Pediatr Infect Dis J. 2011;30(6):e103–e108. [DOI] [PubMed] [Google Scholar]
- 112.Ciarlet M, Sani-Grosso R, Yuan G, et al. Concomitant use of the oral pentavalent human-bovine reassortant rotavirus vaccine and oral poliovirus vaccine. Pediatr Infect Dis J. 2008;27(10):874–880. [DOI] [PubMed] [Google Scholar]
- 113.Harris VC, Armah G, Fuentes S, et al. Significant correlation between the infant gut microbiome and rotavirus vaccine response in Rural Ghana. J Infect Dis. 2017;215(1):34–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Taniuchi M, Platts-Mills JA, Begum S, et al. Impact of enterovirus and other enteric pathogens on oral polio and rotavirus vaccine performance in Bangladeshi infants. Vaccine. 2016;34(27):3068–3075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Clark CE, Fay MP, Chico ME, et al. Maternal helminth infection is associated with higher infant immunoglobulin A titers to antigen in orally administered vaccines. J Infect Dis. 2016;213(12):1996–2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Harris V, Ali A, Fuentes S, et al. Rotavirus vaccine response correlates with the infant gut microbiota composition in Pakistan. Gut Microbes. 2017;0(0):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Yatsunenko T, Rey FE, Manary MJ, et al. Human gut microbiome viewed across age and geography. Nature. 2012;486(7402):222–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Palmer C, Bik EM, DiGiulio DB, et al. Development of the human infant intestinal microbiota. PLoS Biol. 2007;5(7):e177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Valdez Y, Brown EM, Finlay BB. Influence of the microbiota on vaccine effectiveness. Trends Immunol. 2014;35(11):526–537. [DOI] [PubMed] [Google Scholar]
- 120.Pfeiffer JK, Virgin HW. Viral immunity. Transkingdom control of viral infection and immunity in the mammalian intestine. Science (New York, N.Y.). 2016;351(6270):aad5872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hand TW, Dos Santos LM, Bouladoux N, et al. Acute gastrointestinal infection induces long-lived microbiota-specific T cell responses. Science (New York, N.Y.). 2012;337(6101):1553–1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Dubourg G, Lagier J-C, Hüe S, et al. Gut microbiota associated with HIV infection is significantly enriched in bacteria tolerant to oxygen. BMJ Open Gastroenterol. 2016;3(1):e000080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lin A, Bik EM, Costello EK, et al. Distinct distal gut microbiome diversity and composition in healthy children from Bangladesh and the United States. PLoS One. 2013;8(1):e53838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Adlerberth I, Carlsson B, De Man P, et al. Intestinal colonization with Enterobacteriaceae in Pakistani and Swedish hospital-delivered infants. Acta Paediatr Scand. 1991;80(6–7):602–610. [DOI] [PubMed] [Google Scholar]
- 125.Nowrouzian F, Hesselmar B, Saalman R, et al. Escherichia coli in infants’ intestinal microflora: colonization rate, strain turnover, and virulence gene carriage. Pediatr Res. 2003;54(1):8–14. [DOI] [PubMed] [Google Scholar]
- 126.Praharaj I, John SM, Bandyopadhyay R, et al. Probiotics, antibiotics and the immune responses to vaccines. Philos Trans R Soc Lond B Biol Sci. 2015;370(1671):20140144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Isolauri E, Joensuu J, Suomalainen H, et al. Improved immunogenicity of oral D x RRV reassortant rotavirus vaccine by Lactobacillus casei GG. Vaccine. 1995;13(3):310–312. [DOI] [PubMed] [Google Scholar]
- 128.Chattha KS, Vlasova AN, Kandasamy S, et al. Divergent immunomodulating effects of probiotics on T cell responses to oral attenuated human rotavirus vaccine and virulent human rotavirus infection in a neonatal gnotobiotic piglet disease model. J Immunol. 2013;191(5):2446–2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kandasamy S, Chattha KS, Vlasova AN, et al. Lactobacilli and Bifidobacteria enhance mucosal B cell responses and differentially modulate systemic antibody responses to an oral human rotavirus vaccine in a neonatal gnotobiotic pig disease model. Gut Microbes. 2014;5(5):639–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zhang W, Azevedo MSP, Wen K, et al. Probiotic Lactobacillus acidophilus enhances the immunogenicity of an oral rotavirus vaccine in gnotobiotic pigs. Vaccine. 2008;26(29–30):3655–3661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Hammarström L Passive immunity against rotavirus in infants. Acta Paediatrica Int J Paediatrics Suppl. 1999;88(430):127–132. [DOI] [PubMed] [Google Scholar]
- 132.Bogstedt AK, Johansen K, Hatta H, et al. Passive immunity against diarrhoea. Acta Paediatr. 1996;85(2):125–128. [DOI] [PubMed] [Google Scholar]
- 133.Mitra A, Mahalanabis D, Ashraf H, et al. Hyperimmune cow colostrum reduces diarrhoea due to rotavirus: a double-blind, controlled clinical trial. Acta Paediatr. 1995;84(9):996–1001. [DOI] [PubMed] [Google Scholar]
- 134.Caulfield LE, De Onis M, Blössner M, et al. Undernutrition as an underlying cause of child deaths associated with diarrhea, pneumonia, malaria, and measles. Am J Clin Nutr. 2004;80(1):193–198. [DOI] [PubMed] [Google Scholar]
- 135.Pelletier DL, Frongillo EA Jr., Schroeder DG, et al. The effects of malnutrition on child mortality in developing countries. Bull World Health Organ. 1995;73(4):443–448. [PMC free article] [PubMed] [Google Scholar]
- 136.Perez-Schael I, Salinas B, Tomat M, et al. Efficacy of the human rotavirus vaccine RIX4414 in malnourished children. J Infect Dis. 2007;196(4):537–540. [DOI] [PubMed] [Google Scholar]
- 137. Savy M, Edmond K, Fine PE, et al. Landscape analysis of interactions between nutrition and vaccine responses in children. J Nutr. 2009;139(11):2154s–2218s.19793845 • Comprehensively reviews studies examining the association between various measures of malnutrition and vaccine response.
- 138.Mora JR, Iwata M, Von Andrian UH. Vitamin effects on the immune system: vitamins A and D take centre stage. Nat Rev Immunol. 2008;8(9):685–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ibs KH, Rink L. Zinc-altered immune function. J Nutr. 2003;133(5 Suppl 1):1452s–1456s. [DOI] [PubMed] [Google Scholar]
- 140.Kaufman DR, De Calisto J, Simmons NL, et al. Vitamin A deficiency impairs vaccine-elicited gastrointestinal immunity. J Immunol. 2011;187(4):1877–1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Ivanov AP, Dragunsky EM, Chumakov KM. 1,25-dihydroxyvitamin d3 enhances systemic and mucosal immune responses to inactivated poliovirus vaccine in mice. J Infect Dis. 2006;193(4):598–600. [DOI] [PubMed] [Google Scholar]
- 142.Enioutina EY, Visic D, McGee ZA, et al. The induction of systemic and mucosal immune responses following the subcutaneous immunization of mature adult mice: characterization of the antibodies in mucosal secretions of animals immunized with antigen formulations containing a vitamin D3 adjuvant. Vaccine. 1999;17(23–24):3050–3064. [DOI] [PubMed] [Google Scholar]
- 143.Daynes RA, Enioutina EY, Butler S, et al. Induction of common mucosal immunity by hormonally immunomodulated peripheral immunization. Infect Immun. 1996;64(4):1100–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Daynes RA, Araneo BA. The development of effective vaccine adjuvants employing natural regulators of T-cell lymphokine production in vivo. Ann N Y Acad Sci. 1994;730:144–161. [DOI] [PubMed] [Google Scholar]
- 145.David LA, Maurice CF, Carmody RN, et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014;505(7484):559–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Fraker PJ, King LE. Reprogramming of the immune system during zinc deficiency. Annu Rev Nutr. 2004;24(1):277–298. [DOI] [PubMed] [Google Scholar]
- 147.Habib MA, Soofi S, Sheraz A, et al. Zinc supplementation fails to increase the immunogenicity of oral poliovirus vaccine: a randomized controlled trial. Vaccine. 2015;33(6):819–825. [DOI] [PubMed] [Google Scholar]
- 148.Ahmed T, Svennerholm A-M, Tarique AA, et al. Enhanced immunogenicity of an oral inactivated cholera vaccine in infants in Bangladesh obtained by zinc supplementation and by temporary withholding breast-feeding. Vaccine. 2009;27(9):1433–1439. [DOI] [PubMed] [Google Scholar]
- 149.Albert MJ, Qadri F, Wahed MA, et al. Supplementation with Zinc, but not Vitamin A, improves seroconversion to vibriocidal antibody in children given an oral cholera vaccine. J Infect Dis. 2003;187(6):909–913. [DOI] [PubMed] [Google Scholar]
- 150.Karlsen TH, Sommerfelt H, Klomstad S, et al. Intestinal and systemic immune responses to an oral cholera toxoid B subunit whole-cell vaccine administered during zinc supplementation. Infect Immun. 2003;71(7):3909–3913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Campbell DI, Murch SH, Elia M, et al. Chronic T cell-mediated enteropathy in rural west African children: relationship with nutritional status and small bowel function. Pediatr Res. 2003;54(3):306–311. [DOI] [PubMed] [Google Scholar]
- 152.Naylor C, Lu M, Haque R, et al. Environmental enteropathy, oral vaccine failure and growth faltering in infants in Bangladesh. EBioMedicine. 2015;2(11):1759–1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Becker-Dreps S, Vilchez S, Bucardo F, et al. The association between fecal biomarkers of environmental enteropathy and rotavirus vaccine response in Nicaraguan infants. Pediatr Infect Dis J. 2017;36(4):412–416. [DOI] [PubMed] [Google Scholar]
- 154.Groome MJ, Madhi SA. Five-year cohort study on the burden of hospitalisation for acute diarrhoeal disease in African HIV-infected and HIV-uninfected children: potential benefits of rotavirus vaccine. Vaccine. 2012;30 Suppl 1:A173–A178. [DOI] [PubMed] [Google Scholar]
- 155.Pavia AT, Long EG, Ryder RW, et al. Diarrhea among African children born to human immunodeficiency virus 1-infected mothers: clinical, microbiologic and epidemiologic features. Pediatr Infect Dis J. 1992;11(12):996–1003. [DOI] [PubMed] [Google Scholar]
- 156.Chhagan MK, Kauchali S. Comorbidities and mortality among children hospitalized with diarrheal disease in an area of high prevalence of human immunodeficiency virus infection. Pediatr Infect Dis J. 2006;25(4):333–338. [DOI] [PubMed] [Google Scholar]
- 157.Cunliffe NA, Gondwe JS, Kirkwood CD, et al. Effect of concomitant HIV infection on presentation and outcome of rotavirus gastroenteritis in Malawian children. Lancet (London, England). 2001;358(9281):550–555. [DOI] [PubMed] [Google Scholar]
- 158.Steele AD, Cunliffe N, Tumbo J, et al. A review of rotavirus infection in and vaccination of human immunodeficiency virus-infected children. J Infect Dis. 2009;200 Suppl 1:S57–S62. [DOI] [PubMed] [Google Scholar]
- 159.Groome MJ, Page N, Cortese MM, et al. Effectiveness of monovalent human rotavirus vaccine against admission to hospital for acute rotavirus diarrhoea in South African children: a case-control study. Lancet Infect Dis. 2014;14(11):1096–1104. [DOI] [PubMed] [Google Scholar]
- 160.Levin MJ, Lindsey JC, Kaplan SS, et al. Safety and immunogenicity of a live attenuated pentavalent rotavirus vaccine in HIV-exposed infants with or without HIV infection in Africa. AIDS (London, England). 2017;31(1):49–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Uprety P, Lindsey JC, Levin MJ, et al. Inflammation and immune activation in antiretroviral-treated human immunodeficiency virus type 1-infected African infants and rotavirus vaccine responses. J Infect Dis. 2017;215(6):928–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Steele AD, Madhi SA, Louw CE, et al. Safety, reactogenicity, and immunogenicity of human rotavirus vaccine RIX4414 in human immunodeficiency virus-positive infants in South Africa. Pediatr Infect Dis J. 2011;30(2):125–130. [DOI] [PubMed] [Google Scholar]
- 163.Laserson KF, Nyakundi D, Feikin DR, et al. Safety of the pentavalent rotavirus vaccine (PRV), RotaTeq®, in Kenya, including among HIV-infected and HIV-exposed infants. Vaccine. 2012;30 Suppl 1:A61–A70. [DOI] [PubMed] [Google Scholar]
- 164.Patel NC, Hertel PM, Estes MK, et al. Vaccine-acquired rotavirus in infants with severe combined immunodeficiency. N Engl J Med. 2010;362(4):314–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Merck & Co. Patient product information, RotaTeq [Internet]. Available from: www.merck.com. Accessed on January 2016.
- 166.GSK. Package insert and patient information - Rotarix [Internet]. Available from: www.gsk.com. Accessed on January 2016.
- 167.Ion-Nedelcu N, Dobrescu A, Strebel PM, et al. Vaccine-associated paralytic poliomyelitis and HIV infection. Lancet (London, England). 1994;343(8888):51–52. [DOI] [PubMed] [Google Scholar]
- 168.Huang P, Xia M, Tan M, et al. Spike protein VP8* of human rotavirus recognizes histo-blood group antigens in a type-specific manner. J Virol. 2012;86(9):4833–4843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Liu Y, Huang P, Tan M, et al. Rotavirus VP8*: phylogeny, host range, and interaction with histo-blood group antigens. J Virol. 2012;86(18):9899–9910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Nordgren J, Sharma S, Bucardo F, et al. Both Lewis and secretor status mediate susceptibility to rotavirus infections in a rotavirus genotype-dependent manner. Lancet Infect Dis. 2014;59(11):1567–1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Van Trang N, Vu HT, Le NT, et al. Association between norovirus and rotavirus infection and histo-blood group antigen types in Vietnamese children. J Clin Microbiol. 2014;52(5):1366–1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Imbert-Marcille B-M, Barbé L, Dupé M, et al. A FUT2 gene common polymorphism determines resistance to rotavirus A of the P[8] genotype. J Infect Dis. 2014;209(8):1227–1230. [DOI] [PubMed] [Google Scholar]
- 173.Payne DC, Currier RL, Staat MA, et al. Epidemiologic association between FUT2 secretor status and severe rotavirus gastroenteritis in children in the United States. JAMA Pediatr. 2015;169(11):1040–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Hu L, Crawford SE, Czako R, et al. Cell attachment protein VP8* of a human rotavirus specifically interacts with A-type histo-blood group antigen. Nature. 2012;485(7397):256–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Kazi AM, Cortese MM, Yu Y, et al. Secretor and salivary ABO blood group antigen status predict rotavirus vaccine take in infants. J Infect Dis. 2017;215(5):786–789. [DOI] [PubMed] [Google Scholar]
- 176.Sun X, Guo N, Li D, et al. Binding specificity of P[8] VP8* proteins of rotavirus vaccine strains with histo-blood group antigens. Virology. 2016;495:129–135. [DOI] [PubMed] [Google Scholar]
- 177. Kambhampati A, Payne DC, Costantini V, et al. Host genetic susceptibility to enteric viruses: a systematic review and metaanalysis. Lancet Infect Dis. 2016;62(1):11–18. •• Demonstrates the strong strain-specific differences in norovirus and rotavirus susceptibility that have been observed according to histo blood group antigen phenotype. Factors influencing the performance of live oral rotavirus vaccines in low-income settings: an overview.