Abstract
Background
For nonmetastatic castration-resistant prostate cancer (nmCRPC), 3 drugs under patent protection—apalutamide, enzalutamide, and darolutamide—were approved based on randomized, placebo-controlled trials; 1 drug with generic availability, abiraterone acetate, showed efficacy in a single-arm trial and is commonly prescribed. Lacking head-to-head trials, the optimal treatment for nmCRPC is unknown, despite widely varied treatment costs. We compared the efficacy and safety of nmCRPC treatments.
Methods
We searched bibliographic databases, regulatory documents, and trial registries for nmCRPC trials. We included published results and, when available, original data. We performed matching-adjusted indirect comparison and network meta-analysis and compared treatments regarding metastasis-free survival, overall survival, and serious adverse events.
Results
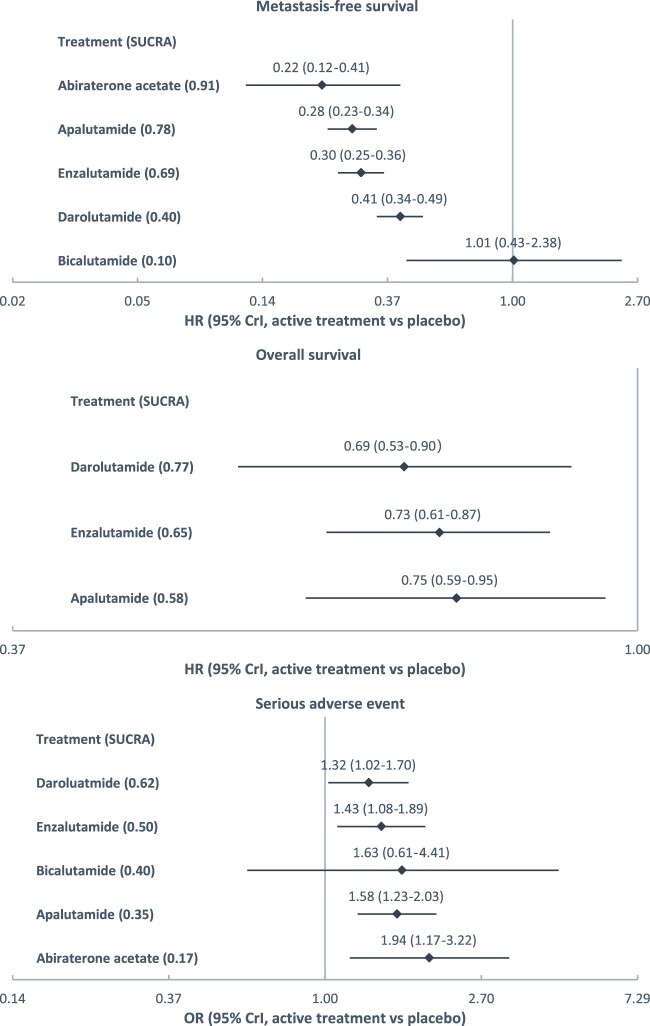
We analyzed 5 trials with 4360 participants. Compared with placebo, abiraterone acetate engendered the lowest hazard of metastasis and death (hazard ratio [HR] = 0.22, 95% credible interval [CrI] = 0.12–0.41), followed by apalutamide (HR = 0.28, 95% CrI = 0.23–0.34), enzalutamide (HR = 0.30, 95% CrI = 0.25–0.36), and darolutamide (HR = 0.41, 95% CrI = 0.34–0.49); darolutamide led to the lowest hazard of death (HR = 0.69, 95% CrI = 0.53–0.90), followed by enzalutamide (HR = 0.73, 95% CrI = 0.61–0.87) and apalutamide (HR = 0.75, 95% CrI = 0.59–0.95); darolutamide resulted in the lowest odds of serious adverse events (odds ratio [OR] = 1.32, 95% CrI = 1.02–1.70), followed by enzalutamide (OR =1.43, 95% CrI = 1.08–1.89), apalutamide (OR = 1.58, 95% CrI = 1.23–2.03), and abiraterone acetate (OR = 1.94, 95% CrI = 1.17–3.22).
Conclusions
For nmCRPC, darolutamide offered optimal efficacy and safety among approved drugs, and abiraterone acetate may offer comparable metastasis-free survival benefit with cost savings from generic availability. Future research is needed to more fully examine the benefit of abiraterone acetate.
Nonmetastatic castration-resistant prostate cancer (nmCRPC) is defined by rising levels of serum prostate-specific antigen (PSA) despite androgen-deprivation therapy in the absence of metastases on conventional imaging (1,2). Among nmCRPC patients, higher PSA levels and shorter PSA doubling time are associated with increased risks of metastases and mortality (3-5). Three androgen receptor inhibitors—apalutamide, enzalutamide, and darolutamide—have been developed as add-on therapy to androgen-deprivation therapy for nmCRPC since 2018. Phase III randomized controlled trials (RCTs) demonstrated their superiority to placebo in delaying PSA progression, metastasis, and death (6–8), leading to their regulatory approval by the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA). An androgen synthesis inhibitor, abiraterone acetate, originally approved for metastatic prostate cancer, was also under development for nmCRPC. In the phase II, single-arm trial (IMAAGEN), patients receiving abiraterone acetate showed delayed PSA progression and metastasis to the same extent that was observed in patients receiving the approved drugs (9). However, abiraterone acetate development for nmCRPC was terminated because of a successful generic challenge in the United States and pending expiry of regulatory exclusivity in Europe (10,11). Nevertheless, abiraterone acetate is commonly used off-label to treat nmCRPC (12–16).
As no RCTs have directly compared these drugs for nmCRPC, guidelines from the US National Comprehensive Cancer Network and the European Society for Medical Oncology currently do not suggest any preference for one of these treatments over the others (1,2). Although previous studies have attempted indirect treatment comparisons, they have been limited to approved drugs and thus have excluded abiraterone acetate (17–20). Furthermore, these studies have failed to estimate relative treatment effects in different patient subgroups and to address nonproportional hazards presented in the underlying trials.
Besides the evidence gap in relative efficacy and safety, these drugs also have widely varying costs because of different patent protection status. For example, the drug acquisition costs for a median 2-year treatment course in the United States range from $34 378 for generic abiraterone acetate to $201 439 for darolutamide (Nubeqa), with enzalutamide (Xtandi) and apalutamide (Erleada) at $177 680 and $190 314, respectively (21). For Europe, similar price disparity is expected in 2022. We compared the efficacy and safety of these drugs for nmCRPC to inform clinical practice and reimbursement policy and to identify evidence gaps for future research.
Methods
Search Strategy and Selection Criteria
We prospectively registered our study with PROSPERO (CRD42020160839) (22). We included parallel-group RCTs and single-arm nmCRPC trials with a median follow-up of at least 12 months. Interventions of interest were apalutamide, enzalutamide, darolutamide, and abiraterone acetate; comparators of interest were any active drug, placebo, and no treatment. We excluded abstracts and trial reports without results because of the insufficient information these would provide for inclusion. We searched bibliographic databases, including MEDLINE, EMBASE, and Cochrane CENTRAL; trial registries, including ClinicalTrials.gov and the European Union Clinical Trials Register; and regulatory documents, including the FDA and EMA review reports, from inception to November 5, 2020 (Supplementary Methods, available online). We did not apply any language or date restriction.
We performed title and abstract screening using Sysrev and full-text screening using Endnote X9. Two investigators (LW and AF) independently performed the screening and data extraction and resolved discrepancies by discussion. Deidentified patient-level data of the 6 eligible trials were requested from 2 trial data-sharing websites, YODA (19) and Vivli (20), and secured for 2 trials, IMAAGEN (9) and STRIVE (19). We included all eligible trials in the systematic review. Only RCTs were included in network meta-analysis except for abiraterone acetate, for which we included the only available, single-arm trial IMAAGEN (9).
Statistical Analysis
For each eligible trial, we extracted information regarding trial design, baseline characteristics, and results (see Supplementary Table 1, available online). We extracted the most recent results in settings where we identified multiple publications reporting the results of a single trial. Our efficacy outcomes of interest were metastasis-free survival (MFS), defined as the time from randomization to radiographic evidence of metastasis or death from any cause, whichever occurs first, and overall survival (OS). Our safety outcome of interest was serious adverse events (SAEs). SAE was defined as any untoward medical occurrence that at any dose results in death, is life-threatening, requires inpatient hospitalization or prolongation of existing hospitalization, results in persistent or clinically significant disability or incapacity, or is a congenital anomaly or birthdefect (23). We assessed the risk of bias for the primary outcome, MFS, using the Cochrane Collaboration tool (2.0) adapted to single-arm trials (24). The overall bias was judged from 5 domains: the randomization process, deviations from intended interventions, missing outcome data, outcome measurement, and selection of reported results. We interpreted our results after having considered the risk of bias assessments (25).
Network meta-analysis facilitates indirect comparisons between treatments that were not compared in head-to-head trials; it typically requires a “connected network” formed by RCTs with shared comparators. As abiraterone acetate for nmCRPC was tested only in the single-arm trial IMAAGEN (9), we connected it to the treatment network by using a matching-adjusted indirect comparison (MAIC) method (26). In addition to connecting abiraterone acetate to the network, MAIC accounts for the risk of bias from the single-arm trial because of a lack of randomization. Thus, we selected an RCT (SPARTAN) (8) from among the included trials that has a population most closely resembling the IMAAGEN population based on baseline characteristics; estimated inverse probability weights for individuals in IMAAGEN to match individuals in SPARTAN, with covariates including age, PSA, Eastern Cooperative Oncology Group (ECOG) performance status, and prior prostatectomy or radiation therapy; applied weights to IMAAGEN so that covariates were balanced among 2 trials as if they were from the same pseudo-RCT; and estimated weighted outcomes of the pseudo-RCT, and included it in the network meta-analysis (see the Supplementary Methods, available online). As an indirect comparison method recommended by the National Institute for Health and Care Excellence of the United Kingdom, MAIC allows inverse probability weights to be estimated with patient-level data in 1 trial—in our case IMAAGEN, and aggregate-level data in the other trial—in our case SPARTAN (26). MAIC uses the method of moments (27) to estimate weights so that the weights exactly balance the mean covariate values between the weighted IMAAGEN population and the SPARTAN population.
We performed Bayesian network meta-analyses using generalized linear models (28). We fitted fixed effects models because treatment comparisons were examined in at most 1 RCT. As primary analyses for OS and MFS, time-invariant hazard ratios (HRs) from individual trials were combined to estimate overall hazard ratios with 95% credible intervals (CrIs). For patient subgroups that were consistently assessed across trials, we performed subgroup network meta-analyses. For SAEs, odds ratios (ORs) estimated from individual trials were combined to estimate the overall odds ratio with 95% credible intervals.
As secondary analyses for OS and MFS, we estimated time-varying hazard ratios using Bayesian parametric survival network meta-analysis and compared expected survival curves across treatments. Specifically, we reconstructed patient-level survival data from published Kaplan-Meier curves using Guyot algorithms (29) and the STATA 16 ipdfc package (30). For IMAAGEN (9) and STRIVE (19), we used the original patient-level data. We fitted a series of first-order fractional polynomial models with power parameter p = (-2,-1,-0.5,0, 0.5,1,2,3), which include common survival distributions such as Weibull (p = 0) and Gompertz (p = 1) (31). We used the deviance information criterion to assess model fit (32).
We used Markov chain Monte Carlo (MCMC) algorithms to estimate treatment effects and diffuse priors to allow observed trial data to inform the estimation (28). We used 4 parallel MCMC chains comprising 100 000 samples in primary analyses and 2 parallel MCMC chains containing 80 000 samples in secondary analyses, after a 5000-sample burn-in. The convergence of MCMC chains was checked by Gelman-Rubin diagnostic statistics (33). Treatment effect sizes (median, 95% CrI) and ranking probabilities were derived from MCMC samples. The surface under the cumulative ranking line (SUCRA) was used to summarize treatment rankings. SUCRA ranges from 0 to 1. The closer SUCRA to 1, the higher the treatment rank. We performed MAIC and primary analyses in R 4.0.3 (31) using sandwich, survival, and gemtc packages (34–36) and secondary analyses in WinBUGS 1.4.3 (37). We have reported the parametric survival network meta-analysis method previously (38).
Results
We identified 10 909 unique study records and screened the full text of 126 publication citations and all trial registrations and regulatory records (Supplementary Figure 1, available online). We included 6 trials in the systematic review: 4 phase III RCTs (6–8,39) involving apalutamide, enzalutamide, darolutamide, bicalutamide, and 2 phase II single-arm trials IMAAGEN (9) and ARN-509-001 (40) involving abiraterone acetate and apalutamide, respectively (Table 1). We excluded ARN-509-001 from the network meta-analysis. Because for apalutamide, the available RCT, SPARTAN, has 1207 patients, whereas the single-arm trial ARN-509-001 has only 51 patients. Including the single-arm trial would only add bias without improving precision. The remaining 5 trials formed the network in Figure 1.
Table 1.
Characteristics and results of included trialsa
| Trial ID | Design (phase) | Main eligibility criteria | Treatments | nmCRPC, No. | Median follow-up, mo | MFS |
OSb |
SAE, n/No. (%) |
|||
|---|---|---|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | Median survival, mo | HR (95% CI) | Median survival, mo | Treatment | Control | ||||||
| ARN-509-001 (40–43) | Single-arm (I/II) | nmCRPC; PSADT ≤ 10 mo or PSA ≥ 8 ng/ml; ECOG 0-1; No history or predisposition of seizure | Apalutamide | 51 | MFS 28.0 | NA | NR | NA | NA | 16/51 (31.4%) | NA |
| SPARTAN (8, 42–49) | RCT (III) | nmCRPC; PSADT ≤10 mo; ECOG 0-1; no history or predisposition of seizure | Apalutamide vs placebo | 1207 (806 vs 401) |
|
0.28 (0.23 to 0.35) | 40.5 vs 16.2 | 0.78 (0.64 to 0.96) | 73.9 vs 59.9 | 199/803 (24.8%) | 92/398 (23.1%) |
| PROSPER (7, 50–55) | RCT (III) | nmCRPC; PSADT ≤10 mo; ECOG 0-1; no history or predisposition of seizure; no active leptomeningeal disease | Enzalutamide vs placebo | 1401 (933 vs 468) |
|
0.30 (0.25 to 0.36) | 36.6 vs 14.7 | 0.73 (0.61 to 0.89) | 67.0 vs 56.3 | 226/930 (24.3%) | 85/465 (18.3%) |
| STRIVE (39, 54, 56)c | RCT (III) | CRPC; PSADT ≤10 mo or PSA ≥5 ng/mL; ECOG 0-1; no history or predisposition of seizure; no active leptomeningeal disease | Enzalutamide vs bicalutamide |
|
|
|
|
NA | NA |
|
|
| ARAMIS (6, 57–60) | RCT (III) | nmCRPC; PSADT ≤10 mo; ECOG 0-1 | Enzalutamide vs placebo | 1509 (955 vs 554) |
|
0.41 (0.34 to 0.50) | 40.4 vs 18.4 | 0.69 (0.53 to 0.88) | NR vs NR | 237/954 (24.8%) | 111/554 (20.0%) |
| IMAAGEN (9, 49)c | Single-arm (II) | nmCRPC; PSADT ≤10 mo or PSA ≥10 ng/ml; ECOG 0-2; medical condition contraindicating prednisone use | Abiraterone acetate + prednisone | 131 | MFS 40 | NA | NR | NA | NA | 47/131 (35.9%) | NA |
Results of MFS and AEs were from primary analyses before unblinding and crossover. (For the single-arm trial IMAAGEN where crossover did not apply, the second data cutoff date of the trial in November 2014 was selected for SAE to allow for comparable treatment duration.) CI = confidence interval; CRPC = nonmetastatic castration-resistant prostate cancer; ECOG = Eastern Cooperative Oncology Group; HR = hazard ratio; MFS = metastasis-free survival; nmCRPC = nonmetastatic castration-resistant prostate cancer; NA = not applicable; NR = not reached; OS = overall survival; PSA = prostate-specific antigen; PSADT = PSA doubling time; RCT = randomized controlled trial; SAE = serious adverse event.
Results of OS were from final analyses with mature OS data after unblinding and crossover.
Results of STRIVE and IMAAGEN trials were from our analysis of patient-level data.
Figure 1.
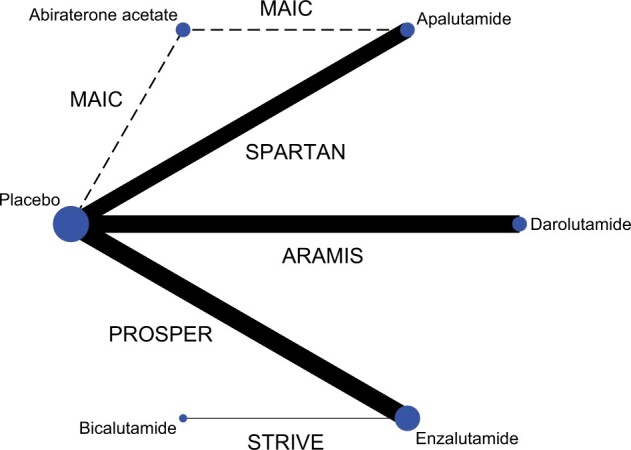
Network graph of treatment comparison. The figure depicts the underlying evidence base of this study. Circles represent competing treatments; solid lines show which treatments have been compared in randomized controlled trials; and the dashed line denotes matching-adjusted indirect comparison (MAIC). The circle size is proportional to the number of trials evaluating each treatment; and the line thickness is proportional to the precision (the inverse of the variance of hazard ratios of metastasis or death estimated from metastasis-free survival) of each comparison. The labels on the lines are trial names.
These 5 trials involved 4360 participants. The median sample size was 1207 (range = 112-1509), the median follow-up duration was 19.9 months (range = 17.9-40) for MFS and 48 months (range = 29-52) for OS. The main eligibility criteria entailed nmCRPC with a PSA doubling time of 10 months or less. Although STRIVE recruited both individuals with nmCRPC and metastatic castration-resistant prostate cancer (39), we only included data from individuals with nmCRPC who had a PSA doubling time of 10 months or less. All 5 trials assessed MFS and SAEs, and 3 trials assessed OS (Table 1). IMAAGEN reported time to radiographic evidence of disease progression (9). With the available patient-level data, including the time to death, we estimated MFS. Efficacy outcomes assessed in trials, along with definitions, are summarized in Supplementary Table 2 (available online).
We detected the risk of bias within all 6 trials included in the review (Table 2). Notably, missing outcome data were a concern for all 6 trials. In each trial, the number of participants with missing data was more than 10.0% of the observed number of events (metastasis or death) and was distributed unevenly between treatment arms. Yet, no method was used to correct for bias and neither was a sensitivity analysis done showing that results were robust against different assumptions about the missing data and its actual value. Furthermore, selective reporting of results may be of concern for all trials except IMAAGEN. In these trials, we found 1) the protocol (including the statistical analysis plan) was finalized after the data cutoff and unblinded analysis, 2) the protocol was not publicly available, or 3) the data cutoff date was not reported. Ideally, the analysis should be specified before unblinded data become available. Otherwise, investigators may cherry-pick the analyses that better support their hypotheses.
Table 2.
Risk of bias within trials
| Trial ID | Treatment | Control | Randomization process | Deviations from intended interventionsa | Missing outcome datab | Measurementof the outcomec | Selection of thereported resultc | Overall biase |
|---|---|---|---|---|---|---|---|---|
| ARN-509-001 | Apalutamide | NA | Some concerns | Low | Some concerns | Some concerns | Some concerns | High |
| SPARTAN | Apalutamide | Placebo | Low | Low | Some concerns | Low | Some concerns | Some concerns |
| PROSPER | Enzalutamide | Placebo | Low | Low | Some concerns | Low | Some concerns | Some concerns |
| STRIVE | Enzalutamide | Bicalutamide | Low | Low | Some concerns | Low | Some concerns | Some concerns |
| ARAMIS | Darolutamide | Placebo | Low | Low | Some concerns | Low | Some concerns | Some concerns |
| IMAAGEN | Abiraterone acetate | NA | NA | Some concerns | Some concerns | Some concerns | Low | High |
Concerns were raised for deviations from intended interventions because the deviation from intended intervention may be because of the awareness of assignment by participants or care deliverers, and efficacy analysis was not based on the intention-to-treat population. NA = not applicable.
Concerns were raised about missing outcome data because 1) the number of patients with missing data was more than 10.0% of the number of events and was distributed unevenly between treatment arms; 2) missing data may relate to the outcome and treatment effect; 3) no method was used to correct for bias; and 4) there was no sensitivity analysis showing that results were robust against different assumptions about missing data and its true value.
Concerns were raised about the measurement of the outcome because outcome assessors were not masked, and the outcome assessment could have been influenced.
Concerns were raised about the selection of the reported results because 1) the trial protocol (including the statistical analysis plan) was finalized after the data cutoff, and the protocol indicated that unblinded outcome data may be made available for analysis; 2) the trial protocol was unavailable; or 3) the data cutoff date was not reported.
Overall bias was judged as “low” if all domains were low, “high” if at least 1 domain was high or some concerns were raised in multiple domains, or “some concerns” otherwise.
Trial populations were largely comparable in terms of baseline characteristics, with SPARTAN (8) bearing a close resemblance to IMAAGEN (see Supplementary Table 3, available online) (9). After MAIC, the weighted IMAAGEN trial and the SPARTAN trial were balanced in mean age and PSA and in percentage compositions of ECOG score and history of prostatectomy or radiation therapy. The crude and weighted baseline characteristics and the distribution of weights are available in the Supplementary Methods (available online). Based on MAIC with placebo, abiraterone acetate was associated with a lower hazard ratio of metastasis or death (HR = 0.22, 95% confidence interval = 0.12 to 0.41) estimated from MFS but increased odds ratio of SAE (OR = 1.94, 95% confidence interval = 1.17 to 3.22).
With the available data, we compared 5 treatments with respect to MFS and 3 treatments with respect to OS. Abiraterone acetate was associated with the lowest of metastasis or death (HR = 0.22, CrI = 0.12–0.41) relative to placebo, followed by apalutamide (HR = 0.28, CrI = 0.23–0.34), enzalutamide (HR = 0.30, CrI = 0.25–0.36), darolutamide (HR = 0.41, CrI = 0.34–0.49), and bicalutamide (HR = 1.01, CrI = 0.43–2.38). The corresponding SUCRAs were 0.91, 0.78, 0.69, 0.40, and 0.10, respectively. Darolutamide was associated with the lowest hazard ratio of death (HR = 0.69, CrI = 0.53–0.90) relative to placebo, followed by enzalutamide (HR = 0.73, CrI = 0.61–0.87] and apalutamide (HR = 0.75, CrI = 0.59–0.95). The corresponding SUCRAs were 0.77, 0.65, and 0.58, respectively (Figure 2).
Figure 2.
Treatment effects relative to placebo estimated from network meta-analysis. SUCRA was used to summarize treatment rankings. SUCRA ranges from 0 to 1. The closer SUCRA to 1, the higher the treatment rank. CrI = credible interval; HR = hazard ratio; OR = odds ratio; SUCRA = surface under the cumulative ranking line.
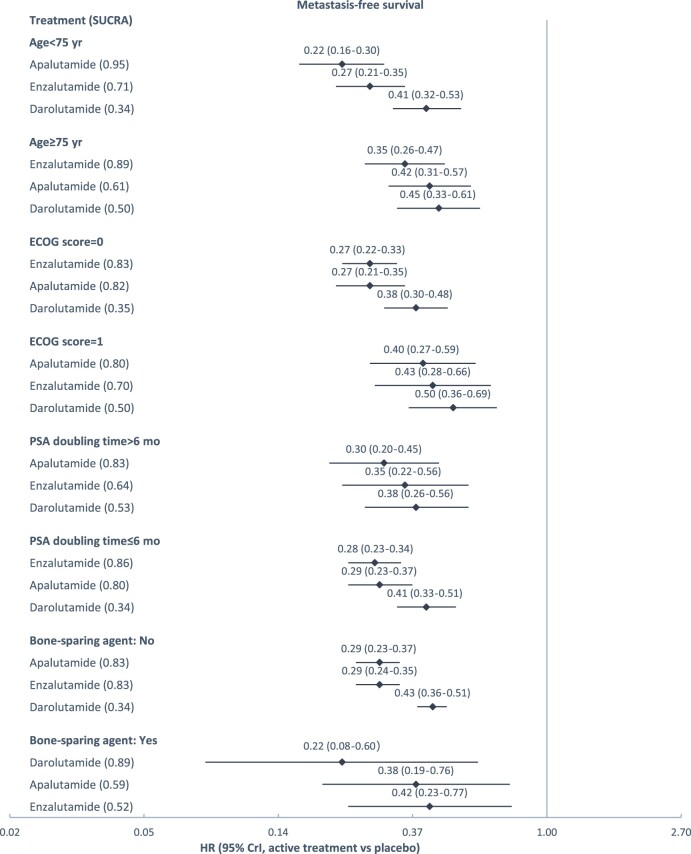
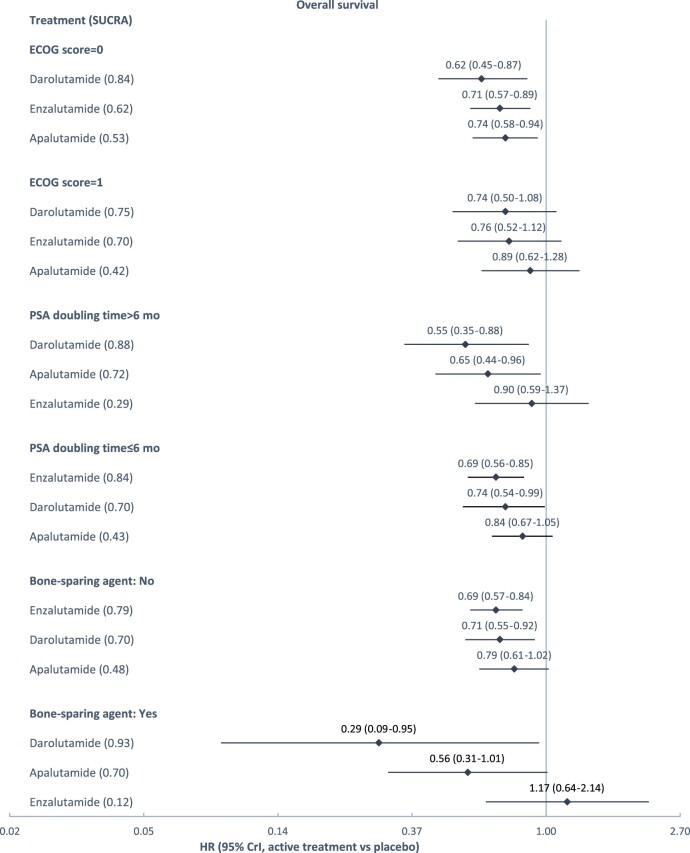
Subgroup network meta-analyses were feasible for the comparison of 3 treatments on MFS and OS, by baseline age (MFS only), ECOG score, PSA doubling time, and bone sparing agent use. The results are presented in Figure 3. Across treatments, better efficacy was observed in younger patients (younger than 75 years of age) and in patients with an ECOG score of 0 (instead of 1). Across patient subgroups, in general, apalutamide and enzalutamide were associated with lower hazard ratios of metastasis or death, and darolutamide was associated with lower hazard ratios of death, relative to placebo.
Figure 3.
Treatment effects relative to placebo estimated from subgroup network meta-analysis. A) Depicts subgroup analyses for metastasis-free survival; (B) depicts subgroup analyses for metastasis-free survival. SUCRA was used to summarize treatment rankings. SUCRA ranges from 0 to 1. The closer SUCRA to 1, the higher the treatment rank. CrI = credible interval; ECOG = Eastern Cooperative Oncology Group; HR = hazard ratio; PSA = prostate-specific antigen; SUCRA = surface under the cumulative ranking line.
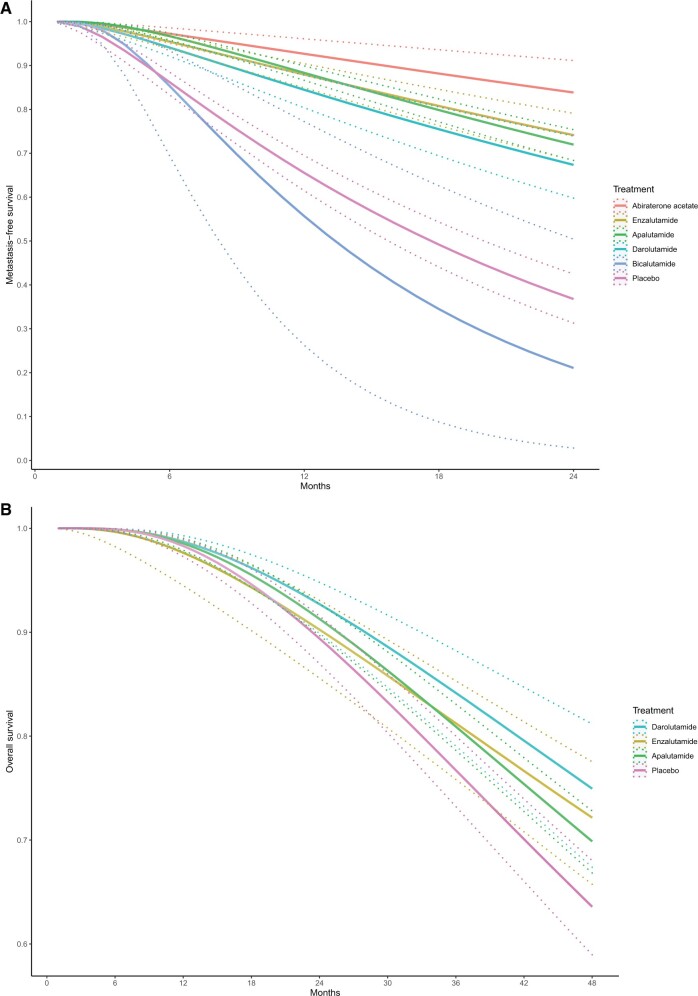
Allowing hazard ratios to change over time, parametric survival network meta-analysis provided consistent treatment rankings. The first-order fractional polynomial model fitted the MFS data best when p = -2 and fitted the OS data best when P = -1. Figure 4 shows the expected MFS and OS curves for each treatment. Abiraterone acetate was associated with the highest MFS probability at 24 months, and darolutamide was associated with the highest OS probability at 48 months. SUCRAs over time are presented in Supplementary Figure 2 (available online).
Figure 4.
Expected survival curves derived from parametric network meta-analysis. A) This graph depicts the expected metastasis-free survival curve. B) The graph depicts the expected overall survival curve. Solid and dashed lines denote the median and 95% credible interval of expected survival probability, respectively.
The risks of SAEs were compared among 5 treatments. Darolutamide was associated with the lowest odds ratio of SAE relative to placebo (1.32, 95% CrI = 1.02–1.70), followed by enzalutamide (1.43, 95% CrI = 1.081.89), bicalutamide (1.63, 95% CrI = 0.61–4.41), apalutamide (1.58, 95% CrI = 1.23–2.03), and abiraterone acetate (1.94, 95% CrI = 1.17–3.22). The corresponding SUCRAs were 0.62, 0.50, 0.40, 0.35, and 0.17, respectively (Figure 2). Bicalutamide had a higher point estimate of odds ratio than apalutamide, and it had a higher SUCRA than apalutamide (ie, ranked higher in terms of safety) because SUCRA takes into account the entire distribution of odds ratio estimates from the MCMC samples, and there were more times that an odds ratio estimate for bicalutamide was lower than that of apalutamide. Ranking probabilities and pairwise comparisons of treatments on MFS, OS, and SAEs are available in Supplementary Figure 3 (available online).
Discussion
We compared treatments for nmCRPC given their common use, variable costs, and the absence of head-to-head trials. To do so, we comprehensively searched for eligible trials, critically appraised trial quality, incorporated RCTs and single-arm trials, and compared treatments based on their efficacy and safety. All 3 FDA- and EMA-approved nmCRPC drugs—apalutamide, enzalutamide, and apalutamide—statistically significantly prolonged OS, with darolutamide providing the largest OS benefit. These 3 drugs and abiraterone acetate also statistically significantly prolonged MFS, with abiraterone acetate providing the largest MFS benefit. All treatments increased SAEs, with darolutamide having the lowest SAE risk. These results suggest that darolutamide has optimal efficacy and safety profile among approved treatments and that abiraterone acetate, a less expensive alternative to approved treatments, may offer comparable value to patients with nmCRPC.
Our findings yield several new insights. First, in contrast to prior reviews (17–20), our analysis included the “forgotten dance partner” (12) abiraterone acetate in the treatment comparisons for nmCRPC (14). As an FDA- and EMA-approved treatment for metastatic castration-sensitive or castration-resistantprostate cancer, abiraterone acetate can delay disease progression, prolong OS, and is well tolerated (61,62). For nmCRPC, we found its MFS benefit promising and safety profile similar to that observed in its approved indications. Although there may be insufficient incentive for the pharmaceutical industry to pursue a phase III RCT validating the long-term benefit of abiraterone acetate, such investments might be worthwhile for the health system. Second, in contrast to prior reviews, we modeled time-varying hazard ratios and validated the robustness of our results based on different assumptions of the time-varying nature of hazard ratios. This approach is important given that nonproportional hazards can be visually spotted from the crossing OS curves in PROSPER (50) and were demonstrated by our parametric survival models. When Kaplan-Meier curves are published, patient-level survival data can be reconstructed to allow the more informative parametric survival network meta-analysis, even if the original trial data are unavailable. Third, the method we used to compare drugs that were only tested in single-arm trials with competitor drugs—a combination of MAIC and network meta-analysis—may be useful for future comparative effectiveness research of cancer treatments, given that as many as one-fifth of pivotal trials supporting the regulatory approvals of oncology drugs were single-arm (63,64). By employing patient-level data and the MAIC method, we created a pseudo-RCT that enabled network meta-analysis in a disconnected treatment network where 1 treatment has only been tested in a single-arm trial. This approach, increasingly possible through patient-level trial data sharing (65), may be preferable to alternative methods such as reference prediction or aggregate level matching because it accounts for the imbalance in patient characteristics induced by single-arm trials (66–68).
Our study has limitations, many related to the underlying trial evidence. First, the trials we examined failed to adjust OS estimates for crossover and subsequent therapies, which may explain the different rankings for darolutamide between MFS and OS. Trials were unblinded after a positive primary analysis for MFS, and patients in the control arm were allowed to cross over to the treatment arm. Different subsequent therapies were given on disease progression based on clinical judgment. Although the unblinding and crossover were inevitable because of ethical concerns and different subsequent treatments were concordant with clinical practice, they may obscure true OS benefits. Several methods are available to adjust OS estimates for crossover and subsequent therapies with patient-level data (69), underscoring the importance of reporting both crude and adjusted OS results. Second, although MAIC was used to account for the imbalance in patient characteristics induced by the single-arm trial, factors that may bias the association between the treatment and outcomes, or residual confounding, cannot be ruled out. An example of such confounding could be that patients’ and clinicians’ judgment of clinical outcomes and adverse events may differ because of their knowledge of the assigned treatment in a single-arm trial as opposed to a double-blinded RCT. The relative effects of abiraterone acetate to other treatments derived from MAIC and network meta-analysis are therefore exploratory and hypothesis generating for future research. Finally, our results are limited by missing data across trials, as well as trial investigators’ neglect to address potential bias. Measures can be taken to reduce missing data in clinical trials at the trial design and conduct stages. For example, a run-in period can be included to ensure that only those who adhere to treatments undergo randomization, efforts should be made to limit the burden of data collection on participants and to gather outcome data after treatment discontinuation, and trial progress should be monitored regarding the acceptable target rates for missing data (70). At the trial analysis stage, appropriate statistical methods can be used to address missing data. For the primary analysis, multiple imputation models are typically recommended, assuming missing data were missing at random (ie, recorded patient characteristics can account for differences in outcomes for observed and missing cases) (70). As this assumption cannot be verified with observed data, sensitivity analyses are often helpful. For example, assuming that the mean outcome differs between observed and missing cases by an offset, investigators can explore the effect on results of a range of offset values that are deemed clinically plausible (70).
Our study has important implications for patients, clinicians, and payers, given the uncertainty about the risk to benefit balance of treatments for nmCRPC, as well as their highly variable costs. Nevertheless, factors beyond those we examined also may inform treatment selection. For example, apalutamide and enzalutamide are associated with increased risks of seizure, ischemic heart disease, falls, and fractures, whereas darolutamide is not (6–8). Patients receiving abiraterone acetate plus prednisone need to be monitored for symptoms and signs of mineralocorticoid excess and adrenocortical insufficiency (9).
For nmCRPC, darolutamide offered optimal efficacy and safety among approved drugs, and abiraterone acetate may offer comparable MFS benefit with cost savings from generic availability. Future research is needed to more fully examine the benefit of abiraterone acetate.
Funding
This work was supported by the Pharmaceutical Research and Manufacturers of America Foundation 2020 Predoctoral Fellowship in Health Outcomes Research and the Ellen B. Gold Scholarship, both awarded to LW. Part of HH’s time was supported by the National Institutes of Health (grant number R00MH111807).
Notes
Role of funders: Funders have no role in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the article for publication.
Disclosures: GA is past chair of FDA’s Peripheral and Central Nervous System Advisory Committee; has served as a paid advisor to IQVIA; is a co-founding principal and equity holder in Monument Analytics, a health-care consultancy whose clients include the life sciences industry as well as plaintiffs in opioid litigation; and is a member of OptumRx’s National P&T Committee. This arrangement has been reviewed and approved by Johns Hopkins University per its conflict-of-interest policies. OB serves as a paid consultant to Genentech. CP serves as a paid consultant to Exelisis.
Author contributions: Conceptualization: LW, CP, OB, and GA. Data curation: LW, LR, and AF. Formal analysis: LW and HH. Funding acquisition: LW, OB, GA, and HH. Investigation: LW, CP, and HH. Methodology: LW and HH. Project administration: LW. Resources: LW, CP, OB, GA, and HH. Software: LW and HH. Supervision: OB, GA, and CP. Validation: HH. Visualization: LW. Writing-original draft: all authors. Writing-review and editing: all authors.
Acknowledgments: This study, carried out under YODA Project # 2019-3886, used data obtained from the Yale University Open Data Access Project, which has an agreement with JANSSEN RESEARCH & DEVELOPMENT, L.L.C.. The interpretation and reporting of research using this data is solely the responsibility of the authors and does not necessarily represent the official views of the Yale University Open Data Access Project or JANSSEN RESEARCH & DEVELOPMENT, L.L.C. This publication is based on research using data from data contributor Pfizer that has been made available through Vivli, Inc. Vivli has not contributed to or approved and is not in any way responsible for the contents of this publication.
Data Availability
As the secondary user of deidentified patient-level clinical trial data, we are not authorized to share the data based on the data use agreements.
Supplementary Material
References
- 1.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Prostate Cancer version 2.2021. https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf. Published February 17, 2021. Accessed May 13, 2021.
- 2. Parker C, Castro E, Fizazi K, et al. Prostate cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2020;31(9):1119–1134. [DOI] [PubMed] [Google Scholar]
- 3. Smith MR, Cook R, Lee KA, Nelson JB.. Disease and host characteristics as predictors of time to first bone metastasis and death in men with progressive castration-resistant nonmetastatic prostate cancer. Cancer. 2011;117(10):2077–2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Smith MR, Kabbinavar F, Saad F, et al. Natural history of rising serum prostate-specific antigen in men with castrate nonmetastatic prostate cancer. J Clin Oncol. 2005;23(13):2918–2925. [DOI] [PubMed] [Google Scholar]
- 5. Smith MR, Saad F, Oudard S, et al. Denosumab and bone metastasis-free survival in men with nonmetastatic castration-resistant prostate cancer: exploratory analyses by baseline prostate-specific antigen doubling time. J Clin Oncol. 2013;31(30):3800–3806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fizazi K, Shore N, Tammela TL, et al. Darolutamide in nonmetastatic, castration-resistant prostate cancer. N Engl J Med. 2019;380(13):1235–1246. [DOI] [PubMed] [Google Scholar]
- 7. Hussain M, Fizazi K, Saad F, et al. Enzalutamide in men with nonmetastatic, castration-resistant prostate cancer. N Engl J Med. 2018;378(26):2465–2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Smith MR, Saad F, Chowdhury S, et al. Apalutamide treatment and metastasis-free survival in prostate cancer. N Engl J Med. 2018;378(15):1408–1418. [DOI] [PubMed] [Google Scholar]
- 9. Ryan CJ, Crawford ED, Shore ND, et al. The IMAAGEN study: effect of abiraterone acetate and prednisone on prostate specific antigen and radiographic disease progression in patients with nonmetastatic castration resistant prostate cancer. J Urol. 2018;200(2):344–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Small EJ, Saad F, Chowdhury S, et al. ; on behalf of the SPARTAN investigators. SPARTAN, a phase 3 double-blind, randomized study of apalutamide (APA) versus placebo (PBO) in patients (PTS) with nonmetastatic castration-resistant prostate cancer (nmCRPC). J Clin Oncol. 2018;36(suppl 6):161. [Google Scholar]
- 11.Johnson and Johnson. Court issues ruling in ZYTIGA® patent infringement litigation. https://www.jnj.com/court-issues-ruling-in-zytiga-patent-infringement-litigation. Published October 26, 2018. Accessed October 18, 2020.
- 12. Klaassen Z, Wallis CJD, Fleshner NE.. Abiraterone acetate for nonmetastatic castration-resistant prostate cancer—the forgotten dance partner? JAMA Oncol. 2019;5(2):144–145. [DOI] [PubMed] [Google Scholar]
- 13. Lowrance WT, Murad MH, Oh WK, Jarrard DF, Resnick MJ, Cookson MS.. Castration-resistant prostate cancer: AUA guideline amendment 2018. J Urol. 2018;200(6):1264–1272. [DOI] [PubMed] [Google Scholar]
- 14.Institute for Clinical and Economic Review. Final evidence report: antiandrogen therapies for nonmetastatic castration-resistant prostate cancer: effectiveness and value. https://icer.org/wp-content/uploads/2020/10/ICER_Prostate_Cancer_Final_Evidence_Report_100418.pdf. Published October 4, 2018. Accessed November 8, 2020.
- 15. Shah R, Botteman M, Waldeck R.. Treatment characteristics for nonmetastatic castration-resistant prostate cancer in the United States, Europe and Japan. Future Oncol. 2019;15(35):4069–4081. [DOI] [PubMed] [Google Scholar]
- 16. Appukkuttan S, Tangirala K, Babajanyan S, Wen L, Simmons S, Shore N.. A retrospective claims analysis of advanced prostate cancer costs and resource use. PharmacoEconomics. 2020;4(3):439–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hird AE, Magee DE, Bhindi B, et al. A systematic review and network meta-analysis of novel androgen receptor inhibitors in non-metastatic castration-resistant prostate cancer. Clin Genitourin Cancer. 2020;18(5):343–350. [DOI] [PubMed] [Google Scholar]
- 18. Kumar J, Jazayeri SB, Gautam S, et al. Comparative efficacy of apalutamide darolutamide and enzalutamide for treatment of non-metastatic castrate-resistant prostate cancer: a systematic review and network meta-analysis. Urol Oncol. 2020. Nov;38(11):826–834. [DOI] [PubMed] [Google Scholar]
- 19. Chowdhury S, Oudard S, Uemura H, et al. Matching-adjusted indirect comparison of the efficacy of apalutamide and enzalutamide with ADT in the treatment of non-metastatic castration-resistant prostate cancer. Adv Ther. 2020;37(1):501–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chowdhury S, Oudard S, Uemura H, et al. Matching-adjusted indirect comparison of health-related quality of life and adverse events of apalutamide versus enzalutamide in non-metastatic castration-resistant prostate cancer. Adv Ther. 2020;37(1):512–526. [DOI] [PubMed] [Google Scholar]
- 21.US Department of Veterans Affairs Office of Procurement, Acquisition and Logistics. Pharmaceutical prices; 2020. https://www.va.gov/opal/nac/fss/pharmPrices.asp. Updated (as of October 1, 2020. Accessed October 18, 2020.
- 22. Wang LB, Alexander C, Paller C, Hong H, Ballreich J, Clinical and cost-effectiveness of competing treatments for advanced prostate cancer: a network meta-analysis and economic evaluation. PROSPERO 2020 CRD42020160839. https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42020160839. Published April 28, 2020. Accessed October 19, 2020.
- 23.The United States Food and Drug Administration. Code of Federal Regulations Title 21 Part 312 Sec. 312.32 IND safety reporting. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=312.32. Published September 29, 2020. Updated April 1, 2020. Accessed October 20, 2020.
- 24. Sterne JAC, Savovic J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019. Aug 28;366:l4898. [DOI] [PubMed] [Google Scholar]
- 25. Higgins JPT TJ, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, eds. Cochrane Handbook for Systematic Reviews of Interventions version 6.1. Cochrane; 2020. www.training.cochrane.org/handbook. Updated September 2020. Accessed December 15, 2020.
- 26. Phillippo DAA, Dias S, Palmer S, Abrams K, Welton N. NICE DSU technical support document 18: methods for population-adjusted indirect comparisons in submissions to NICE. http://nicedsu.org.uk/technical-support-documents/population-adjusted-indirect-comparisons-maic-and-stc/. Published December 2016. Accessed October 23, 2020.
- 27. Signorovitch JE, Wu EQ, Yu AP, et al. Comparative effectiveness without head-to-head trials: a method for matching-adjusted indirect comparisons applied to psoriasis treatment with adalimumab or etanercept. PharmacoEconomics. 2010;28(10):935–945. [DOI] [PubMed] [Google Scholar]
- 28. Dias S, Ades AE, Welton NJ, Jansen JP, Sutton AJ.. Network Meta-Analysis for Decision-Making (Statistics in Practice). Hoboken, NJ: Wiley; 2018. [Google Scholar]
- 29. Guyot P, Ades AE, Ouwens MJ, Welton NJ.. Enhanced secondary analysis of survival data: reconstructing the data from published Kaplan-Meier survival curves. BMC Med Res Methodol. 2012. Feb 1;12:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wei Y, Royston P.. Reconstructing time-to-event data from published Kaplan-Meier curves. Stata J. 2017;17(4):786–802. [PMC free article] [PubMed] [Google Scholar]
- 31. Jansen JP. Network meta-analysis of survival data with fractional polynomials. BMC Med Res Methodol. 2011. May 6;11:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Spiegelhalter DJ, Best NG, Carlin BP, Van Der Linde A.. Bayesian measures of model complexity and fit. J Roy Stat Soc B. 2002;64(4):583–639. [Google Scholar]
- 33. Brooks SP, Gelman A.. General methods for monitoring convergence of iterative simulations. J Comput Graph Stat. 1998;7(4):434–455. [Google Scholar]
- 34. Zeileis A, Köll S, Graham N.. Various versatile variances: an object-oriented implementation of clustered covariances in R. J Stat Softw. 2020;95(1):36. [Google Scholar]
- 35. Therneau T. A package for survival analysis in R. R package version 3.2-7. https://CRAN.R-project.org/package=survival. Published September 28, 2020. Accessed October 23, 2020.
- 36.GEMTC. Network meta-analysis using Bayesian methods. R package version 0.8-7. https://CRAN.R-project.org/package=gemtc. Published August 10, 2020. Accessed October 23, 2020.
- 37. Lunn DJ, Thomas A, Best N, Spiegelhalter D.. WinBUGS–a Bayesian modelling framework: concepts, structure, and extensibility. Stat Comput. 2000;10(4):325–337. [Google Scholar]
- 38. Wang L, Paller CJ, Hong H, De Felice A, Alexander GC, Brawley O.. Comparison of systemic treatments for metastatic castration-sensitive prostate cancer: a systematic review and network meta-analysis. JAMA Oncol. 2021;7(3):412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Penson DF, Armstrong AJ, Concepcion R, et al. Enzalutamide versus bicalutamide in castration-resistant prostate cancer: the STRIVE trial. J Clin Oncol. 2016;34(18):2098–2106. [DOI] [PubMed] [Google Scholar]
- 40. Smith MR, Antonarakis ES, Ryan CJ, et al. Phase 2 study of the safety and antitumor activity of apalutamide (ARN-509), a potent androgen receptor antagonist, in the high-risk nonmetastatic castration-resistant prostate cancer cohort. Eur Urol. 2016;70(6):963–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.US National Library of Medicine ClinicalTrials.gov. Safety, pharmacokinetic and proof-of-concept study of ARN-509 (apalutamide) in castration-resistant prostate cancer (CRPC). https://clinicaltrials.gov/ct2/show/NCT01171898. Published July 29, 2010. Updated March 2, 2021. Accessed May 13, 2021.
- 42.US Food and Drug Administration. Drug approval package: ERLEADA (apalutamide). https://www.accessdata.fda.gov/drugsatfda_docs/nda/2018/Erleada_210951_toc.cfm. Published March 19, 2018. Updated 7/6/2018. Accessed October 24, 2020.
- 43.European Medicines Agency. Committee for Medicinal Products for Human Use (CHMP) assessment report: Erleada (apalutamide). https://www.ema.europa.eu/en/documents/assessment-report/erleada-epar-public-assessment-report_en.pdf. November 15, 2018. Accessed October 24, 2020.
- 44. Saad F, Cella D, Basch E, et al. Effect of apalutamide on health-related quality of life in patients with non-metastatic castration-resistant prostate cancer: an analysis of the SPARTAN randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2018;19(10):1404–1416. [DOI] [PubMed] [Google Scholar]
- 45. Small EJ, Saad F, Chowdhury S, et al. Apalutamide and overall survival in non-metastatic castration-resistant prostate cancer. Ann Oncol. 2019;30(11):1813–1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Uemura H, Satoh T, Tsumura H, et al. Efficacy and safety of apalutamide in Japanese patients with non-metastatic castration-resistant prostate cancer: a subgroup analysis of a randomized, double-blind, placebo-controlled, phase 3 study. Prostate Int. 2020;8(4):190–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Mori K, Mostafaei H, Pradere B, et al. Apalutamide, enzalutamide, and darolutamide for non-metastatic castration-resistant prostate cancer: a systematic review and network meta-analysis. Int J Clin Oncol. 2020;25(11):1892–1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.US National Library of Medicine ClinicalTrials.gov. A study of apalutamide (ARN-509) in men with non-metastatic castration-resistant prostate cancer (SPARTAN). https://clinicaltrials.gov/ct2/show/NCT01946204. Published Update April 5, 2021. Accessed May 13, 2021.
- 49. Smith MR, Saad F, Chowdhury S, et al. Apalutamide and overall survival in prostate cancer. Eur Urol. 2021. Jan;79(1):150–158. [DOI] [PubMed] [Google Scholar]
- 50. Sternberg CN, Fizazi K, Saad F, et al. Enzalutamide and survival in nonmetastatic, castration-resistant prostate cancer. New Engl J Med. 2020;382(23):2197–2206. [DOI] [PubMed] [Google Scholar]
- 51.US National Library of Medicine ClinicalTrials.gov. Safety and efficacy study of enzalutamide in patients with nonmetastatic castration-resistant prostate cancer (PROSPER). https://clinicaltrials.gov/ct2/show/NCT02003924. Published Update April 27, 2021. Accessed May 13, 2021.
- 52.European Union Clinical Trials Register. PROSPER: a multinational, phase 3, randomized, double-blind, placebo-controlled, efficacy and safety study of enzalutamide in patients with nonmetastatic castration-resistant prostate cancer. https://www.clinicaltrialsregister.eu/ctr-search/trial/2012-005665-12/results. Published July 15, 2018. Updated October 31, 2020. Accessed May 13, 2021.
- 53.US Food and Drug Administration. Supplement approval Xtandi (enzalutamide). https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2018/203415Orig1s014ltr.pdf. Published July 13, 2018. Accessed October 24, 2020.
- 54.European Medicines Agency. Committee for Medicinal Products for Human Use (CHMP) assessment report: Xtandi (enzalutamide). https://www.ema.europa.eu/en/documents/variation-report/xtandi-h-c-2639-ii-0039-g-epar-assessment-report-variation_en.pdf. Published September 20, 2018. Accessed October 24, 2020.
- 55. Tombal B, Saad F, Penson D, et al. Patient-reported outcomes following enzalutamide or placebo in men with non-metastatic, castration-resistant prostate cancer (PROSPER): a multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol. 2019;20(4):556–569. [DOI] [PubMed] [Google Scholar]
- 56.US National Library of Medicine ClinicalTrials.gov. Safety and efficacy study of enzalutamide versus bicalutamide in men with prostate cancer (STRIVE). https://clinicaltrials.gov/ct2/show/NCT01664923. Published Update August 14, 2012. Updated January 30, 2019. Accessed October 24, 2020.
- 57.US National Library of Medicine ClinicalTrials.gov. Efficacy and safety study of darolutamide (ODM-201) in men with high-risk nonmetastatic castration-resistant prostate cancer (ARAMIS). https://clinicaltrials.gov/ct2/show/NCT02200614. Published Update May 13, 2021. Accessed May 13, 2021.
- 58.US Food and Drug Administration. Drug approval package: Nubeqa (darolutamide). https://www.accessdata.fda.gov/drugsatfda_docs/nda/2019/212099Orig1s000TOC.cfm. Published July 30, 2019. Accessed October 24, 2020.
- 59.European Medicines Agency. Committee for Medicinal Products for Human Use (CHMP) assessment report: Nubeqa (darolutamide). https://www.ema.europa.eu/en/documents/assessment-report/nubeqa-epar-public-assessment-report_en.pdf. Published January 30, 2020. Accessed October 24, 2020.
- 60. Fizazi K, Shore N, Tammela TL, et al. Nonmetastatic, castration-resistant prostate cancer and survival with darolutamide. New Engl J Med. 2020;383(11):1040–1049. [DOI] [PubMed] [Google Scholar]
- 61. Fizazi K, Tran N, Fein L, et al. Abiraterone acetate plus prednisone in patients with newly diagnosed high-risk metastatic castration-sensitive prostate cancer (LATITUDE): final overall survival analysis of a randomised, double-blind, phase 3 trial. Lancet Oncol. 2019;20(5):686–700. [DOI] [PubMed] [Google Scholar]
- 62. Hoyle AP, Ali A, James ND, et al. Abiraterone in “high-” and “low-risk” metastatic hormone-sensitive prostate cancer. Eur Urol. 2019;76(6):719–728. [DOI] [PubMed] [Google Scholar]
- 63. Naci H, Davis C, Savović J, et al. Design characteristics, risk of bias, and reporting of randomised controlled trials supporting approvals of cancer drugs by European Medicines Agency, 2014-16: cross sectional analysis. BMJ. 2019. Sep 18;366:l5221 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Tibau A, Molto C, Ocana A, et al. Magnitude of clinical benefit of cancer drugs approved by the US food and drug administration. J Natl Cancer Inst. 2018;110(5):486–492. [DOI] [PubMed] [Google Scholar]
- 65. Taichman DB, Sahni P, Pinborg A, et al. Data sharing statements for clinical trials: a requirement of the International Committee of Medical Journal Editors. Lancet. 2017;389(10086):e12–e14. [DOI] [PubMed] [Google Scholar]
- 66. Béliveau A, Goring S, Platt RW, Gustafson P.. Network meta-analysis of disconnected networks: How dangerous are random baseline treatment effects? Res Synth Methods. 2017;8(4):465–474. [DOI] [PubMed] [Google Scholar]
- 67. Leahy J, Thom H, Jansen JP, et al. Incorporating single-arm evidence into a network meta-analysis using aggregate level matching: assessing the impact. Stati Med. 2019;38(14):2505–2523. [DOI] [PubMed] [Google Scholar]
- 68. Thom H, Network meta-analysis on disconnected evidence networks: What can be done? Cochrane Training. https://training.cochrane.org/resource/network-meta-analysis-disconnected-evidence-networks-what-can-be-done. Published August 2020. Accessed November 9, 2020.
- 69. Latimer NA, NICE DSU Technical support document 16: Adjusting survival time estimates in the presence of treatment switching. http://nicedsu.org.uk/technical-support-documents/treatment-switching-tsd/. Published July 2014. Accessed November 10, 2020. [PubMed]
- 70. Little RJ, D’Agostino R, Cohen ML, et al. The prevention and treatment of missing data in clinical trials. New Engl J Med. 2012;367(14):1355–1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
As the secondary user of deidentified patient-level clinical trial data, we are not authorized to share the data based on the data use agreements.