Abstract
Background
Statistically significantly increased cancer incidence has been reported from 3 cohorts of World Trade Center (WTC) disaster rescue and recovery workers. We pooled data across these cohorts to address ongoing public concerns regarding cancer risk 14 years after WTC exposure.
Methods
From a combined deduplicated cohort of 69 102 WTC rescue and recovery workers, a sample of 57 402 workers enrolled before 2009 and followed through 2015 was studied. Invasive cancers diagnosed in 2002-2015 were identified from 13 state cancer registries. Standardized incidence ratios (SIRs) were used to assess cancer incidence. Adjusted hazard ratios (aHRs) were estimated from Cox regression to examine associations between WTC exposures and cancer risk.
Results
Of the 3611 incident cancers identified, 3236 were reported as first-time primary (FP) cancers, with an accumulated 649 724 and 624 620 person-years of follow-up, respectively. Incidence for combined FP cancers was below expectation (SIR = 0.96, 95% confidence interval [CI] = 0.93 to 0.99). Statistically significantly elevated SIRs were observed for melanoma-skin (SIR = 1.43, 95% CI = 1.24 to 1.64), prostate (SIR = 1.19, 95% CI = 1.11 to 1.26), thyroid (SIR = 1.81, 95% CI = 1.57 to 2.09), and tonsil (SIR = 1.40, 95% CI = 1.00 to 1.91) cancer. Those arriving on September 11 had statistically significantly higher aHRs than those arriving after September 17, 2001, for prostate (aHR = 1.61, 95% CI = 1.33 to 1.95) and thyroid (aHR = 1.77, 95% CI = 1.11 to 2.81) cancers, with a statistically significant exposure-response trend for both.
Conclusions
In the largest cohort of 9/11 rescue and recovery workers ever studied, overall cancer incidence was lower than expected, and intensity of WTC exposure was associated with increased risk for specific cancer sites, demonstrating the value of long-term follow-up studies after environmental disasters.
The September 11 terrorist attacks (9/11) on the World Trade Center (WTC) in New York City resulted in building collapses and fires that released myriad airborne contaminants including toxic fumes, known or suspected carcinogens (eg, asbestos, silica, benzene, heavy metals), combustion products (eg, polycyclic aromatic hydrocarbons [PAHs]), polychlorinated biphenyls [PCBs]), and many other types of particles (eg, wood, metal) (1-3). The unprecedented months-long rescue and recovery work exposed thousands of workers to varying intensities and durations of nonradiation-based toxins (4).
For monitoring long-term 9/11 health effects, 3 WTC-exposed cohorts were established: the Fire Department of the City of New York (FDNY) (5), the WTC Health Registry (6), and the General Responder Cohort (GRC) (7). Early assessments of cancer among 9/11 rescue and recovery and clean-up workers (hereafter, rescue and recovery workers) from these 3 cohorts demonstrated excess incidence of some cancers (eg, prostate, thyroid) compared with the general population (8–10). Studies with additional years of follow-up observed greater-than-expected incidence for melanoma-skin (11) and leukemia (12), in addition to prostate and thyroid cancers. However, these studies examined the 3 WTC-exposed cohorts separately and were limited by low case counts. Further, the intensity of WTC-related exposure varied by study cohort and may affect the precision of estimates in cancer studies (13). After nearly 2 decades, the presence and magnitude of increased cancer risk among rescue and recovery workers remain unclear, necessitating further investigation with a larger sample size and longer follow-up.
To address these limitations, a combined and deduplicated cohort of rescue and recovery workers who were members of any of the 3 WTC-exposed cohorts (combined cohort) was created as a collaborative effort among the cohorts and the New York State (NYS) Cancer Registry (14). This is the first report on the overall cancer incidence and risk associated with WTC exposure in this combined cohort, and the largest sample of WTC rescue and recovery workers studied to date.
Methods
Study Design and Patient Eligibility
In this observational cohort study, the rescue and recovery workers in the 3 WTC cohorts were pooled and deduplicated into a combined cohort of 69 102 workers (14). Briefly, records were pooled from FDNY (n = 16 221), WTC Health Registry (n = 29 372), and GRC (n = 33 427), and 9918 duplicates were removed (14).
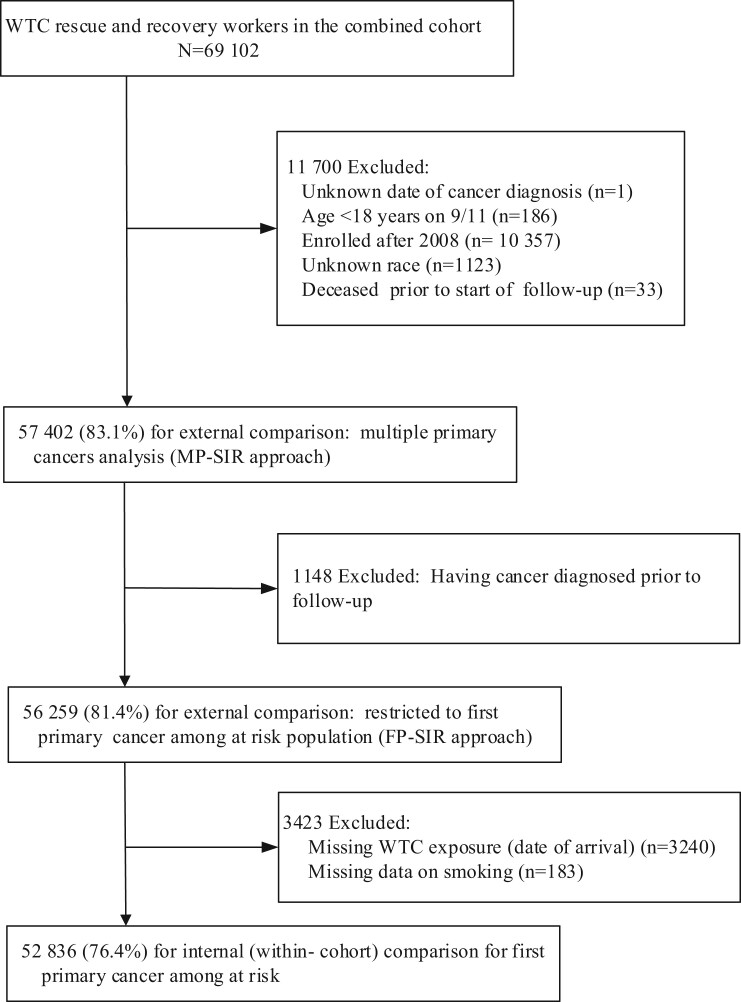
We restricted the present study to those aged 18 years or older on September 11 with known race and ethnicity and enrollment before 2009 (see Figure 1). The enrollment cutoff was selected to mitigate selection bias. We also excluded those who died within 6 months of enrollment. For subsequent analyses of first primary cancers, we further excluded persons who had cancer diagnosed prior to start of follow-up. For analyses of WTC exposures, those with missing exposure data and smoking status were excluded.
Figure 1.
Selection of study sample for data analysis. SIR = standardized incidence ratio; WTC = World Trade Center.
This study was approved by the institutional review boards of the Albert Einstein College of Medicine, New York City Department of Health and Mental Hygiene, NYS Department of Health, and Icahn School of Medicine at Mount Sinai. Registry linkages were approved by the 12 state health department institutional review boards listed below and the University of Medicine and Dentistry of New Jersey .
Cancer Incidence
Cancer incidence was defined as any primary invasive malignant tumor, including in situ bladder cancers, diagnosed during follow-up (2002-2015). Cancers were identified through linkages with 13 US-based state cancer registries based on names, sex, race, birth date, social security number when available, and/or home addresses. The 13 states were Arizona, California, Connecticut, Florida, Massachusetts, New Jersey, New York, North Carolina, Ohio, Pennsylvania, Texas, Virginia, and Washington, where 93% of the combined cohort members resided based on last known residence (14).
All cancers were classified according to the International Classification of Diseases for Oncology (ICD-O-3) (15). The cancer sites were categorized using the Surveillance, Epidemiology, and End Results (SEER) Site Recode ICD-O-3 and World Health Organization 2008 definitions (16), based on primary site and histology. Cancer stage (localized vs regional or distant ) was defined based on SEER summary stage 2000 for cases diagnosed during 2002-2003 and collaborative staging derived SEER summary stage 2000 for cases diagnosed during 2004-2015. If diagnosis month or day was missing, the midpoint (June if month is missing or the15th if day is missing) was assigned.
WTC Exposure
WTC exposures common to the 3 cohorts were derived from self-reported responses collected at study enrollment: 1) date of first arrival to the WTC site (on September 11 , on September 12, between September 13 and 17, or after September 17, 2001); 2) performed tasks on the debris pile from the collapsed towers at the WTC site (yes, no or unknown); and 3) exposed to the dust cloud on September 11 (yes, no or unknown) (14).
Statistical Analyses
External Comparisons. Using standardized incidence ratios (SIRs), we compared the observed cancer incidence in the combined cohort to the expected incidence for all cancer sites combined (hereafter, all-cancers) and by cancer site. Because 81% of cases were identified from the NYS cancer registry, the expected number of cases was calculated based on NYS cancer incidence rates, standardized to age at diagnosis, sex, race and ethnicity (Hispanic and Latino, non-Hispanic White, non-Hispanic Black, American Indian–Alaska Native, and Asian–Pacific Islander), and calendar year (5-year intervals) (17). For members missing ethnicity (14), the North American Association of Central Cancer Registries Hispanic Identification Algorithm was used to assign Hispanic ethnicity (18). SIRs and exact 95% confidence intervals (CI), assuming a Poisson distribution of the observed number of cases, were computed using the SEER*Stat Multiple Primary-SIR session (version 7.0.5; National Cancer Institute, Bethesda, MD).
We estimated SIRs in 2 ways. For both approaches, to exclude individuals who might have enrolled because of preclinical cancer or related symptoms, follow-up started 6 months after September 11 or enrollment date. First, the multiple primaries approach (hereafter, MP-SIR) was used to examine all cancers diagnosed after follow-up began in the entire cohort (n = 57 402); person-time accrued until date of death or December 31, 2015, whichever occurred first . Second, the first primary cancer approach (hereafter, FP-SIR) was used to assess cancer risk in a cancer-free population, and cohort members with any invasive cancer prior to the start of follow-up were excluded. Among the cancer-free cohort (n = 56 259), only first primary cancer diagnoses were counted and person-time accrued until the earliest of first invasive cancer diagnosis, death date, or December 31, 2015. For FP-SIR, the NYS reference rate was recalculated removing individuals with prior invasive cancer diagnoses from the denominator. Using the FP-SIR approach, we also estimated SIRs by specific WTC exposures. Surveillance bias was assessed by stratifying SIRs by cancer stage.
Within Cohort Comparisons. Cox regression models were used to estimate hazard ratios (HR) with 95% confidence intervals for FP cancer risk related to WTC exposure (19). Models were run separately for each type of WTC-exposure measurement, estimating the risk of all-cancers and sites with statistically significantly elevated SIRs from external analyses. We limited the within-cohort analysis of tonsil cancer to non-Hispanic White males because 93% (37 of 40) of cases occurred among this demographic.
Follow-up time was calculated as stated above for FP-SIRs. Models were adjusted for age on September 11, sex, race and ethnicity, smoking status (current, former, never, which was defined based on the questions whether someone smoked at least 100 cigarettes in their lifetime and currently smoked cigarettes every day or some days), and year of enrollment (to adjust for potential selection bias from GRC’s open cohort design). To test proportionality, we assessed the statistical significance of time-dependent covariates by creating interactions of covariates with survival time and included these in the model. If the coefficient of an interaction term was significant (P < .05), proportionality was determined to be violated for that covariate. When age violated the proportional hazard assumption, we controlled for age (5-year intervals) with stratified Cox models (20). To test the exposure-response relationship, we included the date of arrival (from late to early arrival date) as ordinal variable in the multivariable Cox model and examined the statistical significance of the estimate. Analyses were performed using SAS software (v9.4, SAS Institute Inc, Cary, NC). All statistical tests are 2-sided, and statistical significance was indicated if the 95% confidence interval did not contain the null value of 1 or if a P value was less than .05.
Results
Study Sample
The analytic cohort (n = 57 402) was predominantly male (84.1%), non-Hispanic White (71.7%), and mostly (84%) enrolled before 2005 (Table 1). The median age on September 11 was 39 years (interquartile range [IQR] = 13 ), and nearly 40% had ever smoked (16.2% current smokers) at enrollment.
Table 1.
Characteristics of the combined cohort of 57402 rescue and recovery workers exposed to the September 11, 2001 (9/11) World Trade Center (WTC) disaster
| Characteristics | No. (%) |
|---|---|
| Sociodemographics | |
| Age on 9/11, y | |
| 18-24 | 2948 (5.1) |
| 25-29 | 5583 (9.7) |
| 30-34 | 9654 (16.8) |
| 35-39 | 11 810 (20.6) |
| 40-44 | 10 598 (18.5) |
| 45-49 | 7749 (13.5) |
| 50-54 | 4835 (8.4) |
| 55-59 | 2478 (4.3) |
| 60 or older | 1747 (3.0) |
| Sex | |
| Male | 48 251 (84.1) |
| Female | 9151 (15.9) |
| Race/ethnicity | |
| Non-Hispanic (NH) White | 41 130 (71.7) |
| NH-Black | 5614 (9.8) |
| NH-American Indian/Alaska | 160 (0.3) |
| Native | |
| NH-Asian–Pacific Islander | 1134 (2.0) |
| Hispanic, any race | 9364 (16.3) |
| Smoking status at enrollment | |
| Current | 9315 (16.2) |
| Former | 13 363 (23.3) |
| Never | 34 264 (59.7) |
| Unknown/missing | 460 (0.8) |
| Vital status by the end of follow-up | |
| Alive | 55 688 (97.0) |
| Deceased | 1714 (3.0) |
| Year of enrollment | |
| September 11, 2001-2004 | 48 176 (83.9) |
| 2005-2008 | 9226 (16.1) |
| Combined cohort membershipa | |
| FDNY | 15 330 (26.7) |
| GRC | 22 930 (40.0) |
| Remaining in WTCHR | 19 142 (33.4) |
| WTC exposures | |
| Date of arrival at WTC site | |
| September 11, 2001 | 20 948 (36.5) |
| September 12, 2001 | 10 595 (18.5) |
| September 13-17, 2001 | 11 143 (19.4) |
| ≥September 18, 2001 | 11 476 (20.0) |
| Missing | 3240 (5.6) |
| Performed tasks on pile | |
| Yes | 23 086 (40.2) |
| No | 33 725 (58.8) |
| Missing | 591 (1.0) |
| Dust exposure on 9/11 | |
| Yes | 21 704 (37.8) |
| No | 32 462 (56.6) |
| Missing | 3236 (5.6) |
The combined cohort is deduplicated following a hierarchy of the Fire Department of the City of New York (FDNY), the General Responder Cohort (GRC), and then the World Trade Center Health Registry (WTCHR). This results in FDNY including members of GRC and/or WTCHR, GRC including members from WTCHR but not FDNY, and the remaining from WTCHR.
External Comparisons
During the 649 724 person-year follow-up, 3611 incident cancers were identified (3808.2 expected; SIR = 0.95, 95% CI = 0.92 to 0.98) among 57 402 individuals (MP-SIR) with 3236 reported as first primary cancers (3371.1 expected; SIR = 0.96, 95% CI = 0.93 to 0.99; FP-SIR) (Table 2). The total person-years of follow-up among 56 259 rescue and recovery workers who were at risk for FP at the start of the follow-up was 624 620. For site-specific FP cancers, we observed 3 major sites with elevated SIRs: melanoma-skin (SIR = 1.43, 95% CI = 1.24 to 1.64), prostate (SIR = 1.19, 95% CI = 1.11 to 1.26), thyroid (SIR = 1.81, 95% CI = 1.57 to 2.09), and 1 oral cavity and pharynx subsite: tonsil (SIR = 1.40; 95% CI = 1.00 to 1.91). Eight subsites with statistically significantly lower-than-expected rates were identified. MP-SIR results were similar to FP-SIR, except the tonsil cancer SIR was not statistically significant.
Table 2.
Standardized incidence ratio (SIR) and 95% confidence interval (CI), by major cancer site, among WTC rescue and recovery workers with 2 approaches, 2002-2015a
| Cancer site (SEER recode) | All primary cancer | First primary cancer | ||
|---|---|---|---|---|
| (MP-SIR approach)b |
(FP-SIR approach)c |
|||
| Obs. | SIR (95% CI) | Obs. | SIR (95% CI) | |
| All sites | 3611 | 0.95 (0.92 to 0.98) | 3236 | 0.96 (0.93 to 0.99) |
| Oral cavity and pharynx (20010-20100) | 121 | 0.91 (0.76 to 1.09) | 109 | 0.96 (0.79 to 1.16) |
| Tonsil (20070)d | 43 | 1.37 (0.99 to 1.84) | 40 | 1.40 (1.00 to 1.91) |
| Tongue (20020)d | 43 | 1.02 (0.74 to 1.38) | 38 | 1.06 (0.75 to 1.46) |
| Esophagus (21010) | 44 | 0.85 (0.62 to 1.15) | 43 | 0.96 (0.69 to 1.29) |
| Stomach (21020) | 56 | 0.84 (0.64 to 1.09) | 52 | 0.89 (0.66 to 1.16) |
| Colon and rectum (21041-21052) | 233 | 0.74 (0.64 to 0.84) | 212 | 0.76 (0.66 to 0.87) |
| Colon excluding rectum (21041-21049) | 143 | 0.71 (0.60 to 0.84) | 133 | 0.76 (0.64 to 0.90) |
| Rectum and rectosigmoid (21051-21052) | 90 | 0.78 (0.62 to 0.95) | 79 | 0.76 (0.60 to 0.95) |
| Liver and intrahepatic bile duct (21071-21072) | 61 | 0.66 (0.50 to 0.84) | 54 | 0.64 (0.48 to 0.83) |
| Pancreas (21100) | 67 | 0.71 (0.55 to 0.91) | 56 | 0.68 (0.51 to 0.88) |
| Larynx (22020) | 34 | 0.74 (0.52 to 1.04) | 30 | 0.74 (0.50 to 1.05) |
| Lung and bronchus (22030) | 249 | 0.61 (0.53 to 0.69) | 200 | 0.59 (0.51 to 0.67) |
| Melanoma of the skin (25010) | 236 | 1.42 (1.24 to 1.61) | 204 | 1.43 (1.24 to 1.64) |
| Female breast (26000) | 162 | 0.82 (0.70 to 0.96) | 140 | 0.82 (0.69 to 0.96) |
| Corpus uterus and NOS (27020, 27030) | 31 | 0.66 (0.45 to 0.94) | 29 | 0.67 (0.45 to 0.96) |
| Ovary (27040) | 15 | 0.84 (0.47 to 1.38) | 15 | 0.94 (0.53 to 1.55) |
| Prostate (28010) | 1061 | 1.18 (1.11 to 1.25) | 1001 | 1.19 (1.11 to 1.26) |
| Testis (28020) | 37 | 0.81 (0.57 to 1.12) | 35 | 0.82 (0.57 to 1.13) |
| Urinary bladder (including in situ) (29010) | 149 | 0.78 (0.66 to 0.92) | 130 | 0.81 (0.67 to 0.96) |
| Kidney and renal pelvis (29020) | 158 | 0.89 (0.76 to 1.04) | 137 | 0.92 (0.77 to 1.09) |
| Brain and other nervous system (31010, 31040) | 50 | 0.89 (0.66 to 1.17) | 45 | 0.87 (0.63 to 1.16) |
| Thyroid (32010) | 208 | 1.78 (1.54 to 2.03) | 189 | 1.81 (1.57 to 2.09) |
| Hodgkin lymphoma (33011-33012) | 22 | 0.81 (0.51 to 1.23) | 20 | 0.80 (0.49 to 1.23) |
| Non-Hodgkin lymphoma (33041-33042) | 189 | 1.04 (0.90 to 1.20) | 162 | 1.01 (0.86 to 1.18) |
| Multiple myeloma (34000) | 62 | 1.07 (0.82 to 1.37) | 56 | 1.09 (0.82 to 1.42) |
| Leukemia (35011-35043) | 118 | 1.04 (0.86 to 1.24) | 102 | 1.04 (0.85 to 1.26) |
| Mesothelioma (36010) | 9 | 1.38 (0.63 to 2.62) | 8 | 1.51 (0.65 to 2.98) |
Follow-up began 6 months after enrollment, and individuals with non-Hispanic unknown race were excluded. FP = first primary; MP = multiple primaries; NOS = not otherwise specified; Obs. = observed; SEER = Surveillance Epidemiology and End Results; WTC = World Trade Center.
Incidence results based on all invasive (and in situ bladder) cancers diagnosed during follow-up period (MP-SIR approach).
Incidence results based on first primary invasive (and in situ bladder) cancer occurred during follow-up period among at-risk population (FP-SIR approach).
Of a total number of 10 subsites in oral cavity and pharynx, tonsil and tongue accounted for more than 71% of the cases, and the remaining subsites had only 1-8 cases each.
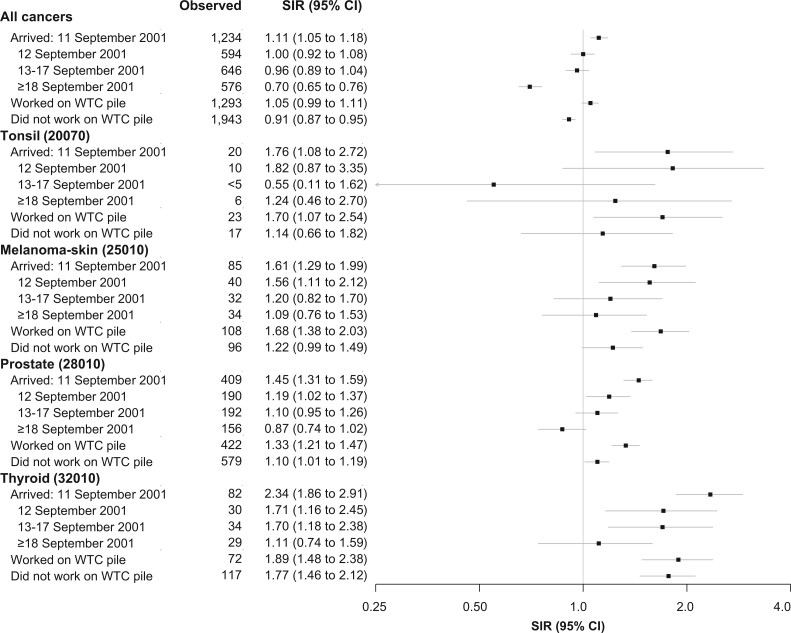
Workers arriving on September 11 had greater-than-expected incidence (FP approach) of all-cancers, melanoma-skin, prostate, thyroid, and tonsil (SIR = 1.11, 95% CI = 1.05 to 1.18; SIR = 1.61, 95% CI = 1.29 to 1.99; SIR = 1.45, 95% CI = 1.31 to 1.59; SIR = 2.34, 95% CI = 1.86 to 2.91; SIR = 1.76, 95% CI = 1.08 to 2.72, respectively) (Figure 2). Elevated SIRs were also observed for those arriving on September 12 for melanoma-skin, prostate, and thyroid cancer but not for all-cancers or tonsil. Work on the pile was associated with statistically significantly elevated SIRs for all 4 cancer subsites.
Figure 2.
Standardized incidence ratio (SIR) and 95% confidence interval (CI), by World Trade Center (WTC) exposure, among WTC rescue and recovery workers for all-cancers and selected cancer sites with elevated SIR, 2002-2015. The SIR for first primary cancer (FP-SIR approach) was used. Follow-up began 6 months after enrollment, and individuals with non-Hispanic unknown race were excluded. All statistical tests were 2-sided.
Stage at cancer diagnosis for FP analyses showed SIRs for regional or distant cancers of the tonsil (SIR = 1.47, 95% CI = 1.05 to 1.18), prostate (SIR = 1.33, 95% CI = 1.13 to 1.55), and thyroid (SIR = 1.82, 95% CI = 1.40 to 2.33) were statistically significantly elevated (Table 3). Elevated SIRs were observed for localized melanoma-skin (SIR = 1.51, 95% CI = 1.28 to 1.78) and all-cancers (SIR = 1.14, 95% CI =1.09 to 1.20) only.
Table 3.
Standardized incidence ratio (SIR) and 95% confidence interval (CI), by cancer stage, among WTC rescue and recovery workers for first primary cancer in all sites combined and selected cancers with elevated SIR, 2002-2015a
| Cancer site (SEER recode) | Localized |
Regional/distant |
||
|---|---|---|---|---|
| Obs. | SIR (95% CI) | Obs. | SIR (95% CI) | |
| All sites | 1838 | 1.14 (1.09 to 1.20) | 1213 | 0.85 (0.80 to 0.90) |
| Tonsil (20070) | <5 | 1.55 (0.42 to 3.96) | 36 | 1.47 (1.03 to 2.04) |
| Melanoma-skin (25010) | 151 | 1.51 (1.28 to 1.78) | 23 | 1.13 (0.72 to 1.70) |
| Prostate (28010) | 785 | 1.27 (1.18 to 1.36) | 167 | 1.33 (1.13 to 1.55) |
| Thyroid (32010) | 121 | 1.84 (1.53 to 220) | 63 | 1.82 (1.40 to 2.33) |
Follow-up began 6 months after enrollment and ended at the earliest of diagnosis date of first primary invasive cancer, date of death, or December 31, 2015. Obs. = Observed; SEER = Surveillance Epidemiology and End Results; WTC = World Trade Center.
Within Cohort Comparisons
Compared with those arriving after September 17, workers arriving on September 11, 12, or from 13-17 were at increased risk of all-cancers (adjusted hazard ratio [aHR] = 1.47, 95% CI = 1.32 to 1.64; aHR = 1.34. 95% CI = 1.19 to 1.51; aHR = 1.32, 95% CI = 1.17 to 1.48, respectively) and prostate cancer (aHR = 1.61, 95% CI = 1.33 to 1.95; aHR = 1.35, 95% CI = 1.09 to 1.68; aHR = 1.25, 95% CI = 1.01 to 1.54, respectively) (Table 4); the test for trend (late to early arrival at the site) was statistically significant for all-cancers and prostate. Only arrival on September 11 was associated with an increased risk of thyroid cancer (aHR = 1.77, 95% CI = 1.11 to 2.81). Although not statistically significant, the adjusted hazard ratio for tonsil cancer was doubled for those arriving on September 11 (aHR = 2.07, 95% CI = 0.87 to 4.92) or September 12 (aHR = 2.10, 95% CI = 0.80 to 5.50) compared with those arriving after September 12. Direct dust exposure was statistically significantly associated with an increased risk of all-cancers (aHR = 1.21, 95% CI = 1.12 to 1.31) and prostate cancer (aHR = 1.19, 95% CI = 1.14 to 1.49) but not thyroid, melanoma-skin, or tonsil cancer.
Table 4.
Risk of selected first primary cancer as a function of WTC exposure among WTC rescue and recovery workers, 2002-2015 (N = 52 836)
| WTC exposures | All sitesa |
Tonsilb |
Melanoma-skinc |
Prostatea |
Thyroid |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. | aHR (95% CI)d | No. | aHR (95% CI)d | No. | aHR (95% CI)d | No. | aHR (95% CI)d | No. | aHR (95% CI)d | |
| Date of arrival at WTC site | ||||||||||
| September 11, 2001 | 1223 | 1.47 (1.32 to 1.64) | 20 | 2.07 (0.87 to 4.92) | 83 | 1.39 (0.89 to 2.16) | 405 | 1.61 (1.33 to 1.95) | 82 | 1.77 (1.11 to 2.81) |
| September 12, 2001 | 589 | 1.34 (1.19 to 1.51) | 9 | 2.10 (0.80 to 5.50) | 40 | 1.47 (0.91 to 2.38) | 189 | 1.35 (1.09 to 1.68) | 30 | 1.39 (0.81 to 2.37) |
| September 13-17, 2001 | 643 | 1.32 (1.17 to 1.48) | 8e | Referent | 32 | 1.14 (0.70 to 1.88) | 190 | 1.25 (1.01 to 1.54) | 34 | 1.49 (0.89 to 2.49) |
| ≥September 18, 2001 | 572 | Referent | 34 | Referent | 155 | Referent | 28 | Referent | ||
| Trend (late to early arrival) | 1.12 (1.08 to 1.15) | 1.23 (0.89 to 1.71) | 1.11 (0.97 to 1.28) | 1.16 (1.09 to 1.23) | 1.17 (1.02 to 1.35) | |||||
| Worked on pile | 1279 | 1.03 (0.95 to 1.11) | 23 | 1.24 (0.59 to 2.60) | 107 | 1.37 (0.99 to 1.91) | 415 | 1.09 (0.95 to 1.25) | 72 | 0.74 (0.52 to 1.06) |
| Direct dust exposure on 9/11 | 1242 | 1.21 (1.12 to 1.31) | 19 | 1.31 (0.66 to 2.59) | 84 | 1.17 (0.86 to 1.59) | 404 | 1.30 (1.14 to 1.49) | 77 | 1.19 (0.87 to 1.63) |
Adjusted for age: included strata of age on 9/11 (in 5-year intervals) for all sites combined and prostate cancer because age violated the Cox proportional hazards assumption; otherwise age on 9/11 was included in the model as a fixed effect. aHR = adjusted hazard ratio; CI = confidence interval; WTC = World Trade Center.
The multivariable analysis of tonsil cancer was limited to male and non-Hispanic White workers because 37 of 39 workers with first-time tonsil cancers were male and non-Hispanic and adjusted for age, smoking status, and year of enrollment. Date of arrival on September 13-17, 2001, was combined with ≥ September 18, 2001 group because of small cell counts.
Race and ethnicity ere categorized into non-Hispanic White vs all others in multivariable analysis for melanoma- skin because 184 of 189 melanoma-skin cases are non-Hispanic White.
Adjusted for race and ethnicity, smoking status at enrollment, year of enrollment, age on 9/11, and sex, unless otherwise indicated.
Includes those with dates of arrival at WTC site of September 13-17 and ≥ September 18, 2001.
After dichotomizing the exposure based on first arrival date to those arriving in 2001 (when fires were still burning) vs those arriving in 2002, we found those arriving in 2001 were at higher risk of prostate cancer (aHR = 2.09, 95% CI = 1.36 to 3.19) and of all-cancers (aHR = 1.76, 95% CI = 1.43 to 2.15) than those arriving in 2002.
Discussion
Using the combined cohort, we observed a lower-than-expected SIR for all-cancers, whereas for the first time, we observed a statistically increased risk for workers arriving on September 11 compared with those arriving later for all-cancers, prostate, and thyroid cancers and an increased risk of all-cancers and prostate cancer for those reporting direct dust exposure. The findings of excess cancer in prostate, melanoma-skin, and thyroid were consistent with previous findings, however, we also observed excess tonsil cancer, a rare cancer that likely would not have been detected among individual cohorts.
In external comparisons, the present study observed excess melanoma-skin, prostate, and thyroid cancers compared with NYS population, consistent with previous findings (8–12). In internal comparisons, early arrival workers who putatively had the greatest intensity of toxic dust exposure were found to be at greater risk of cancer; workers arriving on September 11 were 60% more likely to develop prostate cancer and 77% more likely to develop thyroid cancer than those arriving a week later. Furthermore, the risk of prostate cancer was double for those arriving in 2001 compared with those arriving in 2002 when the smoldering fires had ended, providing additional support for the potential effect of 9/11 exposure on cancer risk. Although the exact etiologic underpinning for these associations is unknown, both direct and indirect pathways exist. The high concentrations of well-established carcinogenic agents (eg, PAHs, PCBs, dioxins, heavy metals) in the dust, smoke, and debris at the WTC site could cause cancer directly and explain the early arrival–cancer association (2,21). There is an emerging body of literature suggesting thyroid cancer is associated with environmental exposures beyond radiation (22), including PCBs (23) and particulate matter (24-27). Lastly, both chronic inflammation, from inflammatory diseases known to occur more frequently in WTC-exposed populations, and chronic stress, from occupational exposures common in this cohort (eg, firefighting, police, construction, transportation), have been associated with altered immune and inflammatory activities and subsequently increased cancer risk (21,28-36). Although the specific mechanisms may be unknown, the consistent evidence of excess incidence of melanoma-skin, prostate, and thyroid cancer warrants continued monitoring and treatment availability for this population.
Rescue and recovery workers had lower-than-expected incidence of all-cancers and 8 cancer sites (colon, rectum, liver, pancreas, lung, female breast, corpus and uterus, bladder) over the 14 years following 9/11, counter to previous reports, except for lung cancer (11-13). One possible explanation is that this population may have benefited from early and consistent access to health care and other support systems since exposure. Eligible workers were offered early access to WTC medical monitoring and treatment programs, which continue to offer care to eligible responders (5,7,37). Secondly, cancer development is a result of the interplay of multiple factors, including stochastic effects, environmental-genetic interactions, healthy worker effects, exposures other than 9/11, and latency periods between exposure and incidence that vary across tumor type (38), all of which may or may not be modified by 9/11 exposure. Lastly, using the more diverse NYS general population as reference for study of a worker population may lead to the underestimation of SIR in certain cancers, as the combined cohort is predominantly White, middle-aged, gainfully employed men (39). Similarly, the use of NYS as referent may also contribute to the underestimation of SIR if the cancer rates in the 12 other states were lower than the NYS rate. The demographic characteristics of the cohort, combined with consistent access to 9/11-related medical monitoring and health care, may mitigate part of the detrimental effects of exposure on cancer incidence. Future research should leverage other worker populations, such as US firefighters (28) and police officers (40), for comparison to better elucidate overall cancer risk.
To explore the contribution of screening practices to our findings (41,42), we examined SIR in early and later stages separately. The results suggest increased screening may play a role with observed excess incidence for all-cancers and melanoma-skin diagnosed at localized stages but not at regional or distant stages. However, the SIRs for prostate and thyroid cancers were similar for both localized and regional/distant stages, suggesting that increased risk is independent of surveillance biases. Among the FDNY cohort, related increases in thyroid cancer diagnoses were reported to be partially due to heightened medical attention and incidental detection (43,44). However, reports on similar clinical characteristics between WTC-exposed and nonexposed hospital-based thyroid cancer cases suggest the potential for more cases than surveillance bias alone would explain (45,46). Studies that better describe the magnitude of difference from the general population because of increased screening and incidental detection on the observed exposure-cancer association are needed.
Certain cancer sites deserve further consideration in future monitoring of this population. Eight persons with w FP mesothelioma were observed, a small, non-statistically significant excess over expected. The consistent finding on deficit of lung cancer (11,12) may be because of lower smoking rate in our study population, as the prevalence of smoking at enrollment (16.2%) was lower than the prevalence in NYS adults (19.9%-23.2%) in a similar time period (47). However, the risk of both cancers remain a major concern in this exposed cohort given their long latency periods (48,49) and numerous known carcinogens well described in WTC dust and fumes (2). Lastly, tonsil and tongue cancers represented more than 70% of cancer in the oral cavity and pharynx site and were examined separately. Forty FP-tonsil cancer cases were identified, which was moderately higher than expected. A recent review described a link between asbestos or PAHs and oral and pharyngeal cancer; it suggested that direct contact with inhaled chemicals could produce chronic inflammation resulting in malignant transformation (50). However, excess tonsil cancer in this study could also be attributable to temporal trends in human papillomavirus–related cancers or to differential patterns of known risk factors (eg, tobacco, alcohol use) (51,52). These potentially important and emergent cancers should be carefully monitored in future WTC analyses.
Limitations are noted. The 9/11 exposures were self-reported and ambient air measurements immediately following 9/11 were unavailable to objectively quantify exposures or validate self-report. However, there is ample evidence for the presence of a wide range of toxic substances in the aftermath of the WTC collapse and use of arrival date aligns with the qualitatively described sequence of outdoor WTC exposures at Ground Zero (4,53,54). We cannot rule out the role of unmeasured confounding for those risk factors unavailable in this study but known to be associated with the reported cancers (eg, family history of cancer, ultraviolet radiation from the sun, diet) or residual confounding because of imperfectly measured risk factors (eg, pack-years smoking) in the reported estimates. Similarly, a large proportion of our study sample comprises firefighters, police, and construction workers, and increased cancer incidence among these occupation-specific groups has been previously reported (8,31,32,55). Occupational details (eg, years of service) were unavailable in this study, making it difficult to rule out the role of occupation in reported estimates (28,56). Although we restricted study inclusion to entry prior to 2009 and delayed person-time accrual by 6 months after enrollment, selection bias remains a concern because of the various reasons for enrollment into the respective cohorts. Notably, cancer coverage began in 2012 under the James Zadroga 9/11 Health and Compensation Act, limiting the likelihood of selection into our study sample for cancer-related care (57). Lastly, incomplete case ascertainment is also a concern, because cancer linkages were conducted in 93% of the combined cohort and cases reported by Veterans Affairs hospitals were not included. We posit these missed cases likely bias our findings toward null.
In the largest post-9/11 cohort of rescue and recovery workers ever studied, although the overall cancer incidence was lower than expected compared with the reference population, higher-than-expected SIRs were observed in melanoma-skin, prostate, thyroid, and tonsil cancers. For the first time, we identified a statistically significant association of early arrival with increased cancer risk of all-cancer sites, prostate, and thyroid cancers in internal comparisons. The combined cohort includes a heterogenous group of rescue and recovery workers with various levels of WTC exposure, allowing for more precise estimations of cancer incidence and risk and the study of rare cancers. Given latency periods between environmental exposure and cancer incidence, future studies will further our understanding of the long-term health effects of 9/11. Finally, our findings demonstrate the value and need for long-term follow-up studies after environmental disasters.
Funding
This work was supported by the National Institute for Occupational Safety and Health at the Centers for Disease Control and Prevention (cooperative agreements U01OH011315, U01 OH011932, U01 OH011681, U01 OH011931, U01 OH011480, and U50/OH009739; and contracts 200-2011-39378, 200-2017-93325, and 200-2017-93326). This work was also supported by the Agency for Toxic Substances and Disease Registry at the Centers for Disease Control and Prevention (cooperative agreement U50/ATU272750); by the National Institutes of Health (grant number P30 CA013330); by the New York City Department of Health and Mental Hygiene; and by the New York State Department of Health.
Additionally, this work was supported in part by cooperative agreement (6NU58DP006309) awarded to the New York State Department of Health by the Centers for Disease Control and Prevention and by the National Cancer Institute at the National Institutes of Health (Contract 75N91018D00005, Task Order 75N91018F00001, grant numbers P30 CA013330 and HHSN261201800009I).
Notes
Role of the funders: The funders of the study had no role in design of the study, data linkage activities, analysis, interpretation, writing of the manuscript, and in the decision to publish the results.
Disclosures: The authors have no disclosures.
Author contributions: JL: Conceptualization, Data curation, Investigation, Formal Analysis, Investigation, Methodology, Validation, Visualization, Writing—original draft, Writing—review & editing. JY: Data curation, Formal Analysis, Methodology, Software, Validation, Writing—review & editing. BZQ: Conceptualization, Formal Analysis, Methodology, Software, Validation, Writing—review & editing. ET: Visualization, Writing—original draft, Writing—review & editing. DGG: Data curation, Formal Analysis, Investigation, Methodology, Validation, Visualization, Writing—review & editing. RZO: Conceptualization, Data curation, Investigation, Methodology, Project administration, Supervision, Writing—review & editing. JEC: Conceptualization, Funding acquisition, Project administration, Supervision, Writing—review & editing. RMB: Conceptualization, Funding acquisition, Investigation, Methodology, Writing—review & editing. MRF: Funding acquisition, Resources, Supervision, Writing—review & editing. ARK: Data curation, Investigation, Methodology, Writing—review & editing. MJS: Project administration, Resources, Supervision, Writing—review & editing. MZS: Data curation, Investigation, Supervision, Writing—review & editing. CRD: Data curation, Investigation, Project administration, Supervision, Writing—review & editing. ACT: Data curation, Funding acquisition, Investigation, Project administration, Supervision, Writing—review & editing. DK: Funding acquisition, Writing—review & editing. DJP: Conceptualization, Funding acquisition, Project administration, Resources, Writing—review & editing. PB: conceptualization, Funding acquisition, Project administration, Writing—review & editing. CBH: Conceptualization, Funding acquisition, Methodology, Project administration, Supervision, Writing—review & editing.
Acknowledgements: We thank the 13 state cancer registries for carrying out record linkages: Bureau of Cancer Epidemiology, NYS Department of Health (DOH); Arizona Cancer Registry, DOHS; California Cancer Registry, Department of Public Health (DPH); Connecticut Tumor Registry, CT DPH; Florida Cancer Registry, FL DOH; Massachusetts Cancer Registry, MA DPH; New Jersey State Cancer Registry, NJ DOH; Rutgers Cancer Institute of New Jersey; North Carolina Central Cancer Registry, State Center for Health Statistics; Ohio Cancer Incidence Surveillance System, OH DOH; Bureau of Health Statistics and Research, Pennsylvania DOH; Texas Cancer Registry, TX Department of State Health Services; Virginia Cancer Registry, VA DOH; and Washington State Cancer Registry, WA DOH.
There are additional acknowledgements specified by individual state cancer registry: “The collection of cancer incidence data used in this study was supported by the California Department of Public Health pursuant to California Health and Safety Code Section 103885; Centers for Disease Control and Prevention’s (CDC) National Program of Cancer Registries, under cooperative agreement 5NU58DP006344; the National Cancer Institute’s Surveillance, Epidemiology and End Results Program under contract HHSN261201800032I awarded to the University of California, San Francisco, contract HHSN261201800015I awarded to the University of Southern California, and contract HHSN261201800009I awarded to the Public Health Institute.”
“The Connecticut Department of Public Health Human Investigations Committee approved this research project, which used data obtained from the Connecticut Department of Public Health.”
“The Florida cancer incidence data used in this report were collected by the Florida Cancer Data System (FCDS), the statewide cancer registry funded by the Florida Department of Health (DOH) and the Centers for Disease Control and Prevention, National Program of Cancer Registries (CDC-NPCR).”
“Cancer incidence data used in these analyses were obtained from the Ohio Cancer Incidence Surveillance System (OCISS), Ohio Department of Health (ODH), a cancer registry partially supported by the National Program of Cancer Registries at the Centers for Disease Control and Prevention (CDC) through Cooperative Agreement Number NU58DP006284.”
“These data were supplied by the Bureau of Health Statistics & Registries, Pennsylvania Department of Health, Harrisburg, Pennsylvania.”
“Cancer incidence data have been provided by the Texas Cancer Registry, Cancer Epidemiology and Surveillance Branch, Texas Department of State Health Services, PO Box 149347, Austin, Texas, 78756.”
Disclaimers: The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of the Centers for Disease Control and Prevention—National Institute for Occupational Safety and Health.
There are additional disclaimers specified by individual state cancer registry: “The ideas and opinions expressed herein are those of the author(s) and do not necessarily reflect the opinions of the State of California, Department of Public Health, the National Cancer Institute, and the Centers for Disease Control and Prevention or their Contractors and Subcontractors.”
“The Connecticut Department of Public Health does not endorse or assume any responsibility for any analyses, interpretations or conclusions based on the data. The authors assume full responsibility for all such analyses, interpretations and conclusions.”
“The views expressed herein are solely those of the author(s) and not necessarily reflect those of the Florida Department of Health (DOH) or the Centers for Disease Control and Prevention, National Program of Cancer Registries (CDC-NPCR).”
“Use of these data does not imply that Ohio Department of Health (ODH) or the Centers for Disease Control and Prevention (CDC) agrees or disagrees with the analyses, interpretations or conclusions in this publication.”
“The Pennsylvania Department of Health specifically disclaims responsibility for any analyses, interpretations or conclusions.”
Data Availability
Data are available upon reasonable request from the corresponding author once permission is granted by the state cancer registries that supplied these data and the request is approved by the steering committee for the combined cohort in accordance with the official data sharing plan.
References
- 1. Lioy PJ, Weisel CP, Millette JR, et al. Characterization of the dust/smoke aerosol that settled east of the World Trade Center (WTC) in lower Manhattan after the collapse of the WTC 11 September 2001. Environ Health Perspect. 2002;110(7):703–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.CDC, NIOSH. World Trade Center chemicals of potential concern and selected other chemical agents; summary of cancer classifications by the National Toxicology Program and International Agency for Research on Cancer. 2012. https://www.cdc.gov/niosh/docs/2012-115/pdfs/2012-115.pdf. Accessed August 26, 2021.
- 3. Connick KD, Enright P, L Middendorf PJ, et al. First Periodic Review of Scientific and Medical Evidence Related to Cancer for the World Trade Center Health Program. Columbia, DC: National Institute for Occupational Safety and Health; 2011.
- 4. Landrigan PJ, Lioy PJ, Thurston G, et al. ; for the NIEHS World Trade Center Working Group. Health and environmental consequences of the world trade center disaster. Environ Health Perspect. 2004;112(6):731–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yip J, Webber MP, Zeig-Owens R, et al. FDNY and 9/11: clinical services and health outcomes in World Trade Center-exposed firefighters and EMS workers from 2001 to 2016. Am J Ind Med. 2016;59(9):695–708. [DOI] [PubMed] [Google Scholar]
- 6. Farfel M, DiGrande L, Brackbill R, et al. An overview of 9/11 experiences and respiratory and mental health conditions among World Trade Center Health Registry enrollees. J Urban Health. 2008;85(6):880–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dasaro CR, Holden WL, Berman KD, et al. Cohort profile: World Trade Center health program general responder cohort. Int J Epidemiol. 2017;46(2):e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zeig-Owens R, Webber MP, Hall CB, et al. Early assessment of cancer outcomes in New York City firefighters after the 9/11 attacks: an observational cohort study. Lancet. 2011;378(9794):898–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Li J, Cone JE, Kahn AR, et al. Association between World Trade Center exposure and excess cancer risk. JAMA. 2012;308(23):2479–2488. [DOI] [PubMed] [Google Scholar]
- 10. Solan S, Wallenstein S, Shapiro M, et al. Cancer incidence in world trade center rescue and recovery workers, 2001-2008. Environ Health Perspect. 2013;121(6):699–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Li J, Brackbill RM, Liao TS, et al. Ten-year cancer incidence in rescue/recovery workers and civilians exposed to the September 11, 2001 terrorist attacks on the World Trade Center. Am J Ind Med. 2016;59(9):709–721. [DOI] [PubMed] [Google Scholar]
- 12. Shapiro MZ, Wallenstein SR, Dasaro CR, et al. Cancer in general responders participating in World Trade Center Health Programs. JNCI Cancer Spectr. 2020;4(1):2003–2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Boffetta P, Zeig-Owens R, Wallenstein S, et al. Cancer in World Trade Center responders: findings from multiple cohorts and options for future study. Am J Ind Med. 2016;59(2):96–105. [DOI] [PubMed] [Google Scholar]
- 14. Brackbill RM, Kahn AR, Li J, et al. Combining three cohorts of World Trade Center rescue/recovery workers for assessing cancer incidence and mortality. Int J Environ Res Public Health. 2021;18:1386. https://doi.org/10.3390/ ijerph18041386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jack A, Percy C, Sobin L, et al. International Classification of Diseases for Oncology: ICD-O. Geneva, Switzerland: World Health Organization; 2000.
- 16.National Cancer Institute: Surveillance Epidemiology and End Results Program. Site Recode B ICD-O-3/WHO 2008 Definition. https://seer.cancer.gov/siterecode_b/icdo3_who2008/#content. Accessed August 26, 2021.
- 17.New York State Cancer Registry. Cancer Incidence and Mortality in New York State, 1976-2016.http://www.health.ny.gov/statistics/cancer/registry/. Accessed June 6, 2021.
- 18. Boscoe FP, Schymura MJ, Zhang X, et al. Heuristic algorithms for assigning Hispanic ethnicity. PLoS One. 2013;8(2):e55689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cox DR. Regression models and life‐tables. J R Stat Soc B (Methodol). 1972;34(2):187–202. [Google Scholar]
- 20. Kleinbaum DG, Klein M.. Survival Analysis. New York, NY: Springer; 2010. [Google Scholar]
- 21.Cancer IAfRo. Painting, Firefighting, and Shiftwork. Lyon, France: International Agency for Research on Cancer Press; 2010.
- 22. Aschebrook-Kilfoy B, Ward MH, Della Valle CT, et al. Occupation and thyroid cancer. Occup Environ Med. 2014;71(5):366–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fiore M, Oliveri Conti G, Caltabiano R, et al. Role of emerging environmental risk factors in thyroid cancer: a brief review. Int J Environ Res Public Health. 2019;16:1185. doi:10.3390/ijerph16071185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yang W, Omaye ST.. Air pollutants, oxidative stress and human health. Mutat Res. 2009;674(1-2):45–54. [DOI] [PubMed] [Google Scholar]
- 25. Lodovici M, Bigagli E.. Oxidative stress and air pollution exposure. J Toxicol. 2011;2011:487074. doi:10.1155/2011/487074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Xing M. Oxidative stress: a new risk factor for thyroid cancer. Endocr Relat Cancer. 2012;19(1):C7–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ameziane El Hassani R, Buffet C, Leboulleux S, et al. Oxidative stress in thyroid carcinomas: biological and clinical significance. Endocr Relat Cancer. 2019;26(3):R131–R143. [DOI] [PubMed] [Google Scholar]
- 28. Daniels RD, Kubale TL, Yiin JH, et al. Mortality and cancer incidence in a pooled cohort of US firefighters from San Francisco, Chicago and Philadelphia (1950-2009). Occup Environ Med. 2014;71(6):388–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mullins JK, Loeb S.. Environmental exposures and prostate cancer. Urol Oncol. 2012;30(2):216–219. [DOI] [PubMed] [Google Scholar]
- 30. Bostwick DG, Burke HB, Djakiew D, et al. Human prostate cancer risk factors. Cancer. 2004;101(10 suppl):2371–2490. [DOI] [PubMed] [Google Scholar]
- 31. Sritharan J, MacLeod J, Harris S, et al. Prostate cancer surveillance by occupation and industry: the Canadian Census Health and Environment Cohort (CanCHEC). Cancer Med. 2018;7(4):1468–1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sritharan J, MacLeod JS, McLeod CB, et al. Prostate cancer risk by occupation in the Occupational Disease Surveillance System (ODSS) in Ontario, Canada. Health Promot Chronic Dis Prev Can. 2019;39(5):178–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. LeMasters GK, Genaidy AM, Succop P, et al. Cancer risk among firefighters: a review and meta-analysis of 32 studies. J Occup Environ Med. 2006;48(11):1189–1202. [DOI] [PubMed] [Google Scholar]
- 34. Coussens LM, Werb Z.. Inflammation and cancer. Nature. 2002;420(6917):860–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Guarino V, Castellone MD, Avilla E, et al. Thyroid cancer and inflammation. Mol Cell Endocrinol. 2010;321(1):94–102. [DOI] [PubMed] [Google Scholar]
- 36. Allavena P, Garlanda C, Borrello MG, et al. Pathways connecting inflammation and cancer. Curr Opin Genet Dev. 2008;18(1):3–10. [DOI] [PubMed] [Google Scholar]
- 37. Savitz DA, Oxman RT, Metzger KB, et al. Epidemiologic research on man-made disasters: strategies and implications of cohort definition for World Trade Center worker and volunteer surveillance program. Mt Sinai J Med. 2008;75(2):77–87. [DOI] [PubMed] [Google Scholar]
- 38. Tomasetti C, Vogelstein B.. Variation in cancer risk among tissues can be explained by the number of stem cell divisions. Science. 2015;347(6217):78–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kirkeleit J, Riise T, Bjorge T, et al. The healthy worker effect in cancer incidence studies. Am J Epidemiol. 2013;177(11):1218–1224. [DOI] [PubMed] [Google Scholar]
- 40. Gu JK, Charles LE, Burchfiel CM, et al. Cancer incidence among police officers in a U.S. northeast region: 1976-2006. Int J Emerg Ment Health. 2011;13(4):279–289. [PMC free article] [PubMed] [Google Scholar]
- 41. Yung J, Li J, Jordan HT, et al. Prevalence of and factors associated with mammography and prostate-specific antigen screening among World Trade Center Health Registry enrollees, 2015-2016. Prev Med Rep. 2018;11:81–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Moir W, Zeig-Owens R, Daniels RD, et al. Post-9/11 cancer incidence in World Trade Center-exposed New York City firefighters as compared to a pooled cohort of firefighters from San Francisco, Chicago and Philadelphia (9/11/2001-2009). Am J Ind Med. 2016;59(9):722–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zeig-Owens R. Diagnostic Procedures Using Radiation and Risk of Thyroid Cancer: Causal Association or Detection Bias? An Examination of Population Cancer Trends and Data from the NYC Fire Department. 2015. https://academicworks.cuny.edu/cgi/viewcontent.cgi?article=2213&context=gc_etds. Accessed August 26, 2021.
- 44. Colbeth HL, Genere N, Hall CB, et al. Evaluation of medical surveillance and incidence of post-September 11, 2001, thyroid cancer in World Trade Center-exposed firefighters and emergency medical service workers. JAMA Intern Med. 2020;180(6):888–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. van Gerwen MAG, Tuminello S, Riggins GJ, et al. Molecular study of thyroid cancer in world trade center responders. Int J Environ Res Public Health. 2019;16:1600. doi:10.3390/ijerph16091600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Tuminello S, van Gerwen MAG, Genden E, et al. Increased incidence of thyroid cancer among World Trade Center first responders: a descriptive epidemiological assessment. Int J Environ Res Public Health. 2019;16:1258. doi:10.3390/ijerph16071258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.New York State Department of Health. BRFSS Brief: Cigarette Smoking among New York State Adults. 2006. https://www.health.ny.gov/statistics/brfss/reports/docs/brfssbrief_smoking_0707.pdf. Accessed June 6, 2021.
- 48. Liu B, van Gerwen M, Bonassi S, et al. ; for the International Association for the Study of Lung Cancer Mesothelioma Task Force. Epidemiology of environmental exposure and malignant mesothelioma. J Thorac Oncol. 2017;12(7):1031–1045. [DOI] [PubMed] [Google Scholar]
- 49. Russi M, Cone J, Malignancies of the respiratory tract and pleura. In: Rosenstock L, Cullen M, eds. Textbook of Clinical Occupational and Environmental Medicine. 2nd ed. Philadelphia, PA: WB Saunders; 2004:543–555. [Google Scholar]
- 50. Paget-Bailly S, Cyr D, Luce D.. Occupational exposures to asbestos, polycyclic aromatic hydrocarbons and solvents, and cancers of the oral cavity and pharynx: a quantitative literature review. Int Arch Occup Environ Health. 2012;85(4):341–351. [DOI] [PubMed] [Google Scholar]
- 51. Rettig EM, Zaidi M, Faraji F, et al. Oropharyngeal cancer is no longer a disease of younger patients and the prognostic advantage of human papillomavirus is attenuated among older patients: analysis of the National Cancer Database. Oral Oncol. 2018;83:147–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Smith EM, Rubenstein LM, Haugen TH, et al. Tobacco and alcohol use increases the risk of both HPV-associated and HPV-independent head and neck cancers. Cancer Causes Control. 2010;21(9):1369–1378. [DOI] [PubMed] [Google Scholar]
- 53. Geyh AS, Chillrud S, Williams DL, et al. Assessing truck driver exposure at the World Trade Center disaster site: personal and area monitoring for particulate matter and volatile organic compounds during October 2001 and April 2002. J Occup Environ Hyg. 2005;2(3):179–193. [DOI] [PubMed] [Google Scholar]
- 54. Lioy PJ, Georgopoulos P.. The anatomy of the exposures that occurred around the World Trade Center site: 9/11 and beyond. Ann N Y Acad Sci. 2006;1076(1):54–79. [DOI] [PubMed] [Google Scholar]
- 55. Woskie SR, Kim H, Freund A, et al. World Trade Center disaster: assessment of responder occupations, work locations, and job tasks. Am J Ind Med. 2011;54(9):681–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Sritharan J, Pahwa M, Demers PA, et al. Prostate cancer in firefighting and police work: a systematic review and meta-analysis of epidemiologic studies. Environ Health. 2017;16(1):124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Centers for Disease Control and Prevention. World Trade Center Health Program Covered Conditions. https://www.cdc.gov/wtc/conditions.html. Updated August 2, 2021. Accessed August 26, 2021.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available upon reasonable request from the corresponding author once permission is granted by the state cancer registries that supplied these data and the request is approved by the steering committee for the combined cohort in accordance with the official data sharing plan.