Abstract
Background
Pancreatic cancer (PC) has a grim prognosis, and an early diagnostic biomarker has been highly desired. The molecular link between diabetes and PC has not been well established.
Methods
Bioinformatics screening was performed for a serum PC marker. Experiments in cell lines (5 PC and 1 normal cell lines), mouse models, and human tissue staining (37 PC and 10 normal cases) were performed to test asprosin production from PC. Asprosin’s diagnostic performance was tested with serums from multi-center cohorts (347 PC, 209 normal, and 55 additional diabetic patients) and evaluated according to PC status, stages, and diabetic status, which was compared with that of CA19-9.
Results
Asprosin, a diabetes-related hormone, was found from the bioinformatics screening, and its production from PC was confirmed. Serum asprosin levels from multi-center cohorts yielded an age-adjusted diagnostic area under the curve (AUC) of 0.987 (95% confidence interval [CI] = 0.961 to 0.997), superior to that of CA19-9 (AUC = 0.876, 95% CI = 0.847 to 0.905), and a cut-off of 7.18 ng/mL, at which the validation set exhibited a sensitivity of 0.957 and a specificity of 0.924. Importantly, the performance was maintained in early-stage and non-metastatic PC, consistent with the tissue staining. A slightly lower performance against additional diabetic patients (n = 55) was restored by combining asprosin and CA19-9 (AUC = 0.985, 95% CI = 0.975 to 0.995).
Conclusions
Asprosin is presented as an early-stage PC serum marker that may provide clues for PC-induced diabetes. Larger prospective clinical studies are warranted to solidify its utility.
Pancreatic cancer (PC) is the fourth-leading cause of cancer-related deaths in developed countries and has a very low 5-year overall survival rate (<10%) (1,2). This dismal prognosis is at least partly due to the silent but rapid progression of PC with early metastasis. In fact, approximately 80% of PC cases are diagnosed at late stages (2,3). Therefore, the early enough detection of PC for resection is the most critical factor for better prognosis, but progress in the field has been sluggish. Contributing to the difficulty of early detection is the lack of reliable noninvasive diagnostic modalities or biomarkers. Currently, the most widely used biomarker is CA19-9, but mainly for assessing the treatment progress of confirmed PC patients rather than for diagnosis (4,5). That is due to the unsatisfactory diagnostic sensitivity and specificity, 79%-81% and 82%-90%, respectively, and its natural absence in people with certain Lewis antigen genotypes (5%-10%) (4,5). Therefore, a sensitive and specific marker that can aid the early diagnosis of PC has been highly desired.
One of the most universal manifestations of PC is impaired glucose homeostasis or diabetes, and there is a bidirectional relationship between PC and diabetes (6,7). On one hand, there is ample epidemiological evidence for increased PC risk (approximately twofold) among people with diabetes (8,9). It has also been suggested that the higher insulin level due to increased blood glucose in diabetes might contribute to the progression and survival of PC cells through insulin-like growth factor 1 (IGF-1) signaling (7). On the other hand, the prevalence of diabetes (approximately 50%-68%) in PC is much higher than that in other solid tumors (approximately 15%-21%) (10). Importantly, pancreatectomy in PC patients with new-onset diabetes can improve blood glucose control despite the large removal of pancreatic tissues (11). Therefore, it has long been suggested that something from PC may impair glucose homeostasis, though the molecular identity of the factor(s) is still elusive.
Asprosin is a proteolytic product from the C-terminal part of profibrillin and is receiving growing interest as an adipose tissue–secreted hormone with glucogenic and orexigenic activities (12-14). People with a rare genetic deficiency of asprosin are extremely lean and exhibit low blood insulin with robust insulin sensitivity (12). Asprosin enhances hepatic glucose output (12), a main factor for increased fasting glucose in type 2 diabetes (15), and high levels of asprosin have been found in diabetes patients (16,17). It has been suggested that an asprosin antagonist might be a novel option against diabetes or insulin resistance (14). However, the roles of asprosin in cancer or in the relationship between PC and diabetes have not been defined. Here, we discovered asprosin as a potential PC marker and tested its performance in differentiating normal and PC groups, including early-stage PC, with a multi-center cohort design. In addition, in vitro and in vivo mouse experiments were performed to determine its relevance to the link between PC and diabetes.
Methods
For the detailed experimental procedures, please refer to the Supplementary Methods (available online).
Study Design and Patient Cohorts
We obtained serum samples for the PC and normal groups from a total of 11 hospital biobanks (total = 556, PC = 347, normal = 209). Written informed consent had been obtained from all the donors by the biobanks. Therefore, a review exemption was obtained from the institutional review board of Seoul National University (reference number E2010/003–010). Of those, 6 hospitals provided PC samples, 2 hospitals normal samples, and 3 hospitals both PC and normal samples (PC = 120, normal = 110). Normal samples were from healthy donors without diabetes or any cancer. Because the samples were from retrospective multi-center cohorts, the sample acquisition and patient recruitment were blinded to the authors and not biased by one particular protocol. Because the biobanks were not aware of the study objective and the authors were not involved in the sample selection from the pools in each biobank, the sampling was as heterogeneous as possible rather than controlled for clinical variables except for the normal, cancer, and diabetic status. Diabetes serum samples, based on clinical diagnosis, were separately obtained from another hospital (n = 55). Among these, 50 patients had long-standing diabetes (≥4 years), and the other 5 patients had 2 and more years of diabetic duration.
Bioinformatics Screening for PC Markers
The gene expression and the meta data for the screening were downloaded from UCSC Xena website (The Cancer Genome Atlas [TCGA] TARGET The Genotype-Tissue Expression [GTEx] cohort , dataset: gene expression RNAseq-RSEM expected_count [DESeq2] standardized), version 2018–05-08, https://xenabrowser.net). Cancer tissue data were from TCGA, and normal tissue data were from both TCGA and GTEx. For PC, the number of cancer samples was 179, and the corresponding normal samples were 169 (4 from TCGA and the others from GTEx). The number of normal and cancer samples for all the other cancer types used in this study is provided in Supplementary Table 1 (available online).
The bioinformatics screening strategy was designed to find a protein with highly differential expression in PC vs normal (for sensitivity) as well as PC vs all of the other 30 cancer types (for specificity). It was applied to the pan-cancer and normal databases with more than 60 000 genes and approximately 12 000 patient samples. Specifically, the candidate marker should meet all the following conditions: 1) the median gene expression difference in PC vs normal tissues should be larger than 4 on the log2 scale (sensitivity criterion); 2) the condition in point 1 above should not be met in any other cancer types in the pan-cancer TCGA (specificity criterion 1); 3) the median expression in PC should be higher than in any other cancer types by at least twofold (specificity criterion 2: 2 exceptions in cancer types allowed to avoid 0 candidate); 4) the median gene expression count should be at least 10 (noise criterion); and 5) the protein should be secreted to blood (serum marker criterion).
Statistical Analysis
Descriptive statistical analyses, including Welch’s t test, Mann-Whitney test, and ordinary 1-way analysis of variance (ANOVA), and receiver operating characteristic (ROC) analysis were performed using Prism (version 9.0.0, GraphPad Software, San Diego, CA, USA). The sensitivity and specificity values were obtained using the shortest Euclidean distance approach on the ROC curve. R (version 4.0.3) was used for multivariable logistic regression (glm function) and the age-adjusted area under the curve (AUC) value (package AROC with the non-parametric Bayesian inference). All statistical tests were 2-sided, and P < .05 was considered statistically significant.
Results
Relevance of Asprosin as a Possible Serum Marker for PC
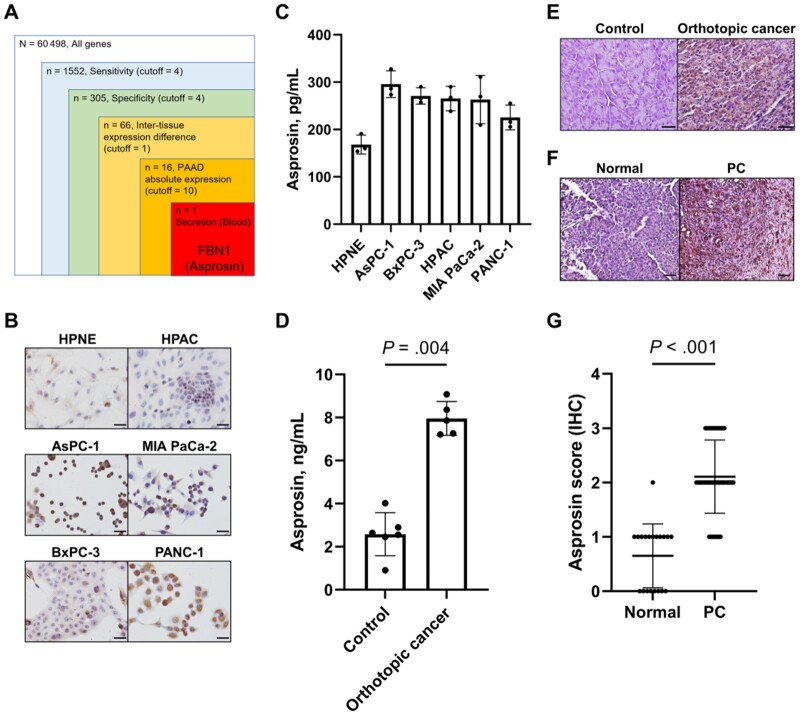
First, we performed bioinformatics screening with stringent criteria to identify a highly sensitive and specific serum protein marker for PC using the TCGA and GTEx databases. Among more than 60 000 genes in approximately 12 000 patient samples in the Xena sequencing data, only 1 met all of the criteria: FBN1 (Figure 1, A). FBN1 is a gene encoding the fibrillin family of proteins, and the FBN1 protein is post-translationally cleaved to yield an extracellular matrix protein fibrillin-1 (N-terminal 2731 residues) and asprosin (C-terminal 140 residues; Supplementary Figure 1, A, available online). Interestingly, asprosin is a blood hormone implicated in insulin resistance (16). Because diabetes and PC are reciprocal risk factors, we focused on asprosin. The expression level of asprosin was higher in the PC cell lines than in a normal pancreatic cell line (Figure 1, B), and asprosin was secreted to the cell media from PC cells (Figure 1, C). In mice, orthotopic transplantation of human PC cells (MIA PaCa-2) to mouse pancreas led to a higher serum asprosin level (Figure 1, D) as well as conspicuous staining in the cancer tissue (Figure 1, E). In humans, staining in PC, noninvolved, and normal pancreatic tissues revealed that the asprosin level was higher in the PC tissues (Figure 1, F and G). Notably, the staining did not differ between early- (stage I and II) and late-stage (III and IV) PC, indicating that the asprosin level increases even in early-stage PC (Supplementary Figure 1, B, available online). Also, the staining was not related to the tumor grade (grades 1, 2, and 3) in PC (Supplementary Figure 1, C, available online). These results suggest that asprosin is secreted from PC and is a potential early marker for it.
Figure 1.
Asprosin's relevance as a possible serum marker for pancreatic cancer. A) Bioinformatics screening was carried out to find a serum marker for pancreatic cancer. FBN1 was identified after 5 steps of screening using The Cancer Genome Atlas and The Genotype-Tissue Expression databases consisting of 31 cancer types and relevant tumor and normal samples from a total of approximately 12 000 patients. B) Immunocytochemistry staining was performed for asprosin in 5 pancreatic cancer (PC) and 1 normal pancreas (HPNE) cell line (scale bar = 50 µm). C) Asprosin levels were measured in cell culture media from B. Asprosin concentrations were determined by enzyme-linked immunosorbent assay (ELISA) after 48-hour incubation of 5 × 104 cells. D) Asprosin levels were measured in serums of control (n = 6) and orthotopic xenograft cancer (n = 5) mice; 2-sided Mann-Whitney test was used because the number of samples for the continuous variable (asprosin concentration) was not enough to guarantee normal distribution. Orthotopic xenograft cancer mice were generated by transplanting MIA PaCa-2 cells to the pancreas. Serum asprosin concentrations were determined by ELISA. E) Representative asprosin immunohistochemistry of pancreas was performed from control and orthotopic xenograft cancer mice (scale bar = 25 µm). F) Representative asprosin immunohistochemistry (IHC) of normal (n = 20, 10 cases) and PC tissues (n = 74, 37 cases) was performed from human patients (scale bar = 25 µm). G) Scores of asprosin staining were obtained from the samples in F; 2-sided Mann-Whitney test was used, because the variable (IHC score: 0, 1, 2, 3) was discrete and thus does not follow normal distribution. Asprosin staining was scored by 4 levels: 0 for none, 1 for weak, 2 for moderate, and 3 for strong staining. Data are presented as the mean ± SD. PAAD = pancreatic adenocarcinoma.
Discrimination of PC and Normal Groups With Serum Asprosin Concentration
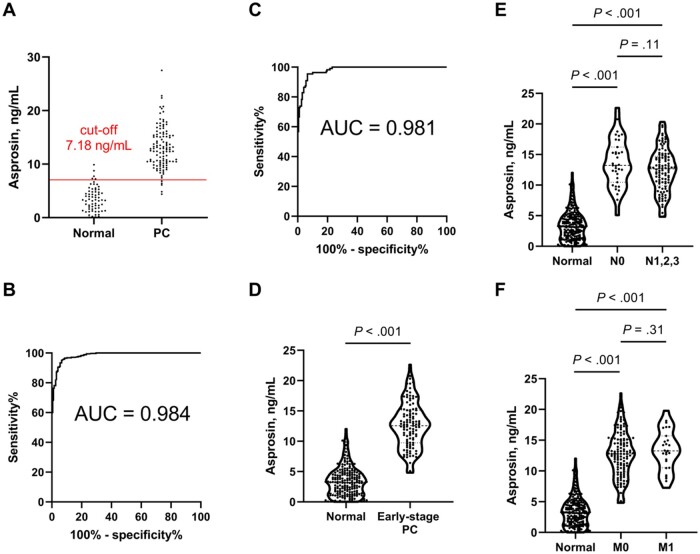
To test the actual marker performance, we obtained serum samples for both the PC and normal groups (PC, n = 347; normal, n = 209; Table 1). Then, two-thirds of the samples were randomly selected from each group and designated as the training set (PC, n = 232; normal, n = 133), and the remaining one-third from each group was designated as the validation set. The asprosin concentration in the training set discriminated PC and normal serum samples with high performance (AUC = 0.982, 95% CI = 0.971 to 0.993; cut-off value 7.18 ng/mL; Supplementary Figure 2, A, available online). This cut-off value predicted the PC status of the samples in the independent validation set with a sensitivity of 0.957 and a specificity of 0.924 (Figure 2, A; Supplementary Table 2, available online). For all of the samples, the asprosin levels were higher in the PC group than in the normal group (mean [SD], normal = 3.38 [2.41] ng/mL vs PC = 13.0 [3.88] ng/mL; P < .001; see Figure 3, A below), and the ROC analysis yielded an AUC of 0.984 (95% CI = 0.976 to 0.992; Figure 2, B). The age-adjusted AUC value (0.987, 95% CI = 0.961 to 0.997) (19,20) was not practically different from the unadjusted value, and the possible effects of age are described in more detail in the Discussion. Compared with CA19-9, asprosin exhibited a superior AUC for the entire-patient analysis (CA19-9’s AUC = 0.876, 95% CI = 0.847 to 0.905; Supplementary Figure 2, B, available online). Overall, it seems that serum asprosin may be suitable for use as a high-performance marker for PC.
Table 1.
Demographic and clinical characteristics of patients
| Sample status | AJCC stage (18) | No. of samples, (%) | Training set, No. | Validation set, No. | Sex |
Median age (range), y | Median BMIa, kg/m2 | |
|---|---|---|---|---|---|---|---|---|
| Male | Female | |||||||
| PC | Total | 347 (56.8) | 232 | 115 | 231 | 116 | 66.0 (29-99) | 21.9 |
| IA | 7 (1.1) | 5 | 2 | 5 | 2 | 65.0 (55-77) | 20.1 | |
| IB | 6 (1.0) | 4 | 2 | 3 | 3 | 70.0 (56-79) | 24.5 | |
| IIA | 18 (2.9) | 12 | 6 | 10 | 8 | 66.0 (44-80) | 22.7 | |
| IIB | 80 (13.1) | 53 | 27 | 55 | 25 | 65.0 (47-86) | 22.6 | |
| Early-stage PC (IA, IB, IIA, IIB) | 111 (18.2) | 74 | 37 | 73 | 38 | 65.0 (44-86) | 22.7 | |
| III | 13 (2.1) | 9 | 4 | 9 | 4 | 67.5 (52-78) | 21.2 | |
| IV | 27 (4.4) | 18 | 9 | 21 | 6 | 64.0 (47-80) | 22.3 | |
| Unknown | 196 (32.1) | 131 | 65 | 128 | 68 | 69.0 (29-99) | 21.7 | |
| Diabetes | NA | 55 (9.0) | 37 | 18 | 31 | 24 | 55.0 (39-72) | 24.7 |
| Normal | NA | 209b (34.2) | 133 | 66 | 113 | 96 | 36.0 (18-73) | 23.1 |
For BMI, 1 out of 27 in PC stage IV, 13 out of 196 in PC with unknown stage, and 16 out of 209 in normal were excluded for data unavailability. AJCC = American Joint Committee on Cancer; BMI = body mass index; NA = not applicable; PC = pancreatic cancer.
Among 209 normal samples, 199 samples were analyzed for asprosin, 207 samples were analyzed for CA19-9, and 197 samples were analyzed for both asprosin and CA19-9 combined. Training and validation set measurements were performed only for asprosin.
Figure 2.
Discrimination between the normal and pancreatic cancer (PC) groups with different stages and metastatic status using serum asprosin concentration. A) Discrimination of normal (n = 66) and PC (n = 115) groups in the validation set with the cut-off value (7.18 ng/mL) from an independent training set was performed using the shortest Euclidean distance method in the receiver operating characteristic (ROC) curve. B) ROC curve was obtained for serum asprosin in the entire cohort (normal, n = 199; PC, n = 347). C) ROC curve and D) violin plot were drawn for serum asprosin in the normal vs early-stage PC group (normal, n = 199; early-stage PC, n = 111); 2-sided Welch’s t test was used, because the number of samples for the continuous variable (asprosin concentration) was large enough (199 vs 111) for normal distribution. E) Violin plot was drawn for serum asprosin in normal vs PC patients with regional lymph node metastasis N score (normal, n = 199; N0, n = 35; N1,2,3, n = 108); 2-sided Welch's t test was used. F) Violin plot was drawn for serum asprosin in normal vs PC patients with distant metastasis M score (normal, n = 199; M0, n = 125; M1, n = 27); 2-sided Welch's t test was used. Violin plots are presented with the median and quartiles. AUC = area under the curve.
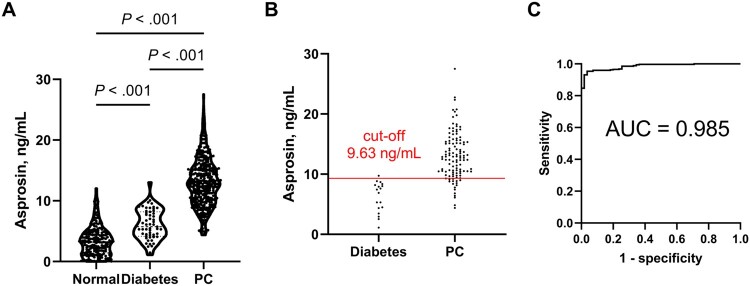
Figure 3.
Discrimination between pancreatic cancer (PC) and diabetes patients with serum asprosin concentration. A) Violin plot was drawn for serum asprosin levels in normal, diabetic, and PC patients (normal, n = 199; diabetic, n = 55; PC, n = 347); 2 -sided Welch’s t test was used. Violin plots are presented with the median and quartiles. B) Discrimination of normal (n = 18) and PC (n = 115) groups in the validation set with the cut-off value (9.63 ng/mL) from an independent training set was performed using the shortest Euclidean distance method in the receiver operating characteristic (ROC) curve. C) ROC curve was obtained for the combination of serum asprosin and CA19-9 in diabetes vs PC comparison. Logistic regression was used for asprosin and CA19-9 as continuous variables. The resulting probability variable was used to construct the ROC curve.
Asprosin for Detecting Early PC
Detection of PC at an early stage is one of the most important factors for effective therapies and better prognosis. Therefore, serum asprosin level was tested for its performance in discriminating early-stage PC from normal samples. It effectively differentiated the early-stage PC (American Joint Committee on Cancer stage I + II, n = 111) from the normal group with the AUC value of 0.981 (95% CI = 0.970 to 0.992, sensitivity = 0.955, and specificity = 0.935; Figure 2, C). Asprosin levels were higher in early-stage PC than in the normal group (mean [SD], normal = 3.38 [2.41] ng/mL vs early-stage PC = 12.6 [3.73] ng/mL, P < .001; Figure 2, D). In addition, all of the subgroups (stage I, n = 13; stage IIA, n = 18; stage IIB, n = 80) exhibited different asprosin levels from that in the normal group (P < .001 for all; Supplementary Figure 3, A, available online). Notably, no difference in asprosin level was found between the early (stages I and II) and late (stages III and IV; Supplementary Figure 3, B, available online) stages. As for metastatic status, both the N0 (no lymph node metastasis) and M0 (no distant metastasis) groups featured higher asprosin levels than did the normal group (mean [SD], normal = 3.38 [2.41] ng/mL vs N0 = 13.6 [3.89] ng/mL vs M0 = 12.6 [3.73] ng/mL; P < .001 for both comparisons to normal; Figure 2, E and F). Moreover, the levels were not different within each N0 vs N1,2,3 (N1,2,3 = 12.4 [3.63] ng/mL, P = .11) or M0 vs M1 (M1 = 13.4 [3.28] ng/mL, P = .31) comparison (Figure 2, E and F). Consistent with the asprosin staining in tissues (see Supplementary Figure 1, B, available online), these results show that asprosin level is high even in early-stage PC and in PC without metastasis, indicating its utility as a marker for early-stage PC.
Discrimination of Diabetes and PC
As stated above, asprosin has been implicated in insulin resistance in diabetes and may well be elevated in diabetes patients. To test the performance of asprosin in the presence of a possible confounding condition, diabetes, we measured asprosin levels in an additional group of 55 diabetic patients. Asprosin’s level in this diabetes group was higher than in the normal group (mean [SD], normal = 3.38 [2.41] ng/mL vs diabetes = 6.07 [2.48] ng/mL vs PC = 13.0 [3.88] ng/mL; P < .001) but lower than in the PC group (P < .001; Figure 3, A), whereas its levels were not different according to the diabetic status among the PC patients (Supplementary Figure 4, A, available online). In addition, the asprosin in the diabetes group was lower than those in both the early- and late-stage PC groups (mean [SD], early-stage PC = 12.57 [3.74] ng/mL vs late-stage PC = 13.17 [3.43] ng/mL; P < .001 for both; Supplementary Figure 4, B, available online). The discrimination between the PC and diabetes groups with the above training and validation set approach yielded an AUC of 0.935 (95% CI = 0.898 to 0.972) and cut-off value of 9.63 ng/mL from the training set (Supplementary Figure 4, C, available online). This cut-off value predicted the PC status of the independent validation set with a sensitivity of 0.835 (96 of 115) and specificity of 0.944 (17 of 18) (Figure 3, B). Therefore, these comparison results exhibited a higher cut-off and a slightly lower performance than those for the normal vs PC comparison (Figure 2, A). In the case of the early-stage PC and diabetes comparison, the performance was again slightly lower (AUC = 0.925, 95% CI = 0.887 to 0.964; Supplementary Figure 4, D, available online).
Along with our cell and mouse results, these results suggest that asprosin, secreted by PC, may be correlated with PC-associated diabetes. Given the decrease in asprosin’s performance under diabetic conditions, we combined asprosin with CA19-9, because the latter was found to be independent of diabetic status (P = .33 for normal vs diabetes; Supplementary Figure 4, E, available online). On logistic regression with the 2 variables against PC status, the combination yielded improved discrimination with a high AUC value of 0.985 (95% CI = 0.975 to 0.995; sensitivity = 0.954 and specificity = 0.964, both asprosin and CA19-9 as continuous variables; Figure 3, C). In the subgroup discrimination between diabetes vs early-stage PC, too, the combination achieved a high AUC of 0.979 (95% CI = 0.961 to 0.998; Supplementary Figure 4, F, available online), indicating its utility for early-stage PC cases with diabetes.
Consideration of Age in the Analysis
It must be mentioned that the PC group was older (median age = 66 years) than the normal group (median age = 36 years) in our cohorts, which, at first glance, could seem problematic regarding the interpretation of asprosin’s performance. The following analysis, however, shows that the effects of age should be negligible, if at all. First, the asprosin level exhibited statistically nonsignificant or even negative correlations with age in each of the normal (Pearson r = −0.21, P = .004), diabetes (Pearson r = 0.21, P = .12), and PC (Pearson r = −0.02, P = .70) groups (Supplementary Figure 5, available online). Therefore, the asprosin level should be the same or even lower in older people, if age affected the asprosin levels. Second, an analysis for the partial correlation between age and asprosin levels, while controlling for the effect of PC status , did not yield any statistical significance (Pearson r = −0.072, P = .09). Third, the age-adjusted AUC value (0.987) (19) was essentially nondifferentiable from the original AUC without age adjustment (0.984). Finally, an ROC analysis of the subset of the entire cohort of matched age (normal: n = 81 with a median age of 53 years; PC: n = 116 with a median age of 55 years; P = .40) gave an AUC value of 0.985 (95% CI = 0.972 to 0.999; Supplementary Figure 6, available online), essentially the same as the value for the entire cohort (AUC = 0.984). Overall, the higher asprosin level in the PC group should not simply be due to the higher age of the group, and the confounding effect of age on AUC is almost nonexistent and therefore can be disregarded for the practical purposes of PC diagnosis with asprosin.
Discussion
In any cancer biomarker study, there is always a possibility of the suggested biomarkers being present in other cancers, which can undermine their specificity. We therefore applied stringent specificity criteria to our bioinformatic screening by including all of the 31 major types of cancer. Also supporting the specificity of asprosin is a recent study that found a lack of any statistically significant difference in asprosin levels among gastric, colorectal, non-small cell lung, small cell lung, and esophageal cancers (21). In addition, asprosin levels were not different between gastric cancer and gastritis (normal control) (21).
A proteolytic enzyme called furin cleaves profibrillin to generate FBN-1 and asprosin (12). Still, furin and FBN-1 are necessary but not sufficient for asprosin secretion, and they are expressed in a wide range of tissues (12,22). This makes the prediction of asprosin secretion based on furin and FBN-1 expressions difficult. Initially suggested as being secreted from adipose tissue (12), asprosin has also been found to be secreted from skin fibroblasts (12), pancreatic beta cells (23), and salivary gland cells (24), and its level is elevated in patients with polycystic ovarian syndrome (25). Therefore, asprosin secretion should be experimentally validated in individual tissues, and in fact, the associated mechanism is an active area of research (14). Our bioinformatics screening was not intended for proving a causal relationship between FBN1 and asprosin secretion; instead, it provided a correlational clue that needed to be tested, which we confirmed in human PC cells both in vitro and in vivo.
Asprosin’s superior performance as a single marker for early-stage PC (AUC = 0.981) relative to others such as CA19-9 (AUC = 0.861; Supplementary Figure 3, C, available online) or thrombospondin 2 (AUC = 0.887) (26) is also noteworthy. Several studies have suggested multi-marker profiles due to the unsatisfactory sensitivity of individual markers (27–29), often without the assurance of their secretion from PC. The benefits of a high-performance single biomarker are evident: low cost and high convenience in measurement; a simple cut-off value for clinical practice; possible application to a simple diagnostic kit. Still, even a near-perfect biomarker would not be recommended for screening of the general public due to the very low incidence of PC (6). Therefore, people with high lifetime risk of PC, such as those with germline mutations, smokers, or those with first-degree PC relatives (26), may best benefit from asprosin. If high-risk patients also have long-term diabetes, the combination with CA19-9 should be given due consideration.
Asprosin has been known to be secreted from adipose tissue and to be implicated in the role of adipose tissue in insulin resistance (12). Asprosin secretion from PC, shown here with cells, mouse models, and human tissues, is consistent with intriguing observations of improved glucose control on pancreatectomy in some PC patients (11). The exact mechanism of this, however, requires further investigation. Because lipoatrophy, loss of fats, is a well-known manifestation of cachexia found in 70%-80% of PC patients (30), the even higher level of asprosin in PC than in the diabetes group, in this study, suggests that the major source of asprosin in PC patients is PC itself rather than adipose tissue. Because PC is highly nutrient avid and glycolytic (31), PC cells might produce asprosin to exploit hepatic glucose output and insulin resistance to meet their own nutritional needs. These possible inter-organ effects and the exact roles of asprosin in PC-associated diabetes should be interesting subjects for future studies.
The limitations of this study include the time dependence of serum asprosin level (12) and several possible confounding conditions. Polycystic ovarian syndrome (25) or other pancreatic conditions such as chronic pancreatitis (32) and intraductal papillary mucinous neoplasm are associated with diabetes and, therefore, might exhibit high asprosin levels. For chronic pancreatitis, insulin-like growth factor (IGF)-1 (33) or other biomarker signatures (28) have been proposed for differentiation from PC and therefore could be used with asprosin. The multi-center retrospective design of this study also has up- and downsides that need to be considered in future, more-targeted studies. The downsides include limited information on clinical characteristics and the effects of potentially confounding variables, whereas the upsides include nonbiased sampling protocol, blinded patient recruitment, and a relatively large number of samples. With these limitations, our study does provide a promising serum biomarker for early-stage PC with an implication in PC-associated diabetes.
Funding
This work was supported by National Research Foundation of Korea grants funded by the Korea government Ministry of Science and ICT (NRF-2018R1A3B1052328 to SP, NRF-2018R1A2A1A05077263, NRF-2019M3E5D1A02069621, and NRF-2021R1A5A2031612 to S-SH) as well as a grant from the Korean Health Technology R&D Project, Ministry of Health and Welfare (HI14C2640 to SCK).
Notes
Role of the funder: The funders had no role in the design of the study; the collection, analysis, or interpretation of the data; the writing of the manuscript, or the decision to submit the manuscript for publication.
Disclosures: Song Cheol Kim is a stockholder of DoAI Inc (Seongnam, Gyeonggi-do, Korea). All other authors declare no conflict of interest.
Author contributions: HN, SK, SK, MSP, HSK, V-HM, JK, HL: Formal analysis, Data curation, Methodology, Investigation. YJS, JHL, WL, S-YK, SHK, SCK: Formal analysis, Writing—review and editing. KHJ: Formal analysis, Funding acquisition, Investigation, Methodology, Supervision Writing—review and editing. S-SH: Formal analysis, Funding acquisition, Methodology, Project administration, Supervision, Writing—original draft, Writing—review and editing. SP: Conceptualization, Formal analysis, Funding acquisition, Methodology, Project administration, Supervision, Writing—original draft, Writing—review and and editing.
Acknowledgements: The biospecimens of PC and normal group for this study were provided by Ajou Human Bio-Resource Bank, Chonnam National University Hospital Biobank, Chungbuk National University Hospital Biobank, Chungnam National University Hospital Biobank, Gyeongsang National University Hospital Biobank, Inje University-Paik Hospital Biobank, Jeonbuk National University Hospital Biobank, Kangwon National University Hospital Biobank, Keimyung University Dongsan Medical Center Biobank, Kyungpook National University Hospital Biobank, and Seoul National University Hospital Human Biobank, members of the National Biobank of Korea, which are supported by the Ministry of Health and Welfare of Korea. The samples of diabetes group were provided by Inha University Hospital. All samples derived from the National Biobank of Korea were obtained with informed consent under institutional review board-approved protocols. We thank Dr Atul Chopra (Baylor College of Medicine) for sharing an asprosin antibody that had been used in our preliminary setup experiments that eventually led to this project.
Disclaimers: The authors assume full responsibility for analyses and interpretation of these data.
Data Availability
The data underlying this article will be shared on reasonable request to the corresponding author.
Supplementary Material
References
- 1. Kleeff J, Korc M, Apte M, et al. Pancreatic cancer. Nat Rev Dis Primers. 2016;2:16022. [DOI] [PubMed] [Google Scholar]
- 2. Mizrahi JD, Surana R, Valle JW, Shroff RT.. Pancreatic cancer. Lancet. 2020;395(10242):2008–2020. [DOI] [PubMed] [Google Scholar]
- 3. Zhou B, Xu JW, Cheng YG, et al. Early detection of pancreatic cancer: where are we now and where are we going? Int J Cancer. 2017;141(2):231–241. [DOI] [PubMed] [Google Scholar]
- 4. Ballehaninna UK, Chamberlain RS.. The clinical utility of serum CA 19-9 in the diagnosis, prognosis and management of pancreatic adenocarcinoma: an evidence based appraisal. J Gastrointest Oncol. 2012;3:105–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bauer TM, El-Rayes BF, Li X, et al. Carbohydrate antigen 19-9 is a prognostic and predictive biomarker in patients with advanced pancreatic cancer who receive gemcitabine-containing chemotherapy: a pooled analysis of 6 prospective trials. Cancer. 2013;119(2):285–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Singhi AD, Koay EJ, Chari ST, Maitra A.. Early detection of pancreatic cancer: opportunities and challenges. Gastroenterology. 2019;156(7):2024–2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Andersen DK, Korc M, Petersen GM, et al. Diabetes, pancreatogenic diabetes, and pancreatic cancer. Diabetes. 2017;66(5):1103–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pang Y, Kartsonaki C, Guo Y, et al. Diabetes, plasma glucose and incidence of pancreatic cancer: a prospective study of 0.5 million Chinese adults and a meta-analysis of 22 cohort studies. Int J Cancer. 2017;140(8):1781–1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ben Q, Xu M, Ning X, et al. Diabetes mellitus and risk of pancreatic cancer: a meta-analysis of cohort studies. Eur J Cancer. 2011;47(13):1928–1937. [DOI] [PubMed] [Google Scholar]
- 10. Aggarwal G, Kamada P, Chari ST.. Prevalence of diabetes mellitus in pancreatic cancer compared to common cancers. Pancreas. 2013;42(2):198–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pannala R, Leirness JB, Bamlet WR, Basu A, Petersen GM, Chari ST.. Prevalence and clinical profile of pancreatic cancer-associated diabetes mellitus. Gastroenterology. 2008;134(4):981–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Romere C, Duerrschmid C, Bournat J, et al. Asprosin, a fasting-induced glucogenic protein hormone. Cell. 2016;165(3):566–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Duerrschmid C, He Y, Wang C, et al. Asprosin is a centrally acting orexigenic hormone. Nat Med. 2017;23(12):1444–1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hoffmann JG, Xie W, Chopra AR.. Energy regulation mechanism and therapeutic potential of asprosin. Diabetes. 2020;69(4):559–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Consoli A. Role of liver in pathophysiology of NIDDM. Diabetes Care. 1992;15(3):430–441. [DOI] [PubMed] [Google Scholar]
- 16. Zhang X, Jiang H, Ma X, Wu H.. Increased serum level and impaired response to glucose fluctuation of asprosin is associated with type 2 diabetes mellitus. J Diabetes Investig. 2020;11(2):349–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Naiemian S, Naeemipour M, Zarei M, et al. Serum concentration of asprosin in new-onset type 2 diabetes. Diabetol Metab Syndr. 2020;12:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Edge SB, Compton CC.. The American Joint Committee on Cancer: the 7th edition of the AJCC Cancer Staging Manual and the future of TNM. Ann Surg Oncol. 2010;17(6):1471–1474. [DOI] [PubMed] [Google Scholar]
- 19. Janes H, Pepe MS.. Adjusting for covariates in studies of diagnostic, screening, or prognostic markers: an old concept in a new setting. Am J Epidemiol. 2008;168(1):89–97. [DOI] [PubMed] [Google Scholar]
- 20. de Carvalho IV, Rodriguez-Alvarez MX. Bayesian nonparametric inference for the covariate-adjusted ROC curve. arXiv.org; 2018. https://arxiv.org/abs/1806.00473. Accessed February 4, 2021.
- 21. Du C, Wang C, Guan X, et al. Asprosin is associated with anorexia and body fat mass in cancer patients. Support Care Cancer. 2021;29(3):1369–1375. [DOI] [PubMed] [Google Scholar]
- 22. Lönnqvist L, Reinhardt D, Sakai L, Peltonen L.. Evidence for furin-type activity-mediated C-terminal processing of profibrillin-1 and interference in the processing by certain mutations. Hum Mol Genet. 1998;7(13):2039–2044. [DOI] [PubMed] [Google Scholar]
- 23. Lee T, Yun S, Jeong JH, Jung TW.. Asprosin impairs insulin secretion in response to glucose and viability through TLR4/JNK-mediated inflammation. Mol Cell Endocrinol. 2019;486:96–104. [DOI] [PubMed] [Google Scholar]
- 24. Ugur K, Aydin S.. Saliva and blood asprosin hormone concentration associated with obesity. Int J Endocrinol. 2019;2019:2521096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Alan M, Gurlek B, Yilmaz A, et al. Asprosin: a novel peptide hormone related to insulin resistance in women with polycystic ovary syndrome. Gynecol Endocrinol. 2019;35(3):220–223. [DOI] [PubMed] [Google Scholar]
- 26. Kim J, Bamlet WR, Oberg AL, et al. Detection of early pancreatic ductal adenocarcinoma with thrombospondin-2 and CA19-9 blood markers. Sci Transl Med. 2017;9(398):eaah55873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mellby LD, Nyberg AP, Johansen JS, et al. Serum biomarker signature-based liquid biopsy for diagnosis of early-stage pancreatic cancer. J Clin Oncol. 2018;36(28):2887–2894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Radon TP, Massat NJ, Jones R, et al. Identification of a three-biomarker panel in urine for early detection of pancreatic adenocarcinoma. Clin Cancer Res. 2015;21(15):3512–3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mayerle J, Kalthoff H, Reszka R, et al. Metabolic biomarker signature to differentiate pancreatic ductal adenocarcinoma from chronic pancreatitis. Gut. 2018;67(1):128–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Poulia KA, Sarantis P, Antoniadou D, et al. Pancreatic cancer and cachexia-metabolic mechanisms and novel insights. Nutrients. 2020;12:1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sousa CM, Kimmelman AC.. The complex landscape of pancreatic cancer metabolism. Carcinogenesis. 2014;35(7):1441–1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Beyer G, Habtezion A, Werner J, Lerch MM, Mayerle J.. Chronic pancreatitis. Lancet. 2020;396(10249):499–512. [DOI] [PubMed] [Google Scholar]
- 33. Wlodarczyk B, Gasiorowska A, Borkowska A, Malecka-Panas E.. Evaluation of insulin-like growth factor (IGF-1) and retinol binding protein (RBP-4) levels in patients with newly diagnosed pancreatic adenocarcinoma (PDAC). Pancreatology. 2017;17(4):623–628. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.