Abstract
The objective of this study is to provide an assessment of allostatic load (AL) burden among US adults across race/ethnicity, gender, and age groups over a 30-year time period. We analyzed data from 50,671 participants of the National Health and Nutrition Examination Survey (NHANES) years 1988 through 2018. AL score was defined as the sum total for abnormal measures of the following components: serum albumin, body mass index, serum C – reactive protein, serum creatinine, diastolic blood pressure, glycated hemoglobin, systolic blood pressure, total cholesterol, and serum triglycerides. We performed modified Poisson regression to estimate the adjusted Relative Risks (aRRs) of allostatic load, and generalized linear models to determine adjusted mean differences accounting for NHANES sampling weights. Among US adults aged 18 or older, the prevalence of high AL increased by more than 45% from 1988 – 1991 to 2015-2018, from 33.5% to 48.6%. By the latest period, 2015 – 2018, Non-Hispanic Black women (aRR: 1.292; 95% CI: 1.290 - 1.293) and Latina women (aRR: 1.266; 95% CI: 1.265 – 1.267) had higher risks of AL than non-Hispanic White women. Similar trends were observed among men. Age-adjusted mean AL score among NH-Black and Latino adults was higher than for NH-Whites of up to a decade older regardless of gender. From 1988 through 2018, Adults aged 40 years old and older had over 2-fold increased risks of high AL when compared to adults 18-29 years old. After 30-years of collective data, racial disparities in allostatic load persist for NH-Black and Latino adults.
Keywords: life-course, cumulative stress, psychosocial stress, race, disparities
INTRODUCTION
Decades of research into the disproportionally higher morbidity and mortality burden among racial minorities, e.g. Non-Hispanic (NH) Blacks and Latinos, relative to NH-White Americans have failed to identify a satisfactory model to explain the disparity or produce effective strategies to address the problem1,2. In fact, for many conditions, disparities have widened in recent years3–8. The fundamental causes of health disparities in the United States (US) are multifactorial, multilevel, and multigenerational; socially marginalized groups endure prolonged psychosocial and physiological challenges that increase their risk of disease, lead to early onset of disease, and accelerate cellular aging3,9–18.
The biological incorporation of the social and material environment in which humans live is termed ‘embodiment’ and serves as a model for re-conceptualizing health disparities not only as the result of differential distribution of health-related risk factors, but as the result of historically contingent and racially patterned exposures, leading to altered susceptibility to exogenous factors11,15,16. For instance, social determinants of health such as income, education and access to healthcare, in addition to exposure to racial discrimination, may directly or indirectly influence health related risk factors such as diet, exercise, smoking, obesity, psychosocial stress and comorbidities3,19,20. The biological consequences of such exposures include dysregulated immune, cardiovascular and metabolic systems, which are typically tightly regulated via the hypothalamic-pituitary-adrenal (HPA) axis in a state of allostasis21, leading to increased risk of complex diseases.
Allostatic load (AL) represents a measure of biological wear and tear due to chronic over-activation of biological systems1,22,23. While allostatic load attempts to characterize the accelerated ageing of biological systems, it is more defined as the cost or the price the organ system pays for an overactive or inefficiently managed stressor 24. Seeman and colleagues composed an AL score based on 10 biological parameters with the purpose of comprehensively characterizing physiological burden on the human body21,25,26. Previously, AL has been used to predict the morbidity of cardiovascular disease (CVD), diabetes mellitus, high BMI, cognitive function, and overall mortality23,27,28. Socioeconomic factors such as higher income or educational status may mitigate AL burden via benefits of resources and social capital, thus reducing biological stress29,30. In addition, male/female sex differences in AL burden have been observed through varying mechanisms including social integration, occupation, socioeconomic position, support groups, and higher self-perceived masculinity31–34. Furthermore, prior research on AL among racial/ethnic minority groups has shown elevated levels of AL among NH-Blacks compared to NH-Whites and among individuals perceiving racial or social discrimination35–43. Disparities in the burden of AL, therefore, provide a useful global measure of embodiment such that higher scores indicate greater exposure to adversity.
While recent studies have examined trends in AL 44, there is limited knowledge on comparisons in the prevalence of AL over the past few decades. The goal of the present analysis is to provide the most robust assessment of AL burden among a representative sample of US adults and to understand the differences attributed to race/ethnicity, gender, and age groups across a 30-year time period from 1988 through 2018. We hypothesize that racial/ethnic minorities will have higher burden of AL, within gender subgroups, and these disparities will persist throughout age groups.
METHODS
Study Design and Participants:
We performed analyses using data from a representative sample of non-institutionalized US residents. The National Health and Nutrition Examination Survey (NHANES) is a nationally representative sample of US adults, where persons aged 60 and older, Latinos and NH-Blacks are oversampled, and weighted analysis generates generalizable estimates 45. The NHANES weighted sample is considered to be representative of the U.S. civilian non-institutionalized population 46. We examined trends in allostatic load over time by establishing multiple time periods; 1988 – 1991, 1991 – 1994, 1999 – 2002, 2003 – 2006, 2007 – 2010, 2011-2014, and 2015-2018 47. NHANES includes demographic, socioeconomic, dietary, and health-related questionnaires, and includes clinical measures of blood pressure, fasting blood glucose, triglycerides and HDL cholesterol, in addition to self-reported medication use for health conditions. We performed analysis among NHANES participants with data on biomarkers and within the fasting subsample. This analysis included all NH White, NH-Black, Latinos participants, as well as those who identified as mixed raced or other race, ages 18 and older: a total of 50,671 participants over the 30-year study period for the main analysis (Figure 1). The Institutional Review Boards considered this study exempt from review because of the use of publicly available, de-identified data.
Figure 1:
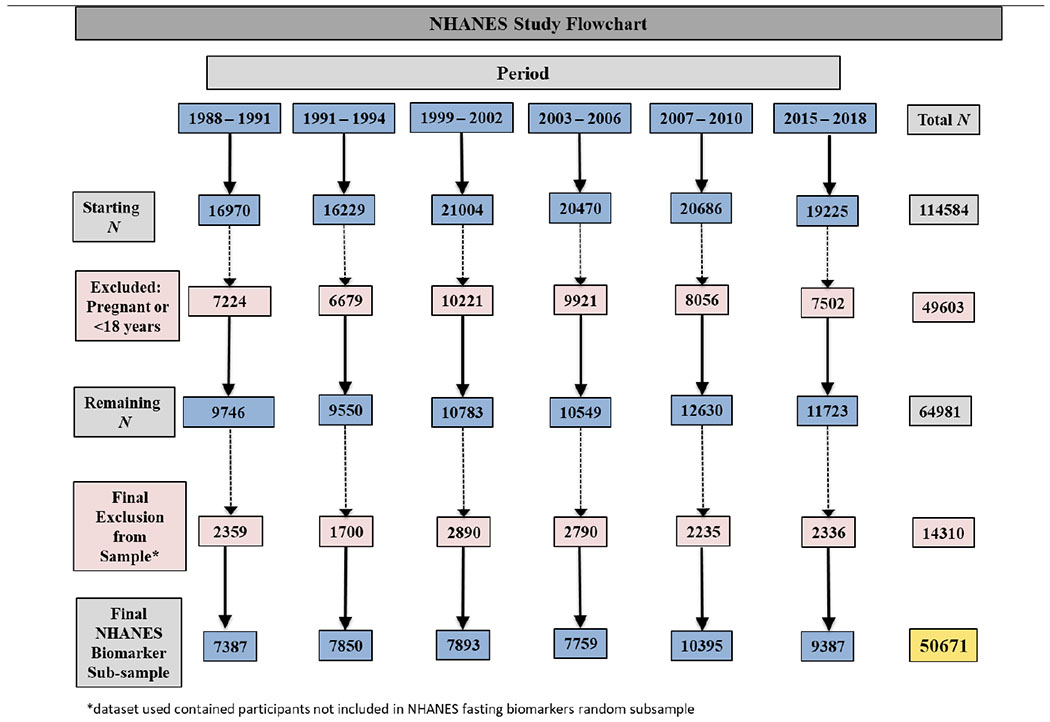
Flowchart of exclusion criteria and final study population of NHANES participants by study periods.
Allostatic Load Definition:
AL has been defined using varying configurations, although most incorporate biomarker measures from three different categories of physiologic functioning: cardiovascular, metabolic, and immune systems48. While there is no consensus definition, we elected to define AL using the Geronimus et al (2006) and Mays et al (2018) taxonomies 38,49. To determine the high-risk thresholds for each AL component, we examined the distribution of each component among the entire study sample with complete biomarker data. High-risk thresholds were determined by either being above the 75th percentile for body mass index (BMI), C – reactive protein (CRP), diastolic blood pressure (DBP), glycated hemoglobin, systolic blood pressure (SBP), total cholesterol, and serum triglycerides; or below the 25th percentile for serum albumin and serum creatinine. Therefore, each NHANES participant was scored as either 1 (high-risk) or 0 (low-risk) based on gender-specific cutoffs for each component (Supplemental Table 1). We calculated total AL score by summing the individual components, and this score ranged from 0 to 9. We further categorized participants with AL score greater or equal to 3 as having high allostatic load 48,49.
Sociodemographic Characteristics:
To assess socio-demographic differences in the prevalence and trends of AL, we evaluated differences by age, race/ethnicity, education, and poverty to income ratio (PIR) (adjusted for inflation),. The NHANES education variable was categorized into: 1) less than high school education; 2) high school graduate/GED/ or equivalent; 3) some college; 4) college graduate or above; and 5) unknown/refused to answer. Poverty income ratio (PIR) was calculated as the ratio of total family income to poverty threshold values (in dollars). Persons who reported having had no income were assigned a zero value for PIR. PIR values less than 1 are considered below the official poverty line, whereas PIR values greater than 1 are above the poverty level 50.
Health Behaviors and Comorbidities:
We evaluated health behaviors that may influence AL score in analysis, including self-reported smoking status. Participants that had not smoked 100 cigarettes in lifetime were categorized as never smokers, while participants with at least 100 cigarettes smoked in lifetime but no current smoke use were categorized as past smokers. Participants with at least 100 lifetime cigarettes used and current smoking use were categorized as current smokers51. We included any self-reported response to a physician-diagnosed history of cancer, as well as self-reported congestive heart failure and heart attack as comorbidities.
Statistical Analysis:
Analyses were performed using NHANES generated sampling statistical strata, clusters, and weights as designated and described in detail in the NHANES methodology handbook 45. NHANES only measures biomarkers among a random sample of participants each survey period, and in turn created subsample weights to account for the probability of being selected into the subsample component, and additional non-response bias. As a result, our analysis focuses on all participants with the fasting subsample weight as we followed the National Center for Health Statistics guidelines for NHANES data, and applied the “least common denominator” approach when deciding the appropriate statistical weights 45. With this approach, we checked the variables of interest in our study and selected the variable that was collected on the smallest number of persons (“least common denominator”) that were our biomarkers: C-reactive protein, albumin, creatinine, glycated hemoglobin, and triglycerides. When a sample is weighted in NHANES it is considered to be representative of the U.S. civilian non-institutionalized population. Only poverty-to-income ratio had missing values, and we categorized those missing as so when performing regression analyses.
Categorical variables were presented as weighted row percentages and continuous variables as mean and associated 95% confidence intervals. The primary outcome of interest was the prevalence of high AL and mean AL score, overall and by interaction terms containing the gender and race/ethnicity variables for the subgroup analyses. For all time periods, the prevalence of high AL and mean AL score stratified by gender-race/ethnicity were estimated. We performed modified Poisson regression models for estimating risk of high AL, stratified by gender-race/ethnicity interaction terms and adjusting for potential confounders including education, age groups, PIR, smoking status, any history of cancer, congestive heart failure, or heart attack52. Weighted generalized linear models associating the mean AL score by gender-race/ethnicity additionally adjusted for possible aforementioned confounders were also conducted. Estimates derived from modified Poisson regression are presented as relative risks (RRs) and associated 95% confidence intervals (CIs), and estimates derived from generalized linear models are presented as mean estimates and differences (from referent group) and associated 95% CIs.
In sensitivity analyses, we conducted regression analysis categorizing participants who reported current use of medications for hypertension, hypercholesterolemia, or pre-diabetes as high-risk for the corresponding AL biomarker 38. Again, NHANES participants were scored one point (high-risk) for the respective condition if they indicated using medications for hypertension (we gave 0.5 points for both systolic and diastolic blood pressure if participant was on antihypertensive, summing to one total point for this medication), hypercholesterolemia, or pre-diabetes38. Prior research has assumed that degradation has already occurred among individuals taking medications, and given our analysis covers multiple time periods among multiple age groups, similar analytic adjustments were made. All statistical analyses were performed using SAS (version 9.4, SAS Institute, Inc.).
RESULTS
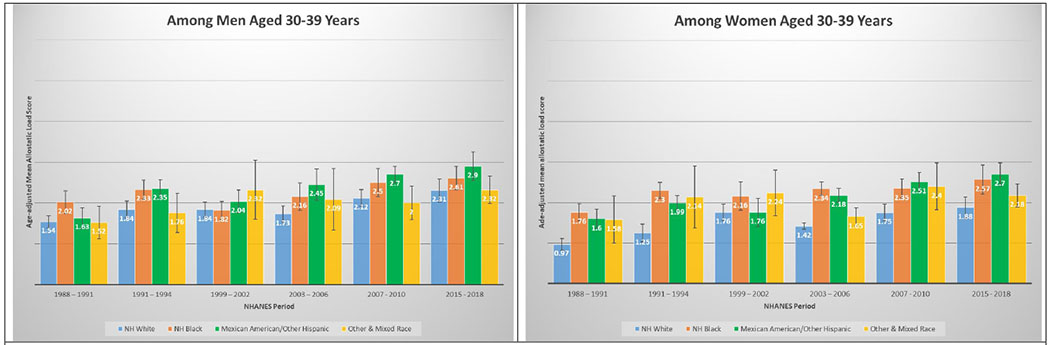
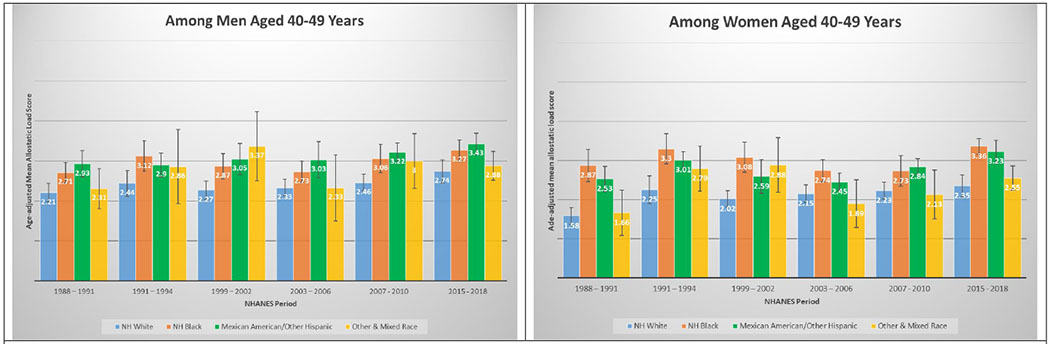
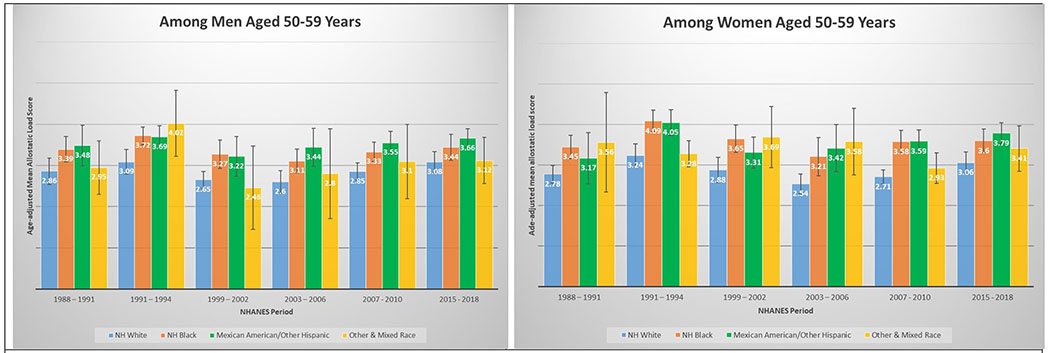
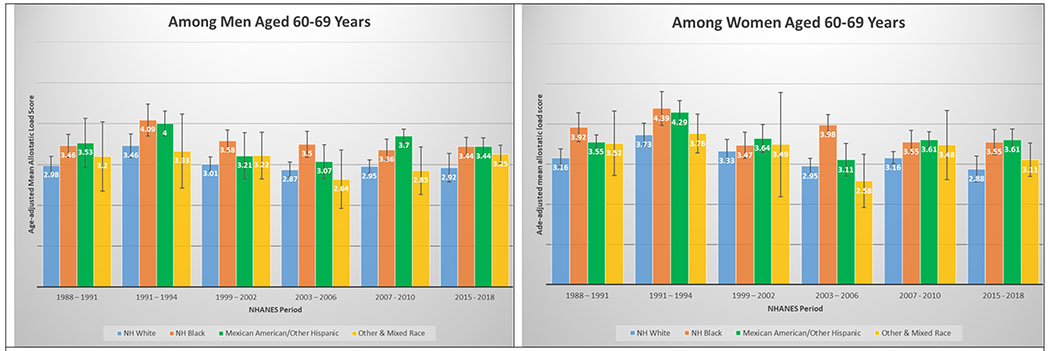
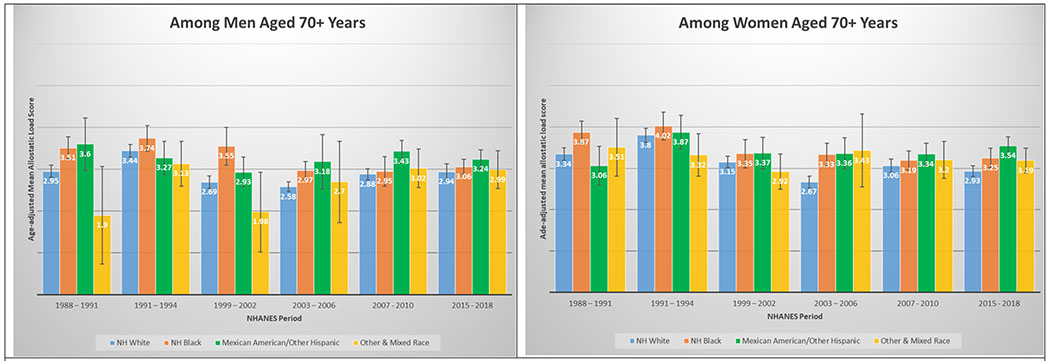
A total of 50,671 NHANES participants between 1988 and 2018 were included in this analysis (Table 1). Table 1 displays the demographic and personal level characteristics by NHANES periods. Over the 30-year observation period, there was an overall 35% increase in mean AL score from 1991 to 2018. The mean AL score was lowest in 1988-1991; remained steady throughout 1991-1994 (mean AL: 2.38), 1999-2002 (mean AL: 2.29), 2003-2006 (mean AL: 2.17), and 2007-2010 (mean AL: 2.44); and peaked in the latest period 2015-2018 (mean AL: 2.62). The distribution of age-adjusted mean AL score by race/ethnicity and time period among males and females is presented in Figure 2. Age-adjusted mean AL score increased among both NH-White males (from 2.18 to 2.55) and females (from 1.97 to 2.44) from 1988-1991 to 2015-2018, respectively. We observed the same elevated trend of age-adjusted mean AL scores among NH-Black males (from 2.75 to 2.86) and females (from 2.69 to 3.04) in the same time periods. Age-adjusted mean AL score was also higher among Latina females compared to males, and increased from 2.48 to 3.10. The age-adjusted mean AL score by race/ethnicity and time period stratified by age groups are presented in Figure 3. While mean AL score increased over time in all racial/ethnic groups, there were clear racial/ethnic differences observed as early as age 18-29 years for both men and women that persisted through ages 70+ years. The distributions of each individual AL component and mean AL score across time periods are presented in Supplemental Tables 1–2. Supplemental Table 2 shows allostatic load components such as obesity (BMI), chronic inflammation (C-reactive protein), creatinine, and elevated glycated hemoglobin all increased over time.
Table 1:
Socio-demographic characteristics, personal health, and medical conditions by National Health Examination Survey (NHANES) study period. Among 50,671 participants an estimated 1,056,925,341 US residents.
| NHANES Period | ||||||
|---|---|---|---|---|---|---|
| 1988 – 1991 | 1991 – 1994 | 1999 – 2002 | 2003 – 2006 | 2007 – 2010 | 2015 – 2018 | |
| Participants (N) | 7,387 | 7,850 | 7,893 | 7,759 | 10,395 | 9,387 |
| Estimated Na (%)b | 155,959,992 (14.76) | 165,955,459 (15.70) | 165,937,416 (15.70) | 168,342,341 (15.93) | 192,397,679 (18.20) | 208,332,545 (19.71) |
| Presented as % (SE) or Mean (95% CI) c | ||||||
| Allostatic Load Total Score d | 1.94 (1.84 – 2.05) | 2.38 (2.28 – 2.48) | 2.29 (2.20 – 2.38) | 2.17 (2.10 – 2.23) | 2.44 (2.37 – 2.51) | 2.62 (2.53 – 2.71) |
| % High Allostatic Load e | 33.47 (1.37) | 43.91 (1.32) | 40.17 (1.07) | 39.15 (0.88) | 44.69 (0.99) | 48.55 (1.22) |
| Male Sex | 48.89 (0.63) | 48.95 (0.86) | 49.71 (0.54) | 49.97 (0.54) | 49.67 (0.45) | 49.19 (0.66) |
| Mean Age in years | 43.77 (42.81 – 44.73) | 44.25 (43.03 – 45.46) | 45.03 (44.35 – 45.70) | 45.51 (44.46 – 46.56) | 46.13 (45.50 – 46.76) | 47.44 (46.52 - 48.36) |
| Age Group | ||||||
| 18 – 29 | 24.70 (1.24) | 23.30 (0.90) | 20.79 (0.86) | 20.33 (0.90) | 21.16 (0.68) | 20.26 (0.86) |
| 30 – 39 | 23.12 (1.07) | 22.95 (0.83) | 20.53 (0.85) | 19.12 (0.78) | 17.29 (0.51) | 16.93 (0.63) |
| 40 – 49 | 17.98 (0.79) | 19.51 (1.08) | 21.71 (0.78) | 21.92 (0.83) | 19.94 (0.60) | 16.42 (0.58) |
| 50 – 59 | 12.43 (0.41) | 12.44 (0.64) | 16.00 (0.59) | 17.16 (0.67) | 18.19 (0.52) | 18.66 (0.78) |
| 60 – 69 | 11.65 (0.66) | 10.98 (0.80) | 10.47 (0.56) | 10.79 (0.56) | 12.10 (0.50) | 15.14 (0.77) |
| 70+ | 10.12 (0.74) | 10.82 (1.00) | 10.50 (0.45) | 10.69 (0.80) | 11.31 (0.40) | 12.59 (0.72) |
| Race/Ethnicity | ||||||
| Non-Hispanic White | 78.04 (2.44) | 75.02 (2.27) | 72.56 (1.82) | 73.15 (2.18) | 69.89 (2.41) | 63.95 (2.40) |
| Non-Hispanic Black | 10.25 (1.21) | 10.74 (1.11) | 9.60 (1.16) | 10.50 (1.27) | 10.24 (0.98) | 10.46 (1.32) |
| Latino | 4.87 (0.57) | 5.26 (0.72) | 13.58 (1.86) | 11.21 (1.33) | 13.72 (1.79) | 15.80 (1.73) |
| Other & Mixed Race | 6.84 (1.32) | 8.98 (1.39) | 4.25 (0.65) | 5.15 (0.52) | 6.15 (0.63) | 9.79 (0.88) |
| Education | ||||||
| < High school | 25.97 (1.46) | 24.21 (1.44) | 21.31 (0.87) | 17.82 (1.02) | 19.92 (0.90) | 12.11 (0.95) |
| High school/GED | 34.34 (1.21) | 33.73 (1.33) | 27.15 (1.08) | 27.22 (0.77) | 25.07 (0.89) | 23.32 (0.93) |
| Some college or Associates degreef | 19.99 (0.75) | 21.15 (1.24) | 27.57 (0.89) | 30.77 (0.85) | 29.03 (0.65) | 31.11 (1.08) |
| College graduate | 19.22 (1.64) | 20.63 (1.26) | 23.82 (1.55) | 24.15 (1.40) | 25.88 (1.25) | 30.63 (2.04) |
| Poverty to Income Ratio (PIR) Group | ||||||
| 1st quartile (0 – 1.11) | 13.25 (0.74) | 13.88 (1.64) | 15.03 (1.10) | 13.47 (0.83) | 15.47 (0.79) | 14.68 (0.77) |
| 2nd quartile (1.11 – 2.08) | 18.92 (1.17) | 19.89 (1.02) | 17.87 (1.18) | 18.41 (0.81) | 17.98 (0.74) | 17.67 (0.77) |
| 3rd quartile (2.08 – 3.77) | 34.79 (1.25) | 28.50 (1.08) | 22.76 (0.77) | 25.75 (0.90) | 22.19 (0.98) | 22.45 (0.95) |
| 4th quartile (3.77 – 11.89) | 25.92 (1.98) | 32.01 (2.24) | 36.08 (1.77) | 37.84 (1.48) | 37.27 (1.22) | 36.20 (1.68) |
| Missing | 7.11 (0.60) | 5.72 (0.52) | 8.26 (0.84) | 4.53 (0.43) | 7.09 (0.60) | 9.01 (0.55) |
| Mean BMI, kg m−2 | 26.11 (25.89 – 26.33) | 26.73 (26.46 – 26.99) | 27.78 (27.50 – 28.06) | 28.18 (27.87 – 28.50) | 28.46 (28.26 – 28.66) | 29.45 (29.08 – 29.82) |
| Current Smoker Status | 30.64 (1.24) | 26.45 (1.20) | 23.82 (0.93) | 24.52 (0.85) | 20.81 (0.81) | 17.81 (0.79) |
| Any Cancer History f | 7.63 (0.48) | 7.44 (0.55) | 7.55 (0.41) | 7.95 (0.40) | 9.04 (0.41) | 10.61 (0.47) |
| Ever Congestive Heart Failure | 2.29 (0.18) | 1.93 (0.24) | 1.97 (0.23) | 2.12 (0.19) | 2.01 (0.18) | 2.17 (0.20) |
| Ever Heart Attack | 3.33 (0.31) | 3.37 (0.35) | 3.20 (0.25) | 3.41 (0.32) | 3.20 (0.23) | 3.37 (0.32) |
Estimated using sampling weights from National Health and Nutrition Examination Survey (NHANES).
Presented as weighted row percentage, describes the total percentage of participants among all study periods.
Presented as column proportion (standard error) or mean (95% confidence intervals) for continuous variables.
Allostatic load total score was calculated as sum total of components based on high-risk thresholds: albumin, BMI, C-reactive protein, creatinine clearance, diastolic blood pressure, glycated hemoglobin, systolic blood pressure, total cholesterol, triglycerides. Score range from 0 to 9.
High Allostatic load is defined as total Allostatic load score greater than or equal to 3 (presented as column percentages and standard errors).
Defined as self-reported response to ever being diagnosed by a doctor or health professional of any cancer or malignancy.
Figures 2.
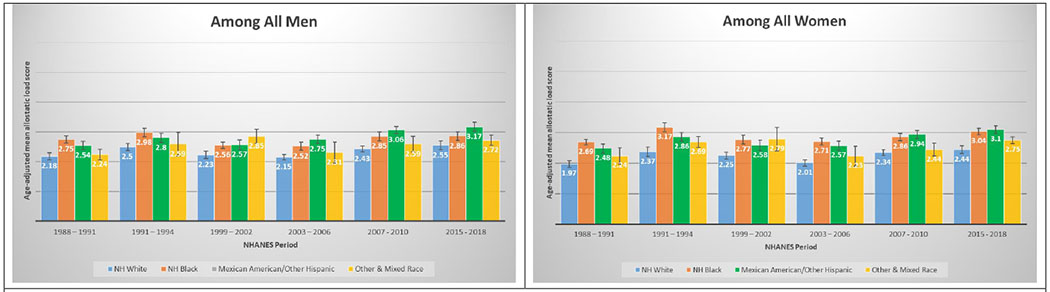
2A and 2B. Age-adjusted mean allostatic load scores among US adults, National Health and Nutrition Examination Survey (NHANES), 1988–2018. Panel A represents age-adjusted mean allostatic load scores among men, and panel B represents age-adjusted mean allostatic load scores among women. Based on the sum total of components with high-risk thresholds: albumin, BMI, C-reactive protein, creatinine clearance, diastolic blood pressure, glycated hemoglobin, systolic blood pressure, total cholesterol, triglycerides. Score range from 0 to 9 for all NHANES years.
Figures 3.
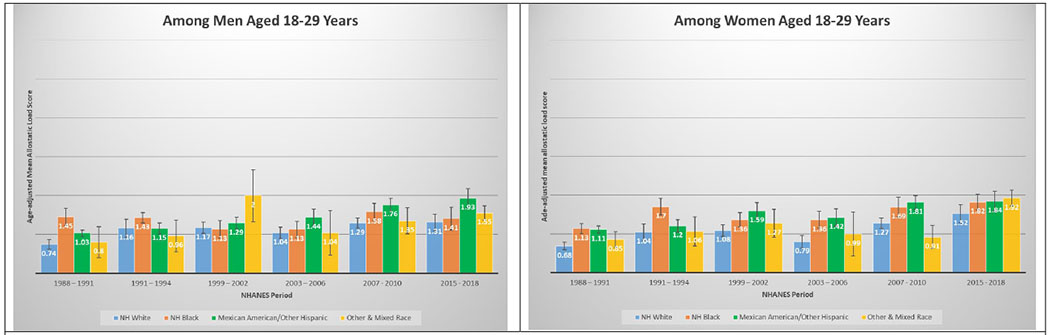
3A and 3B. Age-adjusted mean allostatic load scores among US adults aged 18 – 29, National Health and Nutrition Examination Survey (NHANES), 1988–2018. Panel A represents age-adjusted mean allostatic load scores among men, and panel B represents age-adjusted mean allostatic load scores among women. Based on the sum total of components with high-risk thresholds: albumin, BMI, C-reactive protein, creatinine clearance, diastolic blood pressure, glycated hemoglobin, systolic blood pressure, total cholesterol, triglycerides. Score range from 0 to 9 for all NHANES years.
3C and 3D. Age-adjusted mean allostatic load scores among US adults aged 30 – 39, National Health and Nutrition Examination Survey (NHANES), 1988–2018. Panel A represents age-adjusted mean allostatic load scores among men, and panel B represents age-adjusted mean allostatic load scores among women.
3E and 3F. Age-adjusted mean allostatic load scores among US adults aged 40 – 49, National Health and Nutrition Examination Survey (NHANES), 1988–2010. Panel A represents age-adjusted mean allostatic load scores among men, and panel B represents age-adjusted mean allostatic load scores among women.
3G and 3H. Age-adjusted mean allostatic load scores among US adults aged 50 – 59, National Health and Nutrition Examination Survey (NHANES), 1988–2010. Panel A represents age-adjusted mean allostatic load scores among men, and panel B represents age-adjusted mean allostatic load scores among women.
3I and 3J. Age-adjusted mean allostatic load scores among US adults aged 60 – 69, National Health and Nutrition Examination Survey (NHANES), 1988–2010. Panel A represents age-adjusted mean allostatic load scores among men, and panel B represents age-adjusted mean allostatic load scores among women.
3K and 3L. Age-adjusted mean allostatic load scores among US adults aged 70+, National Health and Nutrition Examination Survey (NHANES), 1988–2010. Panel A represents age-adjusted mean allostatic load scores among men, and panel B represents age-adjusted mean allostatic load scores among women.
In multivariable adjusted models, NH-Black males were at 33% increased risk of high allostatic load (AL score greater or equal to 3) compared to NH-White males in 1988 – 1991 (aRR: 1.332, 95% CI: 1.331 – 1.334; Table 2), and nearly 11% increased risk (aRR: 1.106; 95% CI: 1.105 – 1.107) in 2015 – 2018. Latino males also had significantly higher risks of high AL compared to NH-White males in 1988 – 1991 (aRR: 1.228; 95% CI: 1.225 – 1.230), 2003 – 2006 (aRR: 1.297, 95% CI: 1.295 – 1.298) and 2015-2018 (aRR: 1.259; 95% CI: 1.258 - 1.260). Other & Mixed race males were at significantly increased risks of high AL in 1999-2002 (aRR: 1.527; 95% CI: 1.524 – 1.529), 2007–2010 (aRR: 1.146; 95% CI: 1.145 – 1.147), and 2015-2018 (aRR: 1.120; 95% CI: 1.119 – 1.121). Among females, NH-Blacks had higher risks for high AL compared to NH-Whites regardless of time period, with lowest risk observed at 1999 – 2002 (aRR: 1.198; 95% CI: 1.197 – 1.200) and peaking at nearly 46% higher (aRR: 1.456; 95% CI: 1.455 – 1.458) in 2003 – 2006.. Latina females were also at increased risk of high AL compared to NH-White females regardless of time period, ranging from nearly 32% higher (aRR: 1.316; 95% CI: 1.313 – 1.318) in 1988 – 1991 to only 20% higher (aRR: 1.206; 95% CI: 1.205 – 1.207) in 2003 – 2006, to over 26% higher in 2015-2018 (aRR: 1.266; 95% CI: 1.265 – 1.267). In earliest period, 1988 - 1991, we observed that older age (50 and older compared to those aged 18 – 29), was associated with up to 7-fold higher risk of allostatic load (aRRs: 7.109 for 50 – 59 year olds; 8.052 for 60 – 69 year olds; and 8.196 for 70+ year olds). However, this increased risk slightly attenuated over time and by the 2015-2018 period, and older age (50 and older compared to those aged 18 – 29) was only associated with a 2.5-fold increased risk of high allostatic load (aRRs: 2.546 for 50 – 59 year olds; 2.545 for 60 – 69 year olds; and 2.539 for 70+ year olds). Similar trends were observed for absolute measures of mean AL scores across time periods (Table 3). Sensitivity analysis results when accounting for medications for hypertension, hypercholesterolemia, and pre-diabetes are presented in Supplemental tables (Supplemental Table 3 & 4); results mirrored the main analysis for the effects seen in race-gender groups, and age, although there were larger observed differences.
Table 2:
Multivariable modified Poisson regression presented as Relative Risks (RRs) for higha allostatic load in US adults stratified by race/ethnicity and sex, by National Health and Examination Survey (NHANES) study period. Among 50,671 participants an estimated 1,056,925,341 US residents.
| NHANES Period | ||||||
|---|---|---|---|---|---|---|
| 1988 – 1991 | 1991 – 1994 | 1999 – 2002 | 2003 – 2006 | 2007 – 2010 | 2015 – 2018 | |
| Participants (N) | 7,387 | 7,850 | 7,893 | 7,759 | 10,395 | 9,387 |
| Estimated Nb (%)c | 155,959,992 (14.76) | 165,955,459 (15.70) | 165,937,416 (15.70) | 168,342,341 (15.93) | 192,397,679 (18.20) | 208,332,545 (19.71) |
| Presented as Adjusted Relative Risks (95% CI) d | ||||||
| Race/Ethnicity and Male Sex | ||||||
| Non-Hispanic White | 1.000 (Referent) | 1.000 (Referent) | 1.000 (Referent) | 1.000 (Referent) | 1.000 (Referent) | 1.000 (Referent) |
| Non-Hispanic Black | 1.332 (1.331 – 1.334) | 1.154 (1.153 – 1.155) | 1.131 (1.130 – 1.133) | 1.169 (1.168 – 1.171) | 1.189 (1.188 – 1.190) | 1.106 (1.105 – 1.107) |
| Latino | 1.228 (1.225 – 1.230) | 1.072 (1.070 – 1.073) | 1.038 (1.037 – 1.039) | 1.297 (1.295 – 1.298) | 1.220 (1.219 – 1.221) | 1.259 (1.258 – 1.260) |
| Other & Mixed-Race | 0.932 (0.931 – 0.934) | 0.909 (0.908 – 0.910) | 1.527 (1.524 – 1.529) | 1.105 (1.103 – 1.107) | 1.146 (1.145 – 1.147) | 1.120 (1.119 – 1.121) |
| Race/Ethnicity and Female Sex | ||||||
| Non-Hispanic White | 1.000 (Referent) | 1.000 (Referent) | 1.000 (Referent) | 1.000 (Referent) | 1.000 (Referent) | 1.000 (Referent) |
| Non-Hispanic Black | 1.399 (1.397 – 1.401) | 1.380 (1.379 – 1.381) | 1.198 (1.197 – 1.200) | 1.456 (1.455 – 1.458) | 1.223 (1.221 – 1.224) | 1.292 (1.290 – 1.293) |
| Latina | 1.316 (1.313 – 1.318) | 1.255 (1.253 – 1.257) | 1.048 (1.047 – 1.049) | 1.206 (1.205 – 1.207) | 1.191 (1.189 – 1.192) | 1.266 (1.265 – 1.267) |
| Other & Mixed-Race | 1.154 (1.152 – 1.155) | 1.183 (1.182 – 1.184) | 1.180 (1.178 – 1.182) | 1.144 (1.142 – 1.146) | 1.001 (0.999 – 1.002) | 1.158 (1.157 – 1.160) |
| Age Groups, in years | ||||||
| 18 – 29 | 1.000 (Referent) | 1.000 (Referent) | 1.000 (Referent) | 1.000 (Referent) | 1.000 (Referent) | 1.000 (Referent) |
| 30 – 39 | 2.406 (2.403 – 2.409) | 1.859 (1.857 – 1.861) | 1.700 (1.698 – 1.701) | 1.967 (1.965 – 1.969) | 1.901 (1.900 – 1.903) | 1.634 (1.632 – 1.635) |
| 40 – 49 | 4.596 (4.590 – 4.602) | 2.986 (2.983 – 2.989) | 2.513 (2.511 – 2.516) | 2.905 (2.902 – 2.908) | 2.243 (2.241 – 2.245) | 2.198 (2.196 – 2.199) |
| 50 – 59 | 7.019 (7.010 – 7.028) | 4.256 (4.252 – 4.260) | 3.556 (3.552 – 3.560) | 3.502 (3.498 – 3.506) | 2.975 (2.973 – 2.978) | 2.546 (2.544 – 2.548) |
| 60 – 69 | 8.052 (8.042 – 8.062) | 4.698 (4.694 – 4.703) | 3.900 (3.895 – 3.904) | 3.759 (3.754 – 3.763) | 3.329 (3.325 – 3.332) | 2.545 (2.543 – 2.547) |
| 70+ | 8.196 (8.185 – 8.207) | 4.934 (4.929 – 4.939) | 3.451 (3.447 – 3.455 | 3.328 (3.324 – 3.332) | 3.024 (3.021 – 3.027) | 2.539 (2.537 – 2.542) |
High Allostatic load is defined as total Allostatic load score greater than or equal to 3 (presented as column percentages and standard errors). Allostatic load total score was calculated as sum total of components based on high-risk thresholds: albumin, BMI, C-reactive protein, creatinine clearance, diastolic blood pressure, glycated hemoglobin, systolic blood pressure, total cholesterol, and triglycerides.
Estimated using sampling weights from National Health and Nutrition Examination Survey (NHANES).
Presented as weighted row percentage, describes the total percentage of participants among all study periods.
Presented as Relative Risks and 95% confidence intervals for high allostatic load estimated using modified Poisson regression with robust variance estimation and accounting for NHANES weighting. Adjusted for race, gender, education, age groups, poverty-to-income ratio, smoking status, cancer, congestive heart failure, and heart attack.
Table 3:
Multivariable association between mean allostatic load and participant characteristics in US adults stratified by race and sex, by National Health and Examination Survey (NHANES) study period. Presented as the absolute differences from referent groups. Among 50,671 participants an estimated 1,056,925,341 US residents.
| NHANES Period | ||||||
|---|---|---|---|---|---|---|
| 1988 – 1991 | 1991 – 1994 | 1999 – 2002 | 2003 – 2006 | 2007 – 2010 | 2015 – 2018 | |
| Participants (N) | 7,387 | 7,850 | 7,893 | 7,759 | 10,395 | 9,387 |
| Estimated Na (%)b | 155,959,992 (14.76) | 165,955,459 (15.70) | 165,937,416 (15.70) | 168,342,341 (15.93) | 192,397,679 (18.20) | 208,332,545 (19.71) |
| Presented as Adjusted Mean Differences, β (95% CI) c | ||||||
| Race/Ethnicity and Male Sex | ||||||
| Non-Hispanic White (Referent) | 2.57 (2.35, 2.79) | 3.12 (2.70, 3.53) | 2.40 (2.12, 2.69) | 2.25 (1.92, 2.58) | 2.67 (2.51, 2.82) | 2.97 (2.56, 3.38) |
| Non-Hispanic Black | +0.46 (0.30, 0.61) | +0.31 (0.11, 0.51) | +0.13 (−0.02, 0.27) | +0.25 (0.10, 0.40) | +0.27 (0.09, 0.44) | +0.17 (0.01, 0.33) |
| Latino | +0.20 (0.02, 0.38) | +0.09 (−0.11, 0.29) | +0.14 (−0.07, 0.36) | +0.42 (0.26, 0.59) | +0.42 (0.28, 0.56) | +0.44 (0.25, 0.64) |
| Other & Mixed-Race | +0.03 (−0.19, 0.25) | −0.06 (−0.45, 0.32) | +0.59 (0.39, 0.78) | +0.14 (−0.24, 0.52) | +0.23 (−0.01, 0.46) | +0.13 (−0.04, 0.31) |
| Race/Ethnicity and Female Sex | ||||||
| Non-Hispanic White (Referent) | 2.38 (2.15, 2.60) | 3.01 (2.67, 3.35) | 2.41 (2.12, 2.71) | 2.11 (1.79, 2.44) | 2.58 (2.43, 2.73) | 2.90 (2.47, 3.33) |
| Non-Hispanic Black | +0.60 (0.47, 0.73) | +0.63 (0.41, 0.86) | +0.34 (0.18, 0.51) | +0.58 (0.44, 0.72) | +0.38 (0.22, 0.53) | +0.48 (0.33, 0.63) |
| Latina | +0.32 (0.16, 0.49) | +0.27 (0.03, 0.51) | +0.13 (−0.08, 0.34) | +0.36 (0.17, 0.56) | +0.41 (0.27, 0.55) | +0.50 (0.37, 0.64) |
| Other & Mixed-Race | +0.20 (−0.08, 0.48) | +0.20 (−0.09, 0.49) | +0.42 (0.11, 0.73) | +0.18 (−0.14, 0.50) | +0.11 (−0.07, 0.28) | +0.29 (0.12, 0.47) |
| Age Groups, in years | ||||||
| 18 – 29 (Referent) | 1.24 (1.01, 1.48) | 1.71 (1.23, 2.20) | 1.45 (1.15, 1.76) | 1.24 (0.89, 1.58) | 1.73 (1.56, 1.91) | 2.12 (1.71, 2.53) |
| 30 – 39 | +0.61 (0.47, 0.75) | +0.62 (0.40, 0.83) | +0.58 (0.47, 0.68) | +0.70 (0.59, 0.82) | +0.72 (0.57, 0.86) | +0.71 (0.53, 0.88) |
| 40 – 49 | +1.29 (1.13, 1.45) | +1.47 (1.25, 1.68) | +1.15 (1.00, 1.30) | +1.28 (1.14, 1.43) | +1.13 (0.96, 1.29) | +1.22 (1.06, 1.39) |
| 50 – 59 | +2.14 (1.94, 2.34) | +2.19 (1.99, 2.40) | +1.70 (1.54, 1.86) | +1.68 (1.50, 1.86) | +1.57 (1.40, 1.75) | +1.64 (1.49, 1.80) |
| 60 – 69 | +2.34 (2.17, 2.51) | +2.46 (2.12, 2.81) | +1.97 (1.84, 2.10) | +1.90 (1.74, 2.07) | +1.75 (1.62, 1.89) | +1.45 (1.29, 1.61) |
| 70+ | +2.35 (2.19, 2.53) | +2.43 (2.24, 2.62) | +1.66 (1.53, 1.78) | +1.55 (1.40, 1.70) | +1.52 (1.38, 1.65) | +1.36 (1.17, 1.54) |
Estimated using sampling weights from National Health and Nutrition Examination Survey (NHANES).
Presented as weighted row percentage, describes the total percentage of participants among all study periods.
Presented as multivariable adjusted estimates (mean difference from referent group) from generalized linear models. Adjusted for race, gender, education, age groups, poverty-to-income ratio, smoking status, cancer, congestive heart failure, and heart attack. For example, in 1988 – 1991, non-Hispanic white males are the referent and non-Hispanic black males had adjusted allostatic load scores 0.46 points higher (95% CI: 0.30 – 0.61) than non-Hispanic white males, adjusted for all abovementioned covariates.
DISCUSSION
In a diverse, nationally representative sample of US adults, we observed marked disparities in the burden of AL across race/ethnicity, gender and age groups over a 30-year period. The burden of high AL and mean AL score increased significantly over time; a relative increase of 45.1% and 35.1% from 1988 through 2018, respectively. By 2015-2018, nearly than one in two US adults met criteria for high AL, and mean AL score was highest among both NH-Black and Latina females, followed by Latino males, Other & Mixed race females, and finally NH-Black males. Racial differences in AL score were evident as early as 18 – 29 years old and persisted across age groups over the entire period examined. For instance, age-adjusted mean AL score among NH-Black and Latino males ages 30-39 years was higher than for NH-White males ages 40 – 49, and higher among NH-Black and Latino males ages 40 – 49 than among NH-White males ages 50 – 59 years. Similar patterns were observed among females; age-adjusted AL score among NH-Black and Latino females at age 40 – 49 years were significantly higher than for NH-White females at ages 50 – 59 years.
Higher AL has been associated with increased mortality risk in numerous studies25,53–57; there was an 88% (HR: 1.88, 95% CI: 1.56, 2.26) increased risk of all-cause mortality53, and 55% (HR: 1.55, 95% CI: 1.04, 2.32) increased risk of cardiovascular or diabetes-related mortality observed in previous studies, with stronger associations among NH-Blacks 53. Other studies have also documented a role of AL in health outcomes globally, including China 58, Denmark 59, and Germany 60, highlighting a critical role for AL in health outcomes across diverse population groups. Similar to our findings, other US studies have documented higher mean AL among NH-Blacks compared with Whites starting in early adulthood; Geronimus et al. reported that NH-Black women were consistently more likely than NH-Black men to have high AL score, and poor Whites were less likely than non-poor NH-Blacks to have high AL score61. Our analysis updates the previous results of nationally generalizable NHANES by including Latino adults in the US, a demographic group that has experienced a significant increase from about 5% of the US population in 1988-1992 to almost 14% in 2007-2010. Similar to NH-Blacks, AL score among Latinos has increased over time and is consistently higher among females compared with males. We also observed that after adjusting for race/ethnicity, age and comorbidity status, there was significantly increased odds of high AL score among those with higher versus lower poverty-to-income ratio, and among those with lower versus higher education.
There are several proposed mechanisms that explain the association of high AL and poorer health outcomes. The ‘weathering’ hypothesis, proposed by Geronimus et al in 199261, describes the cumulative impact of socio-economic adversity and political marginalization on individual health, leading to early and disproportionate physiological deterioration. Persistent high-coping effort due to chronic stress has also been shown to result in cumulative wear and tear on the body’s adaptive biological systems. This stress-related physiologic burden was conceptualized as AL by Seeman et al (1997) and McEwen & Seeman (1999) and has been well examined as a measure of weathering given the inclusion of subclinical measures of stress response across several biological systems 21,26. In addition to socio-economic adversity, exposure to racial discrimination over the life-course such as de-facto racial segregation via Jim Crow laws, other forms of adversity such as conflict and social instability, can also induce chronic stress, leading to pathophysiology in cardiovascular, metabolic and immune systems, key components of AL. Consistent and significant associations have also been shown between exposure to structural racism, racial and economic segregation and biological dysregulation – specifically cardiovascular (e.g., hypertension62–66); metabolic (e.g., obesity67,68, diabetes69,70, metabolic syndrome71,72); and inflammatory (e.g., CRP, TNF-alpha and IL-619,20,73–78) systems. In addition, research studies have documented a potential role for epigenetic dysregulation as a consequence of various forms of psychosocial stress (e.g., abuse as a child, active combat), with differential DNA methylation observed in loci associated with HPA axis regulation, hypertension, and immune response 79–81 82–85. Individual responses to chronic stress may also further exacerbate physiological deterioration, for instance, health damaging behavior e.g. smoking, excessive alcohol use among lower SES groups as a coping mechanism for anxiety and stress, or as a consequence of targeted marketing of health damaging commodities, further increasing AL. Recent phenomena such as elevated rates of multiple chronic diseases (e.g. type 2 diabetes, congestive heart failure, hypertension) among US adults including the obesity epidemic86, i.e. rates of adult obesity significantly increasing in approximately 30 years, could also explain the increases of AL over time in our sample of US adults.
These results should be interpreted with a few strengths and limitations. NHANES is a nationally representative, standardized survey on a multitude of health and nutritional related topics. It also utilizes a stratified, multistage sampling scheme, thus the results of this study are generalizable to other US residents and have high validity. Although NHANES collected a wealth of personal and health information on its study participants, there is possibility for residual confounding. For example, unmeasured factors that have been associated with accelerated health declines specifically in NH-Blacks are self-perceived stress, residential segregation, racial discrimination, and institutionalized racism 87. Future work examining the roles of perceived racism and objective measures of racial segregation on the association between race/ethnicity and AL are warranted to further elucidate these observed racial disparities in AL. We did not examine the potential role of acculturation, language proficiency, and country of birth on the race/ethnicity differences in AL burden. However, recent data suggests that age-related gradients in AL are steepest among foreign-born Blacks of both genders and foreign-born Latina women44. Future studies could also examine how AL may be associated with other social determinants of health (e.g. socioeconomic position), self-perceived health status, dietary patterns, or ecological phenomenon and disparities existing within them, such as the steady decline of life expectancy in the US. In addition, while there is no current consensus on which biomarkers are used to calculate AL, we operationalized an AL definition most consistent with prior literature using that NHANES sample48, and which also allowed us to have the most consistent definition throughout the 30-year observation period.
In conclusion, this study examined temporal trends in AL among a large representative sample of US residents across race/ethnicity, gender and age group over a 30-year period. Results confirm that a disproportional burden of AL- a measure of pathophysiological distress- persists among NH-Blacks and Latino adults in the US, an observation that highlights the need for a paradigm shift in studies examining and addressing health disparities. Implementation of culturally-specific, community based participatory research (CBPR) programs88,89 in communities of color can provide a holistic approach in combating these disparities. There is a need to consider the historical and social context in which minorities live, work, and play, as these will have significant and long-lasting impact on health-related risk factors, biological mechanisms and health outcomes.
Supplementary Material
FUNDING
Dr. Moore was supported by the National Institute On Minority Health And Health Disparities of the National Institutes of Health under Award Number K01MD015304. Dr. Akinyemiju was supported by National Cancer Institute of the National Institutes of Health under award number K01TW010271. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
REFERENCES
- 1.Beckie TM. A systematic review of allostatic load, health, and health disparities. Biol Res Nurs. 2012;14(4):311–346. [DOI] [PubMed] [Google Scholar]
- 2.Zavala VA, Bracci PM, Carethers JM, et al. Cancer health disparities in racial/ethnic minorities in the United States. Br J Cancer. 2021;124(2):315–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Krieger N, Waterman PD, Kosheleva A, et al. Racial discrimination & cardiovascular disease risk: my body my story study of 1005 US-born black and white community health center participants (US). PloS one. 2013;8(10):e77174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moore JX, Chaudhary N, Akinyemiju T. Metabolic Syndrome Prevalence by Race/Ethnicity and Sex in the United States, National Health and Nutrition Examination Survey, 1988-2012. Preventing chronic disease. 2017;14:E24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moore JX, Royston KJ, Langston ME, et al. Mapping hot spots of breast cancer mortality in the United States: place matters for Blacks and Hispanics. Cancer causes & control : CCC. 2018;29(8):737–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Graham G Disparities in cardiovascular disease risk in the United States. Curr Cardiol Rev. 2015;11(3):238–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Singh GK, Jemal A. Socioeconomic and Racial/Ethnic Disparities in Cancer Mortality, Incidence, and Survival in the United States, 1950-2014: Over Six Decades of Changing Patterns and Widening Inequalities. Journal of environmental and public health. 2017;2017:2819372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pool LR, Ning H, Lloyd-Jones DM, Allen NB. Trends in Racial/Ethnic Disparities in Cardiovascular Health Among US Adults From 1999-2012. Journal of the American Heart Association. 2017;6(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Non AL, Gravlee CC, Mulligan CJ. Education, genetic ancestry, and blood pressure in African Americans and Whites. Am J Public Health. 2012;102(8):1559–1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gravlee CC. How race becomes biology: embodiment of social inequality. Am J Phys Anthropol. 2009;139(1):47–57. [DOI] [PubMed] [Google Scholar]
- 11.Krieger N. Living and Dying at the Crossroads: Racism, Embodiment, and Why Theory Is Essential for a Public Health of Consequence. Am J Public Health. 2016;106(5):832–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krieger N Public Health, Embodied History, and Social Justice: Looking Forward. Int J Health Serv. 2015;45(4):587–600. [DOI] [PubMed] [Google Scholar]
- 13.Krieger N Theories for social epidemiology in the 21st century: an ecosocial perspective. Int J Epidemiol. 2001;30(4):668–677. [DOI] [PubMed] [Google Scholar]
- 14.Krieger N. Epidemiology and the web of causation: has anyone seen the spider? Soc Sci Med. 1994;39(7):887–903. [DOI] [PubMed] [Google Scholar]
- 15.Krieger N Embodiment: a conceptual glossary for epidemiology. J Epidemiol Community Health. 2005;59(5):350–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krieger N. Discrimination and healht inequities. 2nd ed. New York: Oxford University Press; 2014. [Google Scholar]
- 17.Williams DR, Collins C. Racial residential segregation: a fundamental cause of racial disparities in health. Public Health Rep. 2001;116(5):404–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krieger N Methods for the scientific study of discrimination and health: an ecosocial approach. Am J Public Health. 2012;102(5):936–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cobb RJ, Parker LJ, Thorpe RJ Jr. Self-Reported Instances of Major Discrimination, Race/Ethnicity, and Inflammation Among Older Adults: Evidence from the Health and Retirement Study. J Gerontol A Biol Sci Med Sci. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kershaw KN, Lewis TT, Diez Roux AV, et al. Self-reported experiences of discrimination and inflammation among men and women: The multi-ethnic study of atherosclerosis. Health Psychol. 2016;35(4):343–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McEwen BS, Seeman T. Protective and damaging effects of mediators of stress. Elaborating and testing the concepts of allostasis and allostatic load. Annals of the New York Academy of Sciences. 1999;896:30–47. [DOI] [PubMed] [Google Scholar]
- 22.Mauss D, Li J, Schmidt B, Angerer P, Jarczok MN. Measuring allostatic load in the workforce: a systematic review. Ind Health. 2015;53(1):5–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guidi J, Lucente M, Sonino N, Fava GA. Allostatic Load and Its Impact on Health: A Systematic Review. Psychother Psychosom. 2021;90(1):11–27. [DOI] [PubMed] [Google Scholar]
- 24.McEwen BS. Interacting mediators of allostasis and allostatic load: towards an understanding of resilience in aging. Metabolism: clinical and experimental. 2003;52(10 Suppl 2):10–16. [DOI] [PubMed] [Google Scholar]
- 25.Seeman TE, McEwen BS, Rowe JW, Singer BH. Allostatic load as a marker of cumulative biological risk: MacArthur studies of successful aging. Proc Natl Acad Sci U S A. 2001;98(8):4770–4775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Seeman TE, Singer BH, Rowe JW, Horwitz RI, McEwen BS. Price of adaptation--allostatic load and its health consequences. MacArthur studies of successful aging. Archives of internal medicine. 1997;157(19):2259–2268. [PubMed] [Google Scholar]
- 27.Juster RP, McEwen BS, Lupien SJ. Allostatic load biomarkers of chronic stress and impact on health and cognition. Neurosci Biobehav Rev. 2010;35(1):2–16. [DOI] [PubMed] [Google Scholar]
- 28.McEwen BS. Stress, adaptation, and disease. Allostasis and allostatic load. Ann N Y Acad Sci. 1998;840:33–44. [DOI] [PubMed] [Google Scholar]
- 29.Seeman T, Merkin SS, Crimmins E, Koretz B, Charette S, Karlamangla A. Education, income and ethnic differences in cumulative biological risk profiles in a national sample of US adults: NHANES III (1988-1994). Soc Sci Med. 2008;66(1):72–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lipowicz A, Szklarska A, Malina RM. Allostatic load and socioeconomic status in Polish adult men. J Biosoc Sci. 2014;46(2):155–167. [DOI] [PubMed] [Google Scholar]
- 31.Juster RP, Lupien S. A sex- and gender-based analysis of allostatic load and physical complaints. Gend Med. 2012;9(6):511–523. [DOI] [PubMed] [Google Scholar]
- 32.Juster RP, Pruessner JC, Desrochers AB, et al. Sex and Gender Roles in Relation to Mental Health and Allostatic Load. Psychosom Med. 2016;78(7):788–804. [DOI] [PubMed] [Google Scholar]
- 33.Juster RP, Moskowitz DS, Lavoie J, D’Antono B. Sex-specific interaction effects of age, occupational status, and workplace stress on psychiatric symptoms and allostatic load among healthy Montreal workers. Stress. 2013;16(6):616–629. [DOI] [PubMed] [Google Scholar]
- 34.Juster RP, de Torre MB, Kerr P, Kheloui S, Rossi M, Bourdon O. Sex Differences and Gender Diversity in Stress Responses and Allostatic Load Among Workers and LGBT People. Curr Psychiatry Rep. 2019;21(11):110. [DOI] [PubMed] [Google Scholar]
- 35.Seeman TE, Gruenewald TL, Cohen S, Williams DR, Matthews KA. Social relationships and their biological correlates: Coronary Artery Risk Development in Young Adults (CARDIA) study. Psychoneuroendocrinology. 2014;43:126–138. [DOI] [PubMed] [Google Scholar]
- 36.Upchurch DM, Stein J, Greendale GA, et al. A Longitudinal Investigation of Race, Socioeconomic Status, and Psychosocial Mediators of Allostatic Load in Midlife Women: Findings From the Study of Women’s Health Across the Nation. Psychosom Med. 2015;77(4):402–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.O’Campo P, Schetter CD, Guardino CM, et al. Explaining racial and ethnic inequalities in postpartum allostatic load: Results from a multisite study of low to middle income woment. SSM Popul Health. 2016;2:850–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Geronimus AT, Hicken M, Keene D, Bound J. “Weathering” and age patterns of allostatic load scores among blacks and whites in the United States. Am J Public Health. 2006;96(5):826–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chyu L, Upchurch DM. Racial and ethnic patterns of allostatic load among adult women in the United States: findings from the National Health and Nutrition Examination Survey 1999–2004. J Womens Health (Larchmt). 2011;20(4):575–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cobb RJ, Thomas CS, Laster Pirtle WN, Darity WA Jr. Self-identified race, socially assigned skin tone, and adult physiological dysregulation: Assessing multiple dimensions of “race” in health disparities research. SSM Popul Health. 2016;2:595–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dormire SL. Life Stress, Race, and Abnormal Glucose Metabolism in Postmenopausal Women. J Am Geriatr Soc. 2016;64(9):e46–48. [DOI] [PubMed] [Google Scholar]
- 42.Arévalo SP, Tucker KL, Falcón LM. Life events trajectories, allostatic load, and the moderating role of age at arrival from Puerto Rico to the US mainland. Soc Sci Med. 2014;120:301–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cuevas AG, Wang K, Williams DR, Mattei J, Tucker KL, Falcon LM. The Association Between Perceived Discrimination and Allostatic Load in the Boston Puerto Rican Health Study. Psychosom Med. 2019;81(7):659–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Langellier BA, Fleming PJ, Kemmick Pintor JB, Stimpson JP. Allostatic Load Among U.S.- and Foreign-Born Whites, Blacks, and Latinx. American journal of preventive medicine. 2021;60(2):159–168. [DOI] [PubMed] [Google Scholar]
- 45.Akinyemiju T, Jha M, Moore JX, Pisu M. Disparities in the prevalence of comorbidities among US adults by state Medicaid expansion status. Preventive medicine. 2016;88:196–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Johnson CL, Paulose-Ram R, Ogden CL, et al. National health and nutrition examination survey: analytic guidelines, 1999–2010. Vital and health statistics Series 2, Data evaluation and methods research. 2013(161):1–24. [PubMed] [Google Scholar]
- 47.McQuillan GM, McLean JE, Chiappa M, Corporation H, Lukacs SL. National Health and Nutrition Examination Survey Biospecimen Program: NHANES III (1988-1994) and NHANES 1999-2014. Vital and health statistics Series 2, Data evaluation and methods research. 2015(170):1–14. [PubMed] [Google Scholar]
- 48.Duong MT, Bingham BA, Aldana PC, Chung ST, Sumner AE. Variation in the Calculation of Allostatic Load Score: 21 Examples from NHANES. Journal of racial and ethnic health disparities. 2017;4(3):455–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mays VM, Juster RP, Williamson TJ, Seeman TE, Cochran SD. Chronic Physiologic Effects of Stress Among Lesbian, Gay, and Bisexual Adults: Results From the National Health and Nutrition Examination Survey. Psychosomatic medicine. 2018;80(6):551–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shargorodsky J, Curhan SG, Curhan GC, Eavey R. Change in prevalence of hearing loss in US adolescents. JAMA. 2010;304(7):772–778. [DOI] [PubMed] [Google Scholar]
- 51.Bondy SJ, Victor JC, Diemert LM. Origin and use of the 100 cigarette criterion in tobacco surveys. Tob Control. 2009;18(4):317–323. [DOI] [PubMed] [Google Scholar]
- 52.Zou G A modified poisson regression approach to prospective studies with binary data. American journal of epidemiology. 2004;159(7):702–706. [DOI] [PubMed] [Google Scholar]
- 53.Borrell LN, Dallo FJ, Nguyen N. Racial/ethnic disparities in all-cause mortality in U.S. adults: the effect of allostatic load. Public Health Rep. 2010;125(6):810–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Castagne R, Gares V, Karimi M, et al. Allostatic load and subsequent all-cause mortality: which biological markers drive the relationship? Findings from a UK birth cohort. Eur J Epidemiol. 2018;33(5):441–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Duru OK, Harawa NT, Kermah D, Norris KC. Allostatic load burden and racial disparities in mortality. J Natl Med Assoc. 2012;104(1-2):89–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Karlamangla AS, Singer BH, Seeman TE. Reduction in allostatic load in older adults is associated with lower all-cause mortality risk: MacArthur studies of successful aging. Psychosomatic medicine. 2006;68(3):500–507. [DOI] [PubMed] [Google Scholar]
- 57.Levine ME, Crimmins EM. A comparison of methods for assessing mortality risk. Am J Hum Biol. 2014;26(6):768–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Xu H Multilevel socioeconomic differentials in allostatic load among Chinese adults. Health & place. 2018;53:182–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Christensen DS, Flensborg-Madsen T, Garde E, Hansen AM, Masters Pedersen J, Mortensen EL. Early life predictors of midlife allostatic load: A prospective cohort study. PloS one. 2018;13(8):e0202395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Scheuer S, Wiggert N, Bruckl TM, et al. Childhood abuse and depression in adulthood: The mediating role of allostatic load. Psychoneuroendocrinology. 2018;94:134–142. [DOI] [PubMed] [Google Scholar]
- 61.Geronimus AT. The weathering hypothesis and the health of African-American women and infants: evidence and speculations. Ethn Dis. 1992;2(3):207–221. [PubMed] [Google Scholar]
- 62.Brondolo E, Libby DJ, Denton EG, et al. Racism and ambulatory blood pressure in a community sample. Psychosomatic medicine. 2008;70(1):49–56. [DOI] [PubMed] [Google Scholar]
- 63.Steffen PR, McNeilly M, Anderson N, Sherwood A. Effects of perceived racism and anger inhibition on ambulatory blood pressure in African Americans. Psychosomatic medicine. 2003;65(5):746–750. [DOI] [PubMed] [Google Scholar]
- 64.Thompson HS, Kamarck TW, Manuck SB. The association between racial identity and hypertension in African-American adults: elevated resting and ambulatory blood pressure as outcomes. Ethn Dis. 2002;12(1):20–28. [PubMed] [Google Scholar]
- 65.Beatty Moody DL, Waldstein SR, Tobin JN, Cassells A, Schwartz JC, Brondolo E. Lifetime racial/ethnic discrimination and ambulatory blood pressure: The moderating effect of age. Health Psychol. 2016;35(4):333–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kershaw KN, Osypuk TL, Do DP, De Chavez PJ, Diez Roux AV. Neighborhood-level racial/ethnic residential segregation and incident cardiovascular disease: the multi-ethnic study of atherosclerosis. Circulation. 2015;131(2):141–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cozier YC, Yu J, Coogan PF, Bethea TN, Rosenberg L, Palmer JR. Racism, segregation, and risk of obesity in the Black Women’s Health Study. Am J Epidemiol. 2014;179(7):875–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Thorpe RJ Jr., Parker LJ, Cobb RJ, Dillard F, Bowie J. Association between discrimination and obesity in African-American men. Biodemography Soc Biol. 2017;63(3):253–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bacon KL, Stuver SO, Cozier YC, Palmer JR, Rosenberg L, Ruiz-Narvaez EA. Perceived racism and incident diabetes in the Black Women’s Health Study. Diabetologia. 2017;60(11):2221–2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Reynolds DB, Walker RJ, Campbell JA, Egede LE. Differential effect of race, education, gender, and language discrimination on glycemic control in adults with type 2 diabetes. Diabetes Technol Ther. 2015;17(4):243–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ikram UZ, Snijder MB, Agyemang C, et al. Perceived Ethnic Discrimination and the Metabolic Syndrome in Ethnic Minority Groups: The Healthy Life in an Urban Setting Study. Psychosomatic medicine. 2017;79(1):101–111. [DOI] [PubMed] [Google Scholar]
- 72.Beatty Moody DL, Chang Y, Brown C, Bromberger JT, Matthews KA. Everyday Discrimination and Metabolic Syndrome Incidence in a Racially/Ethnically Diverse Sample: Study of Women’s Health Across the Nation. Psychosomatic medicine. 2018;80(1):114–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Simons RL, Lei MK, Beach SRH, et al. Discrimination, segregation, and chronic inflammation: Testing the weathering explanation for the poor health of Black Americans. Dev Psychol. 2018;54(10):1993–2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Van Dyke ME, Vaccarino V, Dunbar SB, et al. Socioeconomic status discrimination and C-reactive protein in African-American and White adults. Psychoneuroendocrinology. 2017;82:9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Stepanikova I, Bateman LB, Oates GR. Systemic Inflammation in Midlife: Race, Socioeconomic Status, and Perceived Discrimination. Am J Prev Med. 2017;52(1S1):S63–S76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lewis TT, Aiello AE, Leurgans S, Kelly J, Barnes LL. Self-reported experiences of everyday discrimination are associated with elevated C-reactive protein levels in older African-American adults. Brain Behav Immun. 2010;24(3):438–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Martinez AD, Ruelas L, Granger DA. Household fear of deportation in relation to chronic stressors and salivary proinflammatory cytokines in Mexican-origin families post-SB 1070. SSM Popul Health. 2018;5:188–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Carlson S, Borrell LN, Eng C, et al. Self-reported racial/ethnic discrimination and bronchodilator response in African American youth with asthma. PLoS One. 2017;12(6):e0179091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Argentieri MA, Nagarajan S, Seddighzadeh B, Baccarelli AA, Shields AE. Epigenetic Pathways in Human Disease: The Impact of DNA Methylation on Stress-Related Pathogenesis and Current Challenges in Biomarker Development. EBioMedicine. 2017;18:327–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Alikhani-Koopaei R, Fouladkou F, Frey FJ, Frey BM. Epigenetic regulation of 11 beta-hydroxysteroid dehydrogenase type 2 expression. J Clin Invest. 2004;114(8):1146–1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Friso S, Pizzolo F, Choi SW, et al. Epigenetic control of 11 beta-hydroxysteroid dehydrogenase 2 gene promoter is related to human hypertension. Atherosclerosis. 2008;199(2):323–327. [DOI] [PubMed] [Google Scholar]
- 82.Yehuda R, Daskalakis NP, Bierer LM, et al. Holocaust Exposure Induced Intergenerational Effects on FKBP5 Methylation. Biol Psychiatry. 2016;80(5):372–380. [DOI] [PubMed] [Google Scholar]
- 83.Vukojevic V, Kolassa IT, Fastenrath M, et al. Epigenetic modification of the glucocorticoid receptor gene is linked to traumatic memory and post-traumatic stress disorder risk in genocide survivors. J Neurosci. 2014;34(31):10274–10284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Labonte B, Azoulay N, Yerko V, Turecki G, Brunet A. Epigenetic modulation of glucocorticoid receptors in posttraumatic stress disorder. Transl Psychiatry. 2014;4:e368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yehuda R, Flory JD, Bierer LM, et al. Lower methylation of glucocorticoid receptor gene promoter 1F in peripheral blood of veterans with posttraumatic stress disorder. Biol Psychiatry. 2015;77(4):356–364. [DOI] [PubMed] [Google Scholar]
- 86.Mitchell NS, Catenacci VA, Wyatt HR, Hill JO. Obesity: overview of an epidemic. Psychiatr Clin North Am. 2011;34(4):717–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Thorpe RJ Jr., Fesahazion RG, Parker L, et al. Accelerated Health Declines among African Americans in the USA. Journal of urban health : bulletin of the New York Academy of Medicine. 2016;93(5):808–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bevel M, Babatunde OA, Heiney SP, et al. Sistas Inspiring Sistas Through Activity and Support (SISTAS): Study Design and Demographics of Participants. Ethn Dis. 2018;28(2):75–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wilcox S, Saunders RP, Jake-Schoffman D, Hutto B. The Faith, Activity, and Nutrition (FAN) Dissemination and Implementation Study: 24-Month Organizational Maintenance in a Countywide Initiative. Front Public Health. 2020;8:171. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


