Abstract
Sipuleucel-T, an autologous cellular immunotherapy, was approved to treat metastatic castration-resistant prostate cancer in 2010 in the United States. Treatment with sipuleucel-T primes the immune system to target prostate acid phosphatase, which is expressed by prostate cancer cells, potentially leading to lysis of cancer cells. Expanding on previously reported indirect evidence of cell killing with sipuleucel-T treatment, we sought to provide direct evidence of cell lysis through visualization. We used advanced video technology and available samples of peripheral blood mononuclear cells from subjects enrolled in the STAMP trial (NCT01487863). Isolated CD8+ T cells were used as effector cells and cocultured with autologous monocytes pulsed with control or target antigens. Differentially stained effector and target cells were then video recorded during coculture. Here, we present video recordings and analyses of T cells from sipuleucel-T–treated subjects showing—for the first time—direct lysis of cells that express prostate cancer target antigens, prostate acid phosphatase, or prostate-specific antigen.
In this brief report, we share videos illustrating sipuleucel-T–programmed human T cells recognizing and destroying cells that express the prostate cancer target antigens, either prostatic acid phosphatase (PAP), or prostate-specific antigen (PSA). This report expands on a previous report of indirect evidence of cell killing, based on flow cytometry, in samples from men with metastatic castrate-resistant prostate cancer treated with sipuleucel-T (1).
Sipuleucel-T is an autologous cellular immunotherapy approved in 2010 to treat asymptomatic or minimally symptomatic metastatic castrate-resistant prostate cancer (2). Sipuleucel-T treatment comprises 3 infusions given approximately every 2 weeks. Each infusion involves collecting peripheral blood mononuclear cells (PBMCs) via apheresis, then isolating the PBMCs and culturing them ex vivo with PA2024 (a fusion protein, comprising PAP and human granulocyte macrophage colony-stimulating factor), and finally intravenously infusing the resultant product back into the subject.
Cells in this infusion stimulate peripheral immune responses against PAP and PA2024 and increase cytokine production; further, they cause trafficking of T cells to the prostate in the localized setting (2-5). Also, memory cytolytic T-lymphocyte activity against both PA2024 and PAP, as measured by a flow cytometry assay, is induced (1). These immune responses correlate with overall survival (1,6), along with the breadth of posttreatment humoral response (7). To date, although hypothesized, tumor cell lysis has not been demonstrated directly.
We used banked PBMC samples from subjects who displayed week 26 post–sipuleucel-T treatment antibody responses against PA2024, PAP, and PSA during the STAMP study (NCT01487863) (8). The responses of the subjects included in the current assessments are described in Table 1. The study was approved by the institutional review board of each site, and subjects provided informed consent. Samples from 3 timepoints were assessed: baseline (ie, week 0, before sipuleucel-T treatment) and after sipuleucel-T treatment (weeks 6 and 26).
Table 1.
Demonstration of long-term responses after sipuleucel-T treatment in the STAMP study in subjects included in the current assessmentsa
| Subject | Antibody fold-change from week 0 |
|||
|---|---|---|---|---|
| PSA |
PAP |
|||
| Week 6 | Week 26 | Week 6 | Week 26 | |
| Subject #1 | 2.40 | 2.98 | 4.87 | 4.40 |
| Subject #2 | 5.52 | 4.91 | 7.86 | 7.65 |
| Subject #3 | 2.94 | 3.58 | 6.96 | 6.81 |
aResponses are described by prostate-specific antigen (PSA)- and prostatic acid phosphatase (PAP)-antibody fold-changes from week 0 to week 26. Methodology as described in Small et al. (8).
Samples containing cryopreserved PBMCs for each timepoint for each subject were thawed and treated as described previously (1). Next, monocytes were isolated using negative selection techniques (EasySep human monocyte enrichment kit [STEMCELL Technologies, Vancouver, BC, Canada] and CD8+ cell isolation kit [Miltenyl Biotec, Auburn, CA]). Isolated CD8+ T cells were stained with LysoBrite Red (AAT Bioquest, Sunnyvale, CA) to allow subsequent effector cell lysosome visualization (red). Isolated autologous monocytes were either pulsed separately with PAP peptides (20mers with 10-aa overlap from New England Peptide, Gardner, MA) or PSA peptides (15mers with 11-aa overlap from JPT Innovative Peptide Solutions, Berlin, Germany). As control, HER2 peptides (20mers with 10-aa overlap from New England Peptide) or unpulsed monocytes were used to test for specificity or not pulsed (Figure 1). Pulsed and unpulsed monocytes were stained with calcein AM (ThermoFisher Scientific, Waltham, MA) to allow for visualization of target cells (green). Monocytes were incubated with CD8+ T cells at a 1:7 effector to target ratio: 2 x 105 green-stained monocytes were loaded on a well of a chamber slide system (ThermoFisher Scientific) and then incubated (37°C, 5% CO2) for 1 hour before the addition of 1.4 x 106 red-stained CD8+ T cells.
Figure 1.
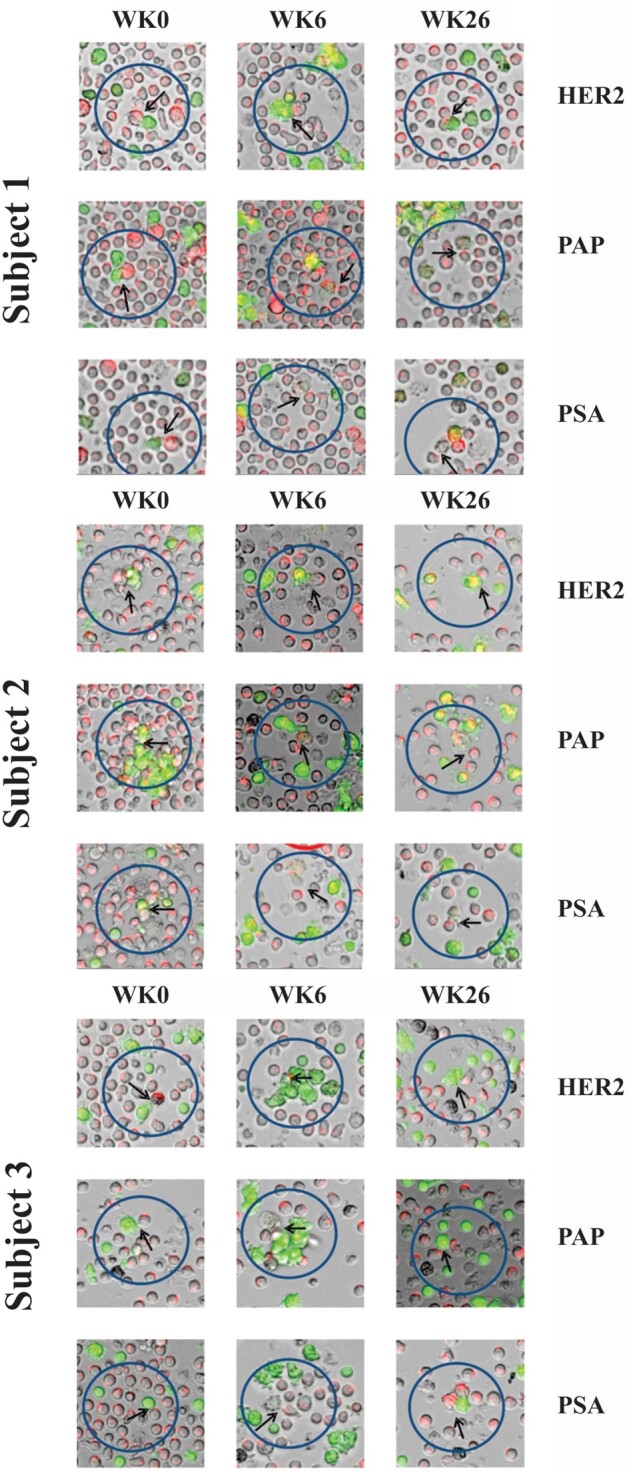
Still images of cell-cell interactions organized by both applied antigen and timepoint. CD8+ T cells (red) interact with target cells (green). The cells are visualized to reflect these colors. Arrowheads indicate effector - target cell interactions. Interactions suggest antigen-specificity and antitumor activity. The arrow tips indicate the contact point of the target and effector cells of interest. During week 0, more red-stained cells are observed. Target cells pulsed with irrelevant antigen HER2 and tested antigens at week 0 retain their green color after interaction with baseline CD8+ T cells. The green color of target cells pulsed with tested antigens disappears after they are killed by sipuleucel-T stimulated CD8+ T cells at week 6 and week 26. PAP = prostatic acid phosphatase; PSA = prostate-specific antigen.
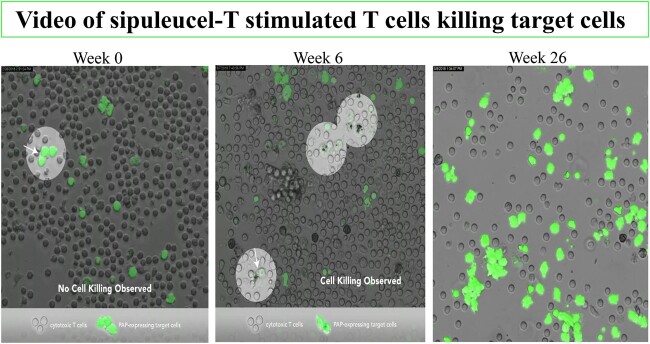
Video.
Three time-lapse videos of cell-cell interactions by timepoint: A) week 0; B) week 6; C) week 26. CD8+ T cells interact with target cells. The green-stained target cells are visualized as white. Effector cells are not highlighted. Arrowheads indicate effector - target cell interactions in sipuleucel-T–treated subjects demonstrating antigen specificity and antitumor activity. The arrow tips indicate the contact point of the target and effector cells of interest. Cell interaction and killing at room temperature were captured using a Leica CRT6500 confocal microscope with LASAF software (Leica Microsystems, Buffalo Grove, IL). The final products were 41-second videos where the sipuleucel-T–stimulated CD8+ T cells were tracked from the time they come in close contact with the green-stained target cells. For purposes of presentation, the videos have been shortened to fit in the file size limitations. Time-lapse recording was performed using the confocal microscope set up as follows: the “XYT” scanning of acquisition mode was activated; 10X objective was set with digital zoom factor at 6; the XY field was set at the largest size format (2048 x 2048 pixels) with a recording speed at 400 Hz; and the time field was set for a duration of 8 hours. After acquisition, images were analyzed, processed, and recorded as time-lapse video sequences using Adobe Premier Pro CC 2018 at a speed of 30 frames per second.
Cell activity at room temperature was then recorded for 8 hours using a Leica CRT6500 confocal microscope with LASAF software (Leica Microsystems, Buffalo Grove, IL) at the largest XY-format size (2048 x 2048 pixels) with a recording speed at 400 Hz. Following acquisition, images were analyzed, processed, and recorded as 41-second videos of time-lapse sequences (30 frames per second) using Adobe Premiere Pro CC 2018 (Adobe Systems).
Red-stained CD8+ T cells were tracked from the time they came in contact with the target cells: the green-stained monocytes. T cells in the posttreatment samples exhibited higher motility compared with week 0 (data not shown). We observed that the killing of the target cells, the green-stained PAP- and PSA-pulsed monocytes, was only seen in the presence of CD8+ T cells and only in the weeks 6 and 26 samples (ie, after treatment with sipuleucel-T; Figure 1 and Video; see Supplementary material to view videos A-C, available online). Cell killing was not observed at week 0 (Figure 1), nor was it seen posttreatment in the absence of CD8+ T cells (data not shown). Cell killing was not observed in the following control settings: 1) in the presence of HER2 peptides, 2) unpulsed conditions, or 3) in samples from a normal donor (data not shown). T cells not exposed to sipuleucel-T did not recognize PAP- and PSA-pulsed target cells, because the T cells did not exhibit prolonged contact with target cells and did not exhibit cell killing. After treatment, T cells displayed serial lysis; namely, individual T cells were able to lyse multiple targets sequentially.
Together, these observations suggest that exposure to sipuleucel-T programs T cells to recognize and lyse cells that express the primary target antigen for sipuleucel-T (eg, PAP) or a secondary prostate cancer antigen (eg, PSA), because lytic activity was only observed in the samples collected after sipuleucel-T treatment. These results also suggest involvement of antigen spread in the response (5,9). Finally, we were able to detect PAP- and PSA-specific lytic activity out to the week 26 mark, which is in agreement with the vast array of immune response data generated so far, suggesting induction of immunological memory.
Other methods can demonstrate the direct killing of target cells by effector cells, albeit with certain technical limitations. Chromium release or similar cytotoxicity assays require large numbers of both effector and target cells (10), limiting their use when assessing cytolytic T-lymphocyte activity in human samples, given both blood draw restrictions and associated costs. Lack of accessibility to major histocompatibility complex-matched tumor cells, either derived from subjects being assessed or cell lines, further limits this type of research. We addressed these limitations by using autologous, peptide-pulsed monocytes as target cells, allowing us to reduce the requisite number of effector cells and eliminate the need for major histocompatibility complex-matched target cell lines. Direct visualization by time-lapse videography allowed us to closely study direct cell-cell interactions, thus providing direct evidence of induced target cell lysis after sipuleucel-T treatment.
In summary, supporting previous results (1), using an ex vivo human-cell model, our results provide evidence that treatment with sipuleucel-T results in immune activation leading to the destruction of cells expressing the prostate cancer target antigens PAP and PSA and support an additional in vivo mechanism of action in subjects receiving sipuleucel-T.
Funding
This work, as well as the original STAMP study, was funded by Dendreon Pharmaceuticals, LLC.
Notes
The role of the funder: Dendreon funded this research, the original clinical study, and the development of this manuscript.
Disclosures: Dr Kibel is supported by DiNovi Family Foundation. Dr Kibel reports grants from Dendreon, during the conduct of the study; personal fees from Merck, from Janssen, from Bayer, from General Electic, from AstraZeneca, from Profound, from Insightec, outside the submitted work. Dr Inman reports grants and personal fees from Genentech/Roche, grants and personal fees from Combat Medical, grants and personal fees from Taris Biomedical, grants and personal fees from Nucleix, grants from FKD Therapies, personal fees from Fergene, grants from QED Therapies, grants from Anchiano Therapies, grants from Abbott laboratories, and grants from Dendreon, outside the submitted work. Dr Pachynski reports personal fees and nonfinancial support from BMS, personal fees from Dendreon, personal fees from EMD Serono/Pfizer, personal fees and nonfinancial support from Genentech/Roche, personal fees from Genomic Health, grants from Janssen, personal fees from Astra-Zeneca, personal fees from Sanofi, personal fees from Merck, personal fees from Jounce Therapeutics, and personal fees from Bayer, outside the submitted work. Dr Vu is a current employee of Dendreon Pharmaceuticals LLC, makers of sipuleucel-T. Dr Sheikh is a current employee of Dendreon Pharmaceuticals LLC, makers of sipuleucel-T. In addition, Dr Sheikh has a patent “Humoral Immune Response Against Tumor Antigens After Treatment With a Cancer Antigen Specific Active Immunotherapy and its Association With Improved Clinical Outcome” issued, and a patent, “Gene Expression Markers for Predicting Overall Survival in Subjects Treated With Sipuleucel-T,” pending. Dr Petrylak reports other from Bellicum Pharmaceuticals, other from TYME, grants and personal fees from AstraZeneca, grants and personal fees from Bayer, personal fees from Exelixis, personal fees from Johnson & Johnson/Janssen, grants and personal fees from Lilly, grants and personal fees from Roche, grants from Agensys, grants from Medivation, grants and personal fees from Bayer, grants and personal fees from Clovis Oncology, grants from Endocyte, grants from Genentech, grants from Innocrin Pharma, grants from MedImmune, grants from Merck, grants from Millennium, grants from Novartis, grants from Progenics, grants and personal fees from Roche, grants from Sanofi, grants and personal fees from Seattle Genetics, other from Celgene, other from Sanofi, grants and personal fees from Ada Cap (Advanced Accelerator Applications), personal fees from Amgen, personal fees from Bicycle Therapeutics, personal fees from Boehringer Ingelheim, grants and personal fees from Bristol Myers Squibb, personal fees from Incyte, grants and personal fees from Mirati, personal fees from Monopteros, personal fees from Pharmacyclics, personal fees from Urogen, grants from BioXcel Therapeutics, grants from Eisai, grants from Replimune, grants and personal fees from Astellas, and grants and personal fees from Pfizer, outside the submitted work.
Author contributions: Conceptualization, NS, TV, AK, and BI; Methodology, NS and TV; Investigation, NS and TV; Writing—Original Draft, NS; Writing—Review & Editing, AK, BI, RP, TV, NS, and DP; Funding Acquisition, NS; Resources, NS; Supervision, NS. All authors reviewed the results and approved the final version of the manuscript.
Prior presentations: Inman B, Vu T, Yu Evan Y, et al. PD14-07 Real-time imaging demonstrating T-cell mediated destruction of prostatic acid phosphatase (PAP)-expressing cells in patients (PTS) treated with sipuleucel-T (SIP-T). J Urology. 2018; 199(4S):Abstract e307. https://doi.org/10.1016/j.juro.2018.02.793.
Supplementary Material
Acknowledgements
The authors thank the investigators and subjects who participated in the STAMP study. Helen M Wilfehrt, PhD, CMPP (Dendreon Pharmaceuticals, LLC) provided medical writing support to the authors for the development of this manuscript.
Data Availability
The data underlying this article will be shared on reasonable request to Dendreon Pharmaceuticals, LLC (mac@dendreon.com).
References
- 1. Antonarakis ES, Small EJ, Petrylak DP, et al. Antigen-specific CD8 lytic phenotype induced by sipuleucel-T in hormone-sensitive or castration-resistant prostate cancer and association with overall survival. Clin Cancer Res. 2018;24(19):4662–4671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kantoff PW, Higano CS, Shore ND, et al. ; IMPACT Study Investigators. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363(5):411–422. 10.1056/NEJMoa1001294 [DOI] [PubMed] [Google Scholar]
- 3. Sheikh NA, Jones LA.. CD54 is a surrogate marker of antigen presenting cell activation. Cancer Immunol Immunother. 2008;57(9):1381–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fong L, Carroll P, Weinberg V, et al. Activated lymphocyte recruitment into the tumor microenvironment following preoperative sipuleucel-T for localized prostate cancer. J Natl Cancer Inst. 2014;106(11):dju268. 10.1093/jnci/dju268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sheikh N, Cham J, Zhang L, et al. Clonotypic diversification of intratumoral T cells following sipuleucel-T treatment in prostate cancer subjects. Cancer Res. 2016;76(13):3711–3718. [DOI] [PubMed] [Google Scholar]
- 6. Sheikh NA, Petrylak D, Kantoff PW, et al. Sipuleucel-T immune parameters correlate with survival: an analysis of the randomized phase 3 clinical trials in men with castration-resistant prostate cancer. Cancer Immunol Immunother. 2013;62(1):137–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. GuhaThakurta D, Sheikh NA, Fan LQ, et al. Humoral immune response against nontargeted tumor antigens after treatment with sipuleucel-T and its association with improved clinical outcome. Clin Cancer Res. 2015;21(16):3619–3630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Small EJ, Lance RS, Redfern CH, et al. Long-term follow-up from STAMP, a phase II trial, evaluating sipuleucel-T and concurrent (CON) vs sequential (SEQ) abiraterone acetate + prednisone in metastatic castration-resistant prostate cancer patients (pts). J Clin Oncol. 2017;35(6_suppl):190–190. [Google Scholar]
- 9. Antonarakis ES, Kibel AS, Adams GW, et al. Immune responses and clinical outcomes in STAND, a randomized phase 2 study evaluating optimal sequencing of sipuleucel-T (sip-T) and androgen deprivation therapy (ADT) in biochemically-recurrent prostate cancer (BRPC) after local therapy failure. J Clin Oncol. 2015;33(15_suppl):5030–5030. [Google Scholar]
- 10. Brunner KT, Mauel J, Cerottini JC, Chapuis B.. Quantitative assay of the lytic action of immune lymphoid cells on 51-Cr-labelled allogeneic target cells in vitro; inhibition by isoantibody and by drugs. Immunology. 1968;14(2):181–196. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to Dendreon Pharmaceuticals, LLC (mac@dendreon.com).